Open Access Article
Open Access ArticleThe efficient biogeneration of Ag and NiO nanoparticles from VPLE and a study of the anti-diabetic properties of the extract
Hongying Gaoa,
Reza Tayebee *b,
Mojtaba Fattahi Abdizadeh*cd,
Esrafil Mansourie,
Maryam Latifniaf and
Zahra Pourmojahedg
*b,
Mojtaba Fattahi Abdizadeh*cd,
Esrafil Mansourie,
Maryam Latifniaf and
Zahra Pourmojahedg
aDepartment of Chinese Medicine, Binzhou City Central Hospital, Binzhou, Shandong Province 251700, China
bDepartment of Chemistry, School of Sciences, Hakim Sabzevari University, Sabzevar, 96179-76487, Iran. E-mail: rtayebee@hsu.ac.ir
cDepartment of Lab Sciences, Faculty of Paramedicine, Sabzevar University of Medical Sciences, Sabzevar, Iran. E-mail: mojtabafattahi@gmail.com
dCellular and Molecular Research Center, Sabzevar University of Medical Sciences, Sabzevar, Iran
eDepartment of Anatomical Sciences, Cellular and Molecular Research Center, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
fDepartment of Gasterointestinal and Liver Disease, Faculty of Medicine, Sabzevar University of Medical Sciences, Sabzevar, Iran
gDiabetes Clinic, Sabzevar University of Medical Sciences, Sabzevar, Iran
First published on 16th January 2020
Abstract
Vitex pseudo-negundo leaf extract (VPLE) is used to mediate the green biosynthesis of Ag and NiO nanoparticles in aqueous solutions under mild conditions. The synthesized nanoparticles, with a narrow size range and good distribution, are characterized by means of powder X-ray diffraction (PXRD), Fourier-transform infrared (FT-IR), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX), and transmission electron microscopy (TEM) techniques. SEM and TEM micrographs proved formation of mostly spherical or ellipsoidal nanoparticles with little agglomeration, and the average particle size was less than 20–35 nm for both types of nanoparticle. Then, the protective role of VPLE toward the liver is assessed in streptozotocin-induced diabetic rats. For this purpose, diabetes is induced in rats through the intraperitoneal injection of streptozotocin, and VPLE is administered via oral gavage for 6 weeks. This study suggests that VPLE can ameliorate biochemical and structural changes in the livers of diabetic rats, showing that VPLE can improve the condition of rats with diabetic hepatopathy via a decrease in oxidative stress and an enhancement in the activity of antioxidant enzymes in the liver.
1. Introduction
Nanomedicine and nanobiotechnology are emerging fields of nanoscience that utilize therapeutic nanoparticles for various biomedical applications.1 The green facilitated synthesis of metallic and metal oxide nanoparticles is an important and growing objective in the nanotechnology field due to the unusual optical, chemical, photochemical and electronic properties of the fabricated nanoparticles.2 Among the various novel eco-friendly scenarios for the production of nanoparticles, plant extracts possess a wide range of biologically active compounds, which have been exploited as top resources for the green synthesis of nanoparticles.3,4 These biosynthetic methods have received more attention than common chemical and physical methodologies due to their ease of synthesis and their eco-friendly and cost-effective nature.5–9 Therefore, biological resources can be used in rapid, effective, simple, and economic ways to produce desired nanoparticles.10 Furthermore, plant extracts behave as both capping and reducing agents during biosynthesis; therefore, these biotemplates can control the sizes, shapes, and morphologies of the prepared nanoparticles through rendering a coordination sphere for capturing metal ions.11 Among the various available nanoparticles, silver and NiO nanoparticles have attracted wide interest due to their numerous applications in industry and academia. Silver nanoparticles have widespread applications in non-linear optics, biolabelling, and optical receptors, as well as in dentistry, catalysis, and the food industry.12 Furthermore, NiO nanoparticles also have a number of important applications in energy storage devices,13 drug delivery, and magnetic resonance imaging.14 Therefore, developing efficient and simple methods for the fabrication of Ag and NiO nanoparticles with controlled size is a favorable target for many scholars.Vitex pseudo-negundo (VPN) is a familiar herb that commonly grows in wasteland as a hedge plant. Numerous studies have shown that the roots, flowers, fruits, and leaves of this plant have great medicinal benefits. The leaves of VPN contain flavonoids, alkaloids, monoterpenes, eurostoside, saturated steroidal and triterpinoidal saponins, amino acids, and aromatic amines.15 This herb is traditionally utilized to cure and treat many deficiencies, such as amenorrhea, corpus luteum insufficiency, dysmenorrhea, hyperprolactinemia, and breastfeeding disorders.16,17 Moreover, extracts from different parts of this plant have antibacterial,18 anti-inflammatory,19 anti-diabetic,20 antioxidant,21 anti-fungal,22 and anti-HIV23 activities. Recently, this herb was reported to show cytotoxic activity against a human breast cancer cell line.24 In a continuation of our previous studies,25 herein, the use of the leaf extract of this plant is demonstrated for the environmentally friendly, fast, cost-effective, and one-step synthesis of Ag and NiO nanoparticles under ambient conditions and without any external additives.
The antidiabetic activities and effects of different nanoparticles on various tissue injuries and tumors are of interest, and they have been investigated by several research groups.26–32 Diabetes mellitus (DM), which is characterized by the existence of chronic hyperglycemia, is known as one of the five main causes of death.33–35 Liver disease is considered as one of the major complications related to oxidative stress disorders like DM36 and ethological studies have provided many evidence that chronic hyperglycemia causes complicated diabetic hepatopathy.37,38 Oxidative stress provides an effective mechanism by which the glycation process, a precise enzyme-controlled process related to glucose metabolism, the hyper-production of free-radical precursors, and a decrease in the efficiency of antioxidants all contribute to a surge in disease severity.37,39 It has been shown that Vitex constituents have protective effects against experimentally induced diabetic complications through increasing the antioxidant enzyme activity. VPN is a studied model that has been employed to produce multiple effective remedies.40–44 We evaluated the hypoglycemic effects of VPLE in an induced diabetes animal model using Wistar male rats. Furthermore, this study examined some potentially important clinical parameters, including antioxidant activities and lipid peroxidation, related to streptozotocin-induced diabetic hepatopathy in the livers of rats and compared the results with treated and untreated groups. The effects of VPLE treatment on liver function in the affected rats were also evaluated. The findings of the present study may provide new alternative herbal medicine approaches for controlling diabetic complications in humans.
2. Results and discussion
2.1. Morphological and characterization studies
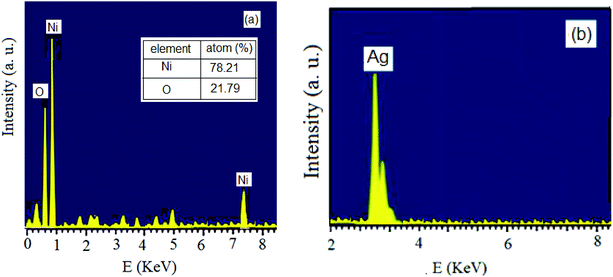
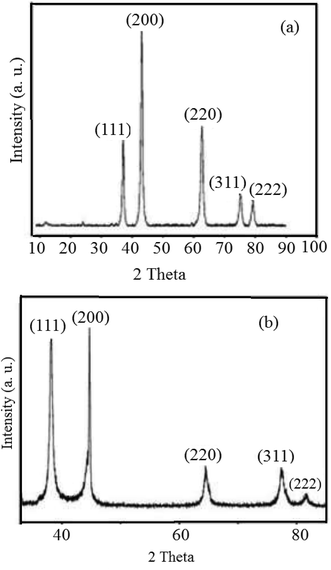
The reduction of Ag+ and Ni2+ ions into nanoparticles was easily demonstrated via the distinct color change from pale yellow-green to dark brown and yellow, respectively. Color changes in aqueous solutions of nanoparticles are due to surface plasmon resonance phenomena. Obviously, the formation of mostly stable complexes between nanoparticles and effective plant extract functional groups can stabilize the prepared particles.45,46 To examine the elemental compositions of the NiO and Ag nanoparticles and ensure the absence of organic elements originating from the plant extract, EDX analysis was conducted (Fig. 1). The obtained profiles of the synthesized nanoparticles confirmed the major presence of only nickel, oxygen, and Ag in the nanoparticles, without any other impurities.Fig. 2 displays the PXRD patterns of NiO and Ag nanoparticles fabricated using VPLE under the outlined experimental conditions. Five sharp and intense Bragg reflections were observed with maxima centered at 2θ values of 37.4, 43.4, 63.1, 75.4, and 79.5° due to the (111), (200), (220), (311) and (222) crystal planes, respectively, of cubic NiO (JCPDS card no. 73-1523) (Fig. 2a). Using the Debye–Scherrer equation, the average size of the NiO nanoparticles was found to be 17–25 nm. Fig. 2b depicts the wide-angle PXRD pattern of the Ag nanoparticles, showing diffraction peaks at 38.2, 44.5, 64.6, 77.5, and 81.7° related, respectively, to the 111, 200, 220, 311, and 222 crystallographic planes of face centered cubic Ag (JCPDS card no. 01-087-0719).47,48 Clearly, the Ag nanoparticles were crystalline and no other impurity phases were found. An average particle size of less than 20 nm was calculated using the Debye–Scherrer equation for the Ag nanoparticles.
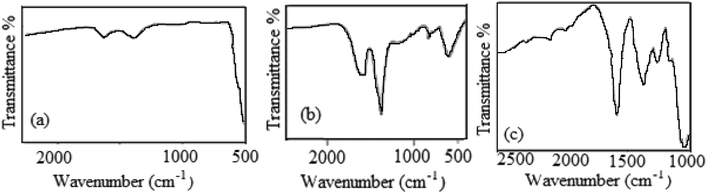
Fig. 3 shows the FT-IR spectra of NiO and Ag nanoparticles in the spectral range of 500–4000 cm−1 after simple calcination to remove the organic components from the leaf extract. The characteristic vibration bands in Fig. 3a at around 500–650 cm−1 are distinctive modes from the NiO phase due to metal–oxygen stretching.49 Additional bands at ∼3500 cm−1 and 1300–1600 cm−1 are due to adsorbed water molecules and surface hydroxyl groups, respectively. It should be mentioned that the non-appearance of any functional groups due to biomolecules from VPLE confirmed the complete combustion of the organic shell materials around the nanoparticles. Fig. 3b shows the FT-IR spectrum of the Ag nanoparticles prepared using VPLE. The interaction of the Ag nanoparticles with the shell biomolecules leads to strong and clear peaks at around 1350–1650 cm−1. The strong peak at ∼650 cm−1 is a characteristic band from Ag nanoparticles. Moreover, a strong and broad band was observed at around 3400 cm−1 in the spectra of both the NiO and Ag nanoparticles, which should be assigned to the hydroxyl groups of adsorbed water molecules.
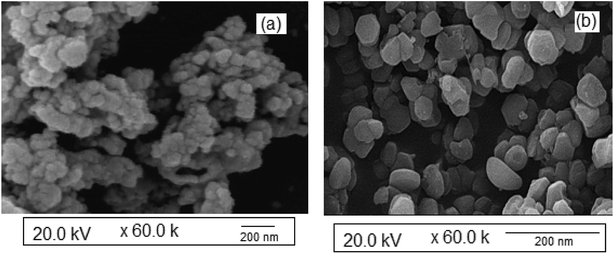
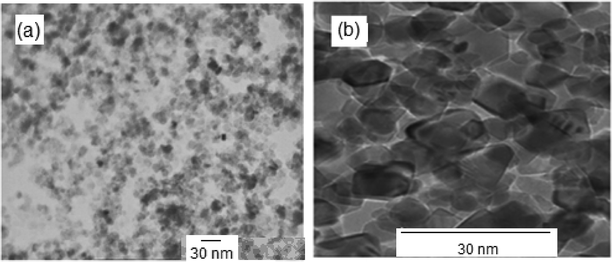
The surface morphology of the synthesized NiO nanoparticles was studied via SEM (Fig. 4b). The particles were almost spherical in shape and some agglomeration was detected. The SEM image in Fig. 4a shows the morphology of the Ag nanoparticles, confirming the presence of almost ellipsoid Ag nanoparticles. The morphologies and sizes of the NiO and Ag nanoparticles were also determined via TEM (Fig. 5). This study confirmed the narrow particle distributions. Fig. 5a shows that the Ag nanoparticles have an almost spherical shape and are well-dispersed. The TEM image reveals a mean diameter of less than 20 nm for the Ag nanoparticles.
2.2. Studying the protective role of VPLE in the livers of streptozotocin-induced diabetic rats
The present study examined the liver-protecting effects of VPLE in an STZ-induced diabetic hepatopathy model. Similar to previous research using other herbal extracts,50 our results indicated that VPLE successfully improved and regulated the FBS, ALT, AST, ALP and Alb levels in diabetic rats at significant levels compared to those left untreated by VPLE (Table 1). We demonstrated that 500 mg kg−1 VPLE was more effective than 250 mg kg−1. The obtained results suggest that VPLE has hypoglycemic activity. These observations validate and support the traditional use of VPLE against such diseases.| Parameter | Group | |||
|---|---|---|---|---|
| Healthy control | Diabetic control | VLHE (250 mg kg−1) | VLHE (500 mg kg−1) | |
| a Results are expressed as mean ± SE (n = 5); * means in diabetic and healthy control groups are significantly different from each other (P < 0.01); ** means in diabetic + VLHE (250 and 500 mg kg−1) and diabetic groups are significantly different from each other (P < 0.05). | ||||
| GLU (mg dl−1) | 94.4 ± 7.54 | 366.2 ± 139.26* | 148.5 ± 20.21** | 139 ± 28.91** |
| ALT (U/l) | 64.2 ± 11.0 | 145 ± 18.15* | 80.4 ± 5.94** | 72.6 ± 5.13** |
| AST (U/l) | 115.2 ± 10.91 | 205 ± 32.18* | 136.4 ± 11.97** | 119.8 ± 17.72** |
| ALP (U/l) | 112.8 ± 12.9 | 237 ± 58.83* | 140 ± 12.16** | 117.6 ± 7.7** |
| Alb (g dl−1) | 3.73 ± 0.26 | 2.19 ± 0.27* | 2.876 ± 0.24** | 3.062 ± 0.26** |
The initial and most important indicators for assessing liver injury are the levels of ALT, AST, ALP and Alb. A probable mechanism explaining the above-mentioned effects would be disturbances in the cellular architecture of diabetics, evoked by severe pro-oxidative stress.38,39 The production of oxidants can induce apoptosis in hepatocytes and can induce oxidative stress, which is an indicator of these disturbances.38 Notably, it is found that hyperglycemia decreases the synthesis and activity of some antioxidizing enzymes, such as SOD and GPx, likely via a glycation process.
In the present study, like other studies51,52 using liver-protecting herbal extracts, we demonstrated that VPLE considerably reduced lipid peroxidation and increased the activities of SOD and GPx in diabetic rats compared to healthy controls (Table 2). VPLE played a protective role against experimentally induced diabetic hepatopathy, which may be due to the inhibition of AGE formation. The inhibitory effects of VPLE could be related to the antioxidant activity of VPLE.38,53,54 The remarkable free radical scavenging activity of VPLE could be a potential reason for its effects on reversing lipid peroxidation levels and on the antioxidant activities of SOD and GPx enzymes.
| Parameter | Group | |||
|---|---|---|---|---|
| Healthy control | Diabetic control | VLHE (250 mg kg−1) | VLHE (500 mg kg−1) | |
| a Results are expressed as mean ± SE (n = 5); * means in diabetic and healthy control groups are significantly different from each other (P < 0.01); ** means in diabetic + VLHE (250 and 500 mg kg−1) and diabetic groups are significantly different from each other (P < 0.05). | ||||
| GPx | 19.79 ± 0.60 | 10.44 ± 1.06* | 18.18 ± 1.02** | 18.24 ± 1.60** |
| SOD | 17.09 ± 0.78 | 11.80 ± 0.75* | 16.54 ± 0.47** | 16.93 ± 0.43** |
| MDA | 17.43 ± 1.09 | 30.18 ± 2.19* | 21.18 ± 1.29** | 20.10 ± 1.36** |
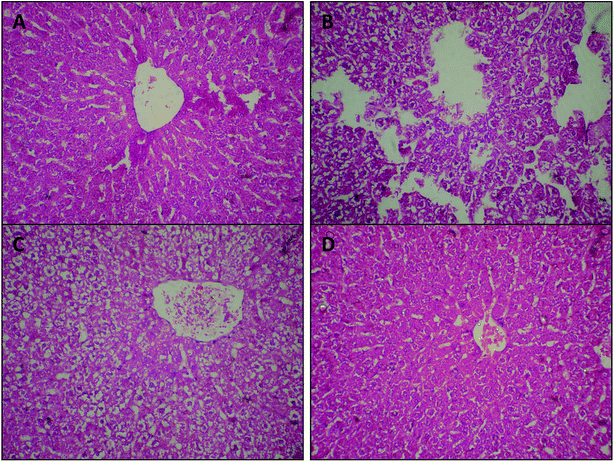
Considering hepatopathy as one of the most important diabetes-induced complications,38,55 we performed histological examinations of H&E stained liver samples and tested pathological tissue variations. Histopathological examinations revealed that VPLE administration (either at a dose of 250 or 500 mg kg−1) may reverse the degeneration of liver tissue structures and lead to the reappearance of hyperplastic hepatocytes in damaged liver, as shown in Fig. 6c and d. On the other hand, mild to rigorous destruction, cellular abnormalities with areas of vascular degeneration, necrosis, and cellular degeneration were detected in liver samples from STZ-induced diabetic rats, compared to the normal control group (Fig. 6b). These findings are consistent with a previous study56 that reported the positive effects of Vitex negundo, which is botanically close to VPN, on liver tissue.
The results in Fig. 6 demonstrate that VPLE at a dose of 250 mg kg−1 leads to some improvements in liver tissue, with moderate areas of cellular restoration and pyknotic nuclei compared to normal and diabetic control groups; however, the destruction of liver structures was still visible (Fig. 6c). On the other hand, 500 mg kg−1 VPLE completely reversed cellular architecture degeneration and led to the reappearance of hyperplastic hepatocytes in the damaged liver (Fig. 6d).
3. Experimental
3.1. Materials, methods, and characterization techniques
Grown leaves of VPN were collected from Sabzevar Province, situated in the east of Iran, during the flowering stage at a rain-free time, and they were air-dried at ambient temperature. AgNO3 (99.98%), nickel nitrate hexahydrate (99.98%), and methanol (CH3OH, 99.9%) were purchased from Merck (Germany). All aqueous solutions were prepared using double-distilled water. Fourier transform infrared (FT-IR) spectra were obtained using an 8700 Shimadzu Fourier transform spectrophotometer in the form of diluted samples (10 wt%) pressed into KBr pellets. PXRD patterns of the prepared nanoparticles were obtained using a computer controlled STOE diffractometer with monochromatic Cu Kα radiation (λ = 1.5406 Å), operating at a voltage and current of 40 kV and 35 mA, respectively, in the angular range of 0–90°. The morphologies and structures of the expected nanoparticles were studied by means of a field emission scanning electron microscope (JEOL, Model JFC-1600) and transmission electron microscope (PHILIPS TECNAI 10) equipped with an energy dispersive X-ray (EDX) analysis instrument.3.2. Preparation of nickel oxide nanoparticles
Air-dried VPN leaves (100 g) were blended with deionized water (1000 ml) in a Clevenger-type apparatus. Then, the obtained green aqueous solution was heated to about 100 °C for 1.5 h. The natural extract solution was filtered properly. Nickel nitrate hexahydrate was used as the nickel precursor without any further purification. In a typical procedure, 20 ml of VPN plant aqueous extract was mixed dropwise with 75 ml of 0.1 mM aqueous Ni(NO3)2 solution under constant stirring at 80 °C for 2 h. Then, the solution was evaporated gently, and the obtained dark green-brown precipitate was transferred to a ceramic container and heated to 400 °C in a furnace for 10 h to obtain an annealed light-yellow powder.3.3. Preparation of silver nanoparticles
Typically, 5 ml of aqueous extract solution was added to 50 ml of 1 mM silver nitrate solution under vigorous stirring at room temperature for 2 h. Finally, the obtained Ag nanoparticles were separated via two centrifugation runs at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min at 4 °C.
000 rpm for 30 min at 4 °C.
3.4. Animal model
Forty healthy Wistar male rats were selected and kept at the animal house of the Sabzevar University of Medical Science under the standard acclimatization conditions (22 ± 3 °C, 12 h light/dark cycle). Then, they were fed, acclimatized, and maintained based on rodent diet guidelines issued by the International Council of Laboratory Animal Science (ICLAS). Their weight was between 180 and 200 g at the time of study.3.5. Studying the protective role of VPLE in the livers of streptozotocin-induced diabetic rats
Forty Wistar male rats were divided into four groups, with ten animals in each group. These included a healthy control group, an untreated streptozotocin-induced diabetic group, and treated diabetic groups, which were separately treated with 250 and 500 mg kg−1 VPLE, respectively, via oral gavage for a 6 week period. Diabetes was induced in rats via the intraperitoneal injection of STZ (Sigma, Millstone, USA) (45 mg kg−1). All rats that showed fasting blood sugar levels of above 200 mg dl−1 were considered as diabetic.4. Conclusions
In summary, we have described a simple biosynthetic method for the preparation of Ag and NiO nanoparticles using aqueous VPLE. Both types of nanoparticle were successfully characterized with different techniques. Therefore, this herb is shown to be a pollutant-free and potent biogenerator of these nanoparticles under aerobic conditions. According to our results, we may claim that VPLE can improve the condition of rats with diabetic hepatopathy via a decrease in oxidative stress and an enhancement in the activities of antioxidant enzymes in the liver.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by Sabzevar University of Medical Sciences and Hakim Sabzevari University.References
- M. V. Yezhelyev, X. Gao, Y. Xing, A. Al-Hajj, S. Nie and R. M. O'Regan, Lancet Oncol., 2006, 7(8), 657–667 CrossRef CAS PubMed.
- F. Mohammadi, M. Yousefi and R. Ghahremanzadeh, Adv. J. Chem., Sect. A, 2019, 2(4), 266–275 CrossRef.
- N. Ahmad, S. Sharma, M. K. Alam, V. N. Singh, S. F. Shamsi and B. R. Mehta, Colloids Surf., B, 2010, 81, 81–86 CrossRef CAS PubMed.
- M. Sathishkumar, K. Sneha, S. W. Won, C. W. Cho, S. Kim and Y. S. Yun, Colloids Surf., B, 2009, 73, 332 CrossRef CAS PubMed.
- B. Mahdavi, S. Saneei, M. Qorbani, M. Zhaleh, A. Zangeneh, M. M. Zangeneh, E. Pirabbasi, N. Abbasi and H. Ghaneialvar, Appl. Organomet. Chem., 2019, 5164 Search PubMed.
- S. S. Shankar, A. Rai, A. Ahmad and M. J. Sastry, J. Colloid Interface Sci., 2004, 275, 496 CrossRef CAS PubMed.
- F. Thema, E. Manikandan and A. Gurib-Fakim, J. Alloys Compd., 2016, 657, 655–661 CrossRef CAS.
- N. Thovhogi, A. Diallo and A. Gurib-Fakim, J. Alloys Compd., 2015, 647, 392–396 CrossRef CAS.
- F. Thema, E. Manikandan and M. Dhlamini, Mater. Lett., 2015, 161, 124–127 CrossRef CAS.
- M. Ovais, A. T. Khalil and A. Raza, Nanomedicine, 2016, 12, 3157–3177 CrossRef PubMed.
- P. Laokul, V. Amornkitbamrung, S. Seraphin and S. Maensiri, Curr. Appl. Phys., 2011, 11, 101 CrossRef.
- S. B. Manjare, S. G. Sharma, V. L. Gurav, M. R. Kunde, S. S. Patil and S. R. Thopate, Asian J. Nanosci. Mater., 2019 DOI:10.26655/AJNANOMAT.2020.1.6.
- N. H. Idirs, J. Z. Wang, S. Chou, C. Zhong, Md. M. Rahman and H. Liu, J. Mater. Res., 2011, 26, 860 CrossRef.
- J. R. Richardson, D. I. Yiagas, B. Turk, K. Forster and M. V. Twigg, J. Appl. Phys., 1991, 70, 6977 CrossRef CAS.
- S. Mohanraj, p. Vanathi, N. Sowbarniga and D. Saravanan, Indian J. Fibre Text. Res., 2012, 37, 389 CAS.
- V. R. Tandon and R. K. Guota, Indian J. Physiol. Pharmacol., 2005, 49(2), 199–205 CAS.
- H. Kirmizibekmez, E. Ariburnu, M. Masullo, M. Festa, A. Capasso, E. Yesilada and S. Piacente, Fitoterapia, 2012, 83(1), 130–136 CrossRef CAS PubMed.
- R. P. Samy, S. Ignacimuthu and A. Sen, J. Ethnopharmacol., 1998, 62, 173–182 CrossRef.
- R. S. Telang, S. Chatterjee and C. Varshneya, Indian J. Pharmacol., 1999, 31, 363–366 Search PubMed.
- R. Manikandan, R. Sundaram, P. Srinivan, S. Beulaja and C. Arulvasu, Int. J. Pharm. Anal., 2009, 2, 4–10 Search PubMed.
- M. Umamaheswari, K. Asokumar, A. Somasundaram, T. Sivashanmugam, V. Subhadradevi and T. K. Ravi, J. Ethnopharmacol., 2007, 109, 547–551 CrossRef PubMed.
- M. I. Alam, J. Ethnopharmacol., 2003, 86, 75–80 CrossRef CAS.
- W. Woradulayapinij, N. Soonthonhareonnon and C. Wiwat, J. Ethnopharmacol., 2005, 101, 84–89 CrossRef.
- C. Arulvasu, D. Prabhu, R. Manikandan, P. Srinivasan, S. Sellamuthu and D. Dinesh, Int. J. Drug Discovery, 2010, 2(1), 1–7 Search PubMed.
- R. Tayebee, E. Filehkesh and V. Amani, Asian J. Chem., 2007, 19(3), 1772 CAS.
- Th. I. Shaheen, M. E. El-Naggar, J. S. Hussein, M. El-Bana, E. Emara, Z. El-Khayat, M. M. G. Fouda, H. Ebaid and A. Hebeish, Biomed. Pharmacother., 2016, 83, 865 CrossRef CAS PubMed.
- S. M. El-Sayed, M. E. El-Naggar, J. Hussein, D. Medhat and M. El-Banna, Colloids Surf., B, 2019, 184, 110465 CrossRef CAS PubMed.
- J. Hussein, M. F. Attia, M. El Bana, S. M. El-Daly, N. Mohamed, Z. El-Khayat and M. E. El-Naggar, Int. J. Biol. Macromol., 2019, 140, 1305 CrossRef CAS PubMed.
- R. A. Hussein, A. A. A. Salama, M. E. El Naggar and G. H. Ali, Biocatal. Agric. Biotechnol., 2019, 20, 101237 CrossRef.
- J. Hussein, M. E. El-Naggar, Y. AbdelLatif, D. Medhat, M. El Bana, E. Refaat and S. Morsy, Colloids Surf., B, 2018, 170, 76 CrossRef CAS PubMed.
- J. Hussein, M. El-Banna, T. Abdel Razik and M. E. El-Naggar, Int. J. Biol. Macromol., 2018, 107, 748 CrossRef CAS PubMed.
- D. Medhat, J. Hussein, M. E. El-Naggar, M. F. Attia, M. Anwar, Y. Abdel Latif, H. F. Booles, S. Morsy, A. Razik Farrag, W. K. B. Khalil and Z. El-Khayat, Biomed. Pharmacother., 2017, 91, 1006 CrossRef.
- M. C. Blendea, M. J. Thompson and S. Malkani, Clin. Diabetes, 2010, 28(4), 139–144 CrossRef.
- K. Kumar, S. Mehershahi, C. Chime, H. Tariq, S. K. Nayudu and S. Chilimuri, Case. Rep. Gastroenterol., 2018, 12(2), 466–472 CrossRef PubMed.
- F. Stickel and D. Schuppan, Dig. Liver Dis., 2007, 39(4), 293–304 CrossRef PubMed.
- H. Yaribeygi, M. T. Mohammadi and A. Sahebkar, Biomed. Pharmacother., 2018, 98, 333–337 CrossRef PubMed.
- S. Bansal, D. Chawla, M. Siddarth, B. D. Banerjee, S. V. Madhu and A. K Tripathi, Clin. Biochem., 2013, 46(1–2), 109–114 CrossRef PubMed.
- J. Mohamed, A. H. Nazratun Nafizah, A. H. Zariyantey and S. B. Budin, Sultan Qaboos Univ. Med. J., 2016, 16(2), 132–141 CrossRef PubMed.
- E. Mansouri, M. Panahi, M. A. Ghaffari and A. Ghorbani, Iran. Biomed. J., 2011, 15(3), 100–106 Search PubMed.
- E. W. C. Chan, S. K. Wong and H. T. Chan, J. Integr. Med., 2018, 16(3), 147–152 CrossRef PubMed.
- T. Movahhed Haghighi, M. J. Saharkhiz, A. R. Khosravi, F. Raouf Fard and M. Moein, Ind. Crops Prod., 2017, 109, 53–59 CrossRef.
- M. Mozdianfard, M. Akhbari and H. Batooli, Nat. Prod. Res., 2012, 26(23), 2162–2167 CrossRef PubMed.
- H. Akhlaghi, J. Chem. Health Risks, 2017, 7, 3 Search PubMed.
- H. ahmadvand, H. amiri, S. Ekbatan Hamadani and S. Bagheri, Sci. Mag. Yafte., 2012, 14(2), 5–13 Search PubMed.
- V. Nayagam, K. Palanisamy and D. Thiraviadoss, Asian J. Nanosci. Mater., 2019, 2(3), 301–313 Search PubMed.
- M. A. Nasseri, M. Shahabi, A. Allahresani and M. Kazemnejadi, Asian J. Green Chem., 2019, 3(3), 382–390 Search PubMed.
- M. B. Ahmad, M. Y. Tay, K. Shameli, M. Z. Hussein and J. J. Lim, Int. J. Mol. Sci., 2011, 12, 4872 CrossRef.
- M. B. Ahmad, K. Shameli, M. Darroudi, W. M. Z. Wan Yunus and N. A. Ibrahim, Am. J. Appl. Sci., 2009, 6, 1909 CrossRef.
- G. E Snopatin, M. Yu, M. Matveeva and G. G. Butsyn, Inorg. Mater., 2006, 42, 1388–1392 CrossRef.
- A. Zarei, G. Vaezi, A. A. Malekirad and M. Abdollahi, Avicenna J. Phytomed., 2015, 5(2), 138–147 Search PubMed.
- F. Hussain, A. Malik, U. Ayyaz, H. Shafique, Z. Rana and Z. Hussain, Asian Pac. J. Trop. Med., 2017, 10(11), 1054–1058 CrossRef.
- N. A. Mat-Rahim, T. H. Lim, N. A. Nor-Amdan and S. AbuBakar, J. Evidence-Based Complementary Altern. Med., 2017, 6125829 Search PubMed.
- S. M. Kalbolandi, A. V. Gorji, H. Babaahmadi-Rezaei and E. Mansouri, Mol. Biol. Rep., 2019, 46(4), 4039–4047 CrossRef.
- E. A. Oshaghi, I. Khodadadi, F. Mirzaei, M. Khazaei, H. Tavilani and M. T. Goodarzi, J. Pharm., 2017, 6081374 Search PubMed.
- R. Kumar, Indian J. Endocrinol. Metab., 2018, 22(4), 552–559 CrossRef CAS.
- Y. Avadhoot and A. C. Rana, Arch. Pharmacal Res., 1991, 14(1), 96–98 CrossRef CAS.
- E. Mansouri, L. Khorsandi and H. A. Abedi, Iran. J. Basic Med. Sci., 2014, 17(6), 460–464 Search PubMed.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS.
- E. Mansouri, M. Panahi, M. A. Ghaffari and A. Ghorbani, Iran. Biomed. J., 2011, 15, 100–106 CAS.
- E. A. Golod and A. B. Gershman, Urologiia i nefrologiia, 1989, 4, 50–55 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |