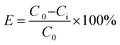Open Access Article
Open Access ArticleA novel silica-supported polyether polysiloxane quaternary ammonium demulsifier for highly efficient fine-sized oil droplet removal of oil-in-water emulsions
Mengjin Zhaiab,
Mian Wub,
Cunying Wangbc and
Xiaobing Li *a
*a
aNational Center for Coal Preparation and Purification Engineering Research, China University of Mining and Technology, No. 1 Daxue Road, South, Xuzhou, Jiangsu 221116, PR China. E-mail: Xiaobing.li@cumt.edu.cn; Fax: +86-516-83885878; Tel: +86-516-83591117
bSchool of Chemical Engineering and Technology, China University of Mining and Technology, Xuzhou, Jiangsu 221116, PR China
cSchool of Chemical Engineering and Technology, Xuzhou College of Industrial Technology, Xuzhou, Jiangsu 221006, PR China
First published on 19th May 2020
Abstract
The existence of fine-sized oil drops that are difficult to coalesce greatly decreases the separation efficiency of produced water from alkali, surfactant, and polymer flooding technology (ASP) containing oil-in-water emulsions. To improve oil–water separation efficiency, a silica-supported polyether polysiloxane quaternary ammonium (abbreviated as PPQA@SiO2) demulsifier was synthesized. The supported demulsifier possesses a rough surface structure and large surface area. In addition, it displays high thermal stability. It was applied for treating the produced water from ASP flooding. The effects of dosage, treatment temperature and treatment time on the oil removal efficiency from ASP produced water were investigated. Owing to the synergetic effect of demulsification and adsorption, the supported demulsifier exhibited an oil removal greater than 92% within 50 min at the initial oil concentration of 300 mg L−1, which is much higher than that of a commercial demulsifier SA001 (40.33%). Furthermore, the demulsification mechanism was explored from the perspective of the zeta potential, mean diameter and size distribution of the oil droplets. The high oil removal efficiency establishes PPQA@SiO2 as a promising candidate for oil–water separation from the ASP flooding produced wastewater.
1. Introduction
ASP (alkaline, surfactant, and polymer) flooding has developed into a significant technology in oil extraction over the past decades. The technique is found to enhance oil recovery by over 20% than that of water flooding (30–50%). The produced water from ASP flooding is an oil-in-water emulsion that cannot be discharged directly into the environment due to its high concentration of emulsification agent, high viscosity and salinity.1,2 In order to meet the demand for the re-injection process and wastewater discharge, the oil-in-water emulsion needs to be demulsified.Several techniques, including filtration, membrane, electrical, biological and chemical treatments, have been employed to achieve the oil–water separation.3–6 Among them, chemical demulsification has been demonstrated to actively destabilize the oil droplets and accelerate oil–water separation.7 A number of chemical demulsifiers have been developed in recent years, such as quaternary ammonium polymers,8 polyether demulsifiers9 and polysiloxane demulsifiers.10 Although the demulsification efficiency has been improved to some extent, it still cannot efficiently treat the produced water from ASP flooding. The main reason is that the surfactants in the produced water compete with the demulsifier for adsorption sites on the oil droplets, resulting in secondary emulsification after demulsifying. As a result, fine-sized oil droplets are highly stable and do not sufficiently coalesce into larger oil droplets.
To solve the above problems, adsorbents need to be introduced to capture oil droplets and accelerate their settling. Currently, the widely used oil adsorbents include inorganic materials, polymer materials, and inorganic/organic composite materials. For example, Yao and his colleagues11 have developed expanded graphite with high porosity and hydrophobicity. These unique properties endow expanded graphite with outstanding adsorption performance for lubricating oils. Kun-Yi Andrew Lin et al.12 reported the highly efficient removal of oil droplets from water using copper-based metal–organic frameworks. In our previous work, we described the adsorption of oil from wastewater by coal and investigated the effect of absorption time, coal type, coal particle size distribution, pH value and oil concentration on the absorption capacity.13 In addition, we explored the absorption mechanism and found that the whole absorption process included two kinds of absorption, a physical process assisted by a chemical one. Later, we demonstrated the adsorption of oil droplets from wastewater by hydrated silica (SiO2·2H2O) and a high absorption capacity was achieved due to the large specific surface area and porosity of silica.14
From the above-mentioned findings, the advantages of both a demulsifier and adsorbent in the O/W demulsification process have been demonstrated. To further enhance the efficiency of the oil–water separation, we consolidated the functions of the demulsifier and adsorbent and synthesized a novel silica-supported polyether polysiloxane quaternary ammonium (PPQA@SiO2) reverse demulsifier by grafting the demulsifier PPQA onto the surface of silica particles. Then, it was characterized by FTIR, XPS, SEM, N2 adsorption and TG analysis and used to treat the produced water from ASP flooding. The effects of dosage, treatment temperature and treatment time on the oil removal efficiency from ASP produced water were investigated. A great oil removal of 92.1% was achieved, which is much higher than that of a commercial SA001 demulsifier (40.33%). This remarkable oil removal performance is due to its rough surface, large surface area and high thermal stability. The demulsification mechanism of the prepared PPQA@SiO2 from the perspective of the zeta potential, mean diameter and size distribution of the residual oil droplets was further explored. Specifically, the demulsifier PPQA played the role of displacement demulsifying and charge neutralization so that the original oil–water interfacial film was ruptured. Meanwhile, the fine-sized oil droplets could be adsorbed onto the surface of silica carrier particles rapidly after demulsifying.15 Finally, the oil-laden silica particles were removed by gravity settling separation. Based on this, this supported demulsifier is a more effective alternative to treat produced water containing fine-sized oil droplets.
2. Experimental
2.1. Materials
Low-hydro silicone oil (PHMS, 0.18% hydrogen content, industrial purity) was purchased from Zhongsheng Silicone Technology Co., Ltd. (Shenzhen, China). Allyl polyoxyethylene polyoxypropylene epoxy ether (abbreviated as epoxy ether, n(EO)/n(PO) = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, molecular weight 500, industrial purity) and allyl polyoxyethylene polyoxypropylene methyl ether (abbreviated as methyl ether, molecular weight 300, industrial purity) were purchased from Hangzhou Hangyuan Technology Co., Ltd. (Hangzhou, China). Chloroplatinic acid, vinyltriethoxysilane (97%) and trimethylamine hydrochloride were purchased from Aladdin Co., Ltd. 2-Propanol, NaOH, NaCl, NaHCO3, CaCl2, MgCl2·6H2O, polyacrylamide (PAM), and sodium dodecylbenzene sulfonate (SDBS) were obtained from Sinopharm Chemical Reagents Co. Ltd. A commercial reverse SA001 demulsifier (Shenai, Nantong, China) was used to compare the demulsification performance with PPQA. All chemicals were used directly without further purification.
1, molecular weight 500, industrial purity) and allyl polyoxyethylene polyoxypropylene methyl ether (abbreviated as methyl ether, molecular weight 300, industrial purity) were purchased from Hangzhou Hangyuan Technology Co., Ltd. (Hangzhou, China). Chloroplatinic acid, vinyltriethoxysilane (97%) and trimethylamine hydrochloride were purchased from Aladdin Co., Ltd. 2-Propanol, NaOH, NaCl, NaHCO3, CaCl2, MgCl2·6H2O, polyacrylamide (PAM), and sodium dodecylbenzene sulfonate (SDBS) were obtained from Sinopharm Chemical Reagents Co. Ltd. A commercial reverse SA001 demulsifier (Shenai, Nantong, China) was used to compare the demulsification performance with PPQA. All chemicals were used directly without further purification.
2.2. Synthesis of supported demulsifier PPQA@SiO2
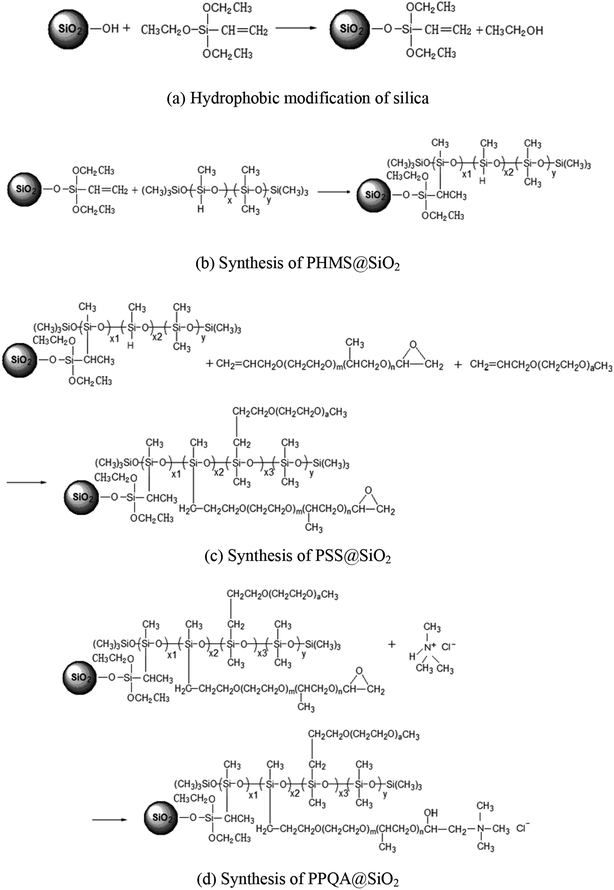
The synthesis steps of the supported demulsifier PPQA@SiO2 are presented in Fig. 1. To improve the hydrophobicity of silica, vinyltriethoxysilane was grafted onto the silica via the silicon coupling reaction,16,17 which is shown in Fig. 1a. 10 mL of vinyltriethoxysilane was dissolved in 10 mL 98% ethanol, hydrolyzed for 20 min at room temperature and then mixed with 6 g silica that had been dispersed in 15 mL 98% ethanol. The mixture was agitated at 50 °C for 5 h. Finally, hydrophobic silica was collected by filtration, washed with ethanol thoroughly and dried in a vacuum oven at 60 °C for 12 h.Next, low-hydro silicone oil (abbreviated as PHMS) was grafted onto the surface of silica via the well-known hydrosilylation reaction,18 which is shown in Fig. 1b. During grafting, 5 g of hydrophobic silica and 4.5 g of PHMS were dispersed in 10 mL of isopropanol, the mixture was stirred at 40 °C for 30 min and then reacted at 80 °C for 5 h using 2 mL of chloroplatinic acid as a catalyzer. Solid products were collected from the mixture by filtration, washed by ethanol thoroughly and dried in a vacuum oven at 80 °C for 12 h, yielding silica-supported PHMS (abbreviated as PHMS@SiO2).
Then, methyl ether and epoxy ether were grafted onto the surface of PHMS@SiO2 via the hydrosilylation reaction.19,20 The process is presented in Fig. 1c. Five grams of PHMS@SiO2, 3.5 g of epoxy ether and 1.1 g of methyl ether were added into 10 mL of isopropanol and stirred at 40 °C for 30 min. Then, the mixture was reacted at 80 °C for 5 h using 2 mL of chloroplatinic acid as a catalyzer. The solid products were collected by filtration, washed with ethanol thoroughly and dried in a vacuum oven at 80 °C for 12 h, yielding silica-supported polyether polysiloxane (abbreviated as PSS@SiO2).
The polyether polysiloxane quaternary ammonium grafted on silica (abbreviated as PPQA@SiO2) was prepared through an epoxy ring-opening reaction.21,22 The process is shown in Fig. 1d. Here, part A (5 g of PSS@SiO2 dispersed in 10 mL of isopropanol) and part B (0.25 g of trimethylamine hydrochloride dissolved in 5 mL of ethanol) were reacted at 80 °C for 6 h. The obtained final products of PPQA@SiO2 were collected by filtration, washed with ethanol thoroughly and dried in a vacuum oven at 80 °C for 12 h (Table 1).
| Full name | Abbreviation |
|---|---|
| Alkali surfactant polymer produced water | ASP produced water |
| Polyether polysiloxane quaternary ammonium | PPQA |
| Low-hydro silicone oil | PHMS |
| Silica-supported PHMS | PHMS@SiO2 |
| Silica-supported polyether polysiloxane | PSS@SiO2 |
| Silica-supported polyether polysiloxane quaternary ammonium | PPQA@SiO2 |
2.3. Characterization of supported demulsifier PPQA@SiO2
The specific surface area and pore structure of PPQA@SiO2 were determined by N2 adsorption at 77 K using an Autosorb-Qi analyzer (MicrotracBEL Inc. Japan). Surface areas were calculated by the Brunauer–Emmett–Teller (BET) method. Pore size distributions were determined from the Barrett–Joyner–Halenda (BJH) method. The chemical structure of the supported demulsifier PPQA@SiO2 sample was determined with a Vector-2 FTIR spectrometer (Bruker Company, Germany). The elemental composition of PPQA@SiO2 was characterized by an X-ray photoelectron spectrometer (XPS) (ESCALAB 250xi, Thermo Fisher Company, USA). The surface morphologies of PPQA@SiO2 were examined with scanning electron microscopy (SEM) (Quanta 250, FEI Company, USA). The thermogravimetric analysis (TGA) of PPQA@SiO2 was performed using a thermogravimetric analyzer (Mettler Toledo, China) in flowing N2 from the ambient temperature to 800 °C at a heating rate of 15 °C min−1. The residual oil content was measured by UV-vis spectroscopy (UV-1900, SHIMADZU CO., LTD., China). The sizes of the residual oil droplets were measured using a potential particle size analyzer (ZetaPALS, China).2.4. Preparation of simulated produced water from ASP flooding
The samples of simulated produced water from ASP flooding were prepared according to the properties and components of the produced water from ASP flooding in Shengli Oilfield, China. The preparation process was divided into three steps: mineralized water preparation, oil droplet mother liquor preparation and oil in water emulsions generation.14 To prepare the mineralized water, 1.167 g NaCl, 2.443 g NaHCO3, 0.007 g Na2SO4, 0.060 g CaCl2, and 0.053 g MgCl2·6H2O were dissolved and diluted in deionized water to a final volume of 1 L. To prepare the oil bead mother liquor, 0.1 g SDBS and 0.1 g PAM were dissolved and diluted with the simulated produced water to a final volume of 1 L. Then, 100 mL of this solution was mixed with 100 g of crude oil and heated at 45 °C for 1 h and emulsified using a high-speed mixer at 2000 rpm for 15 min to produce a mother liquid with an oil concentration of 50 wt%. To obtain the simulated produced water, 0.15 g mother liquid was dosed into 500 mL mineralized water that contained 700 mg L−1 NaOH, 300 mg L−1 SDBS and 500 mg L−1 PAM. After a constant water bath for 20 min, the mixture was emulsified at 8000 rpm by the high-speed mixer for 20 min and the simulated oil in water emulsion was obtained.2.5. Oil removal tests from simulated ASP produced water using demulsifier PPQA@SiO2
For the reverse demulsification experiments, a bottle containing 40 mL simulated ASP produced water was placed into a water bath shaker at 50 °C (the temperature of typically produced water from ASP flooding from Shengli Oilfield ranged 40 to 50 °C) for 30 min.14 Varied dosages of PPQA@SiO2 (0.6–1.6 g L−1) were added into the samples of simulated ASP produced water and the mixture was shaken thoroughly to ensure that PPQA@SiO2 and the emulsions were evenly mixed. The oil removal tests were carried out at different times (30–80 min) and different temperatures (20–70 °C) in a thermostat water bath.In order to compare the level of oil removal from the simulated produced water with PPQA@SiO2 with that of PPQA, SiO2·2H2O, and commercial SA001, 0.50 g L−1 PPQA, 1.0 g L−1 PPQA@SiO2, 0.050 g L−1 SA001, and 1.0 g L−1 SiO2·2H2O were mixed, respectively, with 40 mL of freshly-prepared, simulated produced water in glass vials and then vigorously agitated at 30 °C for 50 min.
After the oil–water delamination, the supernatants of each sample were decanted and the residual oil content was measured by UV-vis spectroscopy at a wavelength of 425 nm. The sizes of the residual oil droplets were also measured using a potential particle size analyzer.
The oil removal ability of the PPQA@SiO2 was determined by measuring the residual oil content of the simulated produced water after treatment.
The oil removal, E, was calculated using the following equation:
3. Results and discussion
3.1. Characterization of the demulsifier PPQA@SiO2
The demulsifier PPQA@SiO2 was obtained through the combination of hydrosilylation and epoxy ring-opening reactions. In order to investigate the chemical composition and structure of the product, FTIR and XPS analyses were carried out. As shown in Fig. 2, the peaks at 3440, 1640, 1099, 803, and 470 cm−1 for SiO2·2H2O are attributed to the vibrations of O–H, Si–OH, and Si–O–Si groups. In the spectrum of PHMS@SiO2, new peaks appear at 2968 and 2163 cm−1, which are assigned to the stretching vibration of –CH3 and Si–H groups.23–25 After grafting PHMS, the intensity of the broad O–H peak at 3440 cm−1 is weakened and the Si–O–Si peaks centered near 1099, 800 and 468 cm−1 are stronger than those in SiO2·2H2O, the reason for which is that O–H groups participate in the hydrosilylation reaction and generate Si–O–Si groups. Compared with the characteristic peaks of PHMS@SiO2, the Si–H peak for PPQA@SiO2 disappears, indicating that the hydrosilylation reaction occurs. In addition, the emergence of a new characteristic peak at 1263 cm−1 (in-plane bending vibration of N–CH3) indicates that the formation of PPQA@SiO2 was successful. In conclusion, PPQA was successfully grafted onto the surface of modified silicone.X-ray photoelectron spectroscopy (XPS) analysis was performed to determine the elemental content and chemical state in the PPQA@SiO2 samples (Fig. 3). The full XPS spectrum reveals the presence of oxygen (54.31%), carbon (14.96%), silicon (29.69%), and nitrogen (1.04%) on the surface of the samples. The high-resolution C1s, N1s, and Si2p spectra are presented in Fig. 3. The C1s spectra can be deconvoluted into four peaks representing the following functional groups: C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.1 eV), C–O (285.6 eV), C–C (284.5 eV) and C–H (283.8 eV). The Si2p spectra are presented in Fig. 3c and can be divided into two peaks at 103.5 and 102.7 eV, which correspond to Si–O–Si and Si–C, respectively. The N1s peak is weak, which implies the low content of nitrogen (Fig. 3d). In general, these data further confirm that PPQA is successfully grafted onto the surface of the silica.
O (288.1 eV), C–O (285.6 eV), C–C (284.5 eV) and C–H (283.8 eV). The Si2p spectra are presented in Fig. 3c and can be divided into two peaks at 103.5 and 102.7 eV, which correspond to Si–O–Si and Si–C, respectively. The N1s peak is weak, which implies the low content of nitrogen (Fig. 3d). In general, these data further confirm that PPQA is successfully grafted onto the surface of the silica.
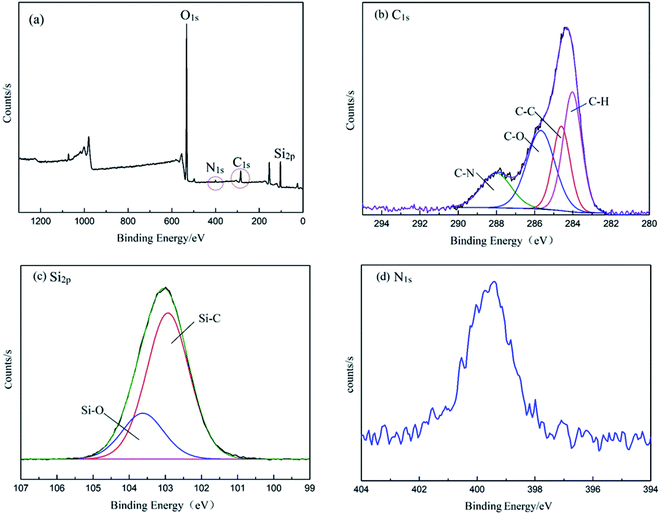 | ||
| Fig. 3 (a) XPS spectra of PPQA@SiO2, (b) high-resolution C1s spectrum, (c) high-resolution Si2p spectrum, and (d) high-resolution N1s spectrum. | ||
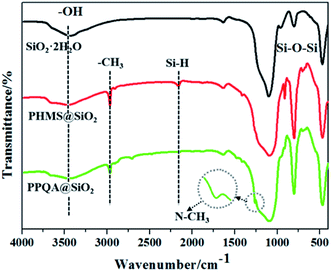
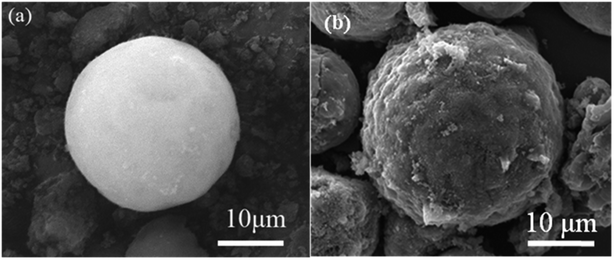
The SEM images of SiO2·2H2O and PPQA@SiO2 are shown in Fig. 4. A very smooth SiO2·2H2O spherical particle with a size of 20 μm is obtained. This is in good agreement with Wang's work.23 After PPQA is attached to the surface of SiO2·2H2O particles, it is clearly observed that the modified surface becomes rougher, which is desired for capturing fine-sized oil droplets from wastewater. In addition, the size of the particle increases to 25 μm, as shown in Fig. 4b.
The surface area and pore structure of the PPQA@SiO2 demulsifier play significant roles in the oil removal performance and were investigated by nitrogen adsorption–desorption analysis. The demulsifier PPQA@SiO2 has a BET specific surface area and a total pore volume of 28.15 m2 g−1 and 0.27 cm3 g−1, respectively, which is slightly smaller than that of silicone due to the fact that PPQA is grafted onto the surface of the carrier particle.23 The large specific surface area endows PPQA@SiO2 with a high adsorption capacity, which is favorable for enhancing the oil–water separation performance. According to the Barrett–Joyner–Halenda (BJH) model, the pore-size distribution is centered at 36 nm, which is much smaller than the size of oil droplets. Thus, the adsorption of oil droplets to PPQA@SiO2 is considered to be a surface phenomenon rather than an interior penetration phenomenon.
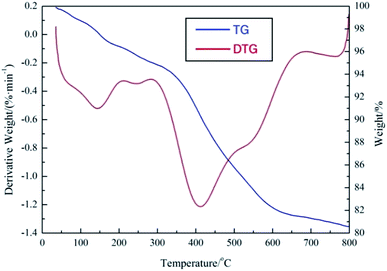
The thermal stability of demulsifier PPQA@SiO2 was studied by TGA in a N2 atmosphere. As shown in Fig. 5, the first weight loss of PPQA@SiO2 occurs at around 50 °C due to the loss of free water and gas adsorbed in the pores of PPQA@SiO2, which is much lower than that of silicone.23,24 There is a second weight loss between 300 °C and 675 °C, indicating that the PPQA@SiO2 structure collapses due to the decomposition of organic groups. The DTG curve of PPQA@SiO2 turns sharply at 415 °C, which verifies that the supported demulsifier decomposes quickly. The results demonstrate that PPQA@SiO2 can be stable under the treatment temperature varied from 30 °C to 60 °C.
3.2. Evaluation of oil removal performance of demulsifier PPQA@SiO2
The oil removal ability of demulsifier PPQA@SiO2 was determined by measuring the residual oil content of the simulated produced water after treatment. The effects of demulsifier dosage, treatment temperature and time on oil removal were investigated through the batch bottle tests. As shown in Fig. 6a, the oil removal increases rapidly with an increase of PPQA@SiO2 dosage. With the PPQA@SiO2 dosage of 0.6 g L−1, the oil removal is 90.6%. Moreover, the oil removal reaches 91.9% when the PPQA@SiO2 dosage increases to 1.0 g L−1. Upon further increasing the dosage of PPQA@SiO2, the oil removal reaches a plateau.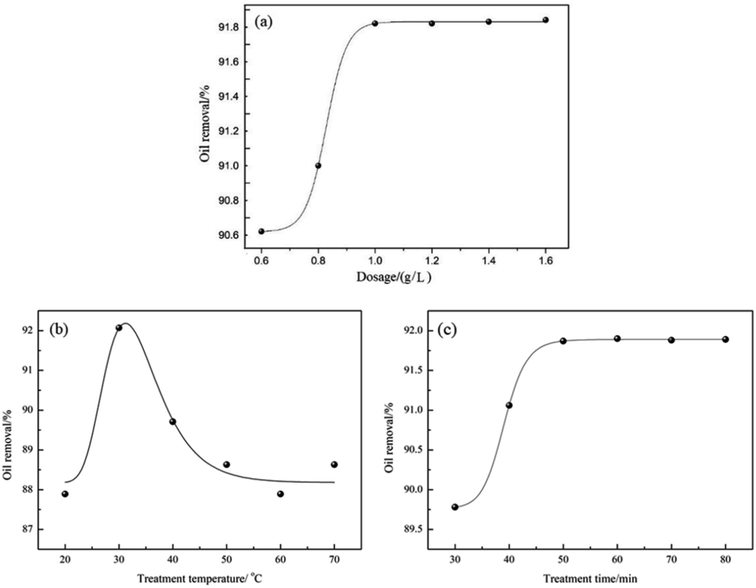 | ||
| Fig. 6 Effects of (a) demulsifier dosage, (b) treatment temperature and (c) treatment time on oil removal from simulated produced water. | ||
The effect of treatment temperature on oil removal from the simulated produced water was investigated using 1.0 g L−1 PPQA@SiO2 for a duration of 60 min. As shown in Fig. 6b, the optimal temperature is near 30 °C where the level of oil removal is 92.1%.
To evaluate the optimal treatment time for PPQA@SiO2 on simulated produced water when using 1.0 g L−1 PPQA@SiO2, the treatment durations were varied between 30 to 80 min at 30 °C. As shown in Fig. 6c, oil removal increases with increasing time. Moreover, oil removal approaches 91.9% with a duration of 50 min, while longer treatment durations do not cause an obvious increase in the oil removal performance. In order to reduce the time cost, 50 min is adopted in the subsequent tests.
3.3. Comparison of oil removal performance of demulsifier PPQA@SiO2 with a commercial demulsifier
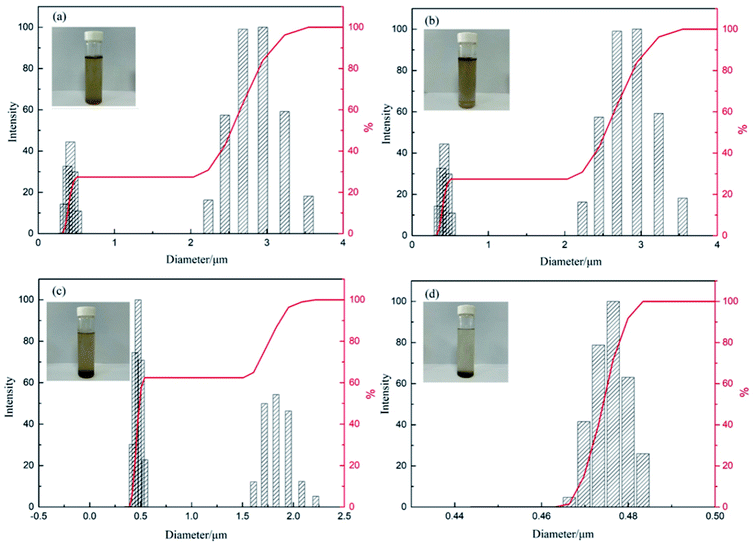
When PPQA@SiO2 is applied to treat the produced water from ASP flooding, it exhibits an oil removal of 92.1%, which is much higher than that of PPQA (60.96%) or SiO2·2H2O (54.44%). Moreover, the oil removal of the prepared PPQA@SiO2 was more than twice that of the commercial SA001 demulsifier (40.33%). This remarkable oil removal performance is attributed to its rough surface, large surface area and high thermal stability. We further explored the demulsification mechanism of the prepared PPQA@SiO2 from the perspective of the zeta potential, mean diameter and size distribution of the residual oil droplets.As presented in Table 2, the mean diameter of the oil droplets in the simulated produced water is 1.857 μm with a zeta potential of −45.51 mV. Since the fine-sized oil droplets repelled each other, the system is highly stable. For that reason, the emulsifier (SDBS) was adsorbing onto oil droplets and formed a stable double electric layer.26 When commercial demulsifiers, PPQA, SiO2, and PPQA@SiO2 were added, the zeta potential of the system increased to −38.32, −27.89, −32.02 and −17.73 mV, respectively. The results indicate that many positively-charged PPQA@SiO2 displaced the demulsifiers adsorbed on oil droplets and neutralized most of the negative charges. Moreover, PPQA@SiO2 adsorbed fine-sized oil droplets through electrostatic attraction.27 However, for commercial demulsifiers, the ability of displacement demulsifying is weak, only a partial negative charge on the oil droplet surfaces was neutralized. Consequently, the residual un-neutralized negative charge prevented the oil droplets from coalescing by maintaining a repulsive force. The results are consistent with our previous report.27
| Parameters | Simulated produced water | Commercial demulsifier | PPQA | SiO2 | PPQA@SiO2 |
|---|---|---|---|---|---|
| Zeta potential/mV | −45.51 | −38.32 | −27.89 | −32.02 | −17.73 |
| Mean diameter/μm | 1.857 | 1.330 | 0.791 | 1.011 | 0.682 |
After the demulsification process, the destabilized small oil droplets coalesced into large oil droplets. When commercial demulsifiers, PPQA, SiO2, and PPQA@SiO2 were added, the mean diameters of residual oil droplets in the simulated produced water were 1.330, 1.011, 0.791 and 0.682 μm, respectively. In addition, as displayed in Fig. 7, the size of the residual oil droplets was centered at 2.8 and 1.7 μm with the addition of commercial demulsifier and PPQA, thus the water sample was muddy. While for SiO2, the size of the residual oil droplets was centered at 0.5 μm. For PPQA@SiO2, the size of the residual oil droplets decreased to less than 0.48 μm. It is inferred that silica carrier particles could capture and adsorb fine-sized oil droplets after demulsifying. Then, the oil-laden silica particles were removed by gravity settling separation, leaving even smaller residual oil drops in the wastewater; thus the water sample was layered, and the upper layer became clear.
In general, the treatment process can be divided into two steps. First, the PPQA could displace emulsifier (SDBS) at the oil–water interface to reduce the strength and stability of the oil–water interfacial film. Meanwhile, it could neutralize the negative surface charges of oil droplets to reduce the electrostatic repulsion and adsorb the fine-sized oil droplets through electrostatic attraction.28 Then, the destabilized small oil droplets coalesced into large oil droplets. Second, silica carrier particles could capture and adsorb fine-sized oil droplets after demulsifying.29 Then, these oil-carrying particles deposit at the bottom of the container. Based on this, the supported demulsifier is more effective for treating the produced water containing fine-sized oil droplets.
4. Conclusions
In summary, we have successfully designed a supported reverse demulsifier PPQA@SiO2 by grafting demulsifier PPQA onto the surface of silica particles. The supported demulsifier showed a rough structure and had a large surface area and high thermal stability. When it was applied to the treatment of simulated produced water from ASP flooding, an oil removal of 92.1% was obtained with a 1 g L−1 dosage over 50 min at 30 °C at the initial oil concentration of 300 mg L−1, and this was much higher than that of the commercial SA001. High oil removal was attributed to the synergistic effect of demulsification and adsorption. To be specific, demulsifier PPQA played the role of displacement demulsifying and charge neutralization; meanwhile, the silica carrier particles could adsorb fine-sized droplets after demulsifying. Ultimately, the oil-carrying silica particles were removed by gravity settling separation. Based on this, the supported reverse demulsifier is more effective for treating produced water containing fine-sized oil droplets. The high oil–water separation efficiency establishes PPQA@SiO2 as a promising candidate for real produced water treatment from ASP flooding.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (No. 51674261).References
- S. H. Si, Z. B. Gong, Z. Yan, Y. Wang, Z. H. Ren, P. F. Liu and S. M. Bu, Emulsification and demulsification factors of produced water from ASP flooding in Xinjiang Oilfield, Oilfield Chem., 2018, 35, 139–143 Search PubMed.
- Z. L. Ren, Y. H. Mou and F. Y. Cui, Mechanism and development trend of crude oil demulsifier, Oil-Gasfield Surface Engineering, 2005, 24, 16–17 Search PubMed.
- X. F. Song, Y. Q. Wu, C. J. Xu and M. Di, Study on the treatment process of produced water by strong alkali ASP flooding, Oil-Gas Field Protection, 2019, 29, 4–8 Search PubMed.
- L. T. Ding, Test of treating produced water from ASP flooding with dissolved gas floatation process, Oil-Gasfield Surface Engineering, 2015, 34, 43–46 Search PubMed.
- Y. Yang, A. Raza, F. Banat and K. Wang, The separation of oil in water (O/W) emulsions using polyether sulfone & nitrocellulose microfiltration membranes, J. Water Process Eng., 2018, 25, 113–117 CrossRef.
- G. F. Zhang, Experiments on treating ASP flooding produced water by microbial method, Oil-Gasfield Surface Engineering, 2016, 35, 28–31 Search PubMed.
- S. Farrukh, A. H. Ibnelwaleed, S. K. Muhammad, A. Waqar, S. S. Abdullah and S. N. Mustafa, Polymeric surfactants and emerging alternatives used in the demulsification of produced water: a review, Polym. Rev., 2018, 58, 63–101 CrossRef.
- L. X. Liu, S. S. Hao, X. C. Wang, M. A. Jing and X. F. Zhao, Synthesis and demulsification of polyquaternium reverse demulsifier, Ind. Wastewater, 2010, 41, 70–73 CAS.
- J. Wang, F. L. Hu and C. Q. Li, Synthesis of dendritic polyether surfactants for demulsification, Sep. Purif. Technol., 2010, 73, 349–354 CrossRef CAS.
- L. W. Liu, G. Rao, C. M. Xie, M. Wang and E. M. Wang, Study on synthesis and properties of oil in water emulsion breaker, Petrochem. Technol., 2014, 43, 1053–1057 CAS.
- T. Yao, Y. G. Zhang and Y. P. Xiao, The effect of environmental factors on the adsorption of lubricating oil onto expanded graphite, J. Mol. Liq., 2016, 218, 611–614 CrossRef CAS.
- K. Y. Andrew L, H. T. Yang and C. Petit, Removing oil droplets from water using a copper-based metal organic frameworks, Chem. Eng. J., 2014, 249, 293–301 CrossRef.
- X. B. Li, C. J. Zhang and J. T. Liu, Adsorption of oil from waste water by coal: characteristics and mechanism, Min. Sci. Technol., 2010, 20, 778–781 CAS.
- J. Li, C. Y. Wang, Q. Tang, M. J. Zhai, Q. Q. Wang and M. Shi, Preparation and application of supported demulsifier PPA@SiO2 for oil removal of oil-in-water emulsion, Sep. Sci. Technol., 2019, 1–12 Search PubMed.
- Z. Y. Wang, M. H. Shen, L. Yu and B. G. Li, Development and performance evaluation of heavy oil soluble demulsifier, Pet. Process. Petrochem., 2012, 47, 53–58 Search PubMed.
- Y. H. Xing, J. Wu, H. Zhang and R. Yang, Process optimization of silica modified by coupling agent, Food Sci. Technol., 2014, 35, 131–134 CAS.
- X. M. Wu and M. Li, Modification of silica and its application progress, Fine Spec. Chem., 2016, 24, 26–29 CAS.
- G. F. Huang, S. B. Qing and S. B. Li, Application of hydrosilylation in preparation and modification of polymers, Silicone Mater., 2000, 14, 23–26 Search PubMed.
- L. W. Liu, R. Guo and M. Wang, Synthesis and demulsification properties of different end-capped polyethers co-modified silicone oil, Silicone Mater., 2015, 29, 18–22 CAS.
- Q. F. Cai, J. Z. Zhou and Z. H. Fu, Study on the relationship between structure and demulsification performance of polyether demulsifier for crude oil, Applied Chemistry for Engineering, 2013, 42, 68–71 CAS.
- M. Y. Li and Z. G. Chen, Synthesis and characterization of polyepichlorohydrin-triethylamine quaternary ammonium salt, China Surfactant Detergent & Cosmetics, 2011, 41, 247–249 Search PubMed.
- C. Y. Wang and R. J. Fang, Synthesis and demulsification evaluation of polyether polyquaternary ammonium salt inverse demulsifier, Chem. Res. Appl., 2015, 27, 1879–1884 CAS.
- B. H. Wang, Y. Z. Xiong, S. X. Wu, M. Jiang, Q. Wang and Q. P. Zhang, Ionic liquid modified silica and characterization of its properties, J. Synth. Cryst., 2017, 46, 1851–1857 CAS.
- W. G. Ren, J. C. Wang, L. P. Chang, W. R. Bao and L. N. Han, Characterization of MTS organic modified silica and its properties, J. Synth. Cryst., 2019, 48, 555–560 CAS.
- X. M. Tan, A. S. Feng and H. Q. Zhao, Study on grafting modification of nano silica surface by silane coupling agent, Zhongguo Fenti Jishu, 2011, 17, 14–17 CAS.
- M. L. Fan, C. H. Nie, H. Du, J. W. Ni, B. H. Wang and X. R. Wang, An insight into the solar demulsification of highly emulsified water produced from oilfields by monitoring the viscosity, zeta potential, particle size and rheology, Colloids Surf., A, 2019, 575, 144–154 CrossRef CAS.
- H. Sun, Q. Q. Wang, X. B. Li and X. He, Novel polyether-polyquaternium copolymer as an effective reverse demulsifier for O/W emulsions: demulsification performance and mechanism, Fuel, 2020, 263, 116770 CrossRef CAS.
- H. T. Bai, Y. Ma, Q. Guo and P. Ren, Simulation study on the stability of oil in water suspension, Fine Petrochem., 2017, 34, 69–72 Search PubMed.
- N. N. Li and X. Q. Lu, Research progress of adsorption of heavy metals in aqueous phase by mesoporous silica, Guangzhou Chem. Ind., 2019, 47, 30–33 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |