Light-responsive vesicles for enantioselective release of chiral drugs prepared from a supra-amphiphilic M-helix†
Tengfei
Yan‡
,
Fei
Li‡
,
Shuaiwei
Qi
,
Jun
Tian
,
Ruizhen
Tian
,
Jinxing
Hou
,
Quan
Luo
 ,
Zeyuan
Dong
,
Zeyuan
Dong
 ,
Jiayun
Xu
and
Junqiu
Liu
,
Jiayun
Xu
and
Junqiu
Liu
 *
*
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun 130012, China. E-mail: junqiuliu@jlu.edu.cn
First published on 28th November 2019
Abstract
A kind of light-responsive vesicle was prepared by aqueous self-assembly of α-CD and an azobenzene-containing M-helical foldamer, which displayed dynamic disassembly–reassembly structural transformation when alternately irradiated by UV and visible light. Distinctively, this vesicle also exhibited enantioselective release abilities toward racemic propranolol (a β-blocker), owing to the M-helical building blocks.
Supra-amphiphiles, whose hydrophilic and hydrophobic moieties are linked by noncovalent forces, have received increasing attention in recent years.1 Owing to their intrinsic reversible and stimuli-responsive nature, supra-amphiphiles were frequently used to fabricate functional materials.2 And noncovalently connected assemblies have displayed a wide range of potential applications in the area of sensors,3 separation,4 catalysis,5 and biomedical systems.6 Among these fields of investigation, drug delivery systems have emerged and have become one of the hottest research fields.7 Numerous vesicles with desirable stimuli-triggered (e.g. pH,8 redox,9 light,10 and thermo11) release capabilities were designed and prepared to help decrease side effects and improve the therapeutic efficacy of some clinical drugs.
However, most of the investigations focused on triggered-release of anti-cancer drugs, and little of the research was on the enantioselective release of chiral drugs, even though over half of the commercialized drugs possess chiral centers.12 As is known, chirality is very crucial for the therapeutic effect of a drug. And homochiral drugs are usually obtained through complicated methods such as plant extraction,13 asymmetric synthesis14 and enantioseparation,15 which make them more expensive than achiral drugs. As far as we know, only very few attempts have reported the enantioselective release behavior of common enantiomers by peptide-based vesicles.16 Owing to the complicated synthesis, purification and poor stability, peptide-based nanocarriers are not very popular among chemical scientists.17 Hence, it is a great challenge to fabricate novel vesicles with enantioseparation abilities.
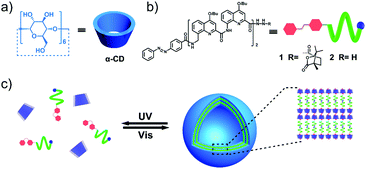
Until recent years, the dramatic development of artificial helices has enabled fabrication of functional materials with enantioselective recognition or separation capabilities. For example, Yashima et al. have designed a switchable enantioseparation system by using the stationary phase of helical polyacetylene to separate the enantiomers of trans-stilbene oxide.18 Huc and coworkers also prepared a kind of homochiral quinoline foldamer grafted silica column, which showed enantioselectivity for both axially and non-axially chiral molecules.19 Nevertheless, fabricating homochiral helix based vesicles with enantioselective release abilities still remains a difficult task and is rarely reported. Herein, we describe a novel type of M-helix based vesicle possessing enantioselective release behaviors toward chiral drugs, which was self-assembled by the supra-amphiphilic complex of α-cyclodextrin (α-CD) and azobenzene containing an M-helical quinoline oligoamide foldamer in water. This kind of vesicle not only displayed light-responsive disassembly–reassembly structural transformation (Fig. 1c) but also exhibited particular enantioselective release performance to racemic propranolol (a chiral drug, Fig. 5a).20 And R-propranolol could preferentially permeate out from the drug-loaded vesicles compared to its S-isomers. Stimuli-responsive nanocarriers are quite common in drug delivery systems, while vesicles with enantioselective release abilities toward chiral drugs are rarely reported.
As the guest molecule, an azobenzene-containing quinoline based oligoamide foldamer (i.e. oligomer 1) was prepared (Fig. 1b), whose synthetic procedure and characterization are clearly described in the ESI† (Scheme S1). This kind of quinoline based serial foldamer possesses a stable helical conformation driven by the intramolecular electrostatic repulsion and hydrogen bonds which has been proved by Huc and coworkers.21 Furthermore, circular dichroism spectrum (CD) and nuclear Overhauser effect spectroscopy (NOESY) measurements were also performed to further confirm the helical structure of 1. As shown in Fig. 2a, a strong negative Cotton effect can be observed at 360 nm, indicating that 1 adopts a left-handed helical conformation in solution. In contrast, the racemic 2 didn’t manifest any CD signal under the same conditions as those for 1. The 2D-NOESY spectrum of 1 showed a strong NOE correlation between protons Ha–Hc, Hb–Hc and Hb–Hd, providing direct evidence for the secondary structure of this quinoline-based artificial helix (Fig. 2b). Correspondingly, α-CD, a toroidal oligosaccharide composed of six glucopyranose residues with a hydrophilic exterior and hydrophobic cavity (Fig. 1a), was selected as the host molecule to recognize the trans azobenzene group of 1 to form a primary building block (Fig. S1, ESI†).22 The self-assembly behaviors of the amphiphilic α-CD⊃1 complex were investigated after dropping 1 to equimolar α-CD at a concentration of 0.05 mM in aqueous solution. Moreover, the CD signal of this M-helix based vesicle revealed a hypochromic shift from 360 nm to 330 nm (Fig. S2, ESI†), suggesting the formation of vesicular self-assemblies.
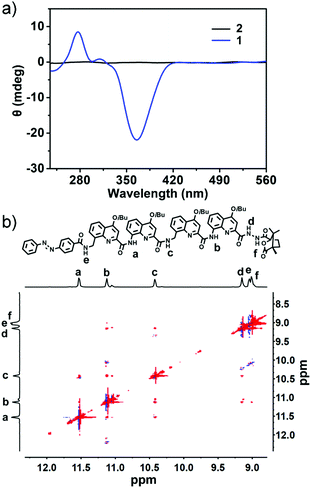 | ||
| Fig. 2 (a) CD spectra of 1 and 2 at a concentration of 1 mM in CH2Cl2. (b) 2D NMR (NOESY) spectrum of 1 in CDCl3. | ||
The detailed morphological information of these assemblies was subsequently investigated using scanning electron microscopy (SEM), transmission electron microscopy (TEM), atomic force microscopy (AFM) and dynamic laser scattering (DLS) instruments, respectively. SEM, AFM and TEM images revealed that the assemblies are typical vesicles with regular spherical structures and well-defined hollow cavities whose average diameter is about 190 nm (Fig. 3a–c). However, the DLS measurement indicated that the diameter of the helix-based vesicles was about 220 nm in aqueous solution, implying that this vesicle has contracted when measuring SEM, TEM and AFM in the dry state. Furthermore, the membrane thickness of vesicles was also clearly visualized from the TEM image and measured to be approximately 28.1 nm (Fig. 3b), indicating a 12 layer membrane according to the calculated length of the host–guest complex of α-CD⊃1 (Fig. S1, ESI†). Additionally, the critical aggregation concentration (CAC) of the vesicles was acquired by measuring the UV absorbance of the host–guest complexed α-CD⊃1 at 330 nm via gradually increasing the concentration from 0.005 mg mL−1 to 0.1 mg mL−1, which was determined to be c.a. 0.031 mg mL−1 (Fig. S3, ESI†). And the binding affinity between α-CD and an azobenzene containing quinoline foldamer was approximately 530 M−1 according to our previously reported literature.23
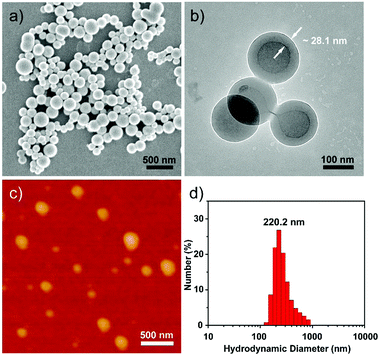 | ||
| Fig. 3 (a) SEM, (b) TEM and (c) AFM images of the M-helix based vesicles. (d) DLS measurement of the vesicles at a concentration of 0.05 mM in aqueous solution. | ||
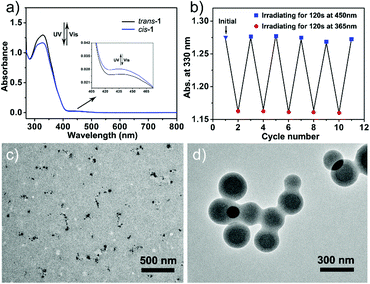
As is known, α-CD can specifically recognize the trans-azobenzene group, while its cis-isomer would be extruded out from the hydrophobic cavity of α-CD due to the mismatched spatial conformation.22 Therefore, we were wondering whether we can control the dynamic disassembly–reassembly transformation of the vesicles via regulating external UV and visible light to control the trans–cis isomerization of the azobenzene group on 1. As expected, when exposed to an UV lamp (365 nm) for 30 min, the vesicles disassembled into irregular aggregates with an average diameter of 100 nm, which were clearly visualized using a TEM instrument (Fig. 4c). In contrast, when this UV-treated aggregate solution was further irradiated by visible light (450 nm) for 30 min, re-assembled vesicles with regular hollow cavities reappeared again (Fig. 4d). By this, we can easily control the disassembly and reassembly transition of the helix-base vesicles by using light as a remote control. The light-sensitive azobenzene moiety on 1 plays a pivotal role in this reversible disassembly–reassembly process (Fig. 1c).
To further underpin the photo-isomerized behaviors of 1, UV-vis spectroscopy was subsequently carried out. As shown in Fig. 4a, when the solution of 1 was irradiated under an UV lamp for 120 s, the characteristic absorption band at 330 nm decreased significantly and another absorption band at 435 nm increased slightly, which could be attributed to the π–π* and n–π* transition of the trans- to cis-azobenzene group.22 In contrast, when the solution was further exposed to visible light for 120 s, the absorption band at 330 nm and 450 nm showed reverse changing tendency, indicating that the photo-isomerizing azobenzene group could be switched between cis and trans conformations. And the reversible isomerization between trans-1 and cis-1 could be reversibly conducted more than five times in N,N-dimethylformamide (DMF) (Fig. 4b). Furthermore, similar UV-vis spectral variation was also detected when the vesicular assemblies were alternately exposed to UV and visible light, respectively (Fig. S4, ESI†).
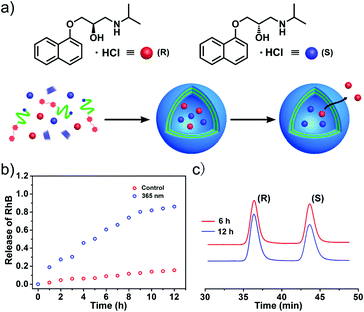
It is well known that hollow nanocapsules with stimuli-responsive abilities are always considered as satisfactory nanocarriers in drug delivery systems. Hence, a frequently-used dye molecule rhodamine B (RhB) was employed as a drug model to estimate the drug loading and triggered release performance of this helix-based vesicle. When RhB was encapsulated into the vesicles during the self-assembly process, we could clearly observe red dots from fluorescence microscopy after sufficient dialysis in water to remove the unloaded RhB molecules (Fig. S5, ESI†). And fluorescence spectroscopy was further carried out to test the stimuli-release properties of the M-helix based vesicle. As shown in Fig. 5b, only about 20% RhB had leaked out from the vesicles after 12 hours when there were no external stimuli. In contrast, the UV-irradiated vesicles displayed much higher release efficiency (approximately 90%) within 12 hours. In addition, this vesicle also exhibited desirable biocompatibility toward 3T3 cells according to 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (Fig. S6, ESI†). It is well known that the cyclodextrin family24 and quinoline derivatives25 are nontoxic and have been utilized in the food and pharmaceutical industries for many years. Light-sensitivity and low-cytotoxicity indicate that this vesicle can serve as a stimuli-responsive drug-carrier.26
As aforementioned, homochiral helices are often used as stationary phases for chiral separation, so we were wondering whether these M-helix based vesicles have enantioselective release abilities toward chiral drugs. Therefore, propranolol was selected as a model drug to examine the enantioselective abilities of these vesicles (Fig. 5a). Firstly, racemic propranolol was encapsulated inside the vesicle during the self-assembly process. After sufficient dialysis, we observed drug-loaded vesicles with dark interiors from the TEM image, suggesting that propranolol was successfully loaded inside the vesicles (Fig. S7, ESI†). In addition, the drug loading efficiency was measured to be about 23.3% by using previously reported methods (Fig. S8, ESI†).9
In order to estimate the enantioselective release behaviors of the M-helix based vesicles, chiral high-performance liquid chromatography (HPLC) was correspondingly applied to monitor the enantiomeric excess (ee, given in %) of the leaked propranolol. As shown in Fig. 5c, the ee value was about 11.2% after the drug-loaded vesicles were allowed to freely diffuse for 6 hours at room temperature. After 12 hours, the ee value appeared to be the maximum and reached approximately 24.2%. This result demonstrated that R-propranolol could preferentially permeate out, and its S-isomer was enantioselectively encapsulated inside the vesicles. In a control experiment, the self-assembly behaviors of 2 and α-CD were investigated by TEM measurements, which revealed similar vesicular structures with an average diameter of 200 nm (Fig. S9a, ESI†). The vesicle self-assembled by α-CD and 2 didn't manifest enantioselectivity toward racemic propranolol after releasing 12 hours (Fig. S9b, ESI†). In addition, the CD family always serves as the stationary phase for chiral separation.27 Therefore, 1H NMR was performed to elucidate the interference of α-CD in the selective release process. When mixed with equimolar α-CD, the resonance peaks of propranolol didn’t show any shift in D2O (Fig. S10a, ESI†). However, when mixed with equimolar β-CD, the resonance peaks of propranolol experienced significant shift variation (Fig. S10b, ESI†), implying that propranolol could only be wrapped inside β-CD rather than α-CD.28 Owing to the small cavity, α-CD cannot recognize propranolol and affect the release process. Therefore, this enantioselective release ability can be attributed to the M-helix based vesicular membrane.
In summary, we have designed and prepared one kind of light-stimuli responsive vesicle, which was self-assembled by the host–guest complex of α-CD and an M-helical quinoline oligoamide foldamer in water. Owing to the photo-sensitive azobenzene group, this vesicle displayed dynamic disassembly–reassembly structural transformation when alternately irradiated by UV and visible light, and could serve as a desirable nanocarrier in drug delivery systems. Interestingly, except for the traditional stimuli-release behaviors, this M-helix based vesicle also exhibited enantioselective release abilities toward racemic propranolol, and R-propranolol could preferentially permeate out from the drug-loaded vesicle with respect to its S-isomer. The M-helix based vesicular membrane was considered to play a crucial role in this selective release process. This M-helix based vesicle could serve as a novel nanocarrier model in the area of chiral drug delivery systems, which still remains an untouched field now.
This work was supported by the National Natural Science Foundation of China (No. 21420102007 and 21574056) and National Key R&D Program of China (Grant No. 2018YFA0901600).
Conflicts of interest
There are no conflicts to declare.Notes and references
- C. Wang, Z. Wang and X. Zhang, Acc. Chem. Res., 2012, 45, 608 CrossRef CAS.
- Y. Chang, Y. Jiao, H. E. Symons, J.-F. Xu, C. F. J. Faul and X. Zhang, Chem. Soc. Rev., 2019, 48, 989 RSC.
- G. Ma and Q. Cheng, Langmuir, 2006, 22, 6743 CrossRef CAS.
- Q. Yan, J. Wang, Y. Yin and J. Yuan, Angew. Chem., Int. Ed., 2013, 52, 5070 CrossRef CAS.
- I. A. Chen, K. Salehi-Ashtiani and J. W. Szostak, J. Am. Chem. Soc., 2005, 127, 13213 CrossRef CAS.
- Q.-D. Hu, G.-P. Tang and P. K. Chu, Acc. Chem. Res., 2014, 47, 2017 CrossRef CAS.
- J. Zhou, G. Yu and F. Huang, Chem. Soc. Rev., 2017, 46, 7021 RSC.
- R. Zhao, Y.-J. Zhou, K.-C. Jie, J. Yang, S. Perrier and F.-H. Huang, Chin. J. Polym. Sci., 2019 DOI:10.1007/s10118-019-2305-1.
- S. Fu, Y. Zhang, S. Guan, Q. Huang, R. Wang, R. Tian, M. Zang, S. Qiao, X. Zhang, S. Liu, X. Fan, X. Li, Q. Luo, C. Hou, J. Xu, Z. Dong and J. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 14281 CrossRef CAS.
- J. Liu, W. Bu, L. Pan and J. Shi, Angew. Chem., Int. Ed., 2013, 52, 4375 CrossRef CAS.
- J. Zhao, P. Zhang, Z. He, Q.-H. Min, E. S. Abdel-Halim and J.-J. Zhu, Chem. Commun., 2016, 52, 5722 RSC.
- X. Zhang, S. Qi, C. Liu and X. Zhao, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2017, 1063, 31 CrossRef CAS.
- G. A. Rather, A. Sharma, S. A. Pandith, V. Kaul, U. Nandi, P. Misra and S. K. Lattoo, Plant Mol. Biol., 2018, 96, 197 CrossRef CAS.
- W. Sangsuwan, B. Kongkathip, P. Chuawong and N. Kongkathip, Tetrahedron, 2017, 73, 7274 CrossRef CAS.
- J. A. Weiss, S. Mohr and M. G. Schmid, Chirality, 2015, 27, 211 CrossRef CAS.
- X. Chen, Y. He, Y. Kim and M. Lee, J. Am. Chem. Soc., 2016, 138, 5773 CrossRef CAS.
- X. Chen, Y. Wang, H. Wang, Y. Kim and M. Lee, Chem. Commun., 2017, 53, 10958 RSC.
- K. Shimomura, T. Ikai, S. Kanoh, E. Yashima and K. Maeda, Nat. Chem., 2014, 6, 429 CrossRef CAS.
- H. Noguchi, M. Takafuji, V. Maurizot, I. Huc and H. Ihara, J. Chromatogr. A, 2016, 1437, 88 CrossRef CAS PubMed.
- M. H. Ghatee and T. Sedghamiz, Chem. Phys., 2014, 445, 5 CrossRef CAS.
- K. Ziach, C. Chollet, V. Parissi, P. Prabhakaran, M. Marchivie, V. Corvaglia, P. P. Bose, K. Laxmi-Reddy, F. Godde, J. M. Schmitter, S. Chaignepain, P. Pourquier and I. Huc, Nat. Chem., 2018, 10, 511 CrossRef CAS.
- Y. Wang, N. Ma, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2007, 46, 2823 CrossRef CAS.
- T. Yan, F. Li, J. Tian, L. Wang, Q. Luo, C. Hou, Z. Dong, J. Xu and J. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 30566 CrossRef CAS.
- G. Chen and M. Jiang, Chem. Soc. Rev., 2011, 40, 2254 RSC.
- P. R. Graves, J. J. Kwiek, P. Fadden, R. Ray, K. Hardeman, A. M. Coley, M. Foley and T. A. J. Haystead, Mol. Pharmacol., 2002, 62, 1364 CrossRef CAS.
- Q. Duan, Y. Cao, Y. Li, X. Hu, T. Xiao, C. Lin, Y. Pan and L. Wang, J. Am. Chem. Soc., 2013, 135, 10542 CrossRef CAS.
- E. Schneiderman and A. M. Stalcup, J. Chromatogr. B: Biomed. Sci. Appl., 2000, 745, 83 CrossRef CAS.
- G. Castronuovo and M. Niccoli, Bioorg. Med. Chem., 2006, 14, 3883 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cc08380d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |