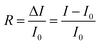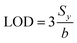Open Access Article
Open Access ArticleVolatile organic compound gas sensors based on methylammonium lead iodide perovskite operating at room temperature†
Anafi Nur'aini a and
Ilwhan Oh
a and
Ilwhan Oh *ab
*ab
aDepartment of IT Convergence Engineering, Kumoh National Institute of Technology, Gumi, Gyeongbuk, South Korea 39177. E-mail: ioh@kumoh.ac.kr
bDepartment of Applied Chemistry, Kumoh National Institute of Technology, Gumi, Gyeongbuk, South Korea 39177
First published on 1st April 2020
Abstract
Methylammonium lead iodide (MAPbI3) perovskite thin film has been successfully applied to a volatile organic compound (VOC) gas sensor that can operate at room temperature. In this study, ∼100 nm-thick MAPbI3 film shows good reversibility and repeatability as a VOC gas sensor. The resistance of the MAPbI3 film substantially decreases when it is exposed to VOC vapour and recovers back to high resistance when the VOC gas is removed. Adsorption of VOC gas molecules to vacancies in MAPbI3 film might lead to charge trap passivation. The VOC sensor based on perovskite thin film is tested in terms of film thickness, applied bias voltage, and polarity of VOC. We expect that our VOC gas sensor based on solution-processed MAPbI3 operating at room temperature has potential to be developed as a low cost and low power smart gas sensor.
Introduction
Gas sensing technology has a crucial role in air condition monitoring,1 safety instruments in laboratories and industry,2 and exhalation analysis for disease diagnosis.3,4 Some key requirements for gas sensors are sensitivity, fast response, room temperature operation, and low-cost fabrication. Regarding the principle of detection, various gas sensor types can be categorized into electrochemical types,5 catalytic types,6 metal oxide types,7–10 and infrared types.10 Recently, due to the emergence of smart sensor technology, the requirement is emphasized on low power and small size devices.11,12 Unfortunately, those requirements are difficult to meet in a specific sensor type. For example, to keep the high operating temperature, metal oxide gas sensor consumes high power although its sensing element can be fabricated in a microscale device.10Volatile organic compound (VOC) gases contribute to air pollution in environment,13 which is more severe in indoor space. Nevertheless, some VOC gases are proven to be disease biomarkers.3,14 In abnormal metabolism, VOC can be produced and disposed through exhales breath.3 Unlike healthy people, lung cancer patients have different VOC profile in exhale breath.14 Moreover, VOCs are omnipresent in our daily lives due to their high volatility and have been commonly used for household products such as paints, aerosol sprays, moth repellents, pesticides, adhesive, and air fresheners. However, VOCs can stimulate health problems such as respiratory diseases,15 eye irritation,16 skin allergy,17 fatigue,18 and central nervous system disorder.19
Several metal oxide semiconductors have been widely utilized as sensing materials in chemical gas sensors, including SnO2,20 Co3O4,21 ZnO,22 and TiO2.23 Traditional metal oxide materials need high temperature (∼250–300 °C) to operate,9 although recently some metal oxide materials are developed to be operated in lower temperature.24 Finding a new semiconductor material for gas sensor that can operate at room temperature is a relevant topic.
Metal halide perovskites are popular as solar cell devices. The efficiency of solar cell based on metal halide perovskite increases from 3.8% in 2009 to 23.3% in 2018.25 Molecular formula of perovskite is AMX3. A is organic cation (CH3NH3+, CH3 CH2NH3+, HC(NH2)2+), M is metal cation (Ge2+, Sn2+, Pb2+), and X is halogen anion (F−, Cl−, Br−, I−).26 Six halogen atoms (X) form octahedral structure with metal M atom located in the centre, while cation A is located at the centre of eight octahedral structure.26 Unlike most of metal oxide semiconductors, organic metal halide perovskites exhibit a relatively high conductivity even at room temperature.27 Moreover, perovskite layer can be fabricated by simple and low-cost solution deposition process.28
Recently, metal halide perovskites have emerged as material for lasers, light-emitting diodes, field-effect transistors, and gas sensors.26,27 In 2013, Zhao's group showed that the colour of MAPbI3 film was bleached when it passed through an open bottle containing 3% NH3 solution.29 The colour change was reversible when MAPbI3 film was taken away from the bottle.29 In other reports, through a chemiresistive technique, MAPbI3 perovskite was utilized as gas sensors toward various chemical vapours, such as ammonia,30 oxygen,31 and NO2.32 When MAPbI3 film was exposed to target gas molecules, its molecules might fill vacancies in MAPbI3 film then substantially elevate conductivity of MAPbI3.30–32 In contrast when an inert gas reaches MAPbI3 film, its molecules will knock out target gas molecules and recreate vacancies, thus conductivity of MAPbI3 decreases.30–32 Those reports indicate that MAPbI3 can detect not only reducing gas but also oxidizing gas.
Modified halide perovskite such as CH3NH3PbI3x(SCN)x was successfully employed as NO2 and acetone gas sensor with high sensitivity.33 Kakavelakis group reported that CH3NH3PbI3−xClx could operate as ozone gas sensor at room temperature.34 On the other hand, Chen and co-workers applied all-inorganic CsPbBr3 materials to detect ethanol, acetone, and oxygen with good sensitivity and stability.35 Nonetheless, organic metal halide perovskite especially MAPbI3 has not been extensively studied for VOCs detection.
In this paper, we fabricate a gas sensor based on a thin film MAPbI3 and show that perovskite-based device is a decent gas sensor toward various VOCs. Through conductivity and photoluminescence measurements, we propose a sensing principle that charge trap passivation model fits better than depletion layer model in traditional metal oxide sensors. Furthermore, we adjust and optimize various experimental parameters such as film applied bias, thickness, and polarity of gas molecules.
Results and discussion
Perovskite-based sensing device was fabricated via conventional microfabrication process (refer to Experimental details section). In brief, a gold interdigitated electrode (IDE) pattern was fabricated using photolithography and wet-etch on a glass substrate (Fig. 1a). Finger width was 100 μm and space width was 50 μm. Perovskite solution was deposited by a spin-coating process. After deposition, perovskite film looked uniform and mirror-like (Fig. 1b). | ||
| Fig. 1 (a) Interdigitated electrode (IDE) (b) perovskite film on the IDE, (c) gas sensing measurement setup. | ||
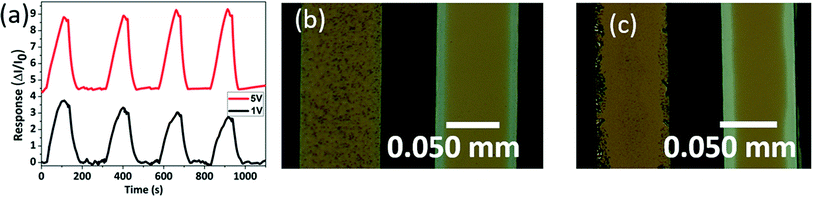
Current–time response of perovskite film gas sensor was measured by applying voltage bias to the interdigitated electrode. Measurement process was conducted at room temperature in a small chamber as shown in Fig. 1c. A known concentration of target gas was injected into the chamber. After measuring sensor response, inert gas was injected to recover perovskite film sensor. Measured current response was transformed into normalized response (R) by following equation:31
Perovskite material was characterized by X-ray diffraction and UV-visible spectroscopy. X-ray diffraction results in Fig. 2a confirms the formation of MAPbI3 tetragonal phase.36 Peaks at 14.08°, 19.98°, 28.4°, 31.92°, and 40.68° correspond to (110), (200), (220), (310), and (400) crystal planes respectively. By Scherrer equation, the average grain was calculated to be 44 nm. As Au interdigitated electrode was employed as a substrate, additional peaks at 38.2° and 44.38° correspond to (111) and (200) Au crystal planes. For UV-visible spectrum in Fig. 2b, absorption onset around 750–800 nm is consistent with the bandgap of MAPbI3. Bandgap energy is determined to be 1.58 eV from (αhv)2 vs. hv graph (Fig. S1†). This value is consistent with reported bandgap of MAPbI3.37
Top-view and cross-section images from SEM are shown in Fig. 3a and b. Fig. 3a shows small grains size and grain boundaries of perovskite film. Perovskite film thickness is ∼100 nm (Fig. 3b) and surface roughness is 15 nm (Fig. S2†). Small grains size is preferred as gas sensing application because of high surface-to-volume ratio, high sensitivity, and fast response. Grain boundaries help target gas to penetrate to perovskite film.
 | ||
| Fig. 3 Scanning electron microscope (SEM) images for (a) top-view, and (b) cross-section of MAPbI3 film. | ||
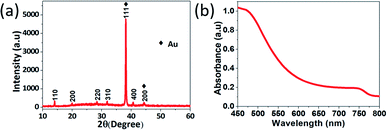
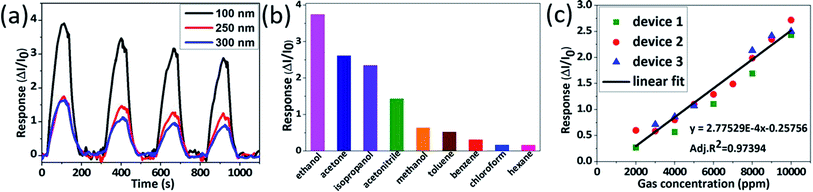
Fig. 4a shows a dynamic response–time graph of perovskite gas sensor towards 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm ethanol vapour. Significant responses emerge when perovskite film is exposed to ethanol vapour and recover to the initial background when ethanol vapour is replaced with inert gas. As shown in Fig. 4a, perovskite film has response time (trs) of 66 seconds and recovery time (trc) of 67 seconds, which are faster than other type gas sensors.38 Perovskite film shows successive responses during four cycles toward ethanol. Thus, ethanol exposure seems not to damage the structural integrity of perovskite film. Interaction between perovskite film and ethanol is assumed to be mainly physisorption and limited on perovskite grain boundaries. However, as measurement repeat, peak heights become lower. This damping probably comes from a slow side reaction between the perovskite crystal and the target gas molecules. We will discuss sensing mechanism in more detail later.
000 ppm ethanol vapour. Significant responses emerge when perovskite film is exposed to ethanol vapour and recover to the initial background when ethanol vapour is replaced with inert gas. As shown in Fig. 4a, perovskite film has response time (trs) of 66 seconds and recovery time (trc) of 67 seconds, which are faster than other type gas sensors.38 Perovskite film shows successive responses during four cycles toward ethanol. Thus, ethanol exposure seems not to damage the structural integrity of perovskite film. Interaction between perovskite film and ethanol is assumed to be mainly physisorption and limited on perovskite grain boundaries. However, as measurement repeat, peak heights become lower. This damping probably comes from a slow side reaction between the perovskite crystal and the target gas molecules. We will discuss sensing mechanism in more detail later.
We further investigate into sensing mechanism of the perovskite gas sensor. As a comparison, the sensing mechanism of conventional metal oxide semiconductor is worth to be considered. Metal oxide semiconductor absorbs oxygen molecules from ambient air.39 Oxygen molecules attract electrons from metal oxide to form O2−, O−, or O2− ions, then form a depletion layer that reduces the conductivity of metal oxide semiconductor.8,9,39 In contrast, our experiment reveals that exposure of perovskite sensor to ambient oxygen increases the conductivity of MAPbI3 film. Fig. S3† shows I–V curve of MAPbI3 film responses toward nitrogen and ambient air under voltage bias −3 V to 3 V. Regarding Fig. S3,† the conductivity of MAPbI3 film is much higher during ambient air exposure than during nitrogen exposure. Similarly, Fig. 4b presents I–V curve of MAPbI3 film that is exposed to nitrogen and ethanol gas under voltage bias −3 V to 3 V. Fig. 4b proves that ethanol exposure substantially promotes the conductivity of perovskite film. This observation proves that the sensing mechanism of MAPbI 3 film is different from conventional metal oxide sensors.
An alternative sensing mechanism involves passivation of charge trap state.31 MAPbI3 is well known to contain iodine vacancies (VI). There are two types of iodine vacancies, VI(a) and VI(b). VI(a) are located in MA-I layers and VI(b) are located in Pb–I layers.40 As MA-I layer is a termination layer of MAPbI3, most of iodine vacancies are located on the surface, interfacial site, and grain boundaries. Due to organic solvent evaporation during annealing process in film formation, a high density of crystal defects can be formed.41 These defects act as trap states. In pristine film, electrons can fall from conduction band into the trap states. As a consequence, perovskite film has low conductivity. Crystal defects on the surface work as active sites. Absorbing target gas molecules can passivate iodine vacancies and release free trapped charges. Therefore, electrons that are trapped in iodine vacancies can be restored into the conduction band. At that moment, the conductivity of perovskite film increases. In contrast, the absorbed inert gas molecules expel the target gas molecules and re-establish crystal defects of perovskite film. This sensing mechanism is supported by the photoluminescence (PL) measurement. Fig. 4c presents PL measurements of perovskite film before and after ethanol gas exposure. Before ethanol exposure, high density of crystal defect is indicated by low PL intensity. After ethanol exposure, crystal defect density becomes lower and crystallinity of perovskite improves, thus PL intensity increases. This result indicates that electronic quality of perovskite film is improved, supporting the trap state passivation hypothesis.34,42
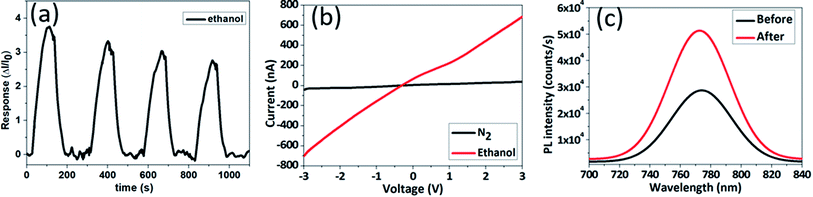
To understand the effect of applied bias on sensor performance, perovskite film was tested under different applied bias. Biasing technique has been applied to improve zinc oxide (ZnO) performance as hydrogen sensor,43 thus this technique might be worked as well for perovskite sensor. Fig. 5a shows response–time graph of MAPbI3 film under 1 V and 5 V. Different applied bias produces a similar trend of response–time graph, but the absolute current of MAPbI3 film under 5 V is one order magnitude higher than under 1 V (Fig. S4†). A higher level of absolute current at 5 V leads to smaller baseline noise level compare to at 1 V. However, at 5 V perovskite film is damaged from ion migration and/or faradaic reactions. As a comparison, Fig. 5c and d show optical image of MAPbI3 film after applying different bias for 10 minutes under similar gas exposure (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm ethanol). MAPbI3 film looks intact under 1 V bias. In contrast, 5 V bias exhibits severe damage to MAPbI3 film especially near cathode, which might be caused by iodide migration and PbI2 formation.44 From these experiments, we understand that sensing response can be improved, but we should keep low voltage bias to prevent perovskite film degradation.
000 ppm ethanol). MAPbI3 film looks intact under 1 V bias. In contrast, 5 V bias exhibits severe damage to MAPbI3 film especially near cathode, which might be caused by iodide migration and PbI2 formation.44 From these experiments, we understand that sensing response can be improved, but we should keep low voltage bias to prevent perovskite film degradation.
We further tried to figure out and optimized perovskite gas sensor. To investigate the effect of perovskite film thickness on sensor response, we fabricated perovskite films from different perovskite precursor concentrations. Perovskite film cross-section and top-view are depicted in Fig. S5 and S6.† Those figures demonstrate that higher concentration produces thicker film and bigger grains size. 0.5 M, 1.0 M, and 1.5 M precursor solution produces perovskite film thickness ∼100 nm, 0–250 nm, and ∼300 nm respectively. Fig. 6a displays a response–time graph of MAPbI3 film with different thickness toward similar gas concentration (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm) exposure at room temperature. Thinner perovskite film produces a higher response. Film thickness has a dependence towards sensitivity of sensing film. The sensitivity drops as the thickness increase.45 Two factors possibly play roles here. First, target gas molecules might penetrate only in the upper part of thicker perovskite film, which produces a lower response. Second, bigger grains in thicker perovskite film lead to lower density of grain boundaries. Therefore, the amount of the target gas molecules that can penetrate the film will be reduced, resulting in a lower response. We have tried to further decrease the film thickness by lowering perovskite concentration. For 0.25 M MAPbI3, the film was not uniform which lead to film damage (Fig. S7a†). As a consequence, this film had a poor response (Fig. S7b†).
000 ppm) exposure at room temperature. Thinner perovskite film produces a higher response. Film thickness has a dependence towards sensitivity of sensing film. The sensitivity drops as the thickness increase.45 Two factors possibly play roles here. First, target gas molecules might penetrate only in the upper part of thicker perovskite film, which produces a lower response. Second, bigger grains in thicker perovskite film lead to lower density of grain boundaries. Therefore, the amount of the target gas molecules that can penetrate the film will be reduced, resulting in a lower response. We have tried to further decrease the film thickness by lowering perovskite concentration. For 0.25 M MAPbI3, the film was not uniform which lead to film damage (Fig. S7a†). As a consequence, this film had a poor response (Fig. S7b†).
As VOC sensor, perovskite gas sensor devices were tested toward a range of typical organic VOCs. We compared MAPbI3 film response to VOC polar gases (ethanol, acetone, isopropanol, acetonitrile, and methanol) and VOC non-polar gases (toluene, benzene, chloroform, and hexane). Among them, ethanol presents the highest response, whereas hexane presents the lowest. Generally, the perovskite sensor is more sensitive to polar gas than non-polar gas. We can assume that polar gas molecules stick stronger to MAPbI3 defect sites, as MAPbI3 perovskite itself is a polar ionic crystal, resulting in higher responses (Fig. 6b).
Multiple devices were fabricated and measured in a range of gas concentrations as shown in Fig. 6c. This result confirms high reproducibility of our gas sensing device. The calibration graph shows good linearity between sensor response and gas concentration. According to Fig. 6b, the sensitivity of perovskite sensor is 3 × 10−4 ppm−1. Limit of detection (LOD) can be calculated by following equation:46
Finally, we have applied the perovskite gas sensor to detect VOC from commercial chemical products such as nail polish remover and soju (famous Korean liquor). Nail polish remover contains 30–60% acetone,47 while soju contains 20–25% ethanol.48 Fig. S8 and S9† show the electrical response of perovskite as it exposed to nail polish remover and soju vapour. Perovskite gas sensor exhibits good response to both commercial products. Those experiments proved that MAPbI3 perovskite gas sensor has a great potential to be utilized as ambient VOC detector in daily life.
Conclusions
In summary, a solution-processed MAPbI3 film was utilized as sensing material in VOCs gas sensor that can respond and recover fast at room temperature. The responses of the perovskite gas sensor originated from the conductivity change in the presence and absence of target gas. A sensing mechanism of the perovskite sensor has been proposed which is different from that of conventional metal oxide gas sensors. Charge trap passivation process by target gas adsorption seems to alter the MAPbI3 film conductivity. Thinner layer and lower applied bias are preferred due to higher electrical response and slow degradation of MAPbI3 film. In addition, selectivity test reveals that the MAPbI3 film is more sensitive to polar VOC gases than to non-polar VOC gases. This work offers an opportunity for MAPbI3 perovskite to be developed as low-cost and low-power gas sensor that can operate at room temperature.Experimental details
Fabrication of interdigitated electrode (IDE)
1 mm thick bare glass (2.5 × 2.5 cm) was cleaned by immersing into piranha solution (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v of H2SO4 and H2O2) for 3 hours at 60 °C and was subsequently treated by oxygen plasma for one minute. Cr (15 nm) and Au (80 nm) were deposited on top of bare glass using a thermal evaporator. Photolithography and wet etch processes were used to form interdigitated electrode pattern. Photoresist AZ GXR 601 was spin-coated above metal film, then followed with soft bake for 90 seconds at 90 °C. After that, the sample was covered by IDE pattern mask and exposed by UV lamp for 16 seconds. The sample was annealed for 90 seconds at 90 °C. Developer AZ MIF 300 was employed to develop for 30 seconds. Next, oxygen plasma treatment was applied for 2 minutes to remove residues. Wet etching processes of Au and Cr were done using Au etchant for 20 seconds and Cr etchant for 15 seconds. Photoresist layer was removed by acetone.
1 v/v of H2SO4 and H2O2) for 3 hours at 60 °C and was subsequently treated by oxygen plasma for one minute. Cr (15 nm) and Au (80 nm) were deposited on top of bare glass using a thermal evaporator. Photolithography and wet etch processes were used to form interdigitated electrode pattern. Photoresist AZ GXR 601 was spin-coated above metal film, then followed with soft bake for 90 seconds at 90 °C. After that, the sample was covered by IDE pattern mask and exposed by UV lamp for 16 seconds. The sample was annealed for 90 seconds at 90 °C. Developer AZ MIF 300 was employed to develop for 30 seconds. Next, oxygen plasma treatment was applied for 2 minutes to remove residues. Wet etching processes of Au and Cr were done using Au etchant for 20 seconds and Cr etchant for 15 seconds. Photoresist layer was removed by acetone.
Perovskite film formation
Perovskite solution was made by CH3NH3I and PbI2 powder (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mol ratio to DMSO in γ-butyrolactone. The perovskite solution was stirred with 300 rpm at 60 °C for minimum 1 hour, then it was filtered using 0.50 μm nylon filter to obtain a clear solution. For deposition process, it was done in a glove box with N2 ambient. 300 μL perovskite solution was dropped on the top of an interdigitated electrode (IDE) and was spun using spin coater with 1000 rpm for 40 seconds and 5000 rpm for 20 seconds. During the last 10 seconds, 800 μL toluene was dropped on the top of perovskite surface as anti-solvent. Perovskite film was annealed at 100 °C for 10 minutes.
1) mol ratio to DMSO in γ-butyrolactone. The perovskite solution was stirred with 300 rpm at 60 °C for minimum 1 hour, then it was filtered using 0.50 μm nylon filter to obtain a clear solution. For deposition process, it was done in a glove box with N2 ambient. 300 μL perovskite solution was dropped on the top of an interdigitated electrode (IDE) and was spun using spin coater with 1000 rpm for 40 seconds and 5000 rpm for 20 seconds. During the last 10 seconds, 800 μL toluene was dropped on the top of perovskite surface as anti-solvent. Perovskite film was annealed at 100 °C for 10 minutes.
Characterization and sensor testing
X-ray diffraction (XRD) pattern of MAPbI3 film was recorded by X-MAX Rigaku diffractometer with Cu Kα radiation from 3° to 60°. Scanning electron microscope (SEM) images of MAPbI3 film were taken by MAIA III-TESCAN. Atomic force microscopy (AFM) was performed by Park SYSTEM/XE-100. UV-visible of UV MAPbI3 film was measured by OPTIZEN 3220 UV absorption spectroscopy with wavelength range from 450 nm to 800 nm. Optical image observation was performed by HIROX/KH-8700 digital microscope.Before gas sensing test, one litre of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm target gas was prepared in a Tedlar bag. Volatile organic compound (liquid) was injected into a Tedlar bag. VOC liquid was diluted by filling the bag with one litre of N2 gas. The amount of VOC (liquid) was calculated correctly correspond to molecular weight of VOC, its density, final volume of dilution, and final concentration.
000 ppm target gas was prepared in a Tedlar bag. Volatile organic compound (liquid) was injected into a Tedlar bag. VOC liquid was diluted by filling the bag with one litre of N2 gas. The amount of VOC (liquid) was calculated correctly correspond to molecular weight of VOC, its density, final volume of dilution, and final concentration.
Gas sensing test was conducted by isolating MAPbI3 film in a cylinder chamber (Vcylinder = 16 ml). Interdigitated electrode was connected to potentiostat electrodes. Potentiostat PalmSens 4 was employed to record electrical response of MAPbI3 film. During gas sensing test, target gas was injected into the chamber for 90 seconds (rate gas 20 ml min−1), then N2 was injected right after target gas was stopped.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
I. O. acknowledges the support from the National Research Foundation (NRF) of Korea (NRF-2016R1A2B4011046).References
- A. L. Clements, W. G. Griswold, A. Rs, J. E. Johnston, M. M. Herting, J. Thorson, A. Collier-Oxandale and M. Hannigan, Sensors, 2017, 17, 2478 CrossRef PubMed.
- M. Willett, Sensors, 2014, 14, 6084–6103 CrossRef PubMed.
- A. H. Jalal, F. Alam, S. Roychoudhury, Y. Umasankar, N. Pala and S. Bhansali, ACS Sens., 2018, 3, 1246–1263 CrossRef PubMed.
- D. Kohl, J. Phys. D: Appl. Phys., 2001, 34, R125–R149 CrossRef.
- M. A. H. Khan, M. V. Rao and Q. Li, Sensors, 2019, 19, 905 CrossRef PubMed.
- J. Liu, T. Han, B. Sun, L. Kong, Z. Jin, X. Huang, J. Liu and F. Meng, Catalysts, 2016, 6, 210 CrossRef.
- A. S. Zuruzi and N. C. MacDonald, Adv. Funct. Mater., 2005, 15, 396–402 CrossRef CAS.
- A. Kolmakov and M. Moskovits, Annu. Rev. Mater. Res., 2004, 34, 151–180 CrossRef.
- C. Wang, L. Yin, L. Zhang, D. Xiang and R. Gao, Sensors, 2010, 10, 2088–2106 CrossRef PubMed.
- G. F. Fine, L. M. Cavanagh, A. Afonja and R. Binions, Sensors, 2010, 10, 5469–5502 CrossRef PubMed.
- D. Antolin, N. Medrano, B. Calvo and F. Perez, Sensors, 2017, 17, 365 CrossRef PubMed.
- V. Jelicic, M. Magno, D. Brunelli, G. Paci and L. Benini, IEEE Sensor. J., 2013, 13, 328–338 Search PubMed.
- H. Huang, Y. Xu, Q. Feng and D. Y. C. Leung, Catal. Sci. Technol., 2015, 5, 2649–2669 RSC.
- S. Das, S. Pal and M. Mitra, J. Med. Biol. Eng., 2016, 36, 605–624 CrossRef PubMed.
- J. Shuai, S. Kim, H. Ryu, J. Park, C. K. Lee, G. B. Kim, V. U. Ultra Jr and W. Yang, BMC Publ. Health, 2018, 18, 528 CrossRef PubMed.
- A. Hempel-Jorgensen, S. K. Kjaergaard, L. Molhave and K. H. Hudnell, Arch. Environ. Health, 1999, 54, 416–424 CrossRef.
- K. Ahn, J. Allergy Clin. Immunol., 2014, 134, 993–999 CrossRef PubMed.
- D. A. Otto, H. K. Hudnell, D. E. House, L. Molhave and W. Counts, Arch. Environ. Health, 1992, 47, 23–30 CrossRef PubMed.
- S. Genc, Z. Zadeoglulari, S. H. Fuss and K. Genc, J. Toxicol., 2012, 2012, 782462 Search PubMed.
- T. Kida, K. Suematsu, K. Hara, K. Kanie and A. Muramatsu, ACS Appl. Mater. Interfaces, 2016, 8, 35485–35495 CrossRef.
- T. Lin, X. Lv, Z. Hu, A. Xu and C. Feng, Sensors, 2019, 19, 233 CrossRef.
- S. Bhatia, N. Verma and R. K. Bedi, Results Phys., 2017, 7, 801–806 CrossRef.
- K. Zakrzewska and M. Radecka, Nanoscale Res. Lett., 2017, 12, 89 CrossRef.
- Z. Li, H. Li, Z. Wu, M. Wang, J. Luo, H. Torun, P. Hu, C. Yang, M. Grundmann, X. Liu and Y. Fu, Mater. Horiz., 2019, 6, 470–506 RSC.
- Z. Zhu, Q. Sun, Z. Zhang, J. Dai, G. Xing, S. Li, X. Huang and W. Huang, J. Mater. Chem. C, 2018, 6, 10121–10137 RSC.
- S. T. Ha, R. Su, J. Xing, Q. Zhang and Q. Xiong, Chem. Sci., 2017, 8, 2522–2536 RSC.
- B. Gebremichael, G. Alemu and G. Tessema Mola, Phys. B, 2017, 514, 85–88 CrossRef.
- A. B. Djurišić, F. Z. Liu, H. W. Tam, M. K. Wong, A. Ng, C. Surya, W. Chen and Z. B. He, Prog. Quantum Electron., 2017, 53, 1–37 CrossRef.
- Y. Zhao and K. Zhu, Chem. Commun., 2014, 50, 1605–1607 RSC.
- C. Bao, J. Yang, W. Zhu, X. Zhou, H. Gao, F. Li, G. Fu, T. Yu and Z. Zou, Chem. Commun., 2015, 51, 15426–15429 RSC.
- M. A. Stoeckel, M. Gobbi, S. Bonacchi, F. Liscio, L. Ferlauto, E. Orgiu and P. Samori, Adv. Mater., 2017, 29, 1702469 CrossRef.
- X. Fu, S. Jiao, N. Dong, G. Lian, T. Zhao, S. Lv, Q. Wang and D. Cui, RSC Adv., 2018, 8, 390–395 RSC.
- Y. Zhuang, W. Yuan, L. Qian, S. Chen and G. Shi, Phys. Chem. Chem. Phys., 2017, 19, 12876–12881 RSC.
- G. Kakavelakis, E. Gagaoudakis, K. Petridis, V. Petromichelaki, V. Binas, G. Kiriakidis and E. Kymakis, ACS Sens., 2018, 3, 135–142 CrossRef CAS.
- H. Chen, M. Zhang, R. Bo, C. Barugkin, J. Zheng, Q. Ma, S. Huang, A. W. Y. Ho-Baillie, K. R. Catchpole and A. Tricoli, Small, 2018, 14, 1702571 CrossRef.
- A. Arakcheeva, V. Svitlyk, E. Polini, L. Henry, D. Chernyshov, A. Sienkiewicz, G. Giriat, A. Glushkova, M. Kollar, B. Náfrádi, L. Forro and E. Horváth, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2019, 75, 361–370 CrossRef.
- A. M. Leguy, P. Azarhoosh, M. I. Alonso, M. Campoy-Quiles, O. J. Weber, J. Yao, D. Bryant, M. T. Weller, J. Nelson, A. Walsh, M. van Schilfgaarde and P. R. Barnes, Nanoscale, 2016, 8, 6317–6327 RSC.
- D. Acharyya and P. Bhattacharyya, Sens. Actuators, B, 2016, 228, 373–386 CrossRef.
- K. Arshak, E. Moore, G. M. Lyons, J. Harris and S. Clifford, Sens. Rev., 2004, 24, 181–198 CrossRef.
- L.-y. Wei, W. Ma, C. Lian and S. Meng, J. Phys. Chem. C, 2017, 121, 5905–5913 CrossRef CAS.
- J. Huang, Y. Yuan, Y. Shao and Y. Yan, Nat. Rev. Mater., 2017, 2, 17042 CrossRef CAS.
- R. Brenes, D. Guo, A. Osherov, N. K. Noel, C. Eames, E. M. Hutter, S. K. Pathak, F. Niroui, R. H. Friend, M. S. Islam, H. J. Snaith, V. Bulović, T. J. Savenije and S. D. Stranks, Joule, 2017, 1, 155–167 CrossRef CAS.
- T. F. Choo, N. U. Saidin and K. Y. Kok, R. Soc. Open Sci., 2018, 5, 172372 CrossRef.
- Z. Xiao, Y. Yuan, Y. Shao, Q. Wang, Q. Dong, C. Bi, P. Sharma, A. Gruverman and J. Huang, Nat. Mater., 2015, 14, 193–198 CrossRef CAS.
- F. Hossein-Babaei and M. Orvatinia, Sens. Actuators, B, 2003, 89, 256–261 CrossRef.
- A. Shrivastava and V. Gupta, Chronicles Young Sci., 2011, 2, 21 CrossRef.
- M. Bernstein, US Pat., 4735798, 1988.
- W. Chung, Alcohol Alcohol., 2004, 39, 39–42 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra10703g |
| This journal is © The Royal Society of Chemistry 2020 |