Photoredox-catalyzed biomass intermediate conversion integrated with H2 production over Ti3C2Tx/CdS composites†
Yue-Hua
Li
ab,
Fan
Zhang
b,
Yan
Chen
ab,
Jing-Yu
Li
ab and
Yi-Jun
Xu
 *ab
*ab
aState Key Laboratory of Photocatalysis on Energy and Environment, College of Chemistry, Fuzhou University, Fuzhou, 350116, P. R. China
bCollege of Chemistry, New Campus, Fuzhou University, Fuzhou, 350116, P. R. China. E-mail: yjxu@fzu.edu.cn
First published on 22nd November 2019
Abstract
Harvesting solar energy to drive highly efficient photocatalytic conversion of renewable biomass and its derivatives to value-added chemicals with the concomitant formation of hydrogen (H2) is a green and promising strategy to cope with the global energy dilemma. In this context, we have reported the facile assembly of uniformly distributed CdS nanoparticles (NPs) on the two-dimensional (2D) platform of Ti3C2Tx MXene nanosheets (NSs) via a low-temperature wet chemistry process, during which tight interfacial contact between CdS and Ti3C2Tx has been realized. The Ti3C2Tx/CdS composites feature remarkable enhancement in the aqueous-phase photoredox conversion of furfural alcohol to furfural and H2 by simultaneously utilizing photoexcited holes and electrons. Mechanistic studies reveal that the Ti3C2Tx MXene acts as an “electron sink” to capture the electrons generated from CdS and the close interfacial connection expedites the separation and transport of photoexcited charge carriers, thereby accelerating the photocatalytic performance of the Ti3C2Tx/CdS composites. We anticipate that this work would provide an instructive paradigm for further rational design of MXene/semiconductor hybrids for photoredox-catalyzed production of value-added products and H2 from biomass intermediates.
1. Introduction
With the escalating demand for fuels and excessive depletion of fossil-based resources, the highly efficient transformations of sustainable resources to value-added chemicals have recently aroused research interest.1–5 As a kind of abundant, eco-friendly, carbon-neutral and renewable carbon source, biomass and its derivatives are potential candidates for fabricating corresponding bioproducts.6,7 For example, furfural alcohol serves as a good intermediate in the manufacture of pentanediol, furoic acid, furfural, ethyl levulinate etc.,6,8,9 among which furfural is a key platform molecule to further produce biofuels and high-value chemical goods, which are widely employed in the agrochemical, plastic, pharmaceutical and oil refining industries.10–12Recently, many conversion routes have been developed to prepare value-added furfural from furfural alcohol. Among these methods, the conventional process of furfural alcohol transformation always requires the utilization of costly catalysts along with harsh reaction conditions, such as toxic oxidants and high temperature and/or pressure.7,13 The electrocatalytic conversion of furfural alcohol coupled with hydrogen (H2) evolution shows efficient performance. But the strong alkaline conditions required have an adverse effect on the selectivity of the product.6,14 Less desirable, the photocatalytic oxidation of furfural alcohol is usually performed under an oxygen atmosphere, in which the photoexcited electrons tend to reduce oxygen molecules to superoxide radicals rather than protons to H2. Besides, the utilization of benzotrifluoride as the solvent fails to fit the theme of sustainable development of green chemistry.15 In this scenario, semiconductor-based photocatalysis in water provides a feasible and green alternative to upgrade furfural alcohol to more valuable furfural and simultaneously produce clean hydrogen energy under an inert atmosphere.1,6
As a new impressive family of two-dimensional (2D) transition metal carbides, carbonitrides or nitrides, MXenes with a general formula of Mn+1XnTx (M represents transition metal, X refers to carbon/nitrogen and T stands for surface terminations, e.g., OH, F, and O), have attracted immense attention.16–18 In view of their strong light-absorbing ability, inherent electrical conductivity and lamellar 2D structure, MXenes can be used as a co-catalyst to enhance the light absorption of composite catalysts and act as an “electron sink” to accelerate the transport of photoexcited electrons, thereby improving the photocatalytic performance.16,19,20 To date, MXenes have been employed in the photocatalytic degradation of pollutants,21 selective organic transformations,16,17 H2 evolution22,23 and carbon dioxide (CO2) reduction.19,24–26 However, there are no reports on the application of MXene/semiconductor composites in photocatalytic biomass intermediate conversion coupled with H2 evolution.
Herein, on the 2D platform of Ti3C2Tx MXene nanosheets (NSs), we have constructed Ti3C2Tx/CdS composites by a low-temperature wet chemistry method and for the first time applied them to an aqueous-phase photocatalytic furfural alcohol conversion reaction, which can simultaneously utilize electrons and holes to enable reduction for H2 evolution and oxidation for furfural production. In this binary composite, due to its appropriate Fermi level position and close interfacial contact, Ti3C2Tx acts as an “electron sink” to extract photogenerated electrons from semiconductor CdS, thus resulting in the higher photocatalytic activity of Ti3C2Tx/CdS composites than bare CdS nanoparticles (NPs). The results of photoelectrochemical tests and photoluminescence (PL) spectra demonstrate that the introduction of Ti3C2Tx into the matrix of CdS exerts a crucial effect on the separation and transport of photoexcited charge carriers, thus leading to boosted photocatalytic performance for furfural alcohol conversion.
2. Experimental
2.1. Materials
Titanium aluminum carbide (Ti3AlC2) was obtained from Forsman (Beijing) Technology Co., Ltd. The other materials, e.g. hydrochloric acid (HCl), cadmium acetate (Cd(CH3COO)2), thioacetamide (CH3CSNH2, TAA), carbon tetrachloride (CCl4), triethanolamine (TEOA), lithium fluoride (LiF), acetonitrile (C2H3N), and ethanol (C2H5OH) were obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). All materials were used as received without further treatment. Deionized (DI) water used in the experimental process was acquired from local sources.2.2. Synthesis of Ti3C2Tx colloid
Ti3C2Tx nanosheets colloid was prepared as described previously.27 For more synthesis details see the “Synthesis of Ti3C2Tx colloid” section in the ESI.†2.3. Synthesis of Ti3C2Tx/CdS composites and CdS nanoparticles (NPs)
Ti3C2Tx colloid (0.19, 0.38, 0.76 and 3.80 mL) was dispersed in 200 mL of DI water via ultrasonication. 12 mL of Cd(CH3COO)2 aqueous solution (0.1 M) and 18 mL of TAA aqueous solution (0.1 M) were sequentially added into the Ti3C2Tx suspension, and then the mixture was placed in an 80 °C oil bath with continuous nitrogen (N2) bubbling. After 5 h, the mixture was cooled down to room temperature naturally, and the sediment was collected by centrifugation and rinsed with absolute ethanol. The as-prepared 0.25%Ti3C2Tx/CdS, 0.5%Ti3C2Tx/CdS, 1%Ti3C2Tx/CdS, and 5%Ti3C2Tx/CdS were dried through vacuum drying. The synthetic process of CdS NPs was consistent with that of Ti3C2Tx/CdS composites, except for the addition ofTi3C2Tx colloids. Details are given in the Materials characterization section in the ESI.†2.4. Photoactivity and recycling tests
Typically, a mixture of furfural alcohol (25 μmol), photocatalyst (10 mg) and DI water (10 mL) was added into a quartz reactor. The reaction solution was bubbled with N2 for 30 min to completely remove the air. The reaction solution was illuminated under visible light (λ > 420 nm) using a 300 W Xe arc lamp (PLS-SXE 300, Beijing Perfectlight), which was placed at a distance of about 3 cm from the quartz reactor. A Thor labs PM100 optical power and energy meter was used to test the light intensity, which was 0.75 W cm−2. A high-performance liquid chromatography system (HPLC, Shimadzu Co., Ltd) equipped with an ultraviolet-visible detector was used to measure the oxidized product of furfural alcohol quantitatively. The detector was set to monitor at 230 nm. The elution (flow rate = 1 mL min−1) composed of 60% DI water and 40% C2H3N was utilized to separate and quantify the furfural alcohol and furfural. We used a syringe to collect 1 mL of gas from the quartz reactor for measurement by using a gas chromatograph (GC 2014C, 5 A molecular sieve column, TCD, argon (Ar) carrier, Shimadzu Co., Ltd). Controlled experiments utilizing different scavengers (scavenger holes: TEOA, scavenger electrons: CCl4) were performed similarly to the above photocatalytic process, except that the scavengers (25 μmol) were added to the reaction solution. The recycling tests of 0.5%Ti3C2Tx/CdS were performed as follows. When a photocatalytic cycle was complete, the catalyst was separated from the reaction system by centrifugation and rinsed with DI water twice. Then, the photocatalyst was dried in a vacuum oven and employed in the next catalytic cycle. Conversion (%) of furfural alcohol is calculated as (C0 – CFA)/C0 × 100% and selectivity (%) of furfural is calculated as CF/(C0 − CFA) × 100%, where C0 is the initial concentration of furfural alcohol, CF is the concentration of the prepared furfural, and CFA is the concentration of residual furfural alcohol.3. Results and discussion
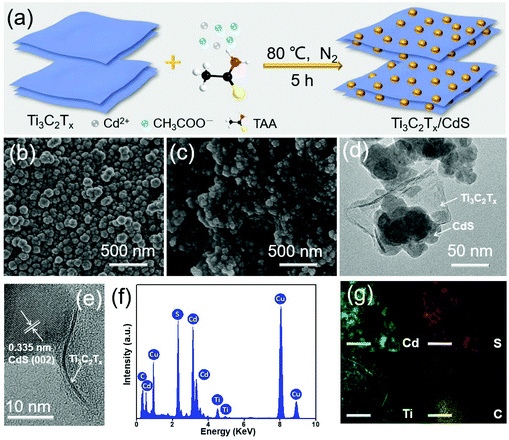
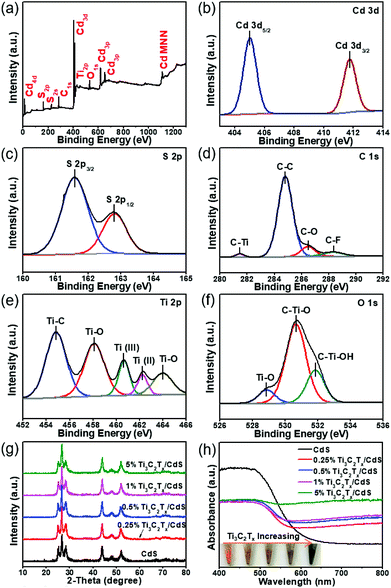
A low-temperature wet chemistry method has been adopted to synthesize Ti3C2Tx/CdS composites, as graphically portrayed in Fig. 1a. After sequentially injecting the cadmium acetate (Cd(CH3COO)2) and thioacetamide (CH3CSNH2, TAA) aqueous solution into the well-dispersed Ti3C2Tx nanosheet (NS) suspension, Cd2+ cations are electrostatically adsorbed on the surface of the negatively charged Ti3C2Tx NSs (−38.81 mV, Fig. S2, ESI†). The following oil bath treatment results in the in situ growth of CdS nanoparticles (NPs) on the Ti3C2Tx NSs, which leads to tight interfacial contact between Ti3C2Tx and CdS. In order to acquire the morphology information, field-emission scanning electron microscopy (FESEM) analyses of the Ti3C2Tx/CdS composites, together with bare CdS for comparison, have been carried out. As shown in Fig. 1b, bare CdS NPs possess a relatively uniform size of about 60 nm. Fig. 1c and Fig. S3 (ESI†) show that the CdS NPs are anchored on the surface of the Ti3C2Tx NSs to construct binary composites. The FESEM images of the Ti3C2Tx/CdS composites also indicate that the introduction of Ti3C2Tx NSs has no remarkable influence on the morphology of CdS NPs. For an in-depth understanding of the microscopic morphology and structure properties of Ti3C2Tx/CdS composites, transmission electron microscopy (TEM) studies have been further performed. Fig. 1d and S4 (ESI†) show that Ti3C2Tx NSs are intimately integrated with the CdS matrix and their sheet-like structure can be distinctly identified from the slightly curled edges. The successful assembly of Ti3C2Tx NSs and CdS NPs can be further authenticated by the high-resolution TEM (HRTEM) results. Fig. 1e shows the close interfacial contact between Ti3C2Tx NSs and CdS NPs and only one distinct lattice fringe with a d-spacing of 0.335 nm, which is ascribed to the (002) crystal facet of hexagonal CdS.28 To determine the elemental composition and distribution in the Ti3C2Tx/CdS composites, energy-dispersive X-ray (EDX) and elemental mapping measurements have been performed. Fig. 1f demonstrates the presence of Ti, C, Cd, and S elements in the Ti3C2Tx/CdS composites. The presence of the Cu element can be ascribed to the utilization of the Cu grid substrate.29 Besides, as shown by the elemental mapping results in Fig. 1g, the Cd, S, Ti and C elements are evenly distributed in the hybrids, illustrating the intimate coupling between CdS and Ti3C2Tx, namely the successful formation of Ti3C2Tx/CdS composites.X-ray photoelectron spectroscopy (XPS) has been conducted to ascertain the chemical valence states of Ti3C2Tx/CdS composites. The survey spectra of Ti3C2Tx/CdS composites (Fig. 2a) confirm that all elements (Cd, S, C, Ti and O) related to Ti3C2Tx and CdS can be detected, which is coincident with the above-mentioned EDX results. Fig. 2b shows that a pair of symmetrical peaks located at 405.2 eV and 411.9 eV correspond to the binding energies of Cd 3d5/2 and Cd 3d3/2, which are attributed to Cd2+ in CdS.30Fig. 2c shows that the binding energies of S 2p3/2 and S 2p1/2 peaks are fixed at 161.5 and 162.7 eV, respectively, which can be attributed to S2− in CdS.31 As clarified in Fig. 2d, the binding energies at 281.5 eV, 284.8 eV, 286.4 eV and 288.5 eV can be respectively assigned to the Ti–C bond, C–C bond, C–O bond and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond.24,32 The spectra of Ti 2p can be deconvoluted into five peaks: Ti–C bond at 454.8 eV, Ti(II) at 462.2 eV, Ti(III) at 460.6 eV, and Ti–O bonds at 464 eV and 458.1 eV (Fig. 2e), which is in accordance with the previous report on Ti3C2Tx.24,33 The regional spectra of O 1s in Fig. 2f show three peaks at 528.8 eV, 530.6 eV and 531.8 eV, which are in good agreement with the Ti–O, C–Ti–O and C–Ti–OH bonds, respectively.32,34 The appearance of relatively weak peaks corresponding to the Ti–O bond could be due to the superficial oxidation of a small amount of Ti3C2Tx upon contact with air.34 The above XPS results clearly validate the successful preparation of Ti3C2Tx/CdS composites by combining Ti3C2Tx NSs and CdS NPs together.
O bond.24,32 The spectra of Ti 2p can be deconvoluted into five peaks: Ti–C bond at 454.8 eV, Ti(II) at 462.2 eV, Ti(III) at 460.6 eV, and Ti–O bonds at 464 eV and 458.1 eV (Fig. 2e), which is in accordance with the previous report on Ti3C2Tx.24,33 The regional spectra of O 1s in Fig. 2f show three peaks at 528.8 eV, 530.6 eV and 531.8 eV, which are in good agreement with the Ti–O, C–Ti–O and C–Ti–OH bonds, respectively.32,34 The appearance of relatively weak peaks corresponding to the Ti–O bond could be due to the superficial oxidation of a small amount of Ti3C2Tx upon contact with air.34 The above XPS results clearly validate the successful preparation of Ti3C2Tx/CdS composites by combining Ti3C2Tx NSs and CdS NPs together.
X-ray diffraction (XRD) patterns have been used to analyze the crystal phase structure of the as-obtained photocatalysts. It can be seen in Fig. 2g that different samples exhibit similar XRD patterns and the peaks located at 24.8°, 26.5°, 28.2°, 43.7°, 47.9° and 51.8° are respectively indexed to the (100), (002), (101), (110), (103) and (112) facet crystal planes of a hexagonal wurtzite-structured phase CdS (JCPDS No. 77-2306).16,35 No apparent characteristic peaks connected with Ti3C2Tx could be observed in the XRD patterns of Ti3C2Tx/CdS composites with different Ti3C2Tx contents, which could be ascribed to the low contents and homogeneous dispersion of Ti3C2Tx in the composites.16 UV–vis diffuse reflectance spectra (DRS) have been used to investigate the optical properties of Ti3C2Tx/CdS composites and bare CdS. Fig. 2h shows that bare CdS exhibits distinct bandgap absorption in the visible light region, and the bandgap value of CdS (2.2 eV) can be calculated by using the Kubelka–Munk function (Fig. S5, ESI†). In addition, with an increase in the Ti3C2Tx content, gradually enhanced light absorption intensity of binary composites can be found in the region of 550–800 nm compared to bare CdS, which could be ascribed to the background light absorption of Ti3C2Tx.16 This is in agreement with the gradually darker color of the samples, which can be observed from the inset of Fig. 2h.
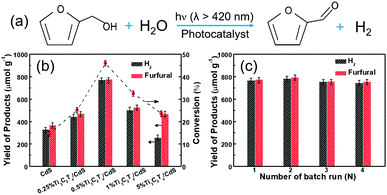
We evaluated the CdS and Ti3C2Tx/CdS composites with different contents of Ti3C2Tx as dual-function catalysts for the photocatalytic conversion of furfural alcohol to furfural coupled with hydrogen (H2) generation under visible light illumination (λ > 420 nm, Fig. 3a) for 4 h. As shown in Fig. 3b, bare CdS exhibits a relatively low activity for H2 generation (328 μmol g−1) and furfural production (368 μmol g−1), on account of the fast recombination of charge carriers. After introducing Ti3C2Tx to form binary composites, the activities of Ti3C2Tx/CdS composites are superior to the bare CdS and a volcano-type relationship between the weight ratios of Ti3C2Tx and the conversion of furfural alcohol is observed. The 0.5%Ti3C2Tx/CdS sample exhibits the highest photocatalytic activity and the yields of H2 and furfural reach up to 773 and 777 μmol g−1, respectively. As sketched in Fig. S6 (ESI†), under visible light illumination for 10 h, the conversion of furfural alcohol over 0.5%Ti3C2Tx/CdS is nearly 100%, while over bare CdS, the conversion is only ca. 37.02%. In addition, the selectivity of bare CdS and Ti3C2Tx/CdS composites with different contents of Ti3C2Tx to furfural is about 93% (Fig. S7, ESI†), indicating that the introduction of Ti3C2Tx has no perceptible effect on the selectivity of liquid phase products. Compared to the reported literature studies,6,36 the as-prepared Ti3C2Tx/CdS composites exhibit good performance. To confirm that this reaction is a photoinduced process, a string of blank experiments with different reaction conditions have been carried out (entries 1–4, Table S1, ESI†). The experiments conducted without visible light irradiation or photocatalysts show no evolution of furfural and H2, indicating that this reaction is really driven by a photocatalytic process. This conclusion can be confirmed by the experimental result of bare Ti3C2Tx, which also exhibits no activity. Considering that photostability is as crucial as photoactivity for the practical use of photocatalysts,37 recycling tests over 0.5%Ti3C2Tx/CdS composites have been performed as sketched in Fig. 3c. The yields of H2 and furfural over the optimal 0.5%Ti3C2Tx/CdS composites remain virtually the same and no obvious decrease in photoactivity can be detected in the 4 reaction cycles for 16 h. The higher photoactivity in the second cycle than in the first cycle could be attributed to the appearance of an induced period at an early stage of illumination.31 The crystal structures and optical properties (Fig. S9a and b, ESI†) of the fresh and used 0.5%Ti3C2Tx/CdS composites have been determined by comparison to evidence that no noticeable change in the Ti3C2Tx/CdS composites is detected after the recycling tests, further validating the good stability of Ti3C2Tx/CdS composites.
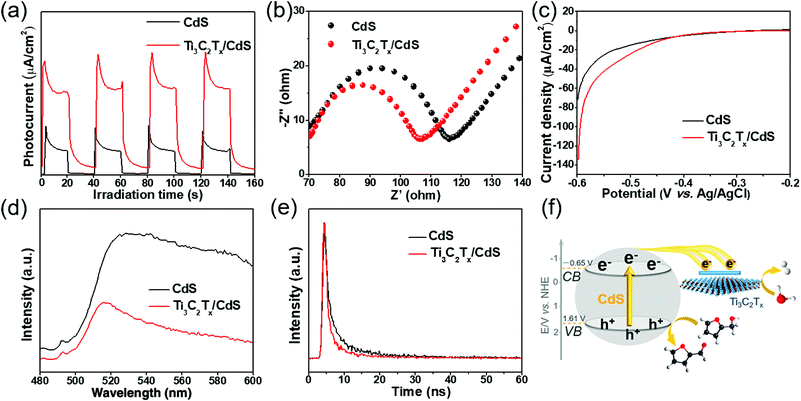
To disclose the origin of the elevated photoactivity of the Ti3C2Tx/CdS composites as compared with bare CdS, a series of photoelectrochemical and photoluminescence (PL) measurements have been conducted to comparatively study the separation and transfer of charge carriers. Fig. 4a shows the transient photocurrent response of Ti3C2Tx/CdS and CdS under intermittent visible light irradiation. An increased transient photocurrent response of Ti3C2Tx/CdS composites can be observed compared to CdS, suggesting the improved separation efficiency of the charge carriers for the Ti3C2Tx/CdS composites.24,26,38 In order to shed light on the interfacial charge transfer resistance between the sample and electrolyte, electrochemical impedance spectroscopy (EIS) has been performed. As shown in Fig. 4b, the Nyquist diagram of Ti3C2Tx/CdS shows a smaller semicircle at a higher frequency than bare CdS, signifying that a lower resistance and a faster charge transfer rate between Ti3C2Tx/CdS and electrolyte solution are achieved compared to those over bare CdS.25,39,40Fig. 4c shows the linear sweep voltammetry (LSV) curves of CdS and Ti3C2Tx/CdS without light illumination, from which it is found that the introduction of Ti2C3Tx into the CdS matrix can decrease the overpotential of H2 evolution and enhance the current density, thus further enhancing the catalytic efficiency.26,41 As shown in the cyclic voltammograms (CV) of these two samples (Fig. S10, ESI†), the current density of Ti3C2Tx/CdS is larger than that of CdS, indicating that more efficient charge transfer of Ti3C2Tx/CdS is obtained compared to that of CdS.39,42 Furthermore, the aforementioned deduction can be clearly confirmed by PL and time-resolved photoluminescence (TRPL) spectra, which are widely employed to reflect the fate of photogenerated charge carriers. As displayed in Fig. 4d, bare CdS exhibits a distinct emission band at ca. 520 nm, which is characteristic of near-band-edge emission.28 When integrating Ti3C2Tx NSs with CdS NPs to construct the binary composites, the PL intensity of Ti3C2Tx/CdS is distinctly decreased. As shown in Fig. 4e and Table S2 (ESI†), the average emission lifetime of Ti3C2Tx/CdS (5.23 ns) is shorter than that of CdS (7.92 ns).43,44 The joint analysis of PL and TRPL results reveals that the cooperation of Ti3C2Tx with CdS retards the recombination of the photoexcited charge carriers from CdS.28,45 The above results together expound that the introduction of Ti3C2Tx is indeed able to improve the separation and migration of the charge carriers, thereby contributing to enhanced photocatalytic performance.
For the purpose of understanding the role of photogenerated active species in this reaction, control experiments have been performed (entries 5–7, Table S1, ESI†). When we replace water with acetonitrile as the reaction solvent, no H2 can be detected, indicating that H2 is derived from water. When the hole scavenger (triethanolamine, TEOA) is added into the photocatalytic reaction system, the production of furfural is restrained to some degree and there is a slight increase in the yield of H2, revealing that the biomass intermediate oxidation reaction is driven by holes and the removal of holes is favourable for the reductive half-reaction.46 When we add the electron scavenger (carbon tetrachloride, CCl4) into the reaction system, H2 evolution is significantly reduced and there is a slight improvement in the production of furfural, indicating that the electrons are mainly used to produce H2 and the removal of electrons facilitates the oxidative half-reaction.46
In light of the aforementioned discussion, a possible mechanism for the photocatalytic furfural alcohol conversion along with the simultaneous H2 production over the Ti3C2Tx/CdS composites is put forward. As illustrated in Fig. 4f, under visible light irradiation, CdS NPs in the Ti3C2Tx/CdS composites are photoexcited to generate electrons and holes. Owing to the low Fermi energy level of Ti3C2Tx and close interfacial contact between CdS and Ti3C2Tx,17,23,47 the electrons can easily migrate to the Ti3C2Tx from the conduction band (CB, −0.65 V vs. normal hydrogen electrode, Fig. S11, ESI†) of CdS, and then participate in the H2 evolution reaction. Simultaneously, the photogenerated holes are accumulated in the valence band (VB, 1.61 V vs. normal hydrogen electrode, Fig. S12, ESI†) of CdS to photocatalytically oxidize furfural alcohol into value-added furfural. As a consequence, the Ti3C2Tx/CdS composites exhibit an enhanced performance for photocatalytic furfural alcohol oxidation coupled with H2 production.
4. Conclusions
In summary, Ti3C2Tx/CdS composites with tight interfacial contact have been successfully fabricated by the in situ growth of CdS NPs on the surface of Ti3C2Tx NSs under mild conditions. Compared to bare CdS, the as-obtained Ti3C2Tx/CdS composites exhibit much improved performance for photoredox-catalyzed conversion of furfural alcohol to the corresponding value-added furfural integrated with H2 production under visible light illumination. In these binary composites, 2D Ti3C2Tx NSs can act as a versatile “electron sink” to extract the photoexcited electrons due to their low Fermi energy level and intimate interfacial contact between Ti3C2Tx and CdS, thereby accelerating the separation of photogenerated electrons and holes and finally resulting in the enhancement of photocatalytic performance. We anticipated that this work on the new application of Ti3C2Tx/CdS composites could open a new doorway to better utilize 2D Ti3C2Tx NSs as promising and efficient co-catalysts in the field of solar-driven biomass conversion.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The support from the Natural Science Foundation (NSF) of Fujian Province for the Distinguished Young Investigator Rolling Grant (2017J07002), the Award Program for Minjiang Scholar Professorship, the National Natural Science Foundation of China (U1463204, 20903023, 21872029, and 21173045), the First Program of Fujian Province for Top Creative Young Talents and the Independent Research Project of State Key Laboratory of Photocatalysis on Energy and Environment (No. 2014A05) is gratefully acknowledged.References
- S. Xu, P. Zhou, Z. Zhang, C. Yang, B. Zhang, K. Deng, S. Bottle and H. Zhu, J. Am. Chem. Soc., 2017, 139, 14775–14782 CrossRef CAS.
- M. Stöcker, Angew. Chem., Int. Ed., 2008, 47, 9200–9211 CrossRef PubMed.
- X. Wu, X. Fan, S. Xie, J. Lin, J. Cheng, Q. Zhang, L. Chen and Y. Wang, Nat. Catal., 2018, 1, 772–780 CrossRef CAS.
- P. Zhang, Y.-J. Guo, J. Chen, Y.-R. Zhao, J. Chang, H. Junge, M. Beller and Y. Li, Nat. Catal., 2018, 1, 332–338 CrossRef CAS.
- T. Wang, M. W. Nolte and B. H. Shanks, Green Chem., 2014, 16, 548–572 RSC.
- G. Han, Y.-H. Jin, R. A. Burgess, N. E. Dickenson, X.-M. Cao and Y. Sun, J. Am. Chem. Soc., 2017, 139, 15584–15587 CrossRef CAS.
- Z. Wang, D. Shen, C. Wu and S. Gu, Green Chem., 2018, 20, 5031–5057 RSC.
- F. Liu, Q. Liu, J. Xu, L. Li, Y.-T. Cui, R. Lang, L. Li, Y. Su, S. Miao, H. Sun, B. Qiao, A. Wang, F. Jérôme and T. Zhang, Green Chem., 2018, 20, 1770–1776 RSC.
- J.-P. Lange, W. D. van de Graaf and R. J. Haan, ChemSusChem, 2009, 2, 437–441 CrossRef CAS.
- A. S. Mamman, J.-M. Lee, Y.-C. Kim, I. T. Hwang, N.-J. Park, Y. K. Hwang, J.-S. Chang and J.-S. Hwang, Biofuels, Bioprod. Biorefin., 2008, 2, 438–454 CrossRef CAS.
- Y. Yang, Z. Du, Y. Huang, F. Lu, F. Wang, J. Gao and J. Xu, Green Chem., 2013, 15, 1932–1940 RSC.
- S. Sitthisa, W. An and D. E. Resasco, J. Catal., 2011, 284, 90–101 CrossRef CAS.
- J. C. Colmenares and R. Luque, Chem. Soc. Rev., 2014, 43, 765–778 RSC.
- B. You, X. Liu, N. Jiang and Y. Sun, J. Am. Chem. Soc., 2016, 138, 13639–13646 CrossRef CAS PubMed.
- H. Wang, Y. Song, J. Xiong, J. Bi, L. Li, Y. Yu, S. Liang and L. Wu, Appl. Catal., B, 2018, 224, 394–403 CrossRef CAS.
- X. Xie, N. Zhang, Z.-R. Tang, M. Anpo and Y.-J. Xu, Appl. Catal., B, 2018, 237, 43–49 CrossRef CAS.
- Y. Chen, X. Xie, X. Xin, Z.-R. Tang and Y.-J. Xu, ACS Nano, 2019, 13, 295–304 CrossRef CAS.
- X. Guo, X. Xie, S. Choi, Y. Zhao, H. Liu, C. Wang, S. Chang and G. Wang, J. Mater. Chem. A, 2017, 5, 12445–12452 RSC.
- Z. Zeng, Y. Yan, J. Chen, P. Zan, Q. Tian and P. Chen, Adv. Funct. Mater., 2019, 29, 1806500 CrossRef.
- Y. Xu, S. Wang, J. Yang, B. Han, R. Nie, J. Wang, J. Wang and H. Jing, Nano Energy, 2018, 51, 442–450 CrossRef CAS.
- T. Cai, L. Wang, Y. Liu, S. Zhang, W. Dong, H. Chen, X. Yi, J. Yuan, X. Xia, C. Liu and S. Luo, Appl. Catal., B, 2018, 239, 545–554 CrossRef CAS.
- G. Jia, Y. Wang, X. Cui and W. Zheng, ACS Sustainable Chem. Eng., 2018, 6, 13480–13486 CrossRef CAS.
- J. Zhang and X. Liu, Phys. Chem. Chem. Phys., 2014, 16, 8655–8660 RSC.
- M. Ye, X. Wang, E. Liu, J. Ye and D. Wang, ChemSusChem, 2018, 11, 1606–1611 CrossRef CAS.
- J. Low, L. Zhang, T. Tong, B. Shen and J. Yu, J. Catal., 2018, 361, 255–266 CrossRef CAS.
- S. Cao, B. Shen, T. Tong, J. Fu and J. Yu, Adv. Funct. Mater., 2018, 28, 1800136 CrossRef.
- M. Ghidiu, M. R. Lukatskaya, M.-Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78 CrossRef CAS.
- B. Han, S. Liu, N. Zhang, Y.-J. Xu and Z.-R. Tang, Appl. Catal., B, 2017, 202, 298–304 CrossRef CAS.
- Y. Shen, S. Zhao, J. Ma, X. Chen, W. Wang, D. Wei, S. Gao, W. Liu, C. Han and B. Cui, J. Alloys Compd., 2016, 664, 229–234 CrossRef CAS.
- M.-Q. Yang, C. Han and Y.-J. Xu, J. Phys. Chem. C, 2015, 119, 27234–27246 CrossRef CAS.
- Y.-S. Xie, L. Yuan, N. Zhang and Y.-J. Xu, Appl. Catal., B, 2018, 238, 19–26 CrossRef CAS.
- Q. Xue, Z. Pei, Y. Huang, M. Zhu, Z. Tang, H. Li, Y. Huang, N. Li, H. Zhang and C. Zhi, J. Mater. Chem. A, 2017, 5, 20818–20823 RSC.
- Z. Ai, Y. Shao, B. Chang, B. Huang, Y. Wu and X. Hao, Appl. Catal., B, 2019, 242, 202–208 CrossRef CAS.
- J. Diao, M. Hu, Z. Lian, Z. Li, H. Zhang, F. Huang, B. Li, X. Wang, D. S. Su and H. Liu, ACS Catal., 2018, 8, 10051–10057 CrossRef CAS.
- J. Ran, G. Gao, F.-T. Li, T.-Y. Ma, A. Du and S.-Z. Qiao, Nat. Commun., 2017, 8, 13907 CrossRef CAS.
- X. He, V. Nguyen, Z. Jiang, D. Wang, Z. Zhu and W.-N. Wang, Catal. Sci. Technol., 2018, 8, 2117–2123 RSC.
- X. Lin, S.-H. Li, K.-Q. Lu, Z.-R. Tang and Y.-J. Xu, New J. Chem., 2018, 42, 14096–14103 RSC.
- K.-Q. Lu, X. Lin, Z.-R. Tang and Y.-J. Xu, Catal. Today, 2019, 335, 294–299 CrossRef.
- X. Xin, S.-H. Li, N. Zhang, Z.-R. Tang and Y.-J. Xu, Appl. Catal., B, 2019, 245, 343–350 CrossRef CAS.
- S.-H. Li, R. Wang, Z.-R. Tang and Y.-J. Xu, Catal. Today, 2019, 335, 160–165 CrossRef CAS.
- L. Yuan, B. Weng, J. C. Colmenares, Y. Sun and Y.-J. Xu, Small, 2017, 13, 1702253 CrossRef.
- R. Wang, K.-Q. Lu, F. Zhang, Z.-R. Tang and Y.-J. Xu, Appl. Catal., B, 2018, 233, 11–18 CrossRef CAS.
- Z. Zhang, Y. Huang, K. Liu, L. Guo, Q. Yuan and B. Dong, Adv. Mater., 2015, 27, 5906–5914 CrossRef CAS.
- W. Bi, L. Zhang, Z. Sun, X. Li, T. Jin, X. Wu, Q. Zhang, Y. Luo, C. Wu and Y. Xie, ACS Catal., 2016, 6, 4253–4257 CrossRef CAS.
- Z. Sun, X. Liu, Q. Yue, H. Jia and P. Du, ChemCatChem, 2016, 8, 157–162 CrossRef CAS.
- T. Zhu, X. Ye, Q. Zhang, Z. Hui, X. Wang and S. Chen, J. Hazard. Mater., 2019, 367, 277–285 CrossRef CAS.
- S. Min and G. Lu, J. Phys. Chem. C, 2011, 115, 13938–13945 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional experimental section and characterization results. See DOI: 10.1039/c9gc03332g |
| This journal is © The Royal Society of Chemistry 2020 |