Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nanosponges for the protection and release of the natural phenolic antioxidants quercetin, curcumin and phenethyl caffeate
Susanna
Guernelli
a,
Alice
Cariola
a,
Andrea
Baschieri
a,
Riccardo
Amorati
 *a and
Paolo
Lo Meo
*a and
Paolo
Lo Meo
 *b
*b
aDipartimento di Chimica “G. Ciamician”, Università degli Studi di Bologna, Via S. Giacomo 11, 40126 Bologna, Italy. E-mail: riccardo.amorati@unibo.it
bDipartimento STEBICEF, Università degli Studi di Palermo, Viale delle Scienze, Ed. 17, 90128 Palermo, Italy. E-mail: paolo.lomeo@unipa.it
First published on 16th September 2020
Abstract
The inclusion of polyphenols into nanoporous materials may significantly improve their application as radical trapping agents for therapeutic purposes. In the present work, nanosponges based on hyper-cross-linked cyclodextrins and calixarenes (NS1–NS3) were used as carriers of three natural phenolic antioxidants: quercetin (Que), curcumin (Cur) and phenethyl caffeate (Phec). Good w/w loadings, namely 7.3% for the Que–NS1 composite, 17.3% for Cur–NS2 and 12.9% for Phec–NS3, were achieved. The release kinetics and the inhibition rate constants (kinh) of the reaction with alkylperoxyl radicals (ROO˙) in 0.1 M phosphate buffer solutions at pH 7.4 were studied and indicated better antioxidant activity as compared to the phenols alone. The three phenols suffered a dramatic reduction of kinh upon increasing their concentration, as a result of their degradation. The inclusion into the nanosponges protected the antioxidants and provided a controlled release in an aqueous medium. Hence, different applications can be envisaged, in food and pharmaceutical technology fields.
Introduction
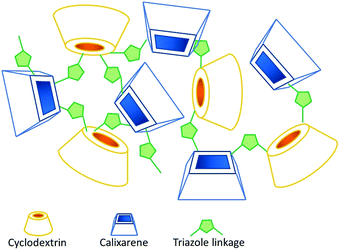
Antioxidant supplementation is considered an effective method to modulate the redox processes that are associated with aging and various pathological conditions.1 Increased oxidative stress and related systemic inflammatory processes are often present in degenerative diseases2 and in abnormal immune responses, such as those recently observed in COVID-19-related pneumonia.3 Food-grade antioxidants from plant-derived extracts are among the first-choice ingredients for antioxidant therapies because they are believed to be safer than novel synthetic drugs, as they are already present in the human diet.4 Natural polyphenols such as curcumin, resveratrol, phenolic acids and flavonoids are among the most popular compounds used as potential modulators of the free radical-induced cellular processes.4,5 However, one important problem that restricts the therapeutic potential of polyphenols is their oxidative instability in water solution and low bioavailability.6,7 The use of nanodelivery systems has allowed the enhancement of the solubility and stability of bioactive phytochemicals.8–10 In particular, the bioavailability can be also 5–10 times higher than that of the native formulation.11 Recent studies report several examples of antioxidants in nanoformulations, such as plant-based bioactive compounds (resveratrol, curcumin, quercetin, genistein, etc.) encapsulated in lipidic or polymeric nanosystems,7 which have shown efficacy in preclinical studies, especially in relation to the increased bioavailability of the antioxidant.10 In general, the development of nanotechnology-based applications for targeted delivery of bioactive phenolic compounds with antioxidant properties to treat chronic diseases is an urgent task of nanomedicine research.12Nanosponges (NSs) are materials with a nanosized porosity13 that represent an emerging tool to improve the solubility and stability of sparingly water soluble drugs.14–16 Nanosponges are hyper-cross-linked polymers or co-polymers made up of typical supramolecular host species, such as cyclodextrins (CDs) or calixarenes (CAs) as the monomers, covalently held together by means of suitable linker units. Native CD NSs can be obtained by reacting the CD synthon with double electrophiles17 such as bis-isocyanates,18 whereas per-halo-CD derivatives can be reticulated by reaction with polyamine nucleophiles.18,19 More recently, CA NSs have been obtained according to a “click” strategy, by reacting propargyloxy-CAs with bis-azidoalkanes under the well-known CuAAC reaction conditions.20 The same approach has been exploited to prepare mixed cyclodextrin–calixarene NS materials (CDCANSs, Fig. 1).21,22 The latter ones present a disordered structure that is permeable to aqueous solutions and able to reversibly absorb and release diverse organic guest species (including dyes19 and antimicrobial agents20), which can be accommodated into both the cavities of the host units and the nanochannels. Of course, the morphological, mechanical and physico-chemical properties of the material critically depend on the chemical nature of both the starting synthon(s) and the linker, as well as on their molar ratio. By a sensible choice of the synthetic strategy, it is possible to obtain materials with tunable properties and, in particular, to achieve sensitivity to external stimuli such as pH variations.19,21–25
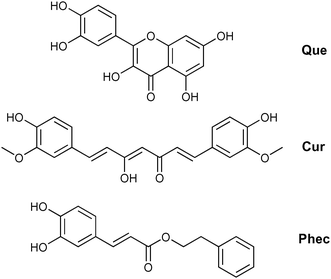
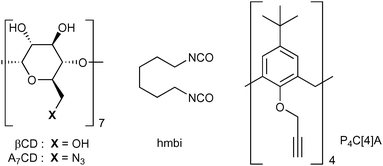
CD-based NSs have been proposed to improve the bioavailability of polyphenols, such as resveratrol,26,27 oxyresveratrol,27,28 curcumin,29 quercetin,30 ferulic acid,31 rutin,32 phlorizin32 and chlorogenic acid.32 However, despite the growing interest toward NSs as carriers of polyphenols, the antioxidant activity of these materials has been seldom investigated.27–29 To the best of our knowledge, the efficiency of the reaction of polyphenols loaded into nanosponges with alkylperoxyl radicals (ROO˙), which are the chain carrying ones of peroxidation in bio-organic systems,9,33 has never been studied in detail. Most popular assays that are used to measure the antioxidant activity of new materials rely on the decay of coloured stable radicals such as 2,2-diphenyl-1-picrylhydrazyl radical (DPPH˙) in alcohol solvents. These methods, although providing an easy estimate useful for screening purposes, have been subjected to criticism34 because they do not describe the ability to inhibit the peroxidation of an oxidizable substrate (for instance, of unsaturated lipids9) and have little relationship with the radicals and solvents involved in biological systems.35 So, in the context of our ongoing studies on the peroxyl radical trapping of nanosized/nanoporous materials,36–38 we were interested in characterizing the antioxidant effect of polyphenols included in NSs. Herein, the preparation and properties of composites constituted by quercetin (Que), curcumin (Cur) or phenethyl caffeate (Phec, Fig. 2) included into CD or mixed CD–CA nanosponges are presented, along with a detailed investigation of their release kinetics and reactivity with alkylperoxyl radicals in a buffered water solution. The polyphenols considered herein are promising therapeutic agents and are representative of some of the main classes of naturally occurring phenols.39–41 In the present work, we chose to test different NSs having diverse chemical nature, in view of the possible tunability of their inclusion and release properties. In particular, we first considered a simple CD-based material obtained by reticulating native β-CD with hexamethylene-bis-isocyanate (hmbi) and then two CD–CA–NS materials obtained by reacting heptakis-(6-azido)-(6-deoxy)-βCD (A7CD) with tetra-propargyloxy-calix[4]arene (P4C[4]A) in either 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 equivalent/equivalent ratio (Fig. 3; the three materials will be herein after indicated as NS1, NS2 and NS3, respectively). The synthesis and properties of the chosen materials have been already reported.22,42,43
2 equivalent/equivalent ratio (Fig. 3; the three materials will be herein after indicated as NS1, NS2 and NS3, respectively). The synthesis and properties of the chosen materials have been already reported.22,42,43
Results and discussion
Loading and release of nanosponges
By means of absorption tests at two different pH values, namely 4.4 and 6.7, we preliminarily verified that the selected materials were able to absorb the guest molecules from aqueous buffered solutions (for the sake of clarity, it is worth mentioning here that all the materials used in this work presented a <150 μm granulometry). The two pH values were chosen for consistency with previous studies and in general have been found to be the most suitable for absorption and release studies. The results obtained (presented in Table 1) show an excellent sequestration efficiency, larger than 80%, in all the cases examined; in particular, a nearly quantitative absorption for curcumin is always observed. In most cases a negligible variation in inclusion efficiency was observed upon changing the pH, with the remarkable exception of Que in materials NS2 and NS3.| pH | NS1 | NS2 | NS3 | |||
|---|---|---|---|---|---|---|
| 4.4 | 6.7 | 4.4 | 6.7 | 4.4 | 6.7 | |
| a Error ± 3%. | ||||||
| Guest | ||||||
| Que | 95 | 95 | 80 | 92 | 85 | 94 |
| Cur | >97 | >97 | >97 | 95 | >97 | >97 |
| Phec | 92 | 93 | 93 | 95 | 90 | 92 |
The latter observation is quite intriguing. In fact, based on the pKa values reported, it can be confidently assumed that the guest is in its undissociated form at both pH values. Conversely, materials NS2 and NS3 assume a positive charge density at the lowest pH value due to the partial protonation of the 1,2,3-triazole linker moieties. Inclusion efficiency is known to be significantly affected by Coulomb interactions and in general by a possible increase in the hydrophilic character of the NS due to the occurrence of a non-null charge density (caused by the protonation or deprotonation of ionisable groups as a function of the pH). Therefore, this effect appears much more effective in the case of Que, which has a more compact and rigid molecule, compared to those in the cases of the more flexible Cur and Phec.
Preliminary absorption tests helped us to plan the preparation of the composites. Indeed, we reasoned that an excessive absorption efficiency, although suggesting possible high loading, could have also resulted in unsatisfactory release over time. Consequently, the choice relevant to the inclusion in a particular NS was made on the basis of a reasonable compromise between the two requirements. So, considering the data reported in Table 1, we decided to prepare three different composites by including Que into material NS1, Cur into NS2 and Phec into NS3. Loadings of the antioxidant agents into the nanosponges were carried out according to the literature (see Experimental) by equilibrating the NS materials with the guest species (a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
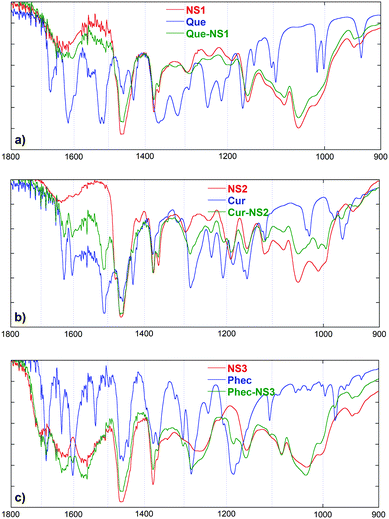
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w starting ratio) in aqueous buffer at pH 4.4. The percent amount of guest actually present in the obtained composites was determined via UV-vis spectrophotometry after having eluted it with methanol. Loadings (w/w) were found to be as large as 7.3% for the Que–NS1 composite, 17.3% for the Cur–NS2 composite and 12.9% for the Phec–NS3 composite. The composites were characterized using FT-IR spectroscopy. A careful inspection of the fingerprint region (Fig. 4) may in principle reveal the presence of the guest embedded into the NS matrix. Of course, the possible detection of signals attributable to the guest is heavily affected by the actual loading. In the case of the Que–NS1 composite, for instance, the presence of the guest is hardly identifiable (due to its low loading) by the very weak bands at ca. 1604 and 1324 cm−1. On the other hand, the presence of Cur in its composite is well outlined by at least three signals of the guest at 1629, 1605 and 1509 cm−1. Similarly, the Phec composite shows fairly intense bands at 1683, 1603, 1301, 1281, 1184, 1106 and 978 cm−1. It is worth noting that all these signals do not show any significant shift with respect to the free guest, indicating the occurrence of only a weak non-specific interaction with the nanosponge matrix.
1 w/w starting ratio) in aqueous buffer at pH 4.4. The percent amount of guest actually present in the obtained composites was determined via UV-vis spectrophotometry after having eluted it with methanol. Loadings (w/w) were found to be as large as 7.3% for the Que–NS1 composite, 17.3% for the Cur–NS2 composite and 12.9% for the Phec–NS3 composite. The composites were characterized using FT-IR spectroscopy. A careful inspection of the fingerprint region (Fig. 4) may in principle reveal the presence of the guest embedded into the NS matrix. Of course, the possible detection of signals attributable to the guest is heavily affected by the actual loading. In the case of the Que–NS1 composite, for instance, the presence of the guest is hardly identifiable (due to its low loading) by the very weak bands at ca. 1604 and 1324 cm−1. On the other hand, the presence of Cur in its composite is well outlined by at least three signals of the guest at 1629, 1605 and 1509 cm−1. Similarly, the Phec composite shows fairly intense bands at 1683, 1603, 1301, 1281, 1184, 1106 and 978 cm−1. It is worth noting that all these signals do not show any significant shift with respect to the free guest, indicating the occurrence of only a weak non-specific interaction with the nanosponge matrix.
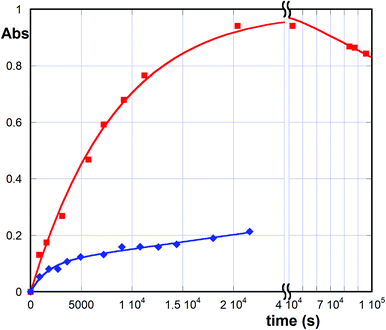
We then verified the release of the guest molecules by the composites under two different conditions (see Experimental): (i) aqueous solution buffered at pH 7.2 and 37 °C, i.e. under conditions consistent with physiological systems; (ii) pH 7.4 aqueous solution containing 10% v/v THF, which are standardized conditions used to study the antioxidant activity (see below). In the first case, we noticed that neither Que nor Cur is appreciably released even after 24 h. Differently, Phec is actually released from its composite, though quite slowly (ca. 3.4% within 6 h). The relevant kinetic profile (Fig. 5, blue line) is the superimposition of a first order process with an apparent kinetic constant k1 = (6 ± 1) × 10−4 s−1 and a zeroth order process with an apparent kinetic constant k0 = (2.5 ± 0.3) × 10−10 M s−1. Noticeably, the occurrence of this peculiar profile can be easily explained admitting that first a little amount of the guest is quickly released from the outer layer of the nanosponge grains, and then a further amount is released from the inner region by a quite slow mass-transfer process. A similar behaviour was observed also under the second set of conditions. In more detail, only a little release amount is found for curcumin and quercetin (less than 2%), and hence we were not able to perform a proper release kinetic experiment. By contrast (Fig. 5, red line), Phec is largely released (up to ca. 80%) in a relatively short time (4 h) according to an apparent pseudo-first-order profile, with k1 = (1.15 ± 0.07) × 10−4 s−1, which can be easily explained considering that the co-solvent THF molecules that are present compete with the guest for the binding sites of the NS material, so that mass-transfer is largely speeded up. However, Phec undergoes slow decomposition after release, as confirmed using a blank experiment on a solution of the free guest under the same conditions (decomposition rates were consistent for the two different experiments).
Antioxidant activity assays
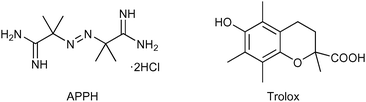
The antioxidant activity was evaluated by measuring the inhibition rate constant (kinh) and the stoichiometry (n) of the reaction between the antioxidants and the alkylperoxyl radicals (ROO˙), which are the most relevant radicals responsible for peroxidation in bioorganic systems.33 The values of kinh were determined by studying the inhibition of the thermally initiated autoxidation of tetrahydrofuran (THF), chosen as the reference oxidizable substrate (Scheme 1), under controlled conditions using pH 7.4 phosphate buffer as the solvent. The reactions were performed at 30 °C using 2,2′-azobis-2-methylpropionamidine dihydrochloride (AAPH, Fig. 6) as the initiator, and the oxygen consumption (Δ[O2]) was measured using a differential pressure transducer.44–47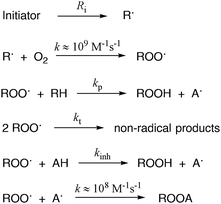 | ||
| Scheme 1 General mechanism of the propagation/inhibition of the autoxidation reaction. RH represents the oxidizable substrate THF. | ||
In the absence of antioxidants, THF autoxidation causes a linear O2 consumption (see for instance the black line in Fig. 7). In the presence of a good antioxidant, THF autoxidation displays an inhibition period, the duration of which depends on the antioxidant concentration and on the rate of radical generation by AAPH (Ri) as shown in eqn (1), while its slope is inversely proportional to the antioxidant efficacy. From the slope of O2 consumption during the inhibition period, we could obtain kinh values by using eqn (1), where AH represents the antioxidant, R0 and Rinh are the slopes of O2 consumption without and with the antioxidant, respectively, 2kt is the termination rate constant of THF (6.6 × 107 M−1 s−1)46 and Ri is the rate of radical generation by AAPH.37 The stoichiometric coefficient n represents the number of radicals trapped by each antioxidant molecule and was determined from the duration of the inhibition period (τ) and the antioxidant concentration using eqn (2).47
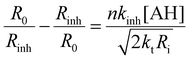 | (1) |
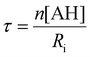 | (2) |
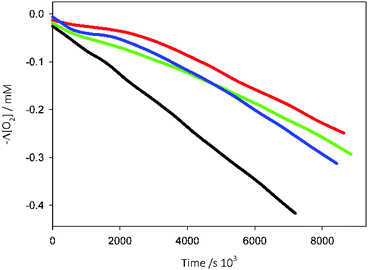
The antioxidant activities of Que, Cur and Phec alone were analysed, to assess their behaviour in the absence of the NSs. Because phenol stability is a critical issue at physiological pH,48 antioxidants were used at a relatively low concentration to avoid long reaction times. All three phenols caused an inhibition of THF oxidation (Fig. 7), accounting for the reaction with ROO˙ radicals. The kinh values obtained using this procedure are reported in Table 2.48Que showed the highest kinh, followed by Phec and then by Cur. All the three antioxidants, however, were less reactive than the antioxidant Trolox (Fig. 6), considered as the standard reference. It is worth pointing out that the kinh values measured herein are higher than those previously measured at a larger THF concentration (3.1 M) for Trolox and Que (kinh = 4.1 × 105 and 1.6 × 105 M−1 s−1, respectively),46,49 indicating the importance of controlling the levels of organic co-solvents when measuring antioxidant activities in an aqueous environment.
Regarding the stoichiometry of radical trapping, it can be noticed that the n coefficient reported in Table 2 in the case of Phec is significantly larger than that measured in non-aqueous systems (in chlorobenzene, n ≈ 2).50 This could be explained by considering that after the reaction with the first two radicals, electrophilic quinonoid structures are formed, which are known to add solvent molecules generating new phenols having antioxidant activity (a possible mechanism is depicted in Scheme 2).51
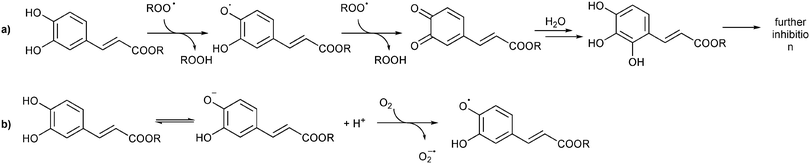 | ||
| Scheme 2 (a) Putative mechanism accounting for the trapping of more than two peroxyl radicals by Phec (adapted from ref. 51); (b) degradation of phenols in water solutions. | ||
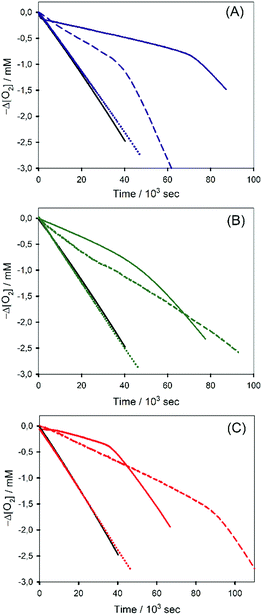
We then investigated the antioxidant activity in the composites (Fig. 8). After having verified that the bare NSs have no appreciable activity of their own (dotted lines in Fig. 8), the observed plots of O2 consumption showed a marked inhibition of THF autoxidation due to the release of the phenols loaded into the NSs. The longer inhibition periods with respect to the experiments presented in Fig. 7 are due to the higher concentrations of the polyphenols. Regression analysis using eqn (1) and (2) (data are collected in Table 3), where [AH] is the molar concentration of the sample of the polyphenols contained in the nanosponges, provided the kinh and n values reported in Table 3. Noticeably, and as previously observed in the case of vitamin C loaded on halloysite nanotubes,52 the kinh values are smaller than those of free antioxidants because the antioxidant is released gradually from the sponges, where it is inactive.
| k inh (M−1 s−1) | n | |
|---|---|---|
| a Average from triplicate experiments. | ||
| Que–NS1 | (2.6 ± 0.3) × 104 | 2.7 ± 0.2 |
| Cur–NS2 | (9.0 ± 0.3) × 103 | 4.0 ± 0.1 |
| Phec–NS3 | (3.2 ± 0.2) × 104 | 4.1 ± 0.1 |
| Conc. Que | (1.1 ± 0.2) × 104 | 6.2 ± 0.2 |
| Conc. Cur | (3.0 ± 0.4) × 103 | >5 |
| Conc. Phec | (8.0 ± 0.2) × 103 | 2.2 ± 0.1 |
In order to understand the application potential of the nanosponges as carriers of antioxidants, we investigated also the antioxidant activity of Phec, Que and Cur at high concentrations, identical to those used in the experiments where antioxidants are included in the nanosponges. The results reported in Table 3 show that the three phenols suffer a dramatic reduction of kinh upon increasing their concentration, which we explain as being due to their degradation that is known to occur in aqueous solution.52–54 It is worth mentioning here that the kinh values determined from the experiments reported in Fig. 8 are to be considered “apparent” inhibition constants, as the slopes of O2 consumption reported in Fig. 8 are influenced by the release from the nanosponges or by the reaction occurring during phenol degradation. Polyphenol decomposition occurs by the deprotonation of phenols and by the reaction of the phenolate with O2, to afford phenoxyl radicals and superoxide, which propagates the oxidative chain (Scheme 2b).52 The results (illustrated in Fig. 8) show that phenols included into nanosponges provided in all cases a more efficient trapping of ROO˙ (i.e. a smaller slope of O2 consumption and, in the case of Que, longer inhibition) than phenols at the same concentration, free in solution. Interestingly, the results reported in Fig. 8 show that polyphenol degradation does not necessarily correspond to a reduction of the inhibition period. Indeed, products possessing phenolic moieties having a residual antioxidant may be formed, as in the case of curcumin in which a phenolic heterocycle is formed after degradation.55 Therefore, we can say that nanosponges not only allow a gradual release of the previously loaded phenols, but also protect them from external agents, increasing their effectiveness over time.
Experimental
Materials and instrumentation
All the reagents and materials needed were used as purchased (Sigma-Aldrich) without further purification. The FT-IR spectra of the nanosponge composites were acquired using an AGILENT Technologies Cary 630 FTIR spectrometer. UV-vis spectra were recorded using a Beckmann-Coulter DU800 spectrophotometer.The nanosponges NS1,42 NS222 and NS323 were prepared, purified and characterized according to literature reports. For information completeness, NS1 contained 0.61 mmoles g−1 of βCD units, NS2 contained 0.46 mmoles g−1 of A7CD units and 0.50 mmoles g−1 of P4C[4]A units, and NS3 contained 0.25 mmoles g−1 of A7CD units and 0.85 mmoles g−1 of P4C[4]A units.
The composites were prepared as follows. The plain nanosponge (80.0 mg) was placed in a vial, wetted with a few drops of methanol, and suspended in 20 mL of an aqueous acetic buffer (0.05 M, pH 4.4). Then 20.0 mg of the guest was dissolved in 1 mL of methanol and the solution was added slowly to the NS suspension under gentle magnetic stirring. The system was kept under stirring at r.t. overnight, and then the product was vacuum filtered off, rapidly washed with drops of ice-cold methanol, and finally dried in vacuo at 50 °C over phosphorus pentoxide for 2 h.
Sequestration and release tests
Sequestration tests were performed as follows. A carefully weighed amount (4.00 ± 0.05 mg) of the nanosponge material was placed in a vial and mixed with 2 mL of a 50 mM solution of the guest in aqueous buffer (I = 0.05 M) at the proper pH value (acetate buffer for pH 4.4, phosphate buffer for pH 6.7). The sample was briefly sonicated to facilitate the dispersion of the material and then mechanically shaken for 90 min. The sample was then centrifuged (5500 rpm for 15 min); the supernatant liquor was carefully pipetted and analyzed (UV-vis) to determine the residual concentration in solution, from which the percent absorption was calculated by trivial algebraic passages.The release tests were performed as follows. A carefully weighed amount (10.00 ± 0.05 mg) of the composite was placed in a dialysis membrane (Medicell International Ltd; MWCO 12–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 with a diameter of 21.5 mm) and dispersed in 1 mL of aqueous phosphate buffer solution (0.05 M and pH 7.2; at pH 7.4 in buffer solution with 10% THF). The membrane was then placed in a round bottom flask containing 20 mL of the release medium at 37 °C under magnetic stirring. At fixed times, 1 mL of the release medium was withdrawn and analyzed spectrophotometrically to determine the concentration of the guest released. Notably, in order to keep the volume of the sample constant, 1 mL of fresh buffer was added each time to replace the withdrawn one.
000 with a diameter of 21.5 mm) and dispersed in 1 mL of aqueous phosphate buffer solution (0.05 M and pH 7.2; at pH 7.4 in buffer solution with 10% THF). The membrane was then placed in a round bottom flask containing 20 mL of the release medium at 37 °C under magnetic stirring. At fixed times, 1 mL of the release medium was withdrawn and analyzed spectrophotometrically to determine the concentration of the guest released. Notably, in order to keep the volume of the sample constant, 1 mL of fresh buffer was added each time to replace the withdrawn one.
Antioxidant activity
The antioxidant activity was evaluated by measuring the O2 consumption during the autoxidation of tetrahydrofuran (THF) initiated by the hydrosoluble azo-initiator AAPH (25 mM) at 30 °C. The initiation rate, Ri, was determined by the inhibition method using (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) as the reference antioxidant: Ri = 2[PMHC]/τ, where τ is the length of the induction period (Ri = 6.2 × 10−9 M s−1).56 The concentration of O2 was measured using an optical oxygen meter (FireStingO2, PyroScience GmbH)57 calibrated by performing a reference THF autoxidation.Conclusions
The goal of this work is represented by the possibility of performing a controlled release in an aqueous medium of natural antioxidants that are sparingly water soluble. In more detail, cyclodextrin- and calixarene-based nanosponges were prepared for the delivery of natural antioxidants, quercetin, curcumin and phenethyl caffeate. The obtained composites were evaluated for guest solubility, guest release and antioxidant properties.Our results positively demonstrate that the use of our nanosponge materials is an interesting approach for the protection and controlled disposal of natural polyphenolic antioxidants. The structure of nanosponges can be easily modulated by changing the relative proportions of the host molecules and of the linkers, to obtain optimal loading and release properties. Kinetic experiments on the reaction with ROO˙ radicals indicate that the inclusion into nanosponges increases the antioxidant activity by blocking the pro-oxidant effect due to phenol degradation. The flexibility of the synthetic method allows us to envisage many future developments involving both the nanosponge scaffold (biocompatibility, biodegradation, material size) and the radical trapping moieties (such as integrating covalently and non-covalently loaded antioxidants with different mechanisms of action). In conclusion, we believe that opportunely designed nanosponges have the potential to give a significant contribution to the development of new highly active antioxidants.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The funding from Bologna University is gratefully acknowledged.Notes and references
- V. Conti, V. Izzo, G. Corbi, G. Russomanno, V. Manzo, F. De Lise, A. Di Donato and A. Filippelli, Front. Pharmacol., 2016, 7, 24, DOI:10.3389/fphar.2016.00024.
- G. Bjørklund and S. Chirumbolo, Nutrition, 2017, 33, 311–321 CrossRef.
- J.-Z. Wang, R.-Y. Zhang and J. Bai, Int. J. Cardiol., 2020, 312, 137–138 CrossRef.
- C. G. Fraga, K. D. Croft, D. O. Kennedy and F. A. Tomás-Barberán, Food Funct., 2019, 10, 514–528 RSC.
- A. Yashin, Y. Yashin, X. Xia and B. Nemzer, Antioxidants, 2017, 6, 70 CrossRef.
- F. Aqil, R. Munagala, J. Jeyabalan and M. V. Vadhanam, Cancer Lett., 2013, 334, 133–141 CrossRef CAS.
- P. Khadka, J. Ro, H. Kim, I. Kim, J. T. Kim, H. Kim, J. M. Cho, G. Yun and J. Lee, Asian J. Pharm. Sci., 2014, 9, 304–316 CrossRef.
- R. Conte, V. Marturano, G. Peluso, A. Calarco and P. Cerruti, Int. J. Mol. Sci., 2017, 18, 709, DOI:10.3390/ijms18040709.
- A. Fontana, S. Guernelli, A. Di Crescenzo, P. Di Profio, F. Palomba, L. De Crescentini, A. Baschieri and R. Amorati, J. Mol. Liq., 2019, 293, 111465 CrossRef.
- A. Vaiserman, A. Koliada, A. Zayachkivska and O. Lushchak, Front. Bioeng. Biotechnol., 2020, 7, 1–19 Search PubMed.
- M. Massaro, C. G. Colletti, B. Fiore, V. La Parola, G. Lazzara, S. Guernelli, N. Zaccheroni and S. Riela, Gold nanoparticles stabilized by modified halloysite nanotubes for catalytic applications, Appl. Organomet. Chem., 2019, 33, 1–11 CrossRef.
- P. Ganesan, G. Karthivashan, S. Y. Park, J. Kim and D. K. Choi, Int. J. Nanomed., 2018, 13, 6109–6121 CrossRef CAS.
- P. Lo Meo, F. Mundo, S. Terranova, P. Conte and D. Chillura Martino, J. Phys. Chem. B, 2020, 124, 1847–1857 CAS.
- R. Cavalli, F. Trotta and W. Tumiatti, J. Inclusion Phenom. Macrocyclic Chem., 2006, 56, 209–213 CrossRef CAS.
- F. Trotta, Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine, John Wiley & Sons, Inc., 2011, pp. 323–342 DOI:10.1002/9780470926819.ch17.
- S. Selvamuthukumar, S. Anandam, K. Kannan and R. Manavalan, J. Pharm. Pharm. Sci., 2012, 15, 103–111 Search PubMed.
- P. K. Shende, F. Trotta, R. S. Gaud, K. Deshmukh, R. Cavalli and M. Biasizzo, J. Inclusion Phenom. Macrocyclic Chem., 2012, 74, 447–454 CrossRef CAS.
- A. L. Taka, K. Pillay and X. Y. Mbianda, Carbohydr. Polym., 2017, 159, 94–107 CrossRef.
- M. Russo, M. L. Saladino, D. Chillura Martino, P. Lo Meo and R. Noto, RSC Adv., 2016, 6, 49941–49953 RSC.
- A. Spinella, M. Russo, A. Di Vincenzo, D. C. Martino and P. Lo Meo, Beilstein J. Org. Chem., 2018, 14, 1498–1507 CrossRef CAS.
- V. Cinà, M. Russo, G. Lazzara, D. Chillura Martino and P. Lo Meo, Carbohydr. Polym., 2017, 157, 1393–1403 CrossRef.
- P. Lo Meo, G. Lazzara, L. Liotta, S. Riela and R. Noto, Polym. Chem., 2014, 5, 4499–4510 RSC.
- M. Massaro, V. Cina, M. Labbozzetta, G. Lazzara, P. Lo Meo, P. Poma, S. Riela and R. Noto, RSC Adv., 2016, 6, 50858–50866 RSC.
- A. Di Vincenzo, M. Russo, S. Cataldo, D. Milea, A. Pettignano and P. Lo Meo, ChemistrySelect, 2019, 4, 6155–6161 CrossRef CAS.
- R. M. Fontana, N. Milano, L. Barbara, A. Di Vincenzo, G. Gallo and P. L. Meo, ChemistrySelect, 2019, 4, 9743–9747 CrossRef CAS.
- K. A. Ansari, P. R. Vavia, F. Trotta and R. Cavalli, AAPS PharmSciTech, 2011, 12, 279–286 CrossRef CAS.
- N. K. Dhakar, A. Matencio, F. Caldera, M. Argenziano, R. Cavalli, C. Dianzani, M. Zanetti, J. M. López-Nicolás and F. Trotta, Pharmaceutics, 2019, 11, 545 CrossRef CAS.
- A. Matencio, N. K. Dhakar, F. Bessone, G. Musso, R. Cavalli, C. Dianzani, F. García-Carmona, J. M. López-Nicolás and F. Trotta, Carbohydr. Polym., 2020, 231, 115763 CrossRef CAS.
- R. Pushpalatha, S. Selvamuthukumar and D. Kilimozhi, J. Drug Delivery Sci. Technol., 2017, 39, 362–371 CrossRef CAS.
- S. Anandam and S. Selvamuthukumar, J. Porous Mater., 2014, 21, 1015–1023 CrossRef CAS.
- Z. Rezaei, S. Khabnadideh, K. Pakshir, Z. Hossaini, F. Amiri and E. Assadpour, Eur. J. Med. Chem., 2009, 44, 3064–3067 CrossRef CAS.
- M. Ramírez-Ambrosi, F. Caldera, F. Trotta, L. Á. Berrueta and B. Gallo, J. Inclusion Phenom. Macrocyclic Chem., 2014, 80, 85–92 CrossRef.
- Z. A. M. Zielinski and D. A. Pratt, J. Org. Chem., 2017, 82, 2817–2825 CrossRef CAS.
- M. C. Foti, J. Agric. Food Chem., 2015, 63, 8765–8776 CrossRef CAS.
- R. Shah, L. A. Farmer, O. Zilka, A. T. M. Van Kessel and D. A. Pratt, Cell Chem. Biol., 2019, 26, 1594 CrossRef CAS.
- M. Massaro, S. Riela, S. Guernelli, F. Parisi, G. Lazzara, A. Baschieri, L. Valgimigli and R. Amorati, J. Mater. Chem. B, 2016, 4, 2229–2241 RSC.
- M. Massaro, R. Amorati, G. Cavallaro, S. Guernelli, G. Lazzara, S. Milioto, R. Noto, P. Poma and S. Riela, Colloids Surf., B, 2016, 140, 505–513 CrossRef CAS.
- C. Viglianisi, A. Scarlini, L. Tofani, S. Menichetti, A. Baschieri and R. Amorati, Sci. Rep., 2019, 9, 17219 CrossRef.
- A. Rauf, M. Imran, I. E. Orhan and S. Bawazeer, Trends Food Sci. Technol., 2018, 74, 33–45 CrossRef CAS.
- V. Pittalà, L. Salerno, G. Romeo, R. Acquaviva, C. Di Giacomo and V. Sorrenti, Curr. Med. Chem., 2018, 25, 4827–4836 CrossRef.
- R. V. Patel, B. M. Mistry, S. K. Shinde, R. Syed, V. Singh and H.-S. Shin, Eur. J. Med. Chem., 2018, 155, 889–904 CrossRef CAS.
- L. D. Wilson, M. H. Mohamed and C. L. Berhaut, Materials, 2011, 4, 1528–1542 CrossRef CAS.
- L. D. Wilson, M. H. Mohamed and J. V. Headley, J. Colloid Interface Sci., 2011, 357, 215–222 CrossRef CAS.
- R. Amorati, A. Baschieri and L. Valgimigli, J. Chem., 2017, 6369358 Search PubMed.
- R. Amorati and L. Valgimigli, J. Agric. Food Chem., 2018, 66, 3324–3329 CrossRef CAS.
- R. Amorati, A. Baschieri, G. Morroni, R. Gambino and L. Valgimigli, Chem. – Eur. J., 2016, 22, 7924–7934 CrossRef CAS.
- E. T. Denisov and I. V. Khudyakov, Chem. Rev., 1987, 87, 1313–1357 CrossRef CAS.
- M. C. Foti, R. Amorati, A. Baschieri and C. Rocco, Biophys. Chem., 2018, 243, 17–23 CrossRef CAS.
- R. Amorati, A. Baschieri, A. Cowden and L. Valgimigli, Biomimetics, 2017, 2, 9 CrossRef.
- C. Spatafora, C. Daquino, C. Tringali and R. Amorati., Org. Biomol. Chem., 2013, 11, 4291–4294 RSC.
- S. Saito, H. Gao and J. Kawabata, Helv. Chim. Acta, 2006, 89, 821–831 CrossRef CAS.
- A. Baschieri, R. Amorati, T. Benelli, L. Mazzocchetti, E. D’Angelo and L. Valgimigli, Antioxidants, 2019, 8, 30, DOI:10.3390/antiox8020030.
- A. Schneider, J. Hemmerlé, M. Allais, J. Didierjean, M. Michel, M. d’Ischia and V. Ball, ACS Appl. Mater. Interfaces, 2018, 10, 7574–7580 CrossRef CAS.
- Y. J. Moon, L. Wang, R. DiCenzo and M. E. Morris, Biopharm. Drug Dispos., 2008, 29, 205–217 CrossRef CAS.
- C. Schneider, O. N. Gordon, R. L. Edwards and P. B. Luis, J. Agric. Food Chem., 2015, 63, 7606–7614 CrossRef CAS.
- L. R. C. Barclay and K. U. Ingold, J. Am. Chem. Soc., 1981, 103, 6478–6485 CrossRef CAS.
- F. Mollica, M. Lucarini, C. Passerini, C. Curati, S. Pavoni, L. Bonoldi and R. Amorati, Antioxidants, 2020, 9, 399 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |