Open Access Article
Open Access ArticleA straightforward synthesis of phenyl boronic acid (PBA) containing BODIPY dyes: new functional and modular fluorescent tools for the tethering of the glycan domain of antibodies†
Gina Elena
Giacomazzo‡
,
Pasquale
Palladino‡
 ,
Cristina
Gellini
,
Cristina
Gellini
 ,
Gianluca
Salerno
,
Veronica
Baldoneschi
,
Alessandro
Feis
,
Gianluca
Salerno
,
Veronica
Baldoneschi
,
Alessandro
Feis
 ,
Simona
Scarano
,
Maria
Minunni
,
Simona
Scarano
,
Maria
Minunni
 and
Barbara
Richichi
and
Barbara
Richichi
 *
*
Department of Chemistry ‘Ugo Schiff’, University of Florence, Via Della Lastruccia 13, Sesto Fiorentino, 50019 FI, Italy. E-mail: barbara.richichi@unifi.it
First published on 30th September 2019
Abstract
We report here on the efficient and straightforward synthesis of a series of modular and functional PBA-BODIPY dyes 1–4. They are an outstanding example of the efficient merge of the versatility of the 3,5-dichloro-BODIPY derivatives and the receptor-like ability of the PBA moiety. The potential bioanalytical applicability of these tools was assessed by measuring the binding to glycan chains of antibodies by a Quartz Crystal Microbalance (QCM).
High performance organic fluorophores (e.g. large molar extinction coefficient, high quantum yield, high photo-stability, large Stokes shift) represent a booming research topic, with a wide range of applications ranging from materials to life sciences.1,2 In this framework, modular and functional probes have become highly sought-after and researchers' attention is manly focused on the fine tuning of optical properties and new functionalities for highly versatile tethering. However, despite the huge amount of effort in this field, only few fluorophores meet all these theoretical claims.3 Moreover, the availability of convenient and few steps synthetic protocols associated with a good overall yield is one of the major bottlenecks that needs to be addressed for ensuring the successful wide applicability of these probes.4 A brilliant example of a versatile fluorophore is the UV-absorbing dye 4,4′-difluoro-4-bora-3a,4a-diaza-s-indacene, known as BODIPY,5 which displays high quantum yield, tunable photo-physical properties and excellent photo-stability.5,6 Strategic structural modifications of BODIPY's molecular architecture offer an unparalleled opportunity to tune its spectroscopic features, and diverse successful functional and bioactive BODIPY-like probes have been proposed and some commercialized, so far.7 3,5-Dichloro-BODIPY dyes are one of the most recent synthetic analogues whose potential versatility has been demonstrated by Dehaen and Boens.8 They showed that appropriate substituents at 3,5 positions of the pyrrole shifted the excitation/emission bands of the corresponding substituted BODIPY derivatives. Thus, the 3,5-dichloro-BODIPY dyes give access, by nucleophilic substitution, to a variety of symmetrical and non-symmetrical BODIPY with several applications including labeling, sensing, energy transfer cassette.1e,8,9 Then, 3,5-dichloro-BODIPY derivatives have been directly employed for the selective detection, in in vitro models, of sulphur containing metabolites.10 Recently, the valuable properties of other BODIPY dyes have been used for saccharides detection by phenyl boronic acid (PBA) moiety introduced at the meso position of the BODIPY core.11 Notably, the dynamic covalent interaction between boronic acid and saccharides has been studied since the pioneering work of Lorand et al.12 and PBA ability to bind 1,2 and 1,3-cis-diols motifs of carbohydrates has been used for the development of synthetic ‘boron-lectins’,13 and lately for the fishing of glycoproteins from complex mixtures, for the site-oriented immobilization of antibodies and for biorthogonal conjugations.14 However, the few examples of PBA-BODIPY probes have been reported to date11 and they are confined to 3,5-dimethyl-BODIPY derivatives that require specific protocols for the further functionalization.
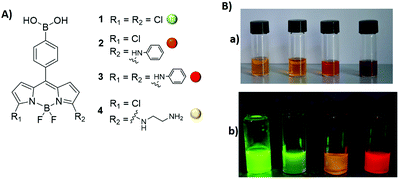
In this context, we report here a straightforward synthetic route to obtain the functional and modular PBA-containing dye 1 and the subsequent late-stage diversification of the architecture of 1. Therefore, we obtained a small family of PBA-BODIPY derivatives with a ‘traffic light’ emission range (Fig. 1) for which we have studied the optical properties in different solvents and in physiological media. Thus, the present approach aims at the outstanding merging of the versatility of the 3,5-dichloro-BODIPY dyes with the presence of a functional PBA at the meso position of the BODIPY core. Finally, we show an example of the formation of the boronate esters between the PBA-BODIPY 4 and the glycan chains of an antibody, i.e. anti-streptavidin monoclonal antibody, by using Quartz Crystal Microbalance.
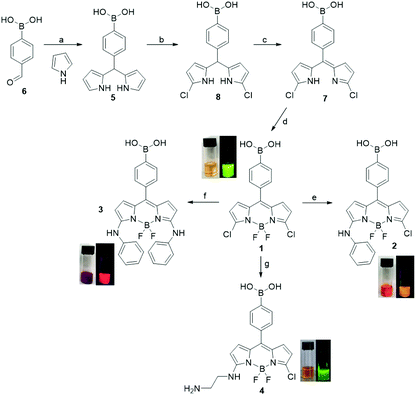
The BODIPY-core is traditionally accessible following a few steps synthetic strategy,8a,15 however, the main concerns are related to the low overall yields of some synthetic steps and to the small scale availability of BODIPY derivatives due to the unstable nature of pyrrole derivatives and/or of some synthetic intermediates.15a,16 In this paper, the boron dipyrromethane dye 1 (Scheme 1) was prepared following the synthetic strategy usually reported for the synthesis of 3,5-dichloro-BODIPY dyes15 by using the commercially available pyrrole-based derivatives and p-substituted benzaldehyde as starting materials.
At first, the meso-PBA substitute dipyrromethane 5 was prepared by acid-catalyzed condensation8a,15a,b of commercially available 4-formylbenzeneboronic acid 6 with neat excess (25 eq.) pyrrole (Scheme 1). In general, the purification of dipyrromethane derivatives is not trivial,15a,b,16 depending on substituents at the meso position, as previously reported.8a,15b A mixture of side products, have been identified and fully characterized,15b and some improvements on the purification steps have been reported15a,b switching from flash chromatography to a bulb-to-bulb distillation followed by recrystallization protocols (yield 27–68%).15b Here, we set up, a straightforward crystallization protocol for the isolation of the pure dipyrromethane 5 (74%) avoiding the low yielding flash chromatography and tedious bulb-to-bulb distillations elsewhere described.15b We have also examined the effect related to the workup of the reaction media (pyrrole![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 25
6, 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, catalytic TFA) on the yield of this synthetic step, by using diverse protocols to refine the formation of 5, observing an higher yield (74% vs. 50%) and an easier removal of side products,15b by the addition of trimethylamine (see ESI†) to neutralize the TFA, and the subsequent concentration to dryness of the reaction mixture. Notably, the use of pure 5 is critical for the overall yield of the two following synthetic steps. The chlorination of 5 (Scheme 1) with N-chlorosuccinimide (NCS) followed by oxidation with 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) afforded the 3,5-disubstututed dipyrromethene 7 (75% over two steps). Finally, 7 was reacted with boron trifluoride diethyl etherate (BF3–OEt2) and N,N′-diisopropylethylamine (DIPEA) to give the green emitting PBA-BODIPY 1 (75%). Dye 1 is amenable to structural modifications at the 3,5 positions of the pyrrole moiety so we prepared a small family of dyes which showed the spectral shift in the absorption and emission bands depending on the substitution pattern (Scheme 1). To demonstrate the versatility of PBA-BODIPY 1 we selected aniline as nucleophile already studied in this kind of reactions.9e,f Thus, the PBA-BODIPY 1 was reacted with an excess of aniline in dichloromethane to give PBA-BODIPY 2 (58%) as pink solid, according to earlier investigations showing that one of the two chlorine atoms is more prone to be involved in the nucleophilic substitution.8,9 Therefore, the di-substitute PBA-BODIPY 3 has been obtained from 3,5-dichloro-BODIPY 1 by using more stringent experimental conditions (neat excess of aniline, 140 °C) as previous reported.9f Finally, mono-substitute PBA-BODIPY 4 was prepared in order to have a functional group on the BODIPY-core useful for further functionalization of the dye for the following studies. Thus, 1 was reacted with a neat excess of 1,2-diaminoethane to afford the yellow emitting PBA-BODIPY 4 (44%).
1 ratio, catalytic TFA) on the yield of this synthetic step, by using diverse protocols to refine the formation of 5, observing an higher yield (74% vs. 50%) and an easier removal of side products,15b by the addition of trimethylamine (see ESI†) to neutralize the TFA, and the subsequent concentration to dryness of the reaction mixture. Notably, the use of pure 5 is critical for the overall yield of the two following synthetic steps. The chlorination of 5 (Scheme 1) with N-chlorosuccinimide (NCS) followed by oxidation with 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) afforded the 3,5-disubstututed dipyrromethene 7 (75% over two steps). Finally, 7 was reacted with boron trifluoride diethyl etherate (BF3–OEt2) and N,N′-diisopropylethylamine (DIPEA) to give the green emitting PBA-BODIPY 1 (75%). Dye 1 is amenable to structural modifications at the 3,5 positions of the pyrrole moiety so we prepared a small family of dyes which showed the spectral shift in the absorption and emission bands depending on the substitution pattern (Scheme 1). To demonstrate the versatility of PBA-BODIPY 1 we selected aniline as nucleophile already studied in this kind of reactions.9e,f Thus, the PBA-BODIPY 1 was reacted with an excess of aniline in dichloromethane to give PBA-BODIPY 2 (58%) as pink solid, according to earlier investigations showing that one of the two chlorine atoms is more prone to be involved in the nucleophilic substitution.8,9 Therefore, the di-substitute PBA-BODIPY 3 has been obtained from 3,5-dichloro-BODIPY 1 by using more stringent experimental conditions (neat excess of aniline, 140 °C) as previous reported.9f Finally, mono-substitute PBA-BODIPY 4 was prepared in order to have a functional group on the BODIPY-core useful for further functionalization of the dye for the following studies. Thus, 1 was reacted with a neat excess of 1,2-diaminoethane to afford the yellow emitting PBA-BODIPY 4 (44%).
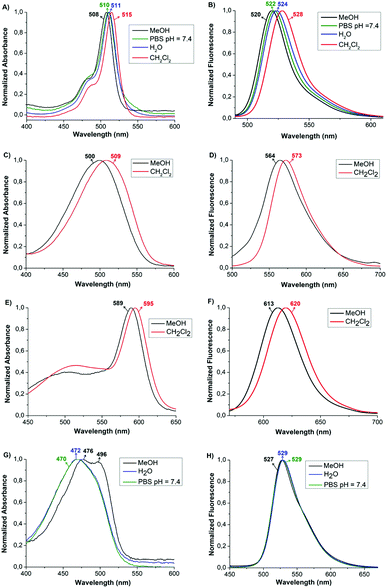
The spectroscopic properties of the PBA-BODIPY dyes 1–4 (Fig. 2, and S1–S11†) were studied in methanol, dichloromethane, water and physiological PBS buffer (pH = 7.4). The spectra reported in the left side of Fig. 2, show the absorption pattern of known PBA-BODIPY derivatives 1–4.8,9,17 In all tested media, the main absorption band attributed to the 0–0 band of the strong S0 → S1 transition is located around 510 nm for PBA-BODIPY 1 and 2 (Fig. 2A, and C) and 590 nm for PBA-BODIPY 3 (Fig. 2E). For PBA-BODIPY 4 (Fig. 2G) the S0 → S1 transition is not the main absorption and is observed around 490 nm. The data are summarized in Table 1. The shoulders at shorter wavelengths for the symmetric dyes 1 and 3, at ∼480 nm and ∼515 nm respectively, have been assigned to the 0–1 vibronic band of the same transition. For PBA-BODIPY 4 the 0–1 vibronic transition is observed at 476 nm in MeOH. In the right side of Fig. 2, the normalized fluorescence spectra of PBA-BODIPY 1–4 are also reported. A reduced solvatochromic effect is observed as both the S0 → S1 absorption and the emission bands are slightly red-shifted in dichloromethane than in more polar media (e.g. MeOH or H2O). The broader full width at half maximum as well as the shape of the absorption and fluorescence bands of asymmetric PBA-BODIPY dyes 2 and 4 are consistent with previously reported amino-containing dyes and they were attributed to the diverse contributes of the proposed Lewis structural formulas.9b The molar extinction coefficient (ε) was assessed in the four different solvents, showing that the highest hyperchromic effect is observed in MeOH (Table 1, Fig. S1–S11†). Finally, the Stokes shift values result moderately low for the symmetric derivatives 1 and 3, while higher value are found for dyes 2 and 4, where symmetry is reduced. The S1 → S0 fluorescence quantum yields (Φf) of 1–4 have been measured in MeOH using Rhodamine 6G as standard reference for 1, 2 and 4 and DODCl for 3 (see ESI†) in the same solvent. High values of Φf have been found for 1 and 3, 0.16 and 0.22 respectively, suggesting that the symmetric presence of bulky substituents on the indacene moiety can stiffen the molecule, promoting radiative decay processes towards the ground state. Moreover, the relatively small Stokes shift observed for both compounds point at a molecular structure that is not much affected by the electronic excitation. On the opposite, the large Stokes shifts measured for the dyes 2 and 4, indicate a non negligible molecular structure variations going from S0 to S1. The less rigid skeleton of these dyes can endorse thermal relaxation, lowering the respective Φf, as shown in Table 1.
| DYE | Solvent | λ abs (nm) | λ em (nm) | Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (cm−1) (cm−1) |
Φ f | ε |
|---|---|---|---|---|---|---|
| 1 | MeOH | 508 | 520 | 454 | 0.16 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 389 ± 246 389 ± 246 |
| PBS | 510 | 522 | 451 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 453 ± 405 453 ± 405 |
||
| H2O | 511 | 524 | 485 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 011 ± 355 011 ± 355 |
||
| CH2Cl2 | 515 | 528 | 478 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 873 ± 395 873 ± 395 |
||
| 2 | MeOH | 500 | 564 | 2270 | 0.0048 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 008 ± 146 008 ± 146 |
| CH2Cl2 | 509 | 573 | 2194 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 701 ± 249 701 ± 249 |
||
| 3 | MeOH | 589 | 613 | 665 | 0.22 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 315 ± 62 315 ± 62 |
| CH2Cl2 | 595 | 620 | 678 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 043 ± 520 043 ± 520 |
||
| 4 | MeOH | 496 | 527 | 1186 | 0.017 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 454 ± 77 454 ± 77 |
| 476 | ||||||
| PBS | ∼494 | 529 | 1340 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 810 ± 94 810 ± 94 |
||
| 470 | ||||||
| H2O | ∼493 | 529 | 1381 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 912 ± 132 912 ± 132 |
||
| 470 |
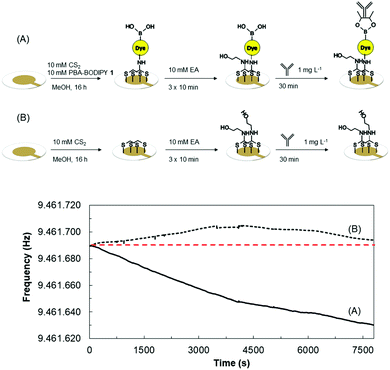
It is well-established that boronic acids can form fast and reversible covalent interactions with 1,2/1,3 cis-diols of carbohydrates generally affording five-/six-membered cyclic boronic esters.13 In this paper, as case sample for the proof of the interaction between our PBA-BODIPY dyes and the glycan unit of glycosylated proteins using Quartz Crystal Microbalance (QCM) for gravimetric sensing. N-Glycosylation site of the Fc regions of monoclonal antibodies (mAbs) have attached significant attention in the last year as site end-on attachment of mAbs in diverse bioanalytical assays,14c,d and for the purification of mAbs from cell mixtures.14e Sialic acid (SA) has been claimed to be the anchoring point of such interactions,14c however the site of PBA-SA interaction is still high debated topic and comprehensive studies combining DFT calculations and NMR spectroscopy have been published.17 Here the binding of rabbit IgG was assessed by monitoring the resonance frequency decrease on 9.5 MHz crystals induced by mass increase on the surface carrying the boronic acid derivative 4 (Fig. 3A).
The gold electrodes sandwiching the quartz crystals were modified as showed in Fig. 3. Quartz crystals were exposed to a 1 mg L−1 solution of a non-glycosylated protein (namely, streptavidin, SAv) in 20 mM Hepes pH 8.5, that was proved to minimize secondary interactions,14c allowing to exclude non-specific binding of SAv to 4 (data not shown). Subsequently, the crystals were exposed to a 1 mg L−1 solution of IgG in the same buffer. Such experiment tested the coupling of glycosylated portion of the mAb to the boronic acid functions of 4. The surfaces were then rinsed with buffer to remove non-specifically bound antibody. Fig. 3 shows the recorded frequency shifts after IgG interaction and baseline equilibration, about 60 Hz for positive control (A) and 1 Hz for negative control (B). Thus, confirming that the antibody was specifically bound to the crystal functionalized with the boronic acid, likely through the glycosylated portions.
In summary, we reported here an efficient methodology for the synthesis of PBA-BODIPY dyes and we demonstrated their tunability in terms of optical properties and capability in tethering of glycans, appearing instrumental and reliable tools for diverse bio-related research fields.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank MIUR-Italy (“Progetto Dipartimenti di Eccellenza 2018–2022′′ allocated to Department of Chemistry “Ugo Schiff”). We thank Dr Annalisa Guerri from the Department of Chemistry, University of Florence (IT) for technical assistance.Notes and references
- (a) Y. Fu and N. S. Finney, RSC Adv., 2018, 51, 29051 RSC; (b) K.-N. Wang, X.-J. Chao, B. Liu, D.-J. Zhou, L. He, X.-H. Zheng, Q. Cao, C.-P. Tan, C. Zhang and Z.-W. Mao, Chem. Commun., 2018, 54, 2635 RSC; (c) P. Gao, W. Pan, N. Li and B. Tang, Chem. Sci., 2019, 10, 6035 RSC; (d) K. Sharma, V. Sharma and S. S. Sharma, Nanoscale Res. Lett., 2018, 13, 381 CrossRef; (e) V. Lakshmi, M. R. Rao and M. Ravikanth, Org. Biomol. Chem., 2015, 13, 2501 RSC.
- (a) L. D Lavis and R. T. Raines, ACS Chem. Biol., 2008, 3, 142 CrossRef and references cited therein; (b) M. A. Reppy, J. Fluoresc., 2008, 18, 461 CrossRef CAS; (c) P. Palladino, V. Castelletto, A. Dehsorkhi, D. Stetsenko and I. W. Hamley, Chem. Commun., 2012, 48, 9774 RSC.
- (a) X. Li, X. Gao, W. Shi and H. Ma, Chem. Rev., 2014, 114, 590 CrossRef CAS; (b) J. O. Escobedo, O. Rusin, S. Lim and R. M. Strongin, Curr. Opin. Chem. Biol., 2010, 14, 64 CrossRef.
- B. Tang, F. Lv, K. Chen, L. Jiao, Q. Liu, H. Wang and E. Hao, Chem. Commun., 2019, 55, 4691 RSC.
- (a) A. Treibs and F. H. Kreuzer, Justus Liebigs Ann. Chem., 1968, 718, 208 CrossRef CAS; (b) L. Jean-Gérard, W. Vasseur, F. Scherninski and B. Andrioletti, Chem. Commun., 2018, 54, 12914 RSC.
- (a) A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891 CrossRef CAS; (b) H. Kowada and K. Kikuchi, Chem. Soc. Rev., 2015, 44, 4953 RSC; (c) N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130 RSC; (d) Y. Ni and J. Wu, Org. Biomol. Chem., 2014, 12, 3774 RSC.
- (a) H. Lu, J. Mack, Y. Yang and Z. Shen, Chem. Soc. Rev., 2014, 43, 4778 RSC; (b) G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184 CrossRef CAS; (c) N. Boens, B. Verbelen and W. Dehaen, Eur. J. Org. Chem., 2015, 6577 CrossRef CAS; (d) K. Krumova and G. Cosa, J. Am. Chem. Soc., 2010, 132, 17560 CrossRef CAS; (e) S. H. Son, S. Daikoku, A. Ohtake, K. Suzuki, K. Kabayama, Y. Ito and O. Kanie, Chem. Commun., 2014, 50, 3010 RSC.
- (a) M. Baruah, W. Qin, N. Basarić, W. M. De Borggraeve and N. Boens, J. Org. Chem., 2005, 70, 4152 CrossRef CAS; (b) T. Rohand, M. Baruah, W. Qin, N. Boens and W. Dehaen, Chem. Commun., 2006, 266 RSC; (c) S. Yin, V. Leen, S. Van Snick, N. Boens and W. Dehaen, Chem. Commun., 2010, 46, 6329 RSC; (d) W. Qin, T. Rohand, M. Baruah, A. Stefan, M. Van der Auweraer, W. Dehaen and N. Boens, Chem. Phys. Lett., 2006, 420, 562 CrossRef CAS; (e) S. Yin, V. Leen, C. Jackers, D. Beljonne, B. Van Averbeke, M. Van der Auweraer, N. Boens and W. Dehaen, Chem.–Eur. J., 2011, 17, 13247 CrossRef CAS; (f) W. Qin, V. Leen, T. Rohand, W. Dehaen, P. Dedecker, M. Van der Auweraer, K. Robeyns, L. Van Meervelt, D. Beljonne, B. Van Averbeke, J. N. Clifford, K. Driesen, K. Binnemans and N. Boens, J. Phys. Chem. A, 2009, 113, 439 CrossRef CAS; (g) W. Qin, V. Leen, W. Dehaen, J. Cui, C. Xu, X. Tang, W. Liu, T. Rohand, D. Beljonne, B. Van Averbeke, J. N. Clifford, K. Driesen, K. Binnemans, M. Van der Auweraer and N. Boens, J. Phys. Chem. A, 2009, 113, 11731 CrossRef CAS.
- (a) J. C. Er, M. K. Tang, C. G. Chia, H. Liew, M. Vendrell and Y.-T. Chang, Chem. Sci., 2013, 4, 2168 RSC; (b) Y. A. Volkova, B. Brizet, P. D. Harvey, A. D. Averin, C. Goze and F. Denat, Eur. J. Org. Chem., 2013, 4270 CrossRef CAS; (c) D. W. Domaille, L. Zeng and C. J. Chang, J. Am. Chem. Soc., 2010, 132, 1194 CrossRef CAS; (d) J. Han, O. Gonzalez, A. Anguilar-Aguilar, E. Pena-Cabrera and K. Burgess, Org. Biomol. Chem., 2009, 7, 34 RSC; (e) N. Dorh, S. Zhu, K. B. Dhungana, R. Pato, F.-T. Luo, H. Liu and A. Tiwari, Sci. Rep., 2015, 5, 18337 CrossRef CAS; (f) E. Fron, E. Coutiño-Gonzalez, L. Pandey, M. Sliwa, M. Van der Auweraer, F. C. De Schryver, J. Thomas, Z. Dong, V. Leen, M. Smet, W. Dehaen and T. Vosch, New J. Chem., 2009, 33, 1490 RSC; (g) T. Rohand, J. Lycoops, S. Smout, E. Braeken, M. Sliwa, M. Van der Auweraer, W. Dehaen, W. M. De Borggraeve and N. Boens, Photochem. Photobiol. Sci., 2007, 6, 1061 RSC; (h) L. Li, B. Nguyen and K. Burgess, Bioorg. Med. Chem. Lett., 2008, 18, 3112 CrossRef CAS.
- L. Y. Niu, Y. S. Guan, Y. Z. Chen, L. Z. Wu, C. H. Tung and Q. Z. Yang, J. Am. Chem. Soc., 2012, 134, 18928 CrossRef CAS.
- (a) B. Liu, N. Novikova, M. C. Simpson, M. S. M. Timmer, B. L. Stocker, T. Sohnel, D. C. Ware and P. J. Brothers, Org. Biomol. Chem., 2016, 14, 5205 RSC; (b) J. Zhai, T. Pan, J. Zhu, Y. Xu, J. Chen, Y. Xie and Y. Qin, Anal. Chem., 2012, 84, 10214 CrossRef CAS; (c) J. S. Hansen, J. F. Petersen, T. Hoeg-Jensen and J. B. Christensen, Tetrahedron Lett., 2012, 53, 5852 CrossRef CAS; (d) J. S. Hansen, T. Hoeg-Jensen and J. B. Christensen, Tetrahedron, 2017, 73, 3010 CrossRef CAS; (e) N. DiCesare and J. B. Lakowicz, Tetrahedron Lett., 2001, 42, 9105 CrossRef CAS.
- J. P. Lorand, J. Org. Chem., 1959, 24, 769 CrossRef CAS.
- (a) X. Wu, X.-X. Chen and Y.-B. Jiang, Analyst, 2017, 142, 1403 RSC; (b) X. Wu, Z. Li, X.-X. Chen, J. S. Fossey, T. D. James and Y.-B. Jiang, Chem. Soc. Rev., 2013, 42, 8032 RSC; (c) X.-T. Zhang, G.-J. Liu, Z.-w. Ning and G.-w. Xing, Carbohydr. Res., 2017, 452, 129 CrossRef CAS; (d) A. Matsumoro and Y. Miyahara, Sci. Technol. Adv. Mater., 2018, 19, 18 CrossRef.
- (a) B. Akgun and D. G. Hall, Angew. Chem., Int. Ed., 2016, 55, 3909 CrossRef CAS; (b) A. Bandyopadhyay and J. Gao, J. Am. Chem. Soc., 2016, 138, 2098 CrossRef CAS; (c) F. Duval, T. A. van Beek and H. Zuilhof, Analyst, 2015, 140, 6467 RSC; (d) H. Y. Song, J. Hobley, X. Su and X. Zhou, Plasmonics, 2014, 9, 851 CrossRef CAS; (e) A. M. Azavedo, A. G. Gomes, L. Borlido, I. F. S. Santos, D. M. F. Prazeres and M. R. Aires-Barros, J. Mol. Recognit., 2010, 23, 569 CrossRef.
- (a) C.-H. Lee and J. S. Lindsey, Tetrahedron, 1994, 50, 11427 CrossRef CAS; (b) B. J. Litter, M. A. Miller, C.-H. Hung, R. W. Wagner, D. F. O'Shea, P. D. Boyle and J. S. Lindsey, J. Org. Chem., 1999, 64, 1391 CrossRef; (c) M. R. Rao, S. M. Mobin and M. Ravikanth, Tetrahedron, 2010, 66, 1728 CrossRef CAS.
- C. Bruckner, V. Karunaratne, S. J. Rettig and D. Dolphin, Can. J. Chem., 1996, 74, 2182 CrossRef CAS.
- (a) J. A. Peters, Coord. Chem. Rev., 2014, 268, 1 CrossRef CAS; (b) S. Nishitani, Y. Maekawa and T. Sakata, ChemistryOpen, 2018, 7, 513 CrossRef CAS; (c) I. R. Vlahov, P. I. Vlahova and R. J. Linhardt, J. Am. Chem. Soc., 1997, 119, 1480 CrossRef CAS; (d) H. Otsuka, E. Uchimura, H. Koshino and T. Okano, J. Am. Chem. Soc., 2003, 125, 3495 Search PubMed; (e) K. Djanashvili, L. Frullano and J. A. Peters, Chem.–Eur. J., 2005, 11, 4010 CrossRef CAS; (f) H. Otsuka, E. Uchimura, H. Koshino, T. Okano and K. Kataoka, J. Am. Chem. Soc., 2003, 125, 3493 CrossRef CAS PubMed; (g) M. Regueiro-Figueroa, K. Djanashvili, D. Esteban-Gòmez, A. de Blas, C. Platas-Iglesias and T. Rodrìguez-Blas, Eur. J. Org. Chem., 2010, 3237 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Protocols for the synthesis of compounds 1–8, fully characterization of compounds 1–8, selected NMR spectra of compounds 1–8. Scalar fluorescence and absorbance spectra, molar extinction coefficients in diverse media of compounds 1–4. Protocols for QCM and QY measurements. See DOI: 10.1039/c9ra07608e |
| ‡ G. G. and P. P. equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2019 |