Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Formation and dissociation of synthetic hetero-double-helix complex in aqueous solutions: significant effect of water content on dynamics of structural change†
Tsukasa Sawato,
Ryosuke Yuzawa,
Higashi Kobayashi,
Nozomi Saito and
Masahiko Yamaguchi *
*
Department of Organic Chemistry, Graduate School of Pharmaceutical Sciences, Tohoku University, Aoba, Sendai 980-8578, Japan. E-mail: yama@m.tohoku.ac.jp; Fax: +81-22-795-6811
First published on 18th September 2019
Abstract
A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of the ethynylhelicene pseudoenantiomers (M)-tetramer and (P)-pentamer, which possess hydrophilic terminal tri(ethyleneglycol) (TEG) groups, changes their structures in the water–THF (10 μM) solvent system between dissociated random-coils and an associated hetero-double-helix upon heating and cooling. A small change in water content between 30 and 33% significantly affects the dynamics of structural changes. At 30% water content, heating to 60 °C causes rapid formation of random-coil and cooling to 10 °C causes the rapid formation of hetero-double-helix, accompanied by repeated changes in Δε at 369 nm between 0 and −2000 cm−1 M−1. Heating and cooling experiments at constant rates between 60 and 10 °C resulted in sigmoidal curves in Δε/temperature profiles, which indicate rapid structural changes. Different phenomena occurred at 33% water content. Heating to 60 °C and cooling to 0 °C initially induced changes in Δε between 0 and −2000 cm−1 M−1, and repeated cycles gradually reduced the range between 0 and −500 cm−1 M−1. Heating and cooling experiments at constant rates between 60 and 10 °C caused small changes in Δε, and repeated cycles at 10 °C gradually increased Δε to −500 cm−1 M−1. These phenomena involved rapid changes in molecular structure and slow structural changes in the water–THF solvent system. The sharp switching of the dynamics of structural changes at water content between 30 and 33% indicated discontinuous structural changes in the hydration of TEG and/or in water clusters in the vicinity of oligomer molecules.
1 mixture of the ethynylhelicene pseudoenantiomers (M)-tetramer and (P)-pentamer, which possess hydrophilic terminal tri(ethyleneglycol) (TEG) groups, changes their structures in the water–THF (10 μM) solvent system between dissociated random-coils and an associated hetero-double-helix upon heating and cooling. A small change in water content between 30 and 33% significantly affects the dynamics of structural changes. At 30% water content, heating to 60 °C causes rapid formation of random-coil and cooling to 10 °C causes the rapid formation of hetero-double-helix, accompanied by repeated changes in Δε at 369 nm between 0 and −2000 cm−1 M−1. Heating and cooling experiments at constant rates between 60 and 10 °C resulted in sigmoidal curves in Δε/temperature profiles, which indicate rapid structural changes. Different phenomena occurred at 33% water content. Heating to 60 °C and cooling to 0 °C initially induced changes in Δε between 0 and −2000 cm−1 M−1, and repeated cycles gradually reduced the range between 0 and −500 cm−1 M−1. Heating and cooling experiments at constant rates between 60 and 10 °C caused small changes in Δε, and repeated cycles at 10 °C gradually increased Δε to −500 cm−1 M−1. These phenomena involved rapid changes in molecular structure and slow structural changes in the water–THF solvent system. The sharp switching of the dynamics of structural changes at water content between 30 and 33% indicated discontinuous structural changes in the hydration of TEG and/or in water clusters in the vicinity of oligomer molecules.
Introduction
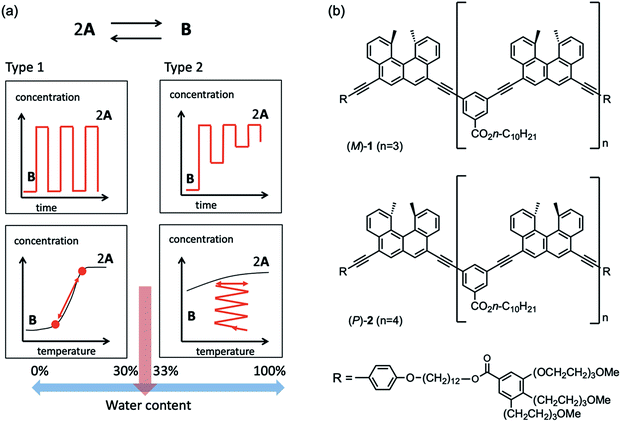
The hetero-double-helix, derived from linear molecules with two different structures, plays pivotal roles in biological double-stranded DNA, which dissociates and associates in water for gene regulation and expression to occur.1,2 Therefore, the development of hetero-double-helix from synthetic molecules in aqueous solution is attractive, because it promotes the understanding and control of such biological events as well as provides novel functional materials.3 Although water-soluble synthetic homo-double-helices have recently been developed4–8 as dimeric aggregates derived from linear molecules with the same structure, the synthesis and dynamic properties of the water-soluble hetero-double-helix have not yet been studied.To develop a water-soluble synthetic hetero-double-helix, the presence of hydrophilic groups is essential. We previously reported that the ethynylhelicene (M)-tetramer (M)-1 with tri(ethyleneglycol) (TEG) groups formed homo-double-helix in mixed solutions of water and organic solvents.9,10 Association and dissociation showed an inverse thermoresponse, in which heating induced association and cooling induced dissociation. Inverse thermoresponses I and II were observed depending on the organic cosolvents used. These unusual thermoresponses were ascribed to the hydration/dehydration of the TEG groups and/or structural changes of water clusters in the vicinity of the oligomer molecules.
It was also found that mixtures of the ethynylhelicene pseudoenantiomers (M)-oligomer and (P)-oligomer with different helicene numbers formed hetero-double-helix in organic solvents.10–12 In this study, a water-soluble hetero-double-helix was developed employing a mixture of pseudoenantiomers ethynylhelicene (M)-tetramer (M)-1 and (P)-pentamer (P)-2, which possess terminal hydrophilic TEG groups (Fig. 1b).
The (M)-1/(P)-2 mixture formed a hetero-double-helix in water-THF solvent system, which switched the dynamics of structural changes with the change in water content from 30 to 33% (Fig. 1a). At 30% water content, repeated heating to 60 °C and cooling to 10 °C changed Δε at 369 nm between 0 and −2000 cm−1 M−1, which rapidly occurred in response to temperature changes. Constant-rate heating and cooling resulted in sigmoidal curves. At 33% water content, heating to 60 °C and cooling to 10 °C initially changed Δε between −2000 and 0 cm−1 M−1, and repeated heating/cooling cycles gradually reduced the range between −500 and 0 cm−1 M−1. Heating and cooling at a constant rate resulted in smaller changes in Δε. These phenomena suggest the involvement of rapid changes in molecular structure and slow structural changes in the water–THF solvent system. The sharp switching of dynamics of structural changes caused by the small change in water content indicates a discontinuous change in the hydration/dehydration of TEG groups and/or water clusters in the vicinity of the oligomers.
Results and discussion
Experiments in water–THF solvent system with 30% water
Hetero-double-helix formation by the (M)-1/(P)-2 mixture was examined using 10 μM in water-THF solvent system with 30% water, which is hereafter referred as 30% water–THF in this paper. The solution was prepared by mixing two solutions of (M)-1 and (P)-2 in 30% water–THF at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Heating the resulting solution to 60 °C produced dissociated random-coil 2A exhibiting a weak Cotton effect, as shown by circular dichroism (CD) spectroscopy (Fig. 2a and S1†). Cooling the solution to 10 °C showed a strong negative Cotton effect at 369 nm with Δε reaching −2000 cm−1 M−1, and the Cotton effect disappeared upon heating the solution to 60 °C. UV-vis spectra showed slightly reduced intensity upon cooling to 10 °C (Fig. 2b and S1†). These results were consistent with the structural changes between 2A and hetero-double-helix B.9 The experiment was also conducted using a 100 μM solution, and CD spectra obtained at 10 °C were very similar to those of the 10 μM solution (Fig. S7†), which indicated that the CD spectrum with Δε reaching −1500 cm−1 M−1 showed the equilibrium shifted to B and essentially contained no 2A. Hetero-double-helix formation by the (M)-1/(P)-2 mixture in water–THF solvent system occurred at μM-order concentrations that was much more extensive than that in organic solvents at mM-order concentrations.11–13 Dynamic light scattering (DLS) analysis (30% water–THF, 10 μM) at 10 and 60 °C showed the formation of particles with average diameters smaller than 10 nm (Fig. S2†). Consequently, no Tyndall effect was observed. Therefore, the thermoresponse of the (M)-1/(P)-2 mixture observed by CD spectroscopy is derived from molecules dispersed in solution that do not form larger aggregates.
1 ratio. Heating the resulting solution to 60 °C produced dissociated random-coil 2A exhibiting a weak Cotton effect, as shown by circular dichroism (CD) spectroscopy (Fig. 2a and S1†). Cooling the solution to 10 °C showed a strong negative Cotton effect at 369 nm with Δε reaching −2000 cm−1 M−1, and the Cotton effect disappeared upon heating the solution to 60 °C. UV-vis spectra showed slightly reduced intensity upon cooling to 10 °C (Fig. 2b and S1†). These results were consistent with the structural changes between 2A and hetero-double-helix B.9 The experiment was also conducted using a 100 μM solution, and CD spectra obtained at 10 °C were very similar to those of the 10 μM solution (Fig. S7†), which indicated that the CD spectrum with Δε reaching −1500 cm−1 M−1 showed the equilibrium shifted to B and essentially contained no 2A. Hetero-double-helix formation by the (M)-1/(P)-2 mixture in water–THF solvent system occurred at μM-order concentrations that was much more extensive than that in organic solvents at mM-order concentrations.11–13 Dynamic light scattering (DLS) analysis (30% water–THF, 10 μM) at 10 and 60 °C showed the formation of particles with average diameters smaller than 10 nm (Fig. S2†). Consequently, no Tyndall effect was observed. Therefore, the thermoresponse of the (M)-1/(P)-2 mixture observed by CD spectroscopy is derived from molecules dispersed in solution that do not form larger aggregates.
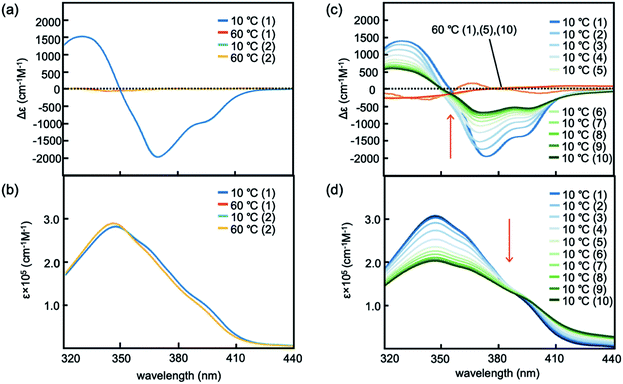 | ||
Fig. 2 (a) CD and (b) UV-vis spectra of (M)-1/(P)-2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in 30% water–THF (10 μM) at 60 and 10 °C. (c) CD and (d) UV-vis spectra of (M)-1/(P)-2 (1 1) in 30% water–THF (10 μM) at 60 and 10 °C. (c) CD and (d) UV-vis spectra of (M)-1/(P)-2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in 33% water–THF (10 μM) at 60 and 10 °C. The numbers of heating/cooling cycles is shown in parentheses. See Fig. S8a† for details of the spectra in (c) and (d). 1) in 33% water–THF (10 μM) at 60 and 10 °C. The numbers of heating/cooling cycles is shown in parentheses. See Fig. S8a† for details of the spectra in (c) and (d). | ||
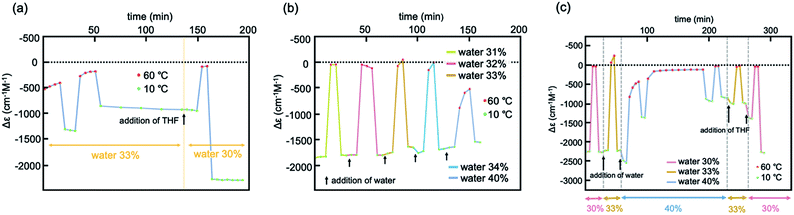
Experiments of repeated and periodic heating to 60 °C and cooling to 10 °C were conducted, and structural changes were monitored on the basis of Δε. Sharp structural changes appeared between 0 and −2000 cm−1 M−1 as shown by the rectangular shapes of Δε/time profiles (Fig. 3a and S6b†), which indicated rapid structural changes between molecules of 2A and B. Other experiments with nonperiodic temperature changes also yielded rectangular shapes of profiles (Fig. S6a†).
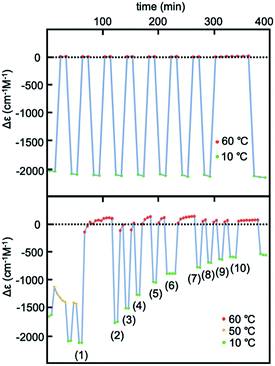 | ||
Fig. 3 Experiments of repeated heating and cooling of (M)-1/(P)-2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) between 60 and 10 °C for Δε at 369 nm in (a) 30% water–THF (10 μM) and for Δε at 376 nm in (b) 33% water–THF (10 μM). 1) between 60 and 10 °C for Δε at 369 nm in (a) 30% water–THF (10 μM) and for Δε at 376 nm in (b) 33% water–THF (10 μM). | ||
Heating and cooling experiments at constant rate were conducted between 60 and 10 °C at a constant rate of 1 °C min−1 in 30% water–THF (Fig. 4a and S16†). Δε at 375 nm decreased from 0 cm−1 M−1 at 60 °C, and reached −2000 cm−1 M−1 at 10 °C. Sigmoidal curves were obtained during heating and cooling with a small thermal hysteresis, which is consistent with the observed rapid structural changes between molecules of 2A and B (Fig. 3a). These phenomena in 30% water–THF are termed type-1 behavior in this paper.
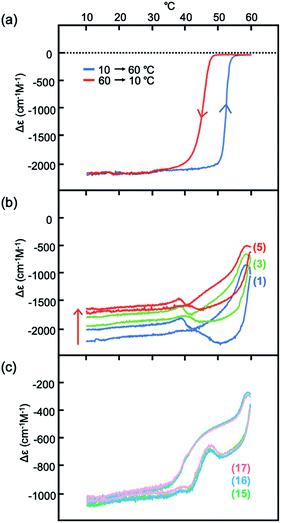 | ||
Fig. 4 Δε (375 nm)/temperature profiles from experiments of heating and cooling of (M)-1/(P)-2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at a constant rate of 1 °C min−1 between 10 and 60 °C, shown by (a) in 30% water–THF (10 μM); (b) in 33% water–THF (10 μM) starting from −2000 cm−1 M−1; (c) enlarged view of (b). The number of heating/cooling cycles is shown in parentheses in (b) and (c). See Fig. S17† for details. 1) at a constant rate of 1 °C min−1 between 10 and 60 °C, shown by (a) in 30% water–THF (10 μM); (b) in 33% water–THF (10 μM) starting from −2000 cm−1 M−1; (c) enlarged view of (b). The number of heating/cooling cycles is shown in parentheses in (b) and (c). See Fig. S17† for details. | ||
Experiments in water–THF solvent system with 33% water
When water content was increased to 33%, different phenomena were observed. A (M)-1/(P)-2 mixture in 33% water–THF (10 μM) was prepared by mixing two solutions of (M)-1 and (P)-2 in 33% water–THF (10 μM) at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio at room temperature. The resulting mixture was heated to 50 °C and cooled to 10 °C, during which a strong negative Cotton effect was observed at 376 nm with Δε reaching −2100 cm−1 M−1 (Fig. S8a†). When heated to 50 °C, Δε increased to −1500 cm−1 M−1. Then, the solution was heated to 60 °C and cooled to 10 °C, during which Δε changed between 0 and −1700 cm−1 M−1. Repeated heating/cooling cycles gradually decreased the Δε range, and Δε at 10 °C reached −500 cm−1 M−1 after 10 cycles (Fig. 2c). UV-vis spectra also showed changes in intensity as a result of repeated heating to 60 °C and cooling to 10 °C (Fig. 2d). The structure of the (M)-1/(P)-2 mixture in 33% water–THF (10 μM) was examined by dynamic light scattering (DLS) analysis at 10, 50, and 60 °C, which showed the formation of particles smaller than 10 nm (Fig. S3†). No Tyndall effect was observed during the process, indicating that the structural changes were molecular events in solution.
1 ratio at room temperature. The resulting mixture was heated to 50 °C and cooled to 10 °C, during which a strong negative Cotton effect was observed at 376 nm with Δε reaching −2100 cm−1 M−1 (Fig. S8a†). When heated to 50 °C, Δε increased to −1500 cm−1 M−1. Then, the solution was heated to 60 °C and cooled to 10 °C, during which Δε changed between 0 and −1700 cm−1 M−1. Repeated heating/cooling cycles gradually decreased the Δε range, and Δε at 10 °C reached −500 cm−1 M−1 after 10 cycles (Fig. 2c). UV-vis spectra also showed changes in intensity as a result of repeated heating to 60 °C and cooling to 10 °C (Fig. 2d). The structure of the (M)-1/(P)-2 mixture in 33% water–THF (10 μM) was examined by dynamic light scattering (DLS) analysis at 10, 50, and 60 °C, which showed the formation of particles smaller than 10 nm (Fig. S3†). No Tyndall effect was observed during the process, indicating that the structural changes were molecular events in solution.
Experiments of repeated heating and cooling between 50 and 10 °C showed Δε (376 nm)/time profiles indicating Δε changes between −1500 and −2200 cm−1 M−1 (Fig. 3b and S8a†). The experiments between 60 and 10 °C showed Δε changes between 0 and −1700 cm−1 M−1, which on repeated heating/cooling cycles between 60 and 10 °C gradually decreased the Δε range between 0 and −500 cm−1 M−1 after 10 cycles, and reached a steady state. Rectangular shapes of Δε/time profiles with slight deviations showed that rapid structural changes occurred between molecules of 2A and B. The gradual decrease in the Δε range with repeated heating/cooling cycles suggests a structural change of the water–THF solvent system.
Constant-rate heating and cooling experiments in 33% water–THF also exhibited dynamics of structural changes different from those in 30% water–THF. A (M)-1/(P)-2 mixture in 33% water–THF (10 μM) with Δε reaching −2000 cm−1 M−1 was obtained at 10 °C; the solution was then heated to 60 °C and cooled to 10 °C at a rate of 1 °C min−1 (Fig. 4b and S17†). Upon heating, Δε not change up to 45 °C, and then increased to −1000 cm−1 M−1 at 60 °C. Upon cooling, Δε decreased to −2000 cm−1 M−1 at 40 °C, and remained constant until 10 °C. The small changes in Δε in 33% water–THF were compared with large changes in 30% water–THF (Fig. 4a). Repeated heating and cooling experiments showed small changes for every cycle, and Δε at 10 °C gradually increased, reaching −1600 cm−1 M−1 after 5 cycles and −1000 cm−1 M−1 after 17 cycles (Fig. 4c). A small thermal hysteresis was observed for every cycle between 40 and 60 °C. The results of the constant-rate heating and cooling experiments in 33% water–THF indicated slow responses of Δε to temperature changes. The phenomena in 33% water–THF are referred to as type-2 behavior in the following discussions. Sharp switching in dynamics of structural changes appeared in the (M)-1/(P)-2 mixture for small changes in water content from 30% for type-1 behavior to 33% for type-2 behavior.
The effect of substrate concentration was examined in 33% water–THF. The structural changes between −1000 and 0 cm−1 M−1 appeared at 5 and 7.5 μM as well as 10 μM, which are the type-2 behavior (Fig. S9 and S10†).
Experiments were conducted in water–THF solvent system with different water contents. In 25% water–THF, heating to 60 °C and cooling to 10 °C showed structural changes between Δε 0 and –2300 cm−1 M−1, which is the type-1 behavior (Fig. S5 and S15†). In 40% water–THF, heating to 50 °C and cooling to 10 °C showed structural changes between Δε −1200 and −1700 cm−1 M−1, which changed between −200 and −1200 cm−1 M−1 upon heating to 60 °C and cooling to 10 °C; these changes are the type-2 behavior (Fig. S11†). Constant-rate temperature change experiments showed similar Δε/temperature profiles of 40% water–THF to those of 33% water–THF (Fig. S18†). An important role of water in the type-1 and type-2 behaviors was confirmed by experiments in THF without water, in which dissociated 2A persisted without formation of B upon heating and cooling (Fig. S4†). These results indicate a sharp switching in dynamics of the structural changes of the (M)-1/(P)-2 mixture at water contents between 30 and 33% owing to the type-1 and -2 behaviors.
Experiments involving changes in water content
In the above experiments, the (M)-1/(P)-2 mixture in 30% water–THF (10 μM) was prepared by mixing two solutions of (M)-1 and (P)-2 in 30% water–THF. Another procedure was examined, in which the water content was changed from 33% to 30% in a single vessel by adding THF to 33% water–THF. A (M)-1/(P)-2 mixture in 33% water–THF (10 μM) was prepared by mixing two solutions of (M)-1 and (P)-2 in 33% water–THF, and the resulting solution showed structural changes between −300 and −1300 cm−1 M−1 upon heating to 60 °C and cooling to 10 °C, which is the type-2 behavior (Fig. 5a and S12†). THF was added to prepare a 30% water–THF solution, and the resulting solution showed structural changes between −2500 and 0 cm−1 M−1, which is the type-1 behavior. Rectangular shapes of structural changes in Δε/time profiles are consistent with rapid structural changes between molecules of 2A and B. These results confirmed the sharp switching of dynamics of the structural changes of the (M)-1/(P)-2 mixture caused by changing the water content from 33 to 30%.A reverse experiment, in which 30% water–THF (10 μM) was changed to 33% water–THF in a single vessel by adding water, showed different phenomena. A (M)-1/(P)-2 mixture in 30% water–THF was prepared by mixing two solutions of (M)-1 and (P)-2 in 30% water–THF; this showed structural changes between −1800 and 0 cm−1 M−1 upon cooling to 10 °C and heating to 60 °C, as the type-1 behavior (Fig. 5b and S13†). Water was added to obtain 31% water–THF solution, which also showed structural changes between −1800 and 0 cm−1 M−1 (type-1 behavior). The type-1 behavior also appeared in 32, 33, and 34% water–THF upon addition of portions of water. Simply increasing of the water content from 30% to 33% did not result in a switch from the type-1 to type-2 behavior. Rectangular shapes associated with structural changes appearing in Δε/time profiles are consistent with rapid structural changes of molecules of (M)-1 and (P)-2.
It was observed that the type-2 behavior appeared as a result of addition of THF to 40% water–THF solution (Fig. 5c and S14†). Water was added to (M)-1/(P)-2 mixture in 30% water–THF to prepare 40% water–THF, which showed structural changes between −1000 and 0 cm−1 M−1 (type-2 behavior). THF was added to prepare 33% water–THF, and the structural changes between −1000 and 0 cm−1 M−1 was observed, which are the type-2 behavior. To switch from the type-1 to type-2 behavior, it is essential to employ complex procedures involving the addition of water to 30% water–THF to prepare 40% water–THF and the addition of THF to prepare 33% water–THF. The phenomenon is consistent with slow structural changes in the water–THF solvent system as a result of changing the water content from 30 to 33%.
Mechanistic model
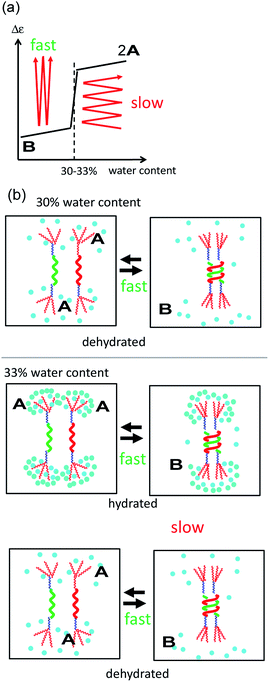
A mechanism underlying the different dynamics of structural changes between 2A and B in 30 and 33% water–THF defined as the type-1 and type-2 behaviors, respectively, is proposed, in which the dynamics of structural changes between molecules of 2A and B and the water–THF solvent system were separately considered (Fig. 6). Structural changes of molecules of 2A and B are rapid both in 30% and 33% water–THF solutions (Fig. 6b, black arrows), which are shown by rectangular shapes and small thermal hysteresis (Fig. 3 and 4). The dynamics of slow structural changes of 33% water–THF was ascribed to the slow structural changes of the solvent system. In 30% water–THF, the equilibrium state at 10 °C is close to Δε −2000 cm−1 M−1 and at 0 cm−1 M−1 at 60 °C, and the structural changes are fast (Fig. 6a). In 33% water–THF, the Δε range gradually narrowed after repeated heating/cooling cycles from 0 and −2000 cm−1 M−1 to 0 and −500 cm−1 M−1, which can be ascribed to the slow structural changes of the 33% water–THF (Fig. 6b). Repeated heating and cooling is required to approach the equilibrium state of in the 33% water–THF. The equilibrium state at 10 °C may be close to −500 cm−1 M−1. Thus, structural changes of molecules of 2A and B are rapid in 30 and 33% water–THF, and structural changes of the 33% water–THF solvent system are slow. Such sharp switching of structural changes of (M)-1/(P)-2 mixtures can be used to sense subtle environmental changes.The change in the structure of the solvent system can be described by the changes in the hydrated/dehydrated state of the TEG groups and/or by the changes in the structure of water clusters. In water–THF solvent system with water content below 30%, hetero-double-helix formation is rapid in response to temperature changes, because the TEG groups are less hydrated. In water–THF solvent system with water content above 33%, interconversion between molecules of 2A and B is rapid, and the hydration–dehydration of TEG groups becomes slow. Alternatively, the structure of water clusters in the vicinity of the molecules of B changes depending on the water content, which slowly responds to temperature changes.
Conclusions
The nature of homo- and hetero-double-helix derived from TEG-containing ethynylhelicene oligomers was compared in water–THF solvent system at 10 μM. A notable difference is the ordinary thermoresponse of hetero-double-helix as observed in this study and the inverse thermoresponses of homo-double-helix as described previously.9,10 It is considered that this difference may be derived from the hydration/dehydration structures of the TEG groups and/or water cluster structures in the vicinity of oligomer molecules. The effect of water content on structural changes of homo- and hetero-double-helix complexes in this study is also notable. Between 30 and 33% water contents, the interconversion between 2A and B showed significant switching in dynamics of the structural changes, as described by the type-1 and type-2 behaviors. It was previously observed that a homo-double-helix formed by (P)-1 showed significant switching in dynamics of structural changes at water contents below and above 30% in water–THF solvent system, which switched from ordinary thermoresponse to inverse thermoresponse II.10 Thus, the nature of structural changes derived from hydration/dehydration of TEG groups and/or water clusters in the vicinity of the oligomer molecules appears to change at water contents of approximately 30%.Mixed solvent systems of water and THF have attracted interest, and the dependence of their thermodynamic properties as a function of water content has been studied. Changes in the properties of water–THF solvent systems appeared at 70% water content, as shown by viscosity, X-ray scattering, molecular aggregation, and fluorescence analyses.14–19 In contrast, a change at 30% water content was observed in this study and in the inverse thermoresponse II,10 which can partly be due to the nature of interactions between TEG groups and water clusters.
To summarize, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of the ethynylhelicene pseudoenantiomers, (M)-tetramer and (P)-pentamer, both of which possess hydrophilic terminal TEG groups, formed hetero-double-helix and random-coils in water–THF solvent system at 10 μM. A small change in water content from 30 to 33% significantly affected dynamics of the structural changes. In 30% water–THF, heating to 60 °C caused the dissociation of hetero-double-helix to random-coils, and cooling to 10 °C caused the formation of hetero-double-helix, which rapidly and repeatedly changed Δε between 0 and −2000 cm−1 M−1. Constant-rate heating and cooling experiment showed rapid changes in Δε. In 33% water–THF, heating to 60 °C and cooling to 10 °C showed changes in Δε between 0 and −2000 cm−1 M−1, and repeated heating and cooling experiments gradually widened the Δε range to 0 and −500 cm−1 M−1. Constant-rate heating and cooling experiments showed small changes in Δε, and Δε at 10 °C gradually increased. The dynamics in the structural changes indicated slow changes of the water–THF solvent system upon heating and cooling. Sharp switching of reaction mechanism between 30 and 33% water contents suggests discontinuous structural changes in TEG hydration/dehydration and/or water clusters in the vicinity of the oligomers in the water–THF solvent system. Water is a complex molecular system,20 the structure of which sensitively and significantly affects molecules depending on the structures of solutes and the water content in mixed solvent systems.
1 mixture of the ethynylhelicene pseudoenantiomers, (M)-tetramer and (P)-pentamer, both of which possess hydrophilic terminal TEG groups, formed hetero-double-helix and random-coils in water–THF solvent system at 10 μM. A small change in water content from 30 to 33% significantly affected dynamics of the structural changes. In 30% water–THF, heating to 60 °C caused the dissociation of hetero-double-helix to random-coils, and cooling to 10 °C caused the formation of hetero-double-helix, which rapidly and repeatedly changed Δε between 0 and −2000 cm−1 M−1. Constant-rate heating and cooling experiment showed rapid changes in Δε. In 33% water–THF, heating to 60 °C and cooling to 10 °C showed changes in Δε between 0 and −2000 cm−1 M−1, and repeated heating and cooling experiments gradually widened the Δε range to 0 and −500 cm−1 M−1. Constant-rate heating and cooling experiments showed small changes in Δε, and Δε at 10 °C gradually increased. The dynamics in the structural changes indicated slow changes of the water–THF solvent system upon heating and cooling. Sharp switching of reaction mechanism between 30 and 33% water contents suggests discontinuous structural changes in TEG hydration/dehydration and/or water clusters in the vicinity of the oligomers in the water–THF solvent system. Water is a complex molecular system,20 the structure of which sensitively and significantly affects molecules depending on the structures of solutes and the water content in mixed solvent systems.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by Grants-in-Aid for Scientific Research (No. 17H03050 and 17H08203) from the Japan Society for the Promotion of Science (JSPS). H. K. thanks the JSPS for Research Fellowship for Young Scientists (No. 17J02496).Notes and references
- D. D Voet and J. G. Voet, Biochemistry, Wiley, New Jersey, 4th edn, 2011 Search PubMed.
- H. Lodish, A. Berk, C. A. Kaiser, M. Krieger, M. P. Scott, A. Bretscher, H. Ploegh, and P. Matsudaira, Molecular Cell Biology, W. H. Freeman and Company, New York, 6th edn, 2008 Search PubMed.
- E. Yashima, N. Ousaka, D. Taura, K. Shimomura, T. Ikai and K. Maeda, Chem. Rev., 2016, 116, 13752–13990 CrossRef CAS PubMed.
- X. de Hatten, D. Asil, R. H. Friend and J. R. Nitschke, J. Am. Chem. Soc., 2012, 134, 19170–19178 CrossRef CAS PubMed.
- H. Goto, H. Katagiri, Y. Furusho and E. Yashima, J. Am. Chem. Soc., 2006, 126, 7176–7178 CrossRef PubMed.
- J. Shang, Q. Gan, S. J. Dawson, F. Rosu, H. Jiang, Y. Ferrand and I. Huc, Org. Lett., 2014, 16, 4992–4995 CrossRef CAS PubMed.
- H. Goto, Y. Furusho and E. Yashima, J. Am. Chem. Soc., 2007, 129, 9168–9174 CrossRef CAS PubMed.
- T. Ben, Y. Furusho, H. Goto, K. Miwa and E. Yashima, Org. Biomol. Chem., 2009, 7, 2509–2512 RSC.
- N. Saito, H. Kobayashi and M. Yamaguchi, Chem. Sci., 2016, 7, 3574–3580 RSC.
- N. Saito, H. Kobayashi and M. Yamaguchi, Tetrahedron, 2017, 73, 6047–6051 CrossRef CAS.
- M. Shigeno, Y. Kushida and M. Yamaguchi, ChemPhysChem, 2015, 16, 2076–2083 CrossRef CAS PubMed.
- M. Shigeno, Y. Kushida and M. Yamaguchi, Chem. Commun., 2016, 52, 4955–4970 RSC.
- N. Saito and M. Yamaguchi, Molecules, 2018, 23, 277–311 CrossRef.
- T. Takamuku, A. Nakamizo, M. Tabata, K. Yoshida, T. Yamaguchi and T. Otomo, J. Mol. Liq., 2003, 103–104, 143–159 CrossRef CAS.
- J. N. Nayak, M. I. Aralaguppi, B. V. K. Naidu and T. M. Aminabhavi, J. Chem. Eng. Data, 2004, 49, 468–474 CrossRef CAS.
- M. Katayama and K. Ozutsumi, J. Solution Chem., 2008, 37, 841–856 CrossRef CAS.
- X. Feng, B. Tong, J. Shen, J. Shi, T. Han, L. Chen, J. Zhi, P. Lu, Y. Ma and Y. Dong, J. Phys. Chem., 2010, 114, 16731–16736 CrossRef CAS PubMed.
- Y. Marcus, J. Mol. Liq., 2012, 166, 62–66 CrossRef CAS.
- S. Indra, B. Guchhait and R. Biswas, J. Chem. Phys., 2016, 144, 124506 CrossRef PubMed.
- E. Brini, C. J. Fennell, M. Fernandez-Serra, B. Hribar-Lee, M. Luksic and K. A. Dill, Chem. Rev., 2017, 117, 12385–12414 CrossRef CAS . Also see, E. E. Meyer, K. J. Rosenberg and J. Israelachvili, Proc. Natl. Acad. Sci., 2006, 103, 15739–15746 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details including general methods, CD, UV-vis, DLS. See DOI: 10.1039/c9ra06073a |
| This journal is © The Royal Society of Chemistry 2019 |