Open Access Article
Open Access ArticleHighly efficient and selective membrane separation of copper from nickel in ammoniacal solution using mixtures of M5640 and BESO as membrane carriers
Hengpan Duanab,
Hongpan Liua,
Chengbo Hua,
Xiangjun Yangb and
Xuequan Wang *c
*c
aInternational Academy of Targeted Therapeutics and Innovation, School of Pharmacy, Chongqing University of Arts and Sciences, Chongqing, 402160, China
bSchool of Chemical Science and Technology, Yunnan University, Kunming, 650091, China
cKey Laboratory of Natural Pharmaceutical and Chemical Biology of Yunnan Province, School of Science, Honghe University, Mengzi, Yunnan 661100, China. E-mail: huolixuanfeng@126.com
First published on 19th May 2020
Abstract
Separation of copper from nickel in ammoniacal/ammonium chloride solution using a flat-sheet supported liquid membrane impregnated with mixtures of Acorga M5640 and bis(2-ethylhexyl)sulfoxide (BESO) was investigated. The crucial parameters influencing copper transport and separation abilities of copper and nickel, such as carrier concentration of M5640 and BESO in the membrane phase, initial concentration of ions in the feed phase, H2SO4 concentration in the strip phase and membrane stability, were discussed. The results show that the mixtures of carriers (20 vol% M5640 + 20 vol% BESO) in the membrane have a considerable antagonistic effect on membrane transport of nickel, but favor copper transport. Nearly all of the copper was transferred from the feed phase to the strip phase after 12 hours with a flux of 2.05 × 10−5 mol m−2 s−1 under the following conditions: 100 mg L−1 each of the copper and nickel dissolved in 1.0 mol L−1 each of ammonia and ammonium chloride solution as the feed phase, 60 g L−1 H2SO4 as the strip phase, and stirring speed of 800 rpm in two aqueous phases. Meanwhile less than 3.8% of the nickel was transported into the strip phase over the same time. Copper and nickel were efficiently separated with a calculated factor of 26.3. Furthermore, satisfactory membrane stability was obtained with at least ten cycle runs in this separation system.
1. Introduction
The ammonia leaching process has been considered as a promising approach for the recovery of copper and nickel from associated minerals and various secondary materials.1 It supports selective extraction of copper and nickel into solutions as their ammine complexes, and simultaneously separates them from the majority of insoluble impurities in ammoniacal solutions, such as calcite and magnesia.2 This developed scheme could enhance the leaching medium utilization and reduce the cost in the later purification process, especially suitable for processing low-grade resources. As a result, the obtained pregnant solutions require an efficient separation of copper and nickel, which remains as a challenging task due to the similar chemical properties of these two metals.3 Many strategies-based solvent extraction containing varieties of commercial extractants have been reported for recovery and separation of copper and nickel from different acid leaching solutions, in hydrometallurgical process. The general conclusion acquired from these researches reveal that copper could be exclusively extracted and then separated with nickel in acid media by using the conventional hydroxyoximes extractants (such as Lix84,4 Lix984,5 Lix84i,6 LK-C2).7 However, almost all of this desired extractants exhibit poor selectivity of extraction in ammoniacal media due to that copper and nickel always were co-extracted into organic phase. In view of this, the procedure of selective stripping with different acid solutions from co-extraction phase was established as a feasible way for the separation of copper and nickel.8 For instance, Sridhar et al.9 investigated that copper and nickel were both extracted by LIX984N form ammoniacal/ammonium carbonate medium, after that nickel and copper in organic phase were severally stripped by 10 g L−1 and 180 g L−1 H2SO4 aqueous solutions in sequence. Unfortunately, this method is also limited by the case if only one of the metals was desired, and it will increase additional stripping stages to remove another metal.10 Therefore, considerable efforts have been devoting to develop more efficient routes for the selectively recovery and separation of copper and nickel from ammoniacal solutions.Currently, a strategy suggested that mixtures of two different extractants can evidently enhance the separation efficiency of copper and nickel based on the antagonistic effects of extraction. Hu et al. modified the extraction ability of extractants LIX84I by Aliquat 336, the mixtures of them made separation efficiency of copper and nickel improved obviously.10 However, compared to the conventional solvent extraction method, the techniques of supported liquid membrane (SLM) also have been extensively studied for recovery and separation of low-concentration metals from aqueous solution because of its superiority combined solvent extraction with membrane separation.11 It offers several advantages including less organic solvent and energy consumption, higher selective separation, and simultaneous extraction and stripping with one step procedure.12 In view of this, Duan et al. studied transport and separation of copper from nickel in ammoniacal/ammonium chloride solution using supported liquid membrane containing antagonistic mixtures of M5640 and TRPO as carrier.13 The results indicated that the transport rate of nickel decreased from 51% to 6.1%, when the carrier concentration of TRPO rose from 0 vol% to 10 vol% at constant 20 vol% M5640, meanwhile copper transport rate only declined slowly during this process. This could lead to the increase of separation factors of copper and nickel. But the transport rate of copper also declined rapidly beyond 10 vol% concentration of TRPO which would result in the poor performance of membrane separation process. This may be owing to the excessive strength of hydrogen-bond interaction between M5640 and TRPO also inhibited the extraction and transport of copper by M5640 carrier.
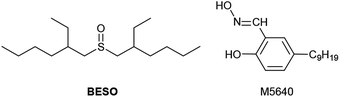
Therefore, in order to obtain the higher separation efficiency of copper and nickel as well as transport rate of copper, bis(2-ethylhexyl)sulfoxide (BESO, Fig. 1) was selected for new modifier of M5640 (Fig. 1) in present work.14 Compared with the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of TRPO, the oxygen atom in the S
O group of TRPO, the oxygen atom in the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of BESO has a lower electronic density due to the fact that the sulfur electronegativity (2.58) is higher than phosphorus (2.19), resulting in a weaker strength of hydrogen bond between O of S
O group of BESO has a lower electronic density due to the fact that the sulfur electronegativity (2.58) is higher than phosphorus (2.19), resulting in a weaker strength of hydrogen bond between O of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group and H of hydroxyl group that belong to M5640.15 The weaker hydrogen-bond interaction between M5640 and BESO could make it easier for M5640 carrier to extract and transport copper, because the hydrogen bond need to be broken before extraction reaction. Thus, selective membrane separation of copper from nickel in ammoniacal solution using mixtures of M5640 and BESO as membrane carrier were studied. The fundamental parameters influencing copper transport and separation abilities of copper and nickel were discussed, including carrier concentration of M5640 and BESO in the membrane phase, initial concentration of ions in the feed phase, H2SO4 concentration in the strip phase and the membrane stability.
O group and H of hydroxyl group that belong to M5640.15 The weaker hydrogen-bond interaction between M5640 and BESO could make it easier for M5640 carrier to extract and transport copper, because the hydrogen bond need to be broken before extraction reaction. Thus, selective membrane separation of copper from nickel in ammoniacal solution using mixtures of M5640 and BESO as membrane carrier were studied. The fundamental parameters influencing copper transport and separation abilities of copper and nickel were discussed, including carrier concentration of M5640 and BESO in the membrane phase, initial concentration of ions in the feed phase, H2SO4 concentration in the strip phase and the membrane stability.
2. Experimental
2.1. Chemicals and materials
The commercial extractant M5640 was supplied by Cognis Co., Ltd. BESO was prepared in our laboratory according to the procedure described earlier.15 Kerosene was used as diluent and all other reagents (CuCl2·2H2O, NiCl2·6H2O, NH3·H2O and NH4Cl) were of analytical grade. The hydrophobic polyvinylidene fluoride13 (PVDF, Millipore Durapore GVHP 4700) was employed as the solid support for a flat-sheet liquid membrane. The initial aqueous solution was composed of 100 mg L−1 each of copper and nickel, and 1.0 mol L−1 each of NH3·H2O and NH4Cl.2.2. Solvent extraction
Solvent extraction was conducted by shaking separating funnel that with equal volumes (10.0 mL) of organic and aqueous phases at the vapor-bathing constant temperature vibrator (25 ± 1 °C, 200 rpm). A total shaking time of 10 minutes was performed to reach the extraction equilibrium. After that, the raffinate phase was analyzed by atomic absorption spectroscopy (AAS, Hitachi Z2000 spectrophotometer).The extraction rate (% E) was estimated as the percentage of metal concentration in the organic phase to its initial concentration in the aqueous phase. The distribution ratios (D) were calculated using the ratio of metal concentration in the organic phase to aqueous phase. The separation factor of copper and nickel (βCu/Ni) was determined according to eqn (1):
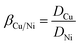 | (1) |
2.3. Transport and separation
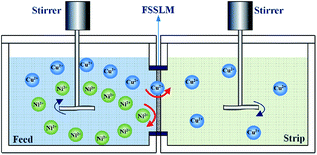
A separation device consisted of two acrylic compartments contacted with the flat-sheet supported liquid membrane (FSSLM, effective membrane area: 11.34 cm2), was shown in Fig. 2.The FSSLM was prepared by impregnating PVDF film with the mixtures of M5640 and BESO in kerosene solution for 24 hours, before being placed in the membrane cell. The feed cell and strip cell were poured into equal volumes (150 mL) of initial metals solution and H2SO4 aqueous solution respectively. Two aqueous phases were stirred at 800 rpm by using the mechanical agitators in order to avoid the ionic concentration polarization of membrane interfaces, and transport experiments were operated in a thermostatic water bath at 25 °C. The concentration of metals in feed phase and strip phase were determined periodically by AAS.
The transport rate of copper (% T) was calculated according to eqn (2).
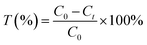 | (2) |
The kinetic model of copper transport through membrane was given by eqn (3):16
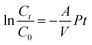 | (3) |
The permeation flux (J, mol m−2 s−1) was used for evaluating copper transport efficiency, which could be calculated by eqn (4):
| J = P × C0 | (4) |
The separation factor (αCu/Ni) of copper and nickel was expressed as eqn (5).17
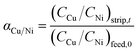 | (5) |
3. Results and discussion
3.1. Solvent extraction studies
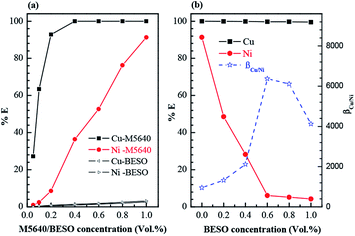
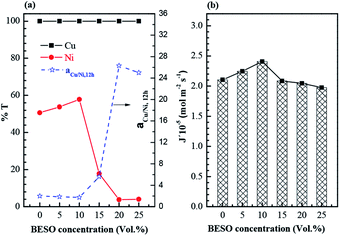
The extraction of copper and nickel from ammoniacal solution was investigated by varying concentrations of M5640 ranging from 0.1 vol% to 1.0 vol%. As shown in Fig. 3(a), copper and nickel were both extracted by sole extractant M5640, nearly all of copper and 91.3% of nickel in initial aqueous phase were transferred to the organic phase as the M5640 concentration increased to 1.0 vol%. The results show that it is difficult to achieve selective extraction and separation of two metals from ammoniacal solution only by using extractant M5640. In view of this, BESO as the desired modifier was used for adjusting the extraction abilities of M5640. Before that, the extraction behavior of sole BESO also was examined. As can be seen from Fig. 3(a), sole BESO as extractant exerted a poor extraction performance compared with M5640.Then, the extraction experiments were performed by varying the BESO concentrations from 0 to 1.0 vol% mixed with a fixed concentration of 1.0 vol% M5640. It can be seen from Fig. 3(b), the nickel extraction rate decreased sharply from 91.3% to 4.3% as the BESO concentration increased from 0 to 1.0 vol%. Meanwhile the extraction efficiency of copper received the neglectable impact, and its extraction rate was consistently over 99.4% with the change of BESO concentrations. A maximum of separation factor of copper and nickel was obtained at a mixed concentration of 0.6 vol% of BESO with 1.0 vol% of M5640. These results suggest that the strategy developed with the mixtures of extractant M5640 and modifier BESO dissolved by kerosene as organic phase could be used for selective extraction of copper and separation with nickel from ammoniacal solution.
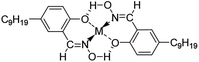
The chelate complex of M5640 and metals (Cu, Ni) is represented on Fig. 4. Before chelation reaction happen, M5640 and BESO tend to co-associate through hydrogen bonding involving the –OH and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups when they were mixed together. Generally, the mixtures of M5640 and BESO may hinder the formation of chelate metal complexes, and reduce kinetics of the metal chelate reaction. That is, this would result in antagonistic effects on extraction of both copper and nickel. However in fact, the antagonistic effect had a negligible impact on copper extraction compared with nickel according to the above experimental result. The distinction of antagonistic effect on two metals provided the theoretical support for efficient separation of copper and nickel from ammoniacal solution.
O groups when they were mixed together. Generally, the mixtures of M5640 and BESO may hinder the formation of chelate metal complexes, and reduce kinetics of the metal chelate reaction. That is, this would result in antagonistic effects on extraction of both copper and nickel. However in fact, the antagonistic effect had a negligible impact on copper extraction compared with nickel according to the above experimental result. The distinction of antagonistic effect on two metals provided the theoretical support for efficient separation of copper and nickel from ammoniacal solution.
3.2. Membrane separation studies
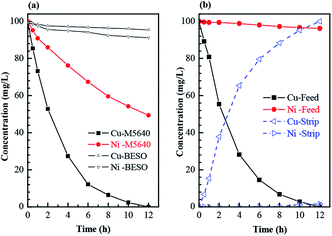
The transport of copper and nickel through FSSLM using M5640, BESO and their mixtures as membrane carriers respectively were studied. Fig. 5(a) shows the concentration of copper and nickel in the feed phase gradually decreased with transport time. Nearly all of copper and 49.4% of nickel were transported from the feed phase to the strip phase after 12 hours by using 20 vol% M5640 as membrane carrier. Besides, the slight decline of metals concentration in feed phase indicated that BESO (20 vol%) could be regarded as the noneffective carrier using for the transport of copper and nickel compared with M5640. However, as can be seen Fig. 5(b), the nickel transport rate decreased significantly from 49.4% to 3.8% over a period of 12 hours, after employing the mixtures of 20 vol% M5640 and 20 vol% BESO as membrane carrier.Meanwhile, high-efficiency transport of copper had hardly changed in this system. The results of membrane separation of copper and nickel agree well with that obtained by above solvent extraction. Subsequently, the specific studies were conducted to evaluate the separation abilities of copper and nickel by using the flat-sheet supported liquid membrane containing the mixtures of M5640 and BESO as carrier.
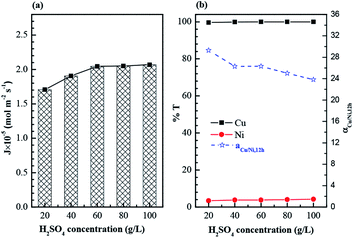
Fig. 7(b) shows that the copper transport rate for a period of 12 hours is consistently high (>99.6%) with the increasing of H2SO4 concentration. Whereas the transport rate of nickel grows slightly, in despite of the fact that the majority of nickel in feed phase cannot be carried by membrane carrier. This also caused a decline of separation factor of copper and nickel. As a result, H2SO4 concentration in strip phase was finally confirmed as 60 g L−1 by taking into consideration both the copper permeation flux and the separation efficiency of copper and nickel.
| [M(NH3)2+n](aq) + 2HL(org) ↔ [ML2](org) + 2[NH+4](aq) + (n − 2)[NH3](aq) | (6) |
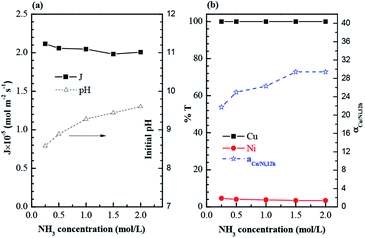
Besides, ammonia also could be co-extracted with copper and nickel by using hydroxyoxime extractants (including M5640 used in this study) in the alkaline region,23 and the higher ammonia concentration may result in the reduction of copper carrying capacity of membrane carrier in membrane phase. This is perhaps the reason of why the transport rate of nickel declined slightly as the increasing of ammonia concentration, then driving up the separation factor of copper and nickel (Fig. 8(b)). In fact, the ammonia content of actual solution derived from different industries is always inequable. In the present work, an ammonia concentration of 1.0 mol L−1 was maintained throughout the experimentation to keep the comparison ability of the results.
| [Cu]feed/[Ni]feed (mg L−1) | Initial flux of copper JCu (mol m−2 s−1) | TCu,12 h% | TNi,12 h% | Separation factor, αcu/Ni,12 h |
|---|---|---|---|---|
| a Conditions: feed phase = 1.0 mol L−1 each of NH3 and NH4+; extractant carrier = 20 vol% M5640 + 20 vol% BESO in kerosene; strip phase = 60 g L−1 H2SO4; stirring speed = 800 rpm. | ||||
| 50/50 | 1.51 × 10−5 | 99.99 | 7.6 | 13.2 |
| 100/100 | 2.05 × 10−5 | 99.99 | 3.8 | 26.3 |
| 300/300 | 3.28 × 10−5 | 93.4 | 4.3 | 21.9 |
| 100/300 | 2.02 × 10−5 | 99.8 | 5.5 | 18.1 |
| 300/100 | 3.37 × 10−5 | 93.8 | 3.4 | 27.6 |
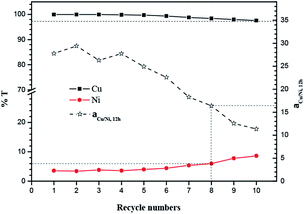
However, it is unexpected that transport of nickel instead increased beyond the sixth transport, and then resulting in the decrease of separation factor of copper and nickel. The reason caused the abnormal phenomenon may be that the loss of BESO in membrane is more easy than M5640. Because it was observed that the visible viscosity of BESO is far below M5640 during the experiment. In this case, it leads to the decline of the ratio of BESO to M5640 in membrane, then result in the rise performance-based extraction nickel in feed/membrane phase interface. On the whole, the present FSSLM system nearly kept stable over six continuous cycles for high-efficiency separation of copper and nickel. Even at the tenth run, the transport rate of nickel was still lower than 8.6%, and together with a separation factor of 11.3. It's worth mentioning that the eighth separation efficiency of copper and nickel (separation factor = 16.4) in this work also exceeded the first operation (separation factor = 16.0) employing M5640/TRPO as carrier.13 These results indicate that the present FSSLM displayed a satisfactory successive performance of copper transport and separation with nickel.
| Methods | Extractants | Cu extraction rate (% ECu) | Ni extraction rate (% ENi) | Separation ratio (ECu/ENi) | References |
|---|---|---|---|---|---|
| Solvent extraction | LIX84I and A336 | 97.66 | 11.39 | 8.57 | 10 |
| M5640 and TRPO | 99.96 | 6.6 | 15.15 | 15 | |
| FSSLM separation | Only M5640 | 99.99 | 51.0 | 1.96 | 13 |
| M5640 and TRPO | 98.40 | 6.1 | 16.04 | 13 | |
| M5640 and BESO | 99.99 | 3.80 | 26.30 | Present work |
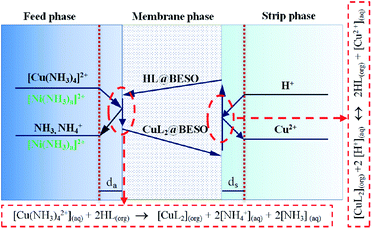
(i) Copper and nickel ammine complexes in the feed phase diffused to the aqueous boundary layer (da) in the feed/membrane phase side.
(ii) A majority of copper were preferentially extracted by membrane carrier (M5640) in the feed/membrane phase interface, following the eqn (7).
| [Cu(NH3)2+4](aq) + 2HL(org) → [CuL2](org) + 2[NH+4](aq) + 2[NH3](aq) | (7) |
(iii) The copper extracted complexes transfer through the membrane toward the membrane/strip phase interface.
(iv) In the membrane/strip phase interface, copper was stripped by H2SO4 solution, according to eqn (8).
| [CuL2](org) + 2[H+](aq) ↔ 2HL(org) + [Cu2+](aq) | (8) |
(v) Cu2+ diffused from the membrane/strip phase interface to the bulk of the strip phase.
(vi) Regenerated carrier HL transfer backed to the feed/membrane phase interface.
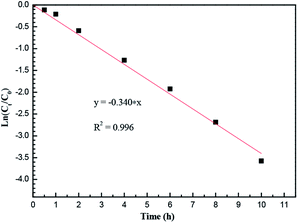
Among these steps, if the interfacial reactions of complexation extraction at the feed/membrane phase interface (ii) and the decomplexation at the membrane/strip phase interface (iv) occur instantaneously relative to other diffusion processes,25 then copper transport efficiency is mainly controlled by the rate of diffusion of the extracted CuL2 complexes through the membrane under the optimal conditions: mixtures of 20 vol% M5640 and 20 vol% BESO in kerosene as carrier, 100 mg L−1 each of the copper and nickel dissolved in 1.0 mol L−1 each of ammonia and ammonium chloride solution as the feed phase, 60 g L−1 H2SO4 as the strip phase, and stirring speed of 800 rpm in two aqueous phases. The transport of copper through the FSSLM follows first-order kinetics. The plot of ln(Ct/C0) versus operation time for copper transport is presented in Fig. 11 and illustrates that ln(Ct/C0) is a linear function of time with a slope of −0.340. Thus, the permeability coefficient (P) was calculated to be 1.26 × 10−5 m s−1.
4. Conclusions
High-efficient and selective membrane separation of copper from nickel in ammoniacal solution using mixtures of Acorga M5640 and bis(2-ethylhexyl)sulfoxide (BESO) as membrane carrier was studied. The preliminary solvent extraction studies illustrated that the mixtures of extractant M5640 and modifier BESO in the organic phase exhibited an obvious inhibiting effect on the nickel extraction. In order to determine the optimum membrane separation conditions of copper and nickel, the crucial parameters influencing copper transport and separation abilities of copper and nickel, such as carrier concentration of M5640 and BESO in the membrane phase, initial concentration of ions in the feed phase, H2SO4 concentration in the strip phase and the membrane stability were discussed. The results show that the mixtures of carrier (20 vol% M5640 + 20 vol% BESO) in membrane favor copper transport, but exert considerable antagonistic effect on membrane transport of nickel. Nearly all of copper were transported from the feed phase to the strip phase after 12 hours with a flux of 2.05 × 10−5 mol m−2 s−1 under the following conditions: 100 mg L−1 each of the copper and nickel dissolved in 1.0 mol L−1 each of ammonia and ammonium chloride solution as the feed phase, 60 g L−1 H2SO4 as the strip phase, and stirring speed of 800 rpm in two aqueous phases. Whereas only less than 3.8% of nickel was transported into strip phase in the meantime. Copper and nickel were efficiently separated with a calculated factor of 26.3. Furthermore, satisfactory membrane stability was obtained with at least ten cycle runs in this separation system. The results achieved in this study indicate that the FSSLM system developed with M5640/BESO as the carrier could be a useful tool for facilitating transport and selective separation of copper from nickel in ammoniacal solutions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank financial supports from the National Natural Science Foundation of China (21602050), Scientific and Technological Research Program of Chongqing Municipal Education Commission (KJQN201801324, KJQN201901310), National Natural Science Foundation of Yongchuan (Ycstc, 2019nb0803) and Chongqing University of Arts and Sciences (Y2019XY18).Notes and references
- (a) Z. Liu, Z. Yin, Y. Chen and L. Xiong, Metall. Mater. Trans. B, 2014, 45, 2027 CrossRef CAS; (b) X. Wang, Q. Chen, H. Hu and Z. Yin, Hydrometallurgy, 2009, 99, 231 CrossRef CAS; (c) X. Meng and K. Han, Miner. Process. Extr. Metall. Rev., 1996, 16, 23 CrossRef CAS; (d) E. Hsu, K. Barmak, A. C. West and A. A. Park, Green Chem., 2019, 21, 919 RSC.
- (a) R. Salhi, Miner. Process. Extr. Metall. Rev., 2010, 119, 147 CrossRef CAS; (b) H.-P. Hu, C.-X. Liu, X.-T. Han, Q.-W. Liang and Q.-Y. Chen, Trans. Nonferrous Metals Soc. China, 2010, 20, 2026 CrossRef CAS.
- L. Li, X. Chen, X. Liu and Z. Zhao, Hydrometallurgy, 2014, 146, 149 CrossRef CAS.
- M. K. Jha, D. Gupta, P. K. Choubey, V. Kumar, J. Jeong and J. Lee, Sep. Purif. Technol., 2014, 122, 119 CrossRef CAS.
- L. Li and H. Zhong, Adv. Mater. Res., 2011, 365, 252 Search PubMed.
- B. R. Reddy and D. N. Priya, Sep. Purif. Technol., 2005, 45, 163 CrossRef CAS.
- X. Zhang, X. Li, H. Cao and Y. Zhang, Sep. Purif. Technol., 2010, 70, 306 CrossRef CAS.
- (a) C. Parija and P. V. R. B. Sarma, Hydrometallurgy, 2000, 54, 195 CrossRef CAS; (b) V. Sridhar, J. K. Verma and N. S. Shenoy, Miner. Eng., 2010, 23, 454 CrossRef CAS.
- V. Sridhar, J. K. Verma and S. N. Kumar, Hydrometallurgy, 2009, 99, 124 CrossRef CAS.
- J. Hu, S. Wang, H. Hu, H. Li, K. Peng, D. Liu, Q. Liu and Q. Chen, Sep. Purif. Technol., 2013, 118, 828 CrossRef CAS.
- (a) F. J. Alguacil, M. Alonso, F. A. Lopez and A. Lopez-Delgado, Desalination, 2011, 281, 221 CrossRef CAS; (b) H. Duan, X. Yuan, Q. Zhang, Z. Wang, Z. Huang, H. Guo and X. Yang, Chem. Pap., 2017, 71, 597 CrossRef CAS.
- S. A. Allahyari, S. J. Ahmadi, A. Minuchehr and A. Charkhi, RSC Adv., 2017, 7, 7413 RSC.
- H. Duan, S. Wang, X. Yang, X. Yuan, Q. Zhang, Z. Huang and H. Guo, Chem. Eng. Res. Des., 2017, 117, 460 CrossRef CAS.
- X. Yang, A. Zou, J. Qiu, S. Wang and H. Guo, Sep. Sci. Technol., 2014, 49, 2495 CrossRef CAS.
- R. Yang, S. Wang, H. Duan, X. Yuan, Z. Huang, H. Guo and X. Yang, Hydrometallurgy, 2016, 163, 18 CrossRef CAS.
- A. Talebi, T. T. Teng, A. F. M. Alkarkhi and N. Ismail, RSC Adv., 2015, 5, 38424 RSC.
- (a) A. Surucu, V. Eyupoglu and O. Tutkun, Desalination, 2010, 250, 1155 CrossRef; (b) A. Surucu, V. Eyupoglu and O. Tutkun, J. Ind. Eng. Chem., 2012, 18, 629 CrossRef CAS.
- F. T. Minhas, M. Shahabuddin, Q. Imdadullah and M. Mujahid, Chimia Rep., 2013, 16, 742 CrossRef CAS.
- F. J. Alguacil, M. Alonso and A. M. Sastre, Chem. Eng. J., 2002, 85, 265 CrossRef CAS.
- H.-D. Zheng, B.-Y. Wang, Y.-X. Wu and Q.-L. Ren, Colloids Surf., A, 2009, 351, 38 CrossRef CAS.
- J. Hu, Q. Chen, H. Hu and B. Qiu, Sep. Purif. Technol., 2012, 95, 136 CrossRef CAS.
- G. Kyuchoukov, M. B. Bogacki and J. Szymanowski, Ind. Eng. Chem. Res., 1998, 37, 4084 CrossRef CAS.
- M. Tanaka and S. Alam, Hydrometallurgy, 2010, 105, 134 CrossRef CAS.
- J. Song, T. Huang, H. Qiu, X. Niu, X. Li, Y. Xie and T. He, Desalination, 2018, 440, 18 CrossRef CAS.
- P. Zaheri, H. Abolghasemi, T. Mohammadi and M. G. Maraghe, Chem. Pap., 2015, 69, 279 CAS.
| This journal is © The Royal Society of Chemistry 2020 |