Open Access Article
Open Access ArticleSynthesis of some new acylhydrazone compounds containing the 1,2,4-triazole structure and their neuritogenic activities in Neuro-2a cells†
Xia Jiangab,
Genyun Tangc,
Jie Yangb,
Jiacheng Dingb,
Hongwei Lin*b and
Xiaoliang Xiang *a
*a
aKey Laboratory of Research and Utilization of Ethnomedicinal Plant Resources of Hunan Province, College of Biological and Food Engineering, Huaihua University, Huaihua 418008, P. R. China. E-mail: jiangxia285333006@163.com; xiangxiaoliang225@163.com
bHunan Engineering Laboratory for Preparation Technology of Polyvinyl Alcohol (PVA) Fiber Material, College of Chemistry and Materials Engineering, Huaihua University, Huaihua 418008, P. R. China. E-mail: 1520224252@qq.com; 1471157054@qq.com; linhongwei1968@163.com
cSchool of Medicine, Hunan Provincial Key Laboratory of Dong Medicine, Hunan University of Medicine, Huaihua, Hunan 418000, P. R. China. E-mail: genyun1983311@126.com
First published on 19th May 2020
Abstract
In the present study, a novel series of acylhydrazone compounds (A0–A10) with the structure of 1,2,4-triazole have been designed and synthesized. In addition, all the synthesized compounds have been evaluated for neuritogenic activity in mouse neuroblastoma (Neuro-2a) cells. Notably, we found that one of these 11 acylhydrazone compounds, compound A5 (2-(4-amino-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-ylthio)-N′-(2-hydroxybenzylidene)-acetohydrazide) displays excellent neuritogenic activity. Moreover, our present study revealed that compound A5 had the ability to induce neurite outgrowth through the PI3K/Akt and MEK-ERK signaling pathway in Neuro-2a cells. These findings suggest that compound A5 might exert neuritogenic effects and thus may be useful for the treatment of neural repair and regeneration.
1. Introduction
The functions of the nervous system are mediated by neural circuits that are formed during brain development.1 Neurite outgrowth is a critical cellular process underlying neural circuit formation.2 It is well known that abnormalities of this process are associated with some neurodegenerative diseases. For instance, neurite atrophy is one of the typical symptoms in early stages of Alzheimer's and Parkinson's diseases.3–5 Furthermore, neurite loss is one of the cardinal features of neuronal injury.6 Therefore, it has been suggested that promoting neurite outgrowth is important when repairing nerve system damage, and it is necessary for the recovery of neuronal functions. So far, various compounds derived from bioactive natural products or chemical synthesis with potential neurite outgrowth have been studied for neuroregeneration.7,8Acylhydrazone Schiff base was synthesized by the condensation of hydrazide with aldehyde or ketone, it has strong coordination ability, a wide spectrum of biological activities and superior performance in various aspects, making it a promising therapeutic drug and functional material.9,10 In recent years, researchers have found that acylhydrazone compounds exhibit many beneficial biological activities, such as anti-bacterial,11–13 anti-inflammatory,14–16 anti-viral17,18 and anti-tumor activities.19–21 However, there are currently few studies on the neuroactivity of acylhydrazone compounds.
In the present study, some new acylhydrazone compounds containing 1,2,4-triazole structure were synthesized, and their neurite outgrowth-promoting activities were investigated in the Neuro-2a cells. This cell line is commonly used as an in vitro model system to study neuronal differentiation, neurite outgrowth and neurotoxicological studies.
2. Results and discussion
2.1 Synthesis of acylhydrazone compounds
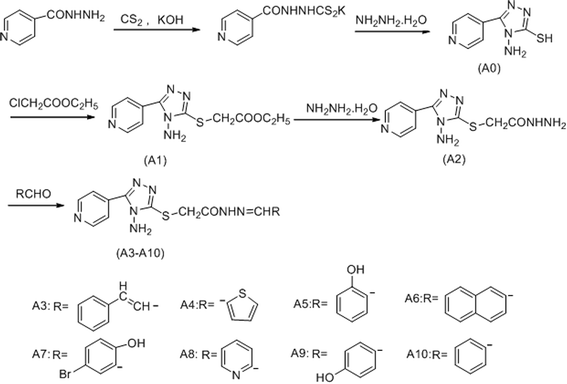
The compound A0–A10 were designed and synthesized via the routes as shown in Scheme 1. Initially, isoniazide and potassium hydroxide were dissolved in ethanol, adding carbon disulfide to obtained potassium 2-isonicotinoylhydrazinecarbodithioate, which reacted with hydrazine hydrate under 143 °C to afford4-amino-5-(pyridin-4-yl)-4H-1,2,4-triazole-3-thiol (A0). Secondly, ethyl 2-(4-amino-5-(pyridin-4-yl)-4H-pyrazol-3-ylthio) acetate (A1) was synthesized by reaction of compound A0 and ethyl chloroacetate in sodium hydroxide solution. Thirdly, hydrazine hydrate and compound A1 were refluxed in methanol to obtain 2-(4-amino-5-(pyridin-4-yl)-4H-pyrazol-3-ylthio) acetohydrazide (A2). Finally, compound A2 was reacted with different structures of aldehydes to gain the target compounds A3–A10. Structural explanations of the final compounds were performed with IR, 1H-NMR, 13C-NMR and GC-MS. In the IR spectra of the compounds, stretching absorptions around 3300 cm−1 proved N–H bone of amino in the triazole and amide. Signals belonging to carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) function appeared at 1651–1764 cm−1 (Amide I band), the absorption peak of another band of amide appeared near 1265 cm−1. The absorption at about 1529 to 1608 cm−1 was registered for C
O) function appeared at 1651–1764 cm−1 (Amide I band), the absorption peak of another band of amide appeared near 1265 cm−1. The absorption at about 1529 to 1608 cm−1 was registered for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds. The out of plane bending belonging to substituted benzene was determined at 794–833 cm−1. In the 1H-NMR spectra, protons belonging to amide (CONH–) were observed as singlet peaks at 9.91 ppm and 11.99 ppm. Protons of methylene bridge (–SCH2) were recorded as singlet peaks between 4.10 ppm and 4.50 ppm. Amino groups on the triazole gave peaks between 6.30 ppm and 6.89 ppm. Protons of carbon–nitrogen double bond in hydrazone (–CONHN
N bonds. The out of plane bending belonging to substituted benzene was determined at 794–833 cm−1. In the 1H-NMR spectra, protons belonging to amide (CONH–) were observed as singlet peaks at 9.91 ppm and 11.99 ppm. Protons of methylene bridge (–SCH2) were recorded as singlet peaks between 4.10 ppm and 4.50 ppm. Amino groups on the triazole gave peaks between 6.30 ppm and 6.89 ppm. Protons of carbon–nitrogen double bond in hydrazone (–CONHN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–) were recorded as singlet peaks between 7.57 ppm and 8.82 ppm. The target compounds A3–A10 had E/Z geometric isomers and cis–trans conformation isomers, so in the absence of coupling, the proton signal peaks of CONH, SCH2, NH2 and N
CH–) were recorded as singlet peaks between 7.57 ppm and 8.82 ppm. The target compounds A3–A10 had E/Z geometric isomers and cis–trans conformation isomers, so in the absence of coupling, the proton signal peaks of CONH, SCH2, NH2 and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH showed splitting. In 13C-NMR, carbons of methylene bridge (–SCH2) were recorded around 34.5 ppm. All aromatic carbons gave peaks from 110 ppm to 159 ppm. C3 of 1,2,4-triazole ring were recorded around 166 ppm and carbonyl group gave peaks over 168 ppm. In the GC-Ms spectra, all masses were matched well with the expected M + H values.
CH showed splitting. In 13C-NMR, carbons of methylene bridge (–SCH2) were recorded around 34.5 ppm. All aromatic carbons gave peaks from 110 ppm to 159 ppm. C3 of 1,2,4-triazole ring were recorded around 166 ppm and carbonyl group gave peaks over 168 ppm. In the GC-Ms spectra, all masses were matched well with the expected M + H values.
2.2. Cytotoxic effects of acylhydrazone compounds on Neuro-2a cells
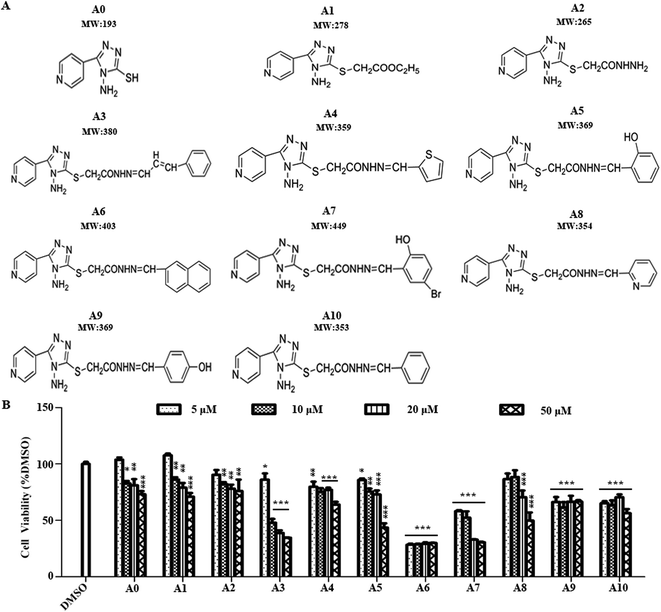
In addition to examination of the effects of acylhydrazone compounds on cell cytotoxicity, cell viability was determined by MTT assay (Fig. 1A). For this experiment, cells were seeded in wells of 96-well plate and maintained in culture medium for 24 h. The cells were then treated with test compounds (5, 10, 20, 50 μM concentration of each compound) for 24 h. As indicated in Fig. 1B, the compounds A0, A1, A2, A3, A4, A5, A7 and A8 showed that the dependence of cell viability on concentration is clearly noticed. Cytotoxic effects according to ISO 10993-5 standard,22 if cell viability was above 80% are considered as non-cytotoxic; weak (60 to 80%); moderate (40 to 60%) and below 40% strong cytotoxicity, respectively. Among the tested compounds, A0, A1, A2, A3, A4, A5 and A8 showed no cytotoxic effect on Neuro-2a cells at 5 μM, whereas, compounds A7, A9 and A10 showed weak cytotoxicity. Notably, compound A6 exhibited strong cytotoxicity at this concentration. Therefore, a concentration of 5 μM was used in subsequent experiments.2.3 Effects of acylhydrazone compounds on neuronal differentiation of Neuro-2a cells
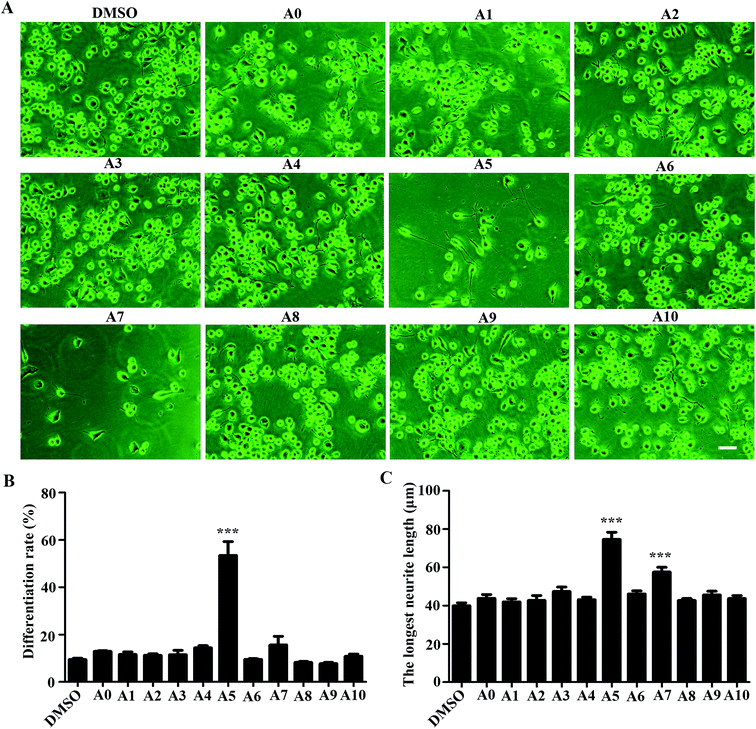
To investigate the influences of acylhydrazone compounds on neuronal differentiation. Neuro-2a cells were cultured in a low-serum (0.5% FBS) medium and treated with compounds A0–A10 (5 μM) for 48 h. As shown in Fig. 2A, morphological changes were captured with phase contrast microscope. The differentiation rate and the longest length of neurite of each cell have been analyzed. As shown in Fig. 2B, only compound A5 apparently induced Neuro-2a cells differentiation rate after 48 h treatment, whereas others were not. Fig. 2C shows that in addition to compound A5, compound A7 also significantly promoted neurite outgrowth.2.4 Effects of compound A5 on neuronal differentiation of Neuro-2a cells
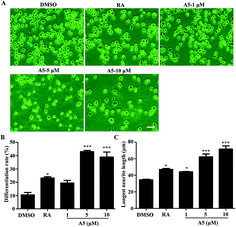
As we revealed that compound A5 had the neuritogenic activity, we then examined in detail the effect of compound A5 on neurite outgrowth in Neuro-2a cells. The cells were treated with compound A5 at the concentration of 1–10 μM and cultured in differentiation medium for 48 h. As a positive control, retinoic acid (RA) was used at the concentration of 10 μM. Fig. 3A shows the untreated cells (DMSO) having a round shape with few neurites and the RA-treated cells apparently displaying long neurites. Here, we compared the effect of the differentiation of Neuro-2a cells induced by A5 and RA. Notably, all aspects we examined, including differentiation rate (Fig. 3B) and the longest neurite length (Fig. 3C), A5 exhibited stronger activities as RA did. Moreover, compound A5 promoted neurite outgrowth in a concentration-dependent manner.2.5 Signaling pathway involved in compound A5-induced neurite outgrowth from Neuro2a cells
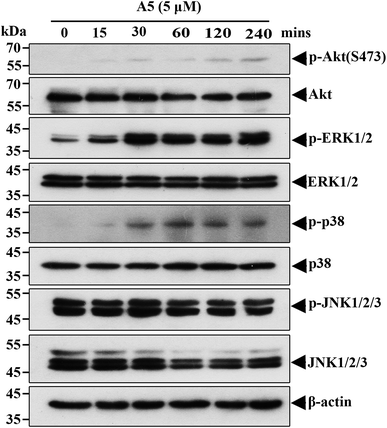
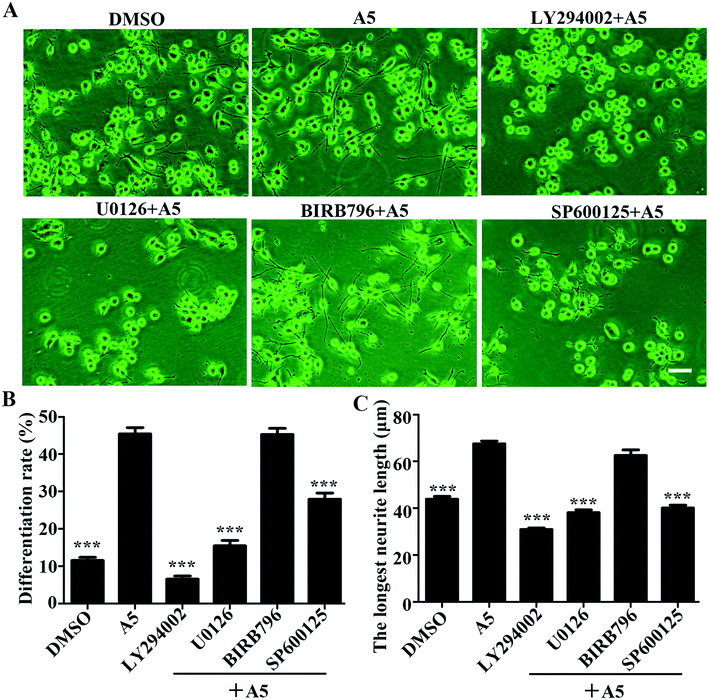
To understand what signaling pathways functioning in the compound A5-induced neurite outgrowth, we treated Neuro-2a cells with A5 for different time points (0–240 min) and examined the levels of activated/phosphorylated forms of different signaling molecules (Fig. 4). Notably, compound A5 markedly activated extracellular signal regulated kinases 1/2 (ERK1/2) and p38 at 30 min treatment and thereafter. Examination of another classical MAPK pathways revealed that c-Jun amino-terminal kinases (JNK) was not affected by compound A5. Besides up-regulating ERK1/2 and p38 pathways, the Akt pathway was slightly induced by A5.To further determine the important signaling molecules that are required for compound A5 promoted neurite outgrowth, we took advantage of a series of pharmacological inhibitors, including LY294002 (PI3K inhibitor), U0126 (MEK1/2 inhibitor), BIRB795 (p38 inhibitor) and SP600125 (JNK inhibitor). We preincubated Neuro-2a cells with each of these inhibitors for 1 h before the treatment of compound A5, and the differentiation rate and the longest neurite length were examined (Fig. 5A). Consistent with the western blot analysis that ERK and Akt were activated by compound A5, the activities of ERK upstream kinases MEKs (MAPK/ERK kinase) and the Akt upstream kinase PI3K were both essentially required for compound A5 induced neurite outgrowth (Fig. 5B and C). By contrast, the activity of p38 was not important for compound A5's effect on promoting neurite outgrowth (Fig. 5B and C). Interestingly, although A5 does not affect the activity of JNK, but inhibition of JNK activity can offset the promotion of A5 on neurite growth (Fig. 5B and C). These results collectively suggest that compound A5 induces neuronal differentiation and neurite extension through activation of MEK-ERK and PI3K-Akt pathways, although the identity of the direct molecular target of A5 awaits further investigation.
3. Materials and methods
3.1 Chemistry
All chemicals in this study were purchased from Shanghai Aladdin Reagent Company. A series of new acylhydrazone compounds containing 1,2,4-triazole (A0–A10) were synthesized by the method previously reported.23,24 The structures of the synthesised compounds were proved by 1H-NMR, 13C-NMR and GC-MS. The 1H-NMR and 13C-NMR spectra were obtained in DMSO-d6 by a Bruker Avance 400 MHz NMR spectrometer at 400 MHz and 100 MHz, respectively. The FT-IR spectra was recorded under ambient conditions in the wave number range of 4000–400 cm−1 using an FT-IR Nicolet (Is5) spectrometer. GC-MS studies were performed on a GCMS-QP 2010 Plus (Shimadzu, Tokyo, Japan). Chemical purities of the compounds were checked by TLC applications performed on silica gel 60F254. The 1H-NMR, 13C-NMR and GC-MS spectra of the compounds available in ESI.†3.2 Synthesis
Step 1: preparation of potassium 2-isonicotinoylhydrazinecarbodithioate. KOH (7.80 g, 0.139 mmol) was added into absolute ethanol (150 mL) and stirred with heating until it dissolved. The reaction mixture was cooled to room temperature. Then, isoniazid (10.04 g, 73.2 mmol) was added into the reaction mixture and 6 mL carbon disulfide (CS2) was slowly added dropwise. The mixture was stirred at room temperature for 10 h. The precipitated product was filtered and dried in vacuo to get the intermediate as a yellow powder.
Step 2: preparation of 4-amino-5-(pyridin-4-yl)-4H-pyrazole-3-thiol (A0). Potassium 2-isonicotinoylhydrazinecarbodithioate (12.99 g, 0.05 mol) was refluxed with 80% hydrazine hydrate (20 mL, 0.41 mol) in oil bath at 143 °C for 4 h. The obtained product was mixed with 20 mL distilled-ice water, and then adjust pH = 5 with concentrated hydrochloric acid. The obtained residue was filtered and dried in vacuo to get the compound A0 as white solid (yield, 77.6%). 1H-NMR (DMSO-d6, 400 MHz) δ: 14.14 (s, 1H, –SH); 8.75–8.77 (d, J = 8, 2H, PyH); 8.02–8.03 (d, J = 4, 2H, PyH); 5.85 (s, 2H, NH2). 13C-NMR (DMSO-d6, 100 MHz):168.08, 151.0, 150.59, 147.79, 133.40, 123.21, 121.05 MS (ESI) m/z: 193 (M+).
2-(4-Amino-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-ylthio)-N′-(3-phenylallylidene) acetohydrazide (A3). Compound A2 (0.21 g, 0.78 mmol) was resolved in 70 mL anhydrous ethanol. Then, cinnamaldehyde (0.18 mL, 1.43 mmol) was slowly added dropwise. The solution was stirred and refluxed at 67 °C for 4 h. The reaction mixture obtained was a clear and transparent solution. The mixture was cooled to room temperature and a solid separates out. Separated solid was filtered, dried and recrystallised by absolute ethanol to give compound A3 as white crystal (yield, 38.1%). 1H-NMR (DMSO-d6, 400 MHz) δ:11.65 (cis), 11.56 (trans) (s, 1H, CONH); 8.70–8.74 (m, 2H, PyH); 7.99–8.01 (d, J = 8, 2H, PyH); 7.57–7.59 (d, J = 8, 1H, N
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 7.37–7.39 (d, J = 8, 2H, –CH
CH); 7.37–7.39 (d, J = 8, 2H, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 7.35 (m, 3H, Ph–H); 7.33 (m, 2H, Ph–H); 6.32 (s, 2H, –NH2); 4.01 (cis), 4.41 (trans) (s, 2H, –SCH2). trans/cis: 5/4; 13C-NMR (DMSO-d6, 100 MHz) δ: 169.05, 164.03, 155.10, 155.02, 152.52, 152.41, 150.58, 150.55, 149.51, 146.87, 139.84, 139.55, 136.27, 134.39, 129.31, 129.26, 127.57, 127.53, 125.77, 125.45, 121.77, 4.69 (trans), 34.63 (cis). MS (ESI) m/z: 380.0 (M+) IR (KBr) ν: 3332, 3201, 1764, 1529.3.
CH); 7.35 (m, 3H, Ph–H); 7.33 (m, 2H, Ph–H); 6.32 (s, 2H, –NH2); 4.01 (cis), 4.41 (trans) (s, 2H, –SCH2). trans/cis: 5/4; 13C-NMR (DMSO-d6, 100 MHz) δ: 169.05, 164.03, 155.10, 155.02, 152.52, 152.41, 150.58, 150.55, 149.51, 146.87, 139.84, 139.55, 136.27, 134.39, 129.31, 129.26, 127.57, 127.53, 125.77, 125.45, 121.77, 4.69 (trans), 34.63 (cis). MS (ESI) m/z: 380.0 (M+) IR (KBr) ν: 3332, 3201, 1764, 1529.3.The syntheses of compounds A4–A10 were carried out by the same procedure as described for the preparation of A3.
2-(4-Amino-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-ylthio)-N′-(thiophen-2-ylmethylene)acetohydrazide (A4). White crystal (yield, 28.41%). 1H-NMR (DMSO-d6, 400 MHz) δ:11.64 (trans), 11.72 (cis) (s, 1H, CONH); 8.73 (s, 2H, PyH); 8.42 (trans), 8.21 (cis) (s, 1H, N
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 7.99 (s, 2H, PyH); 7.64–7.66 (m, 1H, thiophen–H); 7.45–7.48 (m, 1H, thiophen–H); 7.11–7.12 (s, 1H, thiophen–H); 6.31–6.33 (d, J = 8, 2H, –NH2) 4.07(cis), 4.22 (trans) (s, 2H, –SCH2); trans/cis: 10/11; 13C-NMR (DMSO-d6, 100 MHz) δ:168.96, 164.02, 155.15, 155.05, 152.51, 152.41, 150.57, 150.54, 142.73, 139.42, 139.21, 139.07, 134.41, 134.34, 131.65, 131.07, 129.53, 129.07, 128.39, 128.33, 121.78, 34.70 (trans), 34.30 (cis). MS (ESI) m/z: 359.0 (M+) IR (KBr) ν: 3329, 3039, 2930, 1651, 1543, 1265.
CH); 7.99 (s, 2H, PyH); 7.64–7.66 (m, 1H, thiophen–H); 7.45–7.48 (m, 1H, thiophen–H); 7.11–7.12 (s, 1H, thiophen–H); 6.31–6.33 (d, J = 8, 2H, –NH2) 4.07(cis), 4.22 (trans) (s, 2H, –SCH2); trans/cis: 10/11; 13C-NMR (DMSO-d6, 100 MHz) δ:168.96, 164.02, 155.15, 155.05, 152.51, 152.41, 150.57, 150.54, 142.73, 139.42, 139.21, 139.07, 134.41, 134.34, 131.65, 131.07, 129.53, 129.07, 128.39, 128.33, 121.78, 34.70 (trans), 34.30 (cis). MS (ESI) m/z: 359.0 (M+) IR (KBr) ν: 3329, 3039, 2930, 1651, 1543, 1265.
2-(4-Amino-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-ylthio)-N′-(2-hydroxybenzylidene)-acetohydrazide (A5). White flake solid (yield, 78.5%). 1H-NMR (DMSO-d6, 400 MHz) δ: 12.05 (cis), 11.67 (trans) (s, 1H, –ph–OH); 11.02 (trans), 10.11 (cis) (s, 1H, CONH); 8.77 (s, 2H, PyH), 8.03 (s, 2H, PyH); 8.46 (cis), 8.37 (trans) (s, 1H, N
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 7.26–7.33 (m, 2H, Ph–H), 7.59–7.60 (d, J = 4, 1H, Ph–H), 7.72–7.74 (d, J = 8, 1H, Ph–H); 6.37–6.39 (d, J = 8, trans), 6.89–6.96 (m, cis) (2H, –NH2); 4.52 (trans), 4.14 (cis) (s, 2H, –SCH2). trans/cis: 15/16. 13C-NMR (DMSO-d6, 100 MHz) δ: 169.03, 164.08, 157.74, 156.88, 155. 19, 155.14, 152.57, 152.44, 150.61, 150.58, 147.57, 141.69, 134.41, 134.33, 131.98, 131.73, 129.61, 126.71, 121.79, 120.50, 119.90, 119.85, 119.11, 116.82, 116.61, 34.52 (trans), 34.33 (cis). MS (ESI) m/z: 369.0 (M+) IR (KBr) ν: 3425, 3309, 3202, 2930, 1666, 1582, 1265.
CH); 7.26–7.33 (m, 2H, Ph–H), 7.59–7.60 (d, J = 4, 1H, Ph–H), 7.72–7.74 (d, J = 8, 1H, Ph–H); 6.37–6.39 (d, J = 8, trans), 6.89–6.96 (m, cis) (2H, –NH2); 4.52 (trans), 4.14 (cis) (s, 2H, –SCH2). trans/cis: 15/16. 13C-NMR (DMSO-d6, 100 MHz) δ: 169.03, 164.08, 157.74, 156.88, 155. 19, 155.14, 152.57, 152.44, 150.61, 150.58, 147.57, 141.69, 134.41, 134.33, 131.98, 131.73, 129.61, 126.71, 121.79, 120.50, 119.90, 119.85, 119.11, 116.82, 116.61, 34.52 (trans), 34.33 (cis). MS (ESI) m/z: 369.0 (M+) IR (KBr) ν: 3425, 3309, 3202, 2930, 1666, 1582, 1265.
2-(4-Amino-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-ylthio)-N′-(naphthalen-1-ylmethylene)acetohydrazide (A6). White crystal (yield, 79.2%). 1H-NMR (DMSO-d6, 400 MHz) δ: 11.87 (cis), 11.72 (trans) (s, 1H, CONH); 8.72–8.82 (m, 4H, PyH); 8.59–8.62 (d, J = 12, 1H, N
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 7.96–7.98 (m, 4H, naphthalene–H); 7.60–7.64 (m, 3H, naphthalene–H); 6.33–6.35 (d, J = 8, 2H, –NH2); 4.15 (cis), 4.58 (trans) (s, 2H, –SCH2). trans/cis: 5/4; 13C-NMR (DMSO-d6, 100 MHz) δ: 169.37, 164.32, 155.13, 152.47, 150.52, 147.69, 143.90, 134.39, 133.95, 131.16, 130.89, 130.60, 129.75, 129.70, 129.30, 129.25, 128.70, 127.81, 127.47, 126.71, 125.96, 124.96, 124.07, 121.82, 121.75, 34.89 (trans), 34.63 (cis). MS (ESI) m/z: 403.0 (M+) IR (KBr) ν: 3320, 3089, 2930, 1685, 1593, 1357.
CH); 7.96–7.98 (m, 4H, naphthalene–H); 7.60–7.64 (m, 3H, naphthalene–H); 6.33–6.35 (d, J = 8, 2H, –NH2); 4.15 (cis), 4.58 (trans) (s, 2H, –SCH2). trans/cis: 5/4; 13C-NMR (DMSO-d6, 100 MHz) δ: 169.37, 164.32, 155.13, 152.47, 150.52, 147.69, 143.90, 134.39, 133.95, 131.16, 130.89, 130.60, 129.75, 129.70, 129.30, 129.25, 128.70, 127.81, 127.47, 126.71, 125.96, 124.96, 124.07, 121.82, 121.75, 34.89 (trans), 34.63 (cis). MS (ESI) m/z: 403.0 (M+) IR (KBr) ν: 3320, 3089, 2930, 1685, 1593, 1357.
2-(4-Amino-5-(pyridin-4-yl)-4H-pyrazol-3-ylthio)-N′-(5-bromo-2-hydroxybenzylidene)acetohydrazide (A7). White powder (yield, 32.38%). 1H-NMR (DMSO-d6, 400 MHz) δ: 12.06 (cis), 11.66 (trans) (s, 1H, –ph–OH); 11.04 (cis), 10.37 (trans) (s, 1H, CONH); 8.73 (s, 2H, PyH); 8.39 (cis), 8.25 (trans) (s, 1H, N
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 8.0 (s, 2H, PyH); 7.43–7.82 (m, 3H, Ph–H); 6.85 (cis), 6.32 (trans) (s, 2H, –NH2); 4.10 (cis), 4.49 (trans) (s, 2H, –SCH2). trans/cis: 20/19; 13C-NMR (DMSO-d6, 100 MHz) δ: 169.24, 164.28, 155.03, 152.55, 152.39, 150.57, 150.51, 145.21, 139.72, 134.40, 134.12, 133.90, 133.70, 130.70, 128.24, 122.95, 121.77, 121.66, 119.10, 118.87, 111.32, 110.94, 34.45 (trans), 34.41 (cis). MS (ESI) m/z: 449.0 (M+) IR (KBr) ν: 3340, 3279, 2970, 1681, 1550, 1357.
CH); 8.0 (s, 2H, PyH); 7.43–7.82 (m, 3H, Ph–H); 6.85 (cis), 6.32 (trans) (s, 2H, –NH2); 4.10 (cis), 4.49 (trans) (s, 2H, –SCH2). trans/cis: 20/19; 13C-NMR (DMSO-d6, 100 MHz) δ: 169.24, 164.28, 155.03, 152.55, 152.39, 150.57, 150.51, 145.21, 139.72, 134.40, 134.12, 133.90, 133.70, 130.70, 128.24, 122.95, 121.77, 121.66, 119.10, 118.87, 111.32, 110.94, 34.45 (trans), 34.41 (cis). MS (ESI) m/z: 449.0 (M+) IR (KBr) ν: 3340, 3279, 2970, 1681, 1550, 1357.
2-(4-Amino-5-(pyridin-4-yl)-4H-pyrazol-3-ylthio)-N′-(pyridin-2-ylmethylene)acetohydrazide (A8). White powder (yield, 85.1%). 1H-NMR (DMSO-d6, 400 MHz) δ: 11.99 (cis), 11.85 (trans) (s, 1H, CONH); 8.71–8.74 (m, 2H, PyH); 8.58–8.61 (m, 1H, PyH); 8.22 (cis), 8.05 (trans) (s, 1H, N
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 7.81–7.97 (m, 4H, PyH); 7.37–7.44 (m, 1H, PyH); 6.34 (s, 2H, –NH2) 4.12 (cis), 4.51 (trans) (s, 2H, –SCH2); trans/cis: 8/5; 13C-NMR (DMSO-d6, 100 MHz) δ: 172.48, 169.64, 164.54, 155.06, 154.85, 153.39, 153.19, 152.54, 152.43, 150.48, 149.94, 147.78, 144.64, 137.37, 137.29, 134.40, 124.97, 124.79, 121.75, 120.46, 120.30, 34.67 (trans), 34.24 (cis). MS (ESI) m/z: 354.0 (M+) IR (KBr) ν: 3340, 3197, 2930, 1689, 1608, 1377.
CH); 7.81–7.97 (m, 4H, PyH); 7.37–7.44 (m, 1H, PyH); 6.34 (s, 2H, –NH2) 4.12 (cis), 4.51 (trans) (s, 2H, –SCH2); trans/cis: 8/5; 13C-NMR (DMSO-d6, 100 MHz) δ: 172.48, 169.64, 164.54, 155.06, 154.85, 153.39, 153.19, 152.54, 152.43, 150.48, 149.94, 147.78, 144.64, 137.37, 137.29, 134.40, 124.97, 124.79, 121.75, 120.46, 120.30, 34.67 (trans), 34.24 (cis). MS (ESI) m/z: 354.0 (M+) IR (KBr) ν: 3340, 3197, 2930, 1689, 1608, 1377.
2-(4-Amino-5-(pyridin-4-yl)-4H-pyrazol-3-ylthio)-N′-(4-hydroxybenzylidene)acetohydrazide (A9). White powder (yield, 60.2%). 1H-NMR (DMSO-d6, 400 MHz) δ: 11.55 (cis), 11.46 (trans) (s, 1H, –ph–OH); 9.91 (cis), 9.88 (trans) (s, 1H, CONH); 8.73 (s, 2H, PyH), 7.98–8.0 (d, J = 8, 2H, PyH); 8.09 (cis), 7.92 (trans) (s, 1H, N
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 7.50–7.53 (d, J = 12, 2H, Ph–H); 6.80–6.82 (d, J = 8, 2H, Ph–H); 6.31–6.32 (s, 2H, –NH2); 4.06 (cis), 4.46 (trans) (s, 2H, –SCH2); trans/cis: 10/6; 13C-NMR (DMSO-d6, 100 MHz) δ: 168.99, 163.81, 159.96, 159.78, 155.09, 152.40, 150.53, 147.92, 144.62, 134.42, 129.38, 129.08, 125.43, 121.77, 116.16, 34.82 (trans), 34.62 (cis). MS (ESI) m/z: 369.0 (M+) IR (KBr) ν: 3437, 3321, 3190, 1651, 1582, 1265.
CH); 7.50–7.53 (d, J = 12, 2H, Ph–H); 6.80–6.82 (d, J = 8, 2H, Ph–H); 6.31–6.32 (s, 2H, –NH2); 4.06 (cis), 4.46 (trans) (s, 2H, –SCH2); trans/cis: 10/6; 13C-NMR (DMSO-d6, 100 MHz) δ: 168.99, 163.81, 159.96, 159.78, 155.09, 152.40, 150.53, 147.92, 144.62, 134.42, 129.38, 129.08, 125.43, 121.77, 116.16, 34.82 (trans), 34.62 (cis). MS (ESI) m/z: 369.0 (M+) IR (KBr) ν: 3437, 3321, 3190, 1651, 1582, 1265.
2-(4-Amino-5-(pyridin-4-yl)-4H-pyrazol-3-ylthio)-N′-benzylideneacetohydrazide (A10). White powder (yield, 20.13%). 1H-NMR (DMSO-d6, 400 MHz) δ: 11.77 (cis), 11.66 (trans) (s, 1H, CONH); 8.73 (s, 2H, PyH); 8.21 (s, 1H, N
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 7.99–8.03 (d, J = 16, 2H, PyH); 7.69 (s, 2H, Ph–H); 7.42 (s, 3H, Ph–H); 6.32 (s, 2H, –NH2); 4.09 (cis), 4.50 (trans) (s, 2H, –SCH2); trans/cis: 8/5; 13C-NMR (DMSO-d6, 100 MHz) δ: 169.38, 164.20, 155.08, 152.51, 152.41, 150.53, 147.56, 144.27, 134.41, 130.62, 130.43, 129.27, 127.59, 127.34, 121.75, 34.74 (trans), 34.46 (cis). MS (ESI) m/z: 352.0 (M+) IR (KBr) ν: 3250, 3035, 1662, 1552, 1307.
CH); 7.99–8.03 (d, J = 16, 2H, PyH); 7.69 (s, 2H, Ph–H); 7.42 (s, 3H, Ph–H); 6.32 (s, 2H, –NH2); 4.09 (cis), 4.50 (trans) (s, 2H, –SCH2); trans/cis: 8/5; 13C-NMR (DMSO-d6, 100 MHz) δ: 169.38, 164.20, 155.08, 152.51, 152.41, 150.53, 147.56, 144.27, 134.41, 130.62, 130.43, 129.27, 127.59, 127.34, 121.75, 34.74 (trans), 34.46 (cis). MS (ESI) m/z: 352.0 (M+) IR (KBr) ν: 3250, 3035, 1662, 1552, 1307.
3.3 Cell culture
Mouse neuroblastoma Neuro-2a cell line was obtained from ATCC (Manassas, VA, United States) and cultured in MEM medium supplemented with 1% penicillin/streptomycin and 10% heat-inactivated fetal bovine serum at 37 °C in a humidified 5% CO2 atmosphere. Cells were passaged every 3–4 days.3.4 Cell viability assay
Cell viability was determined by MTT reduction assay. For assay, 5 × 103 cells were plated in 96-well microtiter plates and grown for 24 h. Afterwards cells were treated with different concentrations of test compounds (5, 10, 20 and 50 μM). After 24 h incubation, 10 μL of stock MTT (5 mg mL−1) was added to each well and further incubated for four hours, and then the medium in the wells was removed and 200 μL DMSO was added to each well. The absorbance was measured by a microplate reader at 570 nm.Cell viability was shown relative to the control in a graph.3.5 Neurite outgrowth-promoting assay
For neurite outgrowth assay, Neuro-2a cells were seeded into 24-well plates at a density of 1 × 104 cells per mL and grown for 24 h. After 24 h incubation, the culture medium was replaced with differentiation medium (MEM supplied with 0.5% FBS and 1% penicillin/streptomycin) containing different test compounds for 24 h. Neuro-2a cells were captured and counted under a phase contrast microscope. Neurites were defined as a protrusion with lengths longer than one diameter of the cell body. The neurite length of each cell was measured by Image J software.3.6 Protein extraction and western blot analysis
Neuro-2a cells were seeded in 35 mm dish (6 × 104/dish) overnight and then incubated with compound A5 for different time (0–240 min) at 37 °C. Cells were lysed in RIPA buffer containing protease and phosphatase inhibitors and whole cell lysates were quantified using a BCA protein assay kit according to the manufacturer's instructions. Those cell lysates were subsequently separated by SDS-PAGE, and transferred to PVDF membranes. The membranes were probed with primary antibody, and then subsequently with secondary antibody, followed by electrochemiluminescence (ECL) detection. The following primary antibodies were used: P-Akt (Ser473) (#AA329), Akt (#AA326), P-ERK1/2 (#AF1891), ERK1/2 (#AF1051), P-p38 (#AM063), p38 (#AF1111), P-JNK1/2/3 (#AF1762), JNK1/2/3 (#AF1048) and β-actin (#AA128) antibodies. The antibodies and secondary antibody (#A0216, #A0208) were all purchased from Beyotime (Jiangsu, China)3.7 Statistical analysis
The results are expressed as the mean ± standard error of the mean (SEM). Data were subjected to Student's t test or oneway analysis of variance (ANOVA) followed by Tukey's test to assess the differences between the relevant control and each experimental group. A value of P < 0.05 was considered statistically significant.4. Conclusions
In this work, several acylhydrazone derivatives (A0–A10) have been designed and synthesized, among them two newly synthesized compounds (A6, A8). All of the compounds were evaluated for neuritogenic activity in Neuro-2a cells. The pharmacological results indicated that compound A5 exhibit highly potent neuritogenic activity through the PI3K/Akt and MEK-ERK signaling pathway in vitro. The above results suggested that compound A5 hold high potential use for treating neural injury and neurodegenerative diseases.Conflicts of interest
The authors declare that they have no conflict interests.Acknowledgements
This work was financially supported by the Research Foundation of Education Department of Hunan Province, China (No. 17A66, No. 17B208), and the National Natural Science Foundation of China (No. 31900705, No. 81703821), as well as the Foundation of Hunan Double First-rate Discipline Construction Projects of Bioengineering (No. YYZW2019-04).References
- H. Takahashi, K. Matsuda, K. Tabuchi and J. Ko, Central synapse, neural circuit, and brain function, Neurosci. Res., 2017, 116, 1–2 CrossRef PubMed.
- F. M. Gafarov, Neural electrical activity and neural network growth, Neural Network., 2018, 101, 15–24 CrossRef CAS PubMed.
- T. T. Chuang, Neurogenesis in mouse models of Alzheimer's disease, Biochim. Biophys. Acta, 2010, 1802, 872–880 CrossRef CAS PubMed.
- M. W. Marlatt and P. J. Lucassen, Neurogenesis and Alzheimer's disease: Biology and pathophysiology in mice and men, Curr. Alzheimer Res., 2010, 7, 113–125 CrossRef CAS PubMed.
- J. A. Korecka, S. Talbot, T. M. Osborn, S. M. de Leeuw, S. A. Levy, E. J. Ferrari, A. Moskites, E. Atkinson, F. M. Jodelka, A. J. Hinrich, M. L. Hastings, C. J. Woolf, P. J. Hallett and O. Isacson, Neurite Collapse and Altered ER Ca(2+) Control in Human Parkinson Disease Patient iPSC-Derived Neurons with LRRK2 G2019S Mutation, Stem Cell Rep., 2019, 12, 29–41 CrossRef CAS PubMed.
- N. Kurup, P. Sharifnia and Y. Jin, Spatial and temporal dynamics of neurite regrowth, Curr. Opin. Neurobiol., 2013, 23, 1011–1017 CrossRef CAS PubMed.
- S. V. More, S. Koppula, I. S. Kim, H. Kumar, B. W. Kim and D. K. Choi, The role of bioactive compounds on the promotion of neurite outgrowth, Molecules, 2012, 17, 6728–6753 CrossRef CAS PubMed.
- G. Tang, X. Liu, N. Ma, X. Huang, Z. L. Wu, W. Zhang, Y. Wang, B. X. Zhao, Z. Y. Wang, F. C. Ip, N. Y. Ip, W. C. Ye, L. Shi and W. M. Chen, Design and Synthesis of Dimeric Securinine Analogues with Neuritogenic Activities, ACS Chem. Neurosci., 2016, 7, 1442–1451 CrossRef CAS PubMed.
- L. Pol-Fachin, C. A. Fraga, E. J. Barreiro and H. Verli, Characterization of the conformational ensemble from bioactive N-acylhydrazone derivatives, J. Mol. Graphics Modell., 2010, 28, 446–454 CrossRef CAS PubMed.
- C. Maia Rdo, R. Tesch and C. A. Fraga, Acylhydrazone derivatives: a patent review, Expert Opin. Ther. Pat., 2014, 24, 1161–1170 CrossRef PubMed.
- P. Melnyk, V. Leroux, C. Sergheraert and P. Grellier, Design, synthesis and in vitro antimalarial activity of an acylhydrazone library, Bioorg. Med. Chem. Lett., 2006, 16, 31–35 CrossRef CAS PubMed.
- A. C. Lannes, B. Leal, J. S. Novais, V. Lione, G. C. Monteiro, A. L. Lourenco, P. C. Sathler, A. K. Jordao, C. R. Rodrigues, L. M. Cabral, A. C. Cunha, V. Campos, V. F. Ferreira, M. C. de Souza, D. O. Santos and H. C. Castro, Exploring N-acylhydrazone derivatives against clinical resistant bacterial strains, Curr. Microbiol., 2014, 69, 357–364 CrossRef CAS PubMed.
- L. N. Cardoso, M. L. Bispo, C. R. Kaiser, J. L. Wardell, S. M. Wardell, M. C. Lourenco, F. A. Bezerra, R. P. Soares, M. N. Rocha and M. V. de Souza, Anti-tuberculosis evaluation and conformational study of N-acylhydrazones containing the thiophene nucleus, Arch. Pharm., 2014, 347, 432–448 CrossRef CAS PubMed.
- Y. K. da Silva, C. V. Augusto, M. L. de Castro Barbosa, G. M. de Albuquerque Melo, A. C. de Queiroz, T. de Lima Matos Freire Dias, W. B. Junior, E. J. Barreiro, L. M. Lima and M. S. Alexandre-Moreira, Synthesis and pharmacological evaluation of pyrazine N-acylhydrazone derivatives designed as novel analgesic and anti-inflammatory drug candidates, Bioorg. Med. Chem., 2010, 18, 5007–5015 CrossRef PubMed.
- J. C. Silva, R. G. Oliveira Junior, M. G. E. Silva, E. M. Lavor, J. M. D. Soares, S. R. G. Lima-Saraiva, T. C. Diniz, R. L. Mendes, E. B. Alencar Filho, E. J. L. Barreiro, L. M. Lima and J. Almeida, LASSBio-1586, an N-acylhydrazone derivative, attenuates nociceptive behavior and the inflammatory response in mice, PLoS One, 2018, 13, e0199009 CrossRef PubMed.
- J. V. Cerqueira, C. S. Meira, E. S. Santos, L. S. de Aragao Franca, J. F. Vasconcelos, C. K. V. Nonaka, T. L. de Melo, J. M. Dos Santos Filho, D. R. M. Moreira and M. B. P. Soares, Anti-inflammatory activity of SintMed65, an N-acylhydrazone derivative, in a mouse model of allergic airway inflammation, Int. Immunopharmacol., 2019, 75, 105735 CrossRef CAS PubMed.
- B. Tian, M. He, Z. Tan, S. Tang, I. Hewlett, S. Chen, Y. Jin and M. Yang, Synthesis and antiviral evaluation of new N-acylhydrazones containing glycine residue, Chem. Biol. Drug Des., 2011, 77, 189–198 CrossRef CAS PubMed.
- B. Tian, M. He, S. Tang, I. Hewlett, Z. Tan, J. Li, Y. Jin and M. Yang, Synthesis and antiviral activities of novel acylhydrazone derivatives targeting HIV-1 capsid protein, Bioorg. Med. Chem. Lett., 2009, 19, 2162–2167 CrossRef CAS PubMed.
- X. Yu, L. Shi and S. Ke, Acylhydrazone derivatives as potential anticancer agents: Synthesis, bio-evaluation and mechanism of action, Bioorg. Med. Chem. Lett., 2015, 25, 5772–5776 CrossRef CAS PubMed.
- Y. F. He, M. L. Nan, Y. W. Zhao, W. Y. Sun, W. Li and Q. C. Zhao, Design, synthesis and evaluation of antitumor activity of new rotundic acid acylhydrazone derivatives, Z. Naturforsch., C: J. Biosci., 2016, 71, 95–103 CAS.
- S. Rupiani, R. Buonfiglio, M. Manerba, L. Di Ianni, M. Vettraino, E. Giacomini, M. Masetti, F. Falchi, G. Di Stefano, M. Roberti and M. Recanatini, Identification of N-acylhydrazone derivatives as novel lactate dehydrogenase A inhibitors, Eur. J. Med. Chem., 2015, 101, 63–70 CrossRef CAS PubMed.
- F. Miller, U. Hinze, B. Chichkov, W. Leibold, T. Lenarz and G. Paasche, Validation of eGFP fluorescence intensity for testing in vitro cytotoxicity according to ISO 10993-5, J. Biomed. Mater. Res., Part B, 2017, 105, 715–722 CrossRef CAS PubMed.
- Y. An, T. Zhang, H. Jiang, L. Zhang, J. Han and M. X. Yao, Synthesis, Structure and Their Biological Activities of 3-[(5-H/methyl-benzoimidazol-2-yl) methylthio]-5-substituted-1,2, 4-triazol-4-amine Derivatives, Chin. J. Org. Chem., 2012, 32, 1308–1313 CrossRef CAS.
- J. Han, X. X. Zhou, S. B. Chen and Y. An, Synthesis and Biological Activities of Novel Acylhydrazone Containing 1,2,4-Triazole Structure, Chin. J. Org. Chem., 2014, 34, 741–748 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra02880k |
| This journal is © The Royal Society of Chemistry 2020 |