Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Regioselective O/C phosphorylation of α-chloroketones: a general method for the synthesis of enol phosphates and β-ketophosphonates via Perkow/Arbuzov reaction†
Yuepeng Cao,
Zhenhua Gao,
Junchen Li,
Xiaojing Bi,
Ling Yuan,
Chengxin Pei,
Yongbiao Guo * and
Enxue Shi*
* and
Enxue Shi*
State Key Laboratory of NBC Protection for Civilian, Beijing 102205, P. R. China. E-mail: van87120@126.com; exshi@sina.com
First published on 11th August 2020
Abstract
A regioselective O/C phosphorylation of α-chloroketones with trialkyl phosphites was performed for the first time, which employed solvent-free Perkow reaction and NaI-assisted Arbuzov reaction under mild conditions respectively. Versatile enol phosphates were prepared in good to excellent yields as well as β-ketophosphinates.
Introduction
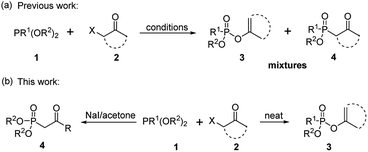
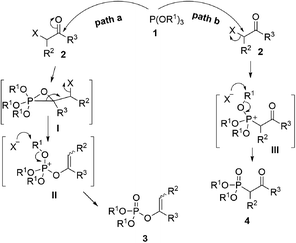
As one of the most utilized classic reactions, Perkow reaction1,2 offers an efficient way to obtain pharmacologically and biologically important enol phosphates through simple condensation of trialkyl phosphites and α-haloketones.3–13 However, the reaction generally gives a mixture of enol phosphates and β-ketophosphonates generating from the competitive O/C phosphorylation (Scheme 1a), which proceeds mainly through the following two possible reaction pathways of α-haloketones (Fig. 1): (i) path a, the nucleophilic P atom of trialkyl phosphite attacks the carbonyl C atom of α-haloketone forming a triatomic heterocyclic I which followed by the ring cleavage transforms into a phosphonium salt II and then by the O-demethylation gives the enol phosphates product 3 (Perkow reaction mechanism); (ii) path b, the nucleophilic P atom attacks the halocarbon of α-haloketone to generate a phosphonium salt III, followed by the O-demethylation to give the β-ketophosphonate product 4 (Arbuzov reaction mechanism).14–19 To the date, highly regioselective O/C phosphorylation of α-haloketones with trialkyl phosphites remains unavailable. Herein, we wish to report a simple but efficient method for regiospecific synthesis of enol phosphates and β-ketophosphonates respectively with good to excellent yields under quite mild conditions only by using the most easily-available and economic α-chloroketones as the starting metarial (Scheme 1b).Results and discussion
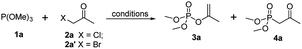
Examination and optimization of the reaction parameters were explored using trimethyl phosphite 1a and 1-bromopropan-2-one 2a′ as the model substrates. The reaction without solvent used at 40 °C proceeded to give enol phosphate 3a and β-ketophosphonate 4a in 50% yield respectively (Table 1, entry 1). Encouraged by this result, a systematic screening of the reaction conditions was carried out to examine the regioselectivity (Table 1). A solvent screen revealed that lower-polar solvents could indeed facilitate the regioselectivity of Perkow reaction (entries 2–7), whereas higher-polar solvent could favour the occuration of Arbuzov reaction (entries 7–11). And the highest regioselectivity (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for Perkow reaction was obtained in p-xylene with yields of 45%. Based on the knowledge of the mechanism of Perkow reaction, we assumed that using 1-chloropropan-2-one 2a instead of 2a′ should improve the selectivity. Unfortunately, the reaction became very sluggish (entry 12). But unexpectedly, the solvent-free reaction of 1a and 2b gave the 3a not only in good yield but with excellent selectivity (entry 13). Moreover, when lowering the temperature just from 40 to 30 °C, the regioselectivity could increase to as high as 94
1) for Perkow reaction was obtained in p-xylene with yields of 45%. Based on the knowledge of the mechanism of Perkow reaction, we assumed that using 1-chloropropan-2-one 2a instead of 2a′ should improve the selectivity. Unfortunately, the reaction became very sluggish (entry 12). But unexpectedly, the solvent-free reaction of 1a and 2b gave the 3a not only in good yield but with excellent selectivity (entry 13). Moreover, when lowering the temperature just from 40 to 30 °C, the regioselectivity could increase to as high as 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (entry 14) though the reaction became some lower reactive (50% yield vs. 70%). Raising the reaction temperature to 50 °C or 90 °C, which prospectively improved the reaction rate significantly, would obviously decrease the selectivity (entries 15 and 16). Further control experiments revealed that the optimized reaction conditions should be usage of 1.2 equiv. of 1a, providing the target compound in 80% yield with 50
1 (entry 14) though the reaction became some lower reactive (50% yield vs. 70%). Raising the reaction temperature to 50 °C or 90 °C, which prospectively improved the reaction rate significantly, would obviously decrease the selectivity (entries 15 and 16). Further control experiments revealed that the optimized reaction conditions should be usage of 1.2 equiv. of 1a, providing the target compound in 80% yield with 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 regioselectivity (entry 17).
1 regioselectivity (entry 17).
| Entry | X | Temp. (°C) | Solvent | T (h) | Yield (%) of 3ab | 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4ab 4ab |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (2.4 mmol), 2a or 2a′ (2 mmol), in neat or 5 mL solvent.b Yield and regioselectivity of 3a and 4a were determined by 1H NMR.c 1.2 equiv. of 1a used.d 1.5 equiv. of 1a used. | ||||||
| 1 | Br | 40 | — | 3 | 50 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | Br | 40 | p-Xylene | 72 | 45 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | Br | 40 | o-Xylene | 72 | 45 | 6.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | Br | 40 | m-Xylene | 72 | 40 | 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | Br | 40 | PhMe | 72 | 30 | 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | Br | 40 | PhH | 72 | 15 | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | Br | 40 | CH2Cl2 | 72 | 50 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | Br | 40 | Et2O | 72 | 15 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | Br | 40 | THF | 72 | 20 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 10 | Br | 40 | 1,4-Dioxane | 72 | 30 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 11 | Br | 40 | Acetone | 72 | 25 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 12 | Cl | 40 | p-Xylene | 36 | Trace | — |
| 13 | Cl | 40 | — | 36 | 70 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | Cl | 30 | — | 36 | 50 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1 |
| 15 | Cl | 50 | — | 24 | 73 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 16 | Cl | 90 | — | 10 | 70 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 17c | Cl | 40 | — | 36 | 80 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 18d | Cl | 40 | — | 36 | 80 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Under the optimized conditions, the scope of the regioselective Perkow reaction of trimethyl phosphite 1a and various α-chloroketone (2a–k) was investigated (Scheme 2). Generally, both the steric and electronic substituents on aliphatic and aromatic α-chloroketones seem to have negligible effects on the regioselectivity. Substrates bearing either an electron-donating (OMe) or electron-withdrawing groups (Br, Cl and F) at the 4-position of the phenyl ring were all converted to their corresponding products 3b–3g in excellent yields (88% to 95%) with high regioselectivities (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to >99
1 to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), albeit the α-chloroketone with electron-donating OMe required a higher ratio of 1a
1), albeit the α-chloroketone with electron-donating OMe required a higher ratio of 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2b (3
2b (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). However, the electronic effects may have great influence on the reaction rate leading to the reaction times of α-chloroketone with electron-withdrawing group (Ph, Br, Cl and F) significantly reduced to 10 h. But the positions of substituents on phenyl ring showed almost no effect (entry 3h and 3i vs. 3f). It is worth noting that the electronic substituents on the halocarbon C could significantly determine the reaction efficiency as shown by chloro-substituted (3l) and 2,2-difluoro-substituted (3m) substrates with only 2 h and 1 h reaction time, while the methyl-substituted substrates (3j) and (3k) with 48 h and 24 h reaction time. For cyclical substrates, the corresponding products were obtained in excellent regioselectivities but much different yields (3n) and (3o) under the optimized conditions. Gratifyingly, when the reaction temperature was increased 90 °C, 81% yield without loss of regioselectivity could be achieved after 48 h reaction time (3o).
1). However, the electronic effects may have great influence on the reaction rate leading to the reaction times of α-chloroketone with electron-withdrawing group (Ph, Br, Cl and F) significantly reduced to 10 h. But the positions of substituents on phenyl ring showed almost no effect (entry 3h and 3i vs. 3f). It is worth noting that the electronic substituents on the halocarbon C could significantly determine the reaction efficiency as shown by chloro-substituted (3l) and 2,2-difluoro-substituted (3m) substrates with only 2 h and 1 h reaction time, while the methyl-substituted substrates (3j) and (3k) with 48 h and 24 h reaction time. For cyclical substrates, the corresponding products were obtained in excellent regioselectivities but much different yields (3n) and (3o) under the optimized conditions. Gratifyingly, when the reaction temperature was increased 90 °C, 81% yield without loss of regioselectivity could be achieved after 48 h reaction time (3o).
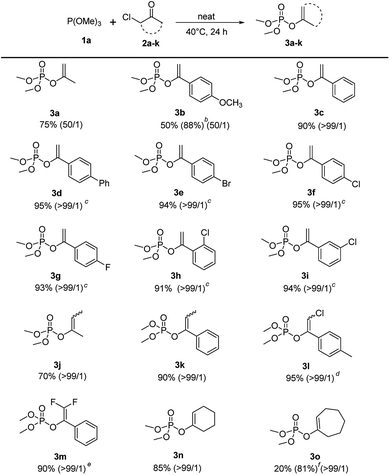 | ||
| Scheme 2 Scope of α-haloketones.a aReaction conditions: 1a (2.4 mmol), 2a–o (2 mmol), 40 °C, 24 h. b1a (6 mmol). c10 h. d2 h. e1 h. f90 °C, 48 h. | ||
Encouraged by those results, we next optimized the Arbuzov reaction conditions (Table 2). Although compared with 1-chloropropan-2-one and 1-bromopropan-2-one, 1-iodopropan-2-one exhibited superior regioselectivity (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of Arbuzov reaction, the yield of 4a was poor (entry 3 vs. entries 1 and 2) due to that 1-iodopropan-2-one would decompose under solvent-free conditions. However, since higher-polar solvent of actone exhibited good regioselectivity of Arbuzov reaction (Table 1, entry 11), we hypothesized that if 1-iodoketone could be generated in situ by treatment of 2a with NaI in acetone (Finkelstein reaction20–23), which may be beneficial to the conversion favouring Arbuzov reaction. Following experiments verified our assumption that 4a was obtained in 85% yield with 26
1) of Arbuzov reaction, the yield of 4a was poor (entry 3 vs. entries 1 and 2) due to that 1-iodopropan-2-one would decompose under solvent-free conditions. However, since higher-polar solvent of actone exhibited good regioselectivity of Arbuzov reaction (Table 1, entry 11), we hypothesized that if 1-iodoketone could be generated in situ by treatment of 2a with NaI in acetone (Finkelstein reaction20–23), which may be beneficial to the conversion favouring Arbuzov reaction. Following experiments verified our assumption that 4a was obtained in 85% yield with 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 regioselectivity (entry 5). Further screening of reaction temperature revealed that 50 °C was the most satisfactory condition (entry 7, 85%, 35
1 regioselectivity (entry 5). Further screening of reaction temperature revealed that 50 °C was the most satisfactory condition (entry 7, 85%, 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
| Entry | X | Temp. (°C) | NaI | Solvent | T (h) | Yield (%) of 4ab | 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3ab 3ab |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (2.4 mmol), 2a, 2a′ or 2a′′ (2 mmol), NaI (0 or 2 mmol) neat or 5 mL acetone, 30–60 °C, 2–30 h.b Yield and regioselectivity of 4a and 3a were determined by 1H NMR. | |||||||
| 1 | Cl | 40 | — | — | 36 | <2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
| 2 | Br | 40 | — | — | 3 | 50 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | I | 40 | — | — | 2 | 25 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | Br | 40 | — | Acetone | 30 | 63 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 5 | Cl | 40 | 1 eq. | Acetone | 4 | 85 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | Cl | 45 | 1 eq. | Acetone | 2 | 84 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | Cl | 50 | 1 eq. | Acetone | 2 | 85 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1 |
| 8 | Cl | 60 | 1 eq. | Acetone | 2 | 87 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
With the optimized reaction conditions in hand, the scope of this reaction was explored with trimethyl phosphite 1a and various α-chloroketones (2a–k) to prepare β-ketophosphonates 4a–k (Scheme 3). Substrates bearing either an electron-donating (OMe) or electron-withdrawing groups (Br, Cl and F) at the 4-position on the phenyl ring were converted to their corresponding Arbuzov products 4b–4i in good to excellent yields (67% to 93%) and moderate to excellent regioselectivities (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 84
1 to 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Notably, the electronic effects and the positions of substituents on the phenyl ring have some effect on the selectivity. For example, electron-donating and 4-position groups on the phenyl ring were more beneficial (4b vs. 4e–g and 4f vs. 4h,i) for Arbuzov reaction. Encouraged by these results, we next tested the generality of the reaction regarding the substituent groups on the halocarbon of α-haloketones. However, the reaction proceeded sluggishly and only trace of the product 4j–4o were detected. It was analyzed that the steric effects of substituents have a significantly effect on the in situ iodation reaction.
1). Notably, the electronic effects and the positions of substituents on the phenyl ring have some effect on the selectivity. For example, electron-donating and 4-position groups on the phenyl ring were more beneficial (4b vs. 4e–g and 4f vs. 4h,i) for Arbuzov reaction. Encouraged by these results, we next tested the generality of the reaction regarding the substituent groups on the halocarbon of α-haloketones. However, the reaction proceeded sluggishly and only trace of the product 4j–4o were detected. It was analyzed that the steric effects of substituents have a significantly effect on the in situ iodation reaction.
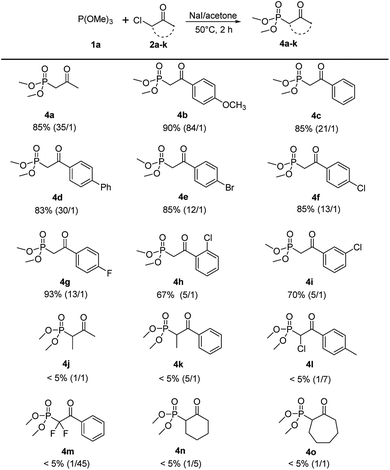 | ||
| Scheme 3 Scope of α-haloketones.a aReaction conditions: 1a (2.4 mmol), 2a–k (2 mmol), NaI (2 mmol), 5 mL acetone, 50 °C, 2 h. | ||
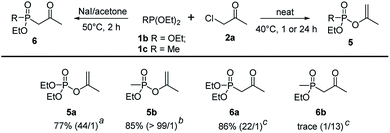
To further extend the substrate scope, triethyl phosphite 1b and diethyl methylphosphonite 1c were examined in the regioselective Perkow![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Arbuzov reaction system. Under the optimized conditions, triethyl phosphite gave its corresponding Perkow product 5a and Arbuzov product 6a in good yields (77% and 86%) and regioselectivities (44
Arbuzov reaction system. Under the optimized conditions, triethyl phosphite gave its corresponding Perkow product 5a and Arbuzov product 6a in good yields (77% and 86%) and regioselectivities (44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 22
1 and 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) respectively, which was similar to trimethyl phosphite 1a. It was noteworthy that the reaction activity of 1c was much greater than 1b (1 h vs. 24 h) giving product 5b in 85% yield with very high regioselectivity (>99
1) respectively, which was similar to trimethyl phosphite 1a. It was noteworthy that the reaction activity of 1c was much greater than 1b (1 h vs. 24 h) giving product 5b in 85% yield with very high regioselectivity (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). However, the substrate 1c gave disappointingly trace amount of 6b under the standard reaction conditions of Arbuzov reaction (Scheme 4).
1). However, the substrate 1c gave disappointingly trace amount of 6b under the standard reaction conditions of Arbuzov reaction (Scheme 4).
Conclusions
In summary, regioselective synthesis of enol phosphates and β-ketophosphonates via Perkow/Arbuzov reaction of phosphites only with α-haloketones in good to excellent yields has been firstly achieved at our laboratory. The success of methylphosphonite also suggests that phosphinites and amino phosphites, as well as α-halothioketones, may be also applicable in this reaction system in the future study.Experimental section
General information
Reagents and solvents were purchased from common commercial suppliers and were used without further purification. Column chromatography was generally performed on silica gel (200–300 mesh). Melting points were determined with a Büchi B-545 melting-point apparatus. 600 MHz 1H NMR and 150 MHz 13C NMR spectra were recorded on Varian VMS-600 spectrometers respectively. The chemical shifts are reported in ppm (δ scale) relative to internal tetramethylsilane, and coupling constants are reported in hertz (Hz). High-resolution mass spectra (HRMS) were obtained on a Agilent 6502 Q-TOF HPLC and mass spectrometry.General procedure for the synthesis of enol phosphates 3
A mixture of 1 (2.4 mmol) and 2 (2 mmol) was stirred at 40 °C under nitrogen atmosphere for 1–48 h and then concentrated under reduced pressure. The resulting orange gum was purified by column chromatography on a silica gel column [eluting with PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i-PrOH (20
i-PrOH (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)] to obtain 3 as colorless oil or white solid.
1)] to obtain 3 as colorless oil or white solid.
General procedure for the synthesis of β-ketophosphonates 4
A mixture of 1 (2.4 mmol), 2 (2 mmol) and NaI (2 mmol) in dry actone was stirred at 50 °C under nitrogen atmosphere for 2 h and then concentrated under reduced pressure. The resulting orange gum was purified by column chromatography on a silica gel column [eluting with PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i-PrOH (10
i-PrOH (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)] to obtain 4 as colorless oil or white solid.
1)] to obtain 4 as colorless oil or white solid.
Conflicts of interest
There are no conflicts to declare.References
- W. Perkow, K. Ullerich and F. Meyer, Neue Phosphorsaureester mit pupillenverengender Wirkung, Naturwissenschaften, 1952, 39, 353 CrossRef.
- W. Perkow, Umsetzungen mit Alkylphosphiten. I. Mitteil.: Umlagerungen bei der Reaktion mit Chloral und Bromal, Chem. Ber., 1954, 81, 755–758 CrossRef.
- J. F. Allen and O. H. Johnson, The Synthesis of Monovinyl Esters of Phosphorus(V) Acids, J. Am. Chem. Soc., 1955, 77, 2871–2875 CrossRef CAS.
- F. Cramer, Neuere Methoden der präparativen organischen Chemie III 2. Darstellung von Estern, Amiden und Anhydriden der Phosphorsäure, Angew. Chem., 1960, 72, 236–249 CrossRef CAS.
- B. Kübel, Neue acetonylsubstituierte Azole I. 5-Acetonyl-1,2,4-oxadiazole, Monatsh. Chem., 1982, 113, 781–792 CrossRef.
- S. C. Welch, J. M. Assercq, J. P. Loh and S. A. Glase, 3-Chloro-2-[(diethoxyphosphoryl)oxy]-1-propene: a new reagent for a one-pot cyclopentenone annelation. Synthesis of desoxyallethrolone, cis-jasmone, and methylenomycin B., J. Org. Chem., 1987, 52, 1440–1450 CrossRef CAS.
- A. D. Piscopio and S. Shakya. e-EROS Encyclopedia of Reagents for Organic Synthesis. 2005, pp. 1–8 Search PubMed.
- Y. N. Mitrasov and M. A. Frolova, Phosphorus-Containing Small Rings: XII.1 Reaction of 3-Phenyl-2,2-dichlorocyclobutanone with Esters and Amidoesters of Phosphorus(III) Acids, Russ. J. Gen. Chem., 2010, 80, 2525–2526 CrossRef CAS.
- C. T. Walsh, T. E. Benson, D. H. Kim and W. J. Lees, The Versatility of Phosphoenolpyruvate and its Vinyl Ether Products in Biosynthesis, Chem. Biol., 1996, 3, 83–91 CrossRef CAS PubMed.
- R. K. Malla, S. Bandyopadhyay, C. D. Spilling, S. Dutta and C. M. Dupureur, The First Total Synthesis of (±)-Cyclophostin and (±)-Cyclipostin P: Inhibitors of the Serine Hydrolases Acetyl Cholinesterase and Hormone Sensitive Lipase, Org. Lett., 2011, 13, 3094–3097 CrossRef CAS PubMed.
- A. Madani, J. N. Ridenour, B. P. Martin, R. R. Paudel, A. A. Basir, V. L. Moigne, J. L. Herrmann, S. Audebert, L. Camoin, L. Kremer, C. D. Spilling, S. Canaan and J. F. Cavalier, Cyclipostins and cyclophostin analogs are multi-target inhibitors that impair growth of Mycobacterium abscessus, ACS Infect. Dis., 2019, 5, 1597–1610 CrossRef CAS PubMed.
- A. D. S. Santosa, M. Cordierb and L. E. Kaïm, Nef–Perkow–Mumm Cascade Towards Imido Phosphate Derivatives, Synlett, 2017, 28, 2637–2641 CrossRef.
- P. M. Krizková, S. Prechelmacher, A. Roller and F. Hammerschmidt, Chemical Synthesis of (RP)- and (SP)-[16O,17O,18O]Phosphoenol, J. Org. Chem., 2017, 82, 10310–10318 CrossRef PubMed.
- I. J. Borowitz and R. Virkhaus, On the Mechanism of the Reactions of α-Bromoketones with Triphenylphosphine, J. Am. Chem. Soc., 1963, 85, 2183 CrossRef CAS.
- P. A. Chopard, V. M. Clark, R. F. Hudoson and A. J. Kirby, The Mechanism of the Reaction Between Trialkyl Phosphites and α-Halogenated Ketones, Tetrahedron, 1965, 21, 1961–1970 CrossRef CAS.
- I. J. Borowitz, S. Firstenberg, G. B. Borowitz and D. Schuessler, Organophosphorus Chemistry. XVII. Kinetics and Mechanism of the Perkow reaction, J. Am. Chem. Soc., 1972, 94, 1623–1628 CrossRef CAS.
- M. Sekine, K. Okimoto, K. Yamada and T. Hata, Silyl phosphites. 15. Reactions of silyl phosphites with α-halo carbonyl compounds. Elucidation of the mechanism of the Perkow reaction and related reactions with confirmed experiments, J. Org. Chem., 1981, 46, 2097–2107 CrossRef CAS.
- R. M. Aminova, G. A. Shamov, L. I. Savostina and V. F. Mironov, Mechanism of the Reaction of 2-Methoxybenzo[d][1,3,2]dioxaphosphinin-4-one with Chloral by Quantum-Chemical Calculations, Russ. J. Gen. Chem., 2006, 76, 906–914 CrossRef CAS.
- A. E. Ferao, Reaction of Chloroacetone with Trimethylphosphite: Perkow Versus Michaelis Arbuzov Reaction Paths, J. Phys. Chem. A, 2017, 121, 6517–6522 CrossRef PubMed.
- H. Finkelstein, Darstellung organischer Jodide aus den entsprechenden Bromiden und Chloriden, Ber. Dtsch. Chem. Ges., 1910, 43, 1528–1532 CrossRef CAS.
- M. Mandal, H. Yun, G. B. Dudley, S. Lin, D. S. Tan and S. J. Danishefsky, Total Synthesis of Guanacastepene A: A Route to Enantiomeric Control, J. Org. Chem., 2005, 70, 10619–10637 CrossRef CAS PubMed.
- V. R. Sirivolu, S. K. V. Vernekar, T. Ilina and N. S. Myshakina, Clicking 3′-Azidothymidine into Novel Potent Inhibitors of Human Immunodeficiency Virus, J. Med. Chem., 2013, 56, 8765–8780 CrossRef CAS PubMed.
- C. Drescher, M. Keller, O. Potterat, M. Hamburger and R. Bruckner, Structure-Elucidating Total Synthesis of the (Polyenoyl)tetramic Acid Militarinone C, Org. Lett., 2020, 22, 2559–2563 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra05140c |
| This journal is © The Royal Society of Chemistry 2020 |