Open Access Article
Open Access ArticleChemical grafting of cholesterol on monomer and PDMMLA polymers, a step towards the development of new polymers for biomedical applications
Elnaz Gholizadeh†
a,
Rima Belibel†b,
Thomas Bachelarta,
Chérifa Bounadjia and
Christel Barbaud *a
*a
aUniversité Sorbonne Paris Nord, Laboratory for Vascular Transitional Science (LVTS), INSERM UMR 1148, Villetaneuse, F-93430, France. E-mail: barbaud@sorbonne-paris-nord.fr; Tel: +33 149403357
bKymiaNova, Châtenay Malabry, F-92290, France
First published on 2nd September 2020
Abstract
Racemic α,α,β-trisubstituted β-lactones are the monomer units of poly((R,S)-3,3-dimethylmalic acid) (PDMMLA) derivatives, new biopolyesters showing great potential for biomedical applications. Using different groups during the synthesis of these β-lactones allows a tailored synthesis of PDMMLA copolymers with adjustable hydrophilic/phobic ratio. The degradation kinetics of the employed material is one of the most important criteria in the development of bioresorbable implants. The degradation time of PDMMLA derivatives can be controlled using different β-lactones of different hydrophilicity levels during the polymerization stage. Furthermore, PDMMLA has chemically available groups on its side chain allowing to graft functional groups on the polymer via covalent bonds. In this work, following a Steglich esterification protocol, the chemical grafting of cholesterol was carried out on a PDMMLA monomer derived β-lactone as well as on homopolymer PDMMLA–H, and copolymer PDMMLAH40-co-Hex60 (PDMMLA 40/60). Nuclear magnetic resonance (NMR) analyses of the products confirm and quantify the grafting ratio. 100% of cholesterol grafting has been realized on the homopolymer PDMMLA–H giving PDMMLA–Chol, and 10% on the copolymer PDMMLA 40/60, giving PDMMLAH30-ter-Chol10-ter-Hex60 (PDMMLA–Chol 30/10/60) as wished. Fourier-transform infrared (FT-IR) spectra, elemental analysis on the β-lactones and thermogravimetric analyses on the polymers also confirm the chemical modification of the products.
Introduction
Racemic α,α,β-trisubstituted β-lactones are synthetic molecules (Scheme 1), serving as monomers in the synthesis of biodegradable polyesters: poly((R,S)-3,3-dimethylmalic acid) PDMMLA derivatives. These are new statistical biopolyesters, studied for their interesting properties to serve in medical treatments. The main aim in studying PDMMLA derivatives is to develop a polymer for cardiovascular metallic stent coating. Previous works proved the great potential of these polymers for this application in terms of mechanical properties,1,2 degradation kinetics,3 and cell response.4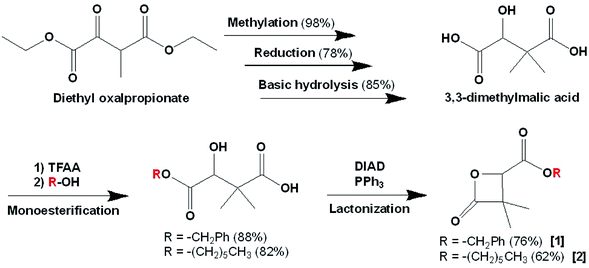 | ||
| Scheme 1 Synthetic pathway of the α,α,β-lactones: 4-benzyloxycarbonyl-3,3-dimethyl-2-oxetanone (benzylic β-lactone) [1] 4-hexyloxycarbonyl-3,3-dimethyl-2-oxetanone (hexylic β-lactone) [2]. | ||
PDMMLA derivatives are synthesized through an anionic ring opening polymerization (ROP) of the β-lactones5 (Scheme 2). The use of different groups in the synthesis of the monomers, gives different hydrophilicity levels to the synthesized polymers. The increase in benzylic β-lactone [1] (the benzylic group is hydrolyzed into a carboxylic acid later) increases the hydrophilicity of the final polymer, which changes its degradation kinetics. The ability to control the degradation time of this biopolymer is an advantage compared to currently used biopolymers.6
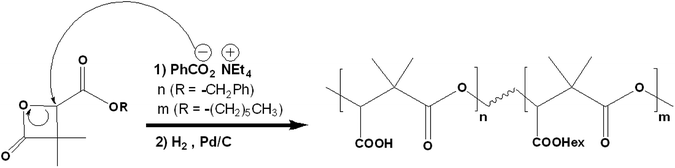 | ||
| Scheme 2 Synthesis of PDMMLA polymers through a ROP of the β-lactones. The final copolymer contains carboxylic acid groups (–COOH) and hexylic ester groups (–COOHex). | ||
Previous work has shown that the degradation products of PDMMLA are non-toxic and bio-assimilable.3 In addition, the presence of available carboxylic acid groups on the side chain of PDMMLA makes the covalent grafting of active drugs possible, which is promising regarding the controlled release of the drug in order to limit an intra-stent restenosis after implantation.
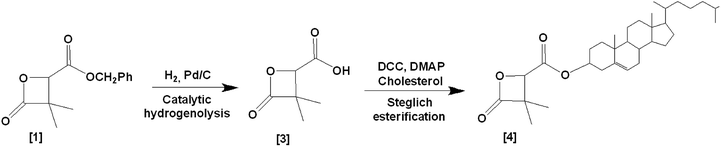
In order to prove the possibility and efficacy of the chemical grafting, we first started with the grafting of cholesterol (Chol) on 4-carboxyl-3,3-dimethyl-2-oxetanone (acidic β-lactone) [3], synthesized here for the first time with the catalytic hydrogenolysis of the benzylic β-lactone [1]. We realized the grafting reaction following the Steglich esterification protocol. Cholesterol is not an active drug, but a model molecule used in this work, only to prove the feasibility of the grafting. Its simple structure compared to that of real drugs allows easier analysis of the obtained data. Furthermore, cholesterol possesses only one hydroxyl group, which makes evident that the reaction will take place involving this very group7,8 to give 4-cholesteryloxycarbonyl-3,3-dimethyl-2-oxetanone (cholesterolic β-lactone) [4] (Scheme 3).
Steglich esterification is used to form an ester bond between a carboxylic acid and a hydroxyl (alcohol) group. N,N′-dicyclohexylcarbodiimide (DCC) activates the carboxyl group, forming an O-acylisourea intermediate.9 The esterification of the carboxylic acid is improved by addition of a catalytic amount of 4-dimethylaminopyridine (DMAP), which leads to a more effective reaction.10,11 In our case, the esterification takes place involving the carboxylic acid group of the monomer and/or the polymer and the available hydroxyl group of cholesterol.
Following the same protocol, we realized the grafting on PDMMLA polymers. In order to determine the maximal grafting percentage, the reaction has been carried out on the 100% hydrophilic homopolyester, PDMMLA–H (PDMMLA 100/0); then, on the 40% hydrophilic copolymer, PDMMLAH40-co-Hex60 (PDMMLA 40/60) in order to obtain the theoretical terpolymer PDMMLAH30-ter-Chol10-ter-Hex60 (PDMMLA 10/30/60).
A study on the degradation kinetics of a series of PDMMLA derivatives showed that the copolymer with 30% of hydrophilic groups (PDMMLA 30/70) has the fastest degradation kinetics in a 6 months’ time interval.3 In order to accelerate this process, we synthesized a more hydrophilic copolymer, PDMMLA 40/60, which degrades even faster in order to prevent the post-implantation complications due to the presence of the polymer. On the other hand, our team has proved a good cell behavior on the PDMMLA 30/70.4 By grafting 10% of an active agent, we aim to conserve 30% of hydrophilicity as in the case of PDMMLA 30/70, and accelerate the degradation of the remaining polymer after the complete release of the active agent.
1H NMR technique was used to confirm the reaction and the grafting ratio. FT-IR spectra, elemental analysis on the β-lactones and thermogravimetric analyses on the polymers also confirm the chemical modification of the products.
Results and discussion
Chemical grafting of cholesterol on the β-lactone and PDMMLAs was carried out via Steglich esterification. 1H analysis of the products confirms the reaction via the modification of chemical shifts of the significant groups. Fig. 1 shows the 1H NMR spectra of cholesterol, acidic β-lactone [3], and cholesterolic β-lactone [4].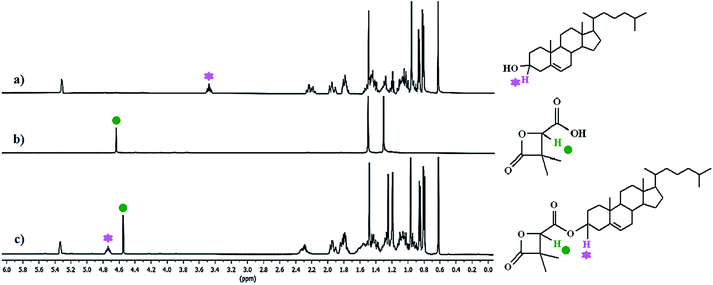 | ||
| Fig. 1 1H NMR spectra of: (a) cholesterol, (b) acidic β-lactone [3], (c) cholesterolic β-lactone [4] in CDCl3, 400 MHz. | ||
Signals at 3.55 ppm (Fig. 1a) and 4.67 ppm (Fig. 1b) correspond to indicated protons on cholesterol and the β-lactone [3]. Changes in chemical shifts of these groups integrating for 1H each show that a chemical modification has taken place between these molecules with 100% conversion efficiency (Fig. 1c).
Regarding the polymers, chemical shift changes are different from the monomer despite similar electronic effects. This can be explained by the difference in mobility of the molecules. Indeed, the polymer chain is more flexible and mobile than the planar and rigid structure of the β-lactone. In the case of polymers, integral values of the peaks allow to calculate the grafting percentage.
For PDMMLA–Chol, one can observe 100% of cholesterol grafting since the integral values for the peaks at 3.40 ppm (H3 on cholesterol) and 5.23 ppm (Hb on the polymer) are 1.04 and 1.05 respectively. Regarding the terpolymer, calculations using the integral values of the peaks at 3.45 ppm (H3 integrating for 0.09H) and 5.28 ppm (Hb and H6 integrating for 1.11H), give 10% of grafted cholesterol as wanted.
FT-IR analysis of the products before and after cholesterol grafting confirm also the esterification. With the β-lactone for example, the –OH stretching band of cholesterol at 3450 cm−1 disappears completely when grafted, indicating that the hydroxyl group was transformed to an ester. Furthermore, the absorption occurring at 1222 cm−1 corresponds to the C–O stretching of the new ester bond (Fig. 2). Similar results were observed with the polymers, confirming the esterification in all cases.
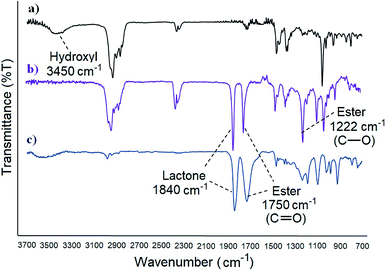 | ||
| Fig. 2 FT-IR spectra of: (a) cholesterol, (b) cholesterolic β-lactone [4], (c) acidic β-lactone [3]. | ||
Elemental analysis of a molecule gives information about its chemical composition. Calculating the molar percentage of each present element in the molecule allows to confirm the expected composition of the sample. Indeed, experimental values obtained in the case of new cholesterolic β-lactone give coherent results with calculated theoretical values (Cth = 76.90%, Cexp = 76/32% and Hth = 10.21%, Hexp = 10.43%), showing that the expected composition is obtained and the esterification has taken place successfully.
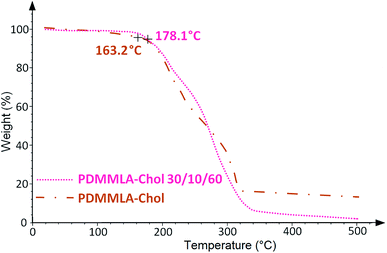
TGA measurements of a polymer give information about the thermal stability of the polymer and its degradation temperature Td.12 From the determined values of Td, we can thus define the maximum temperature lower than the Td to carry out the DSC analysis of the copolymers without degrading them. DSC technique provides information about the thermal properties of a polymer, mainly the glass transition temperature Tg which shows structural changes in the backbone and melting temperature Tm.13,14 Fig. 3 and 4 show the TGA and DSC thermograms of the grafted polymers respectively. Table 1 gathers the values obtained by TGA and DSC measurements.
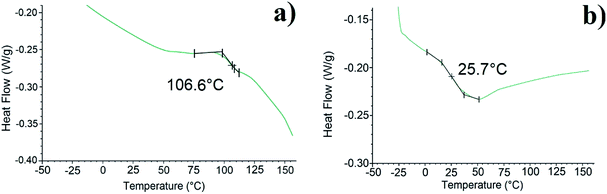 | ||
| Fig. 4 DSC curves of (a) PDMMLA–Chol, (b) PDMMLA–Chol 30/10/60, zoomed in on the inclination point of the second heating curve. | ||
Grafting cholesterol on the acidic β-lactone has increased its Tm from 48 to 160 °C. Regarding the polymers, they all show a Td above 150 °C. High Td value facilitates manipulation under heating conditions if necessary, without thermal degradation of the polymer. The increase of Tg in all cases indicates an addition of rigidity to the structure of the polymers. A significant increase is observed for the homopolymer (from 69.9 to 106.6 °C) since there is 100% of grafting. As for the terpolymer, the Tg increase is slight and less important (from 23.7 °C to 25.7 °C) since there is only 10% of grafted cholesterol.
Experimental
Materials and methods
Cholesterol was purchased from Sigma Aldrich Chemical Co. All other chemicals were purchased from Alfa Aesar chemical Co and employed as received.Anhydrous tetrahydrofuran (THF) was distilled on sodium-benzophenone. In all other cases, the commercially available reagent-grade solvents were employed without purification. All reactions with anhydrous organic solvents were performed under nitrogen atmosphere. All glass apparatuses were kept one night in a drying-oven at 100 °C.
Thin Layer Chromatography (TLC) was performed using plates coated with Merck silica gel 60 Fi254 of 0.25 mm thickness. The TLC plates were first revealed under UV light (254 nm wavelength) then with p-anisaldehyde stain containing absolute ethanol (93 mL), p-anisaldehyde (2.5 mL), concentrated sulfuric acid (3.5 mL) and concentrated acetic acid (1 mL). Flash chromatography (FC) was carried out using silica gel (C–C 35–70 μm, 60 Å).
Nuclear magnetic resonance: 1H and 13C NMR spectra were recorded by a BRUKER AM-400 MHz spectrometer, using CDCl3 as solvent. Chemical shifts (δ) are given in ppm.
Infrared: FT-IR spectra were recorded on AVATAR 370 FT-IR Thermo Nicolet OMNI-sampler ATR Smart Accessory (Ge, DTGS). Absorption bands are given in cm−1.
Melting point of the solid compound was determined using a Stuart SMP11 melting point apparatus.
Thermogravimetric analysis (TGA) measurements of the polymers were registered using a TGA Q50 analyzer. Samples were heated from 10 °C to 500 °C with a heating rate of 10°C min−1 under N2 atmosphere.
Glass transition temperature (Tg) of the samples was measures using differential scanning calorimetry (DSC) technique on a DSC Q2000 analyzer. Polymers were put in the furnace and heated from −25 °C to 160 °C (the maximal temperature was adjusted for each sample according to the Td value obtained from the TGA experiment in the aim to avoid degrading the copolymer during the first run), cooled down to −25 °C, and reheated in order to realize the second heating cycle. The heating rate was set to 10°C min−1 and the Tg value was collected from the inclination point on the second heating curve.
Size exclusion chromatography (SEC) was realized at room temperature for the determination of absolute molecular weights of the polymers. A high performance size exclusion chromatography (HPSEC) was coupled to a multi-angle laser light scattering detector (MALLS), a viscosimeter and a differential refractive index (dRI) detector. THF was used as the carrier phase and was filtered through a 0.1 μm filter unit (Millipore, Billerica, USA), It was degassed (DGU-20 A3R Shimadzu, Kyoto Japan) and eluted at a 0.5 mL min−1 flow rate (LC10Ai Shimadzu, Kyoto Japan). 100 μL of a 0.2 μm-filtered sample solution (C = 10 mg mL−1) were injected with an automatic injector (SIL-20A HT Shimadzu, Kyoto Japan). The column packing was a divinylbenzene gel. The MALLS photometer, a miniDawn TREOS from Wyatt Technology Inc. (Sanata Barbara, CA, USA) was provided with a fused silica cell and a Ga–As laser (λ = 665.8 nm). The whole collected data: light scattering (LS), dRI were analyzed using the Astra v6.0.6 software package. Molar masses were obtained with a Zimm order 1 method. The concentration of each eluted fraction was determined with dRI (RID10A Shimadzu, Kyoto Japan) according to the measured values of dn/dc (0.05 mL g−1).15
β-lactone synthesis
As shown in the Scheme 1, α,α,β-trisubstituted β-lactones with benzylic ester and hexylic ester groups were prepared in five steps from the precursor diethyl oxalpropionate, following the protocols described in the literature.16–18Polymer synthesis
The synthesis of the homopolymer19 and the copolymer5 was realized as described in the literature, using the β-lactones [1] and [2] as monomers, and tetraethyl-ammonium benzoate (Et4N+ PhCOO−) as initiator. A monomer/initiator ratio (M/I) of 5 × 103 was applied for all polymers.Conclusion
The purpose of this work was to study the possibility and efficiency of grafting functional groups on the chemically modifiable side chain of PDMMLA polymers. This grafting can be carried out on PDMMLAs with different functional groups and drugs having a hydroxyl, amine or sulfur function for various biomedical applications. For this reason, chemical grafting study on PDMMLAs was carried out with cholesterol having a simple chemical structure and only one functional group (–OH). A β-lactone derived from the monomer units of PDMMLA and polymers having 100% and 40% –COOH groups on their respective side chains (PDMMLA–H and PDMMLA 40/60, respectively) were used in this work. An ester bond was formed between the carboxylic acid group of the monomer/polymer and the hydroxyl group of the cholesterol following a Steglich esterification. NMR analysis allowed to confirm and quantify the grafting percentage, which prove the efficiency of the practiced grafting procedure (100% concordance with theoretical grafting percentage). 1H NMR and FT-IR spectra show evident results confirming the grafting. TGA and DSC measurements show also the modification brought to the polymers due to the addition of the cholesterol to the basic structure of each polymer. Increases in glass transition temperature are a good indication to the addition of rigidity to the polymers' structure.PDMMLA is a biocompatible and biodegradable polymer, developed in our team for biomedical applications. These experiments allowed to confirm the possibility of covalent grafting on PDMMLA derivatives (homopolymer or copolymer). Future works will be oriented to grafting a veritable active drugs on these polymers in order to improve healing conditions in medical intervention cases.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Nadia Bouchemal (Université Sorbonne Paris Nord, UFR SMBH – CSPBAT-UMR 7244, group NBD) and Véronique Bennevault (Sorbonne Université, IPCM-UMR 7610, group LCP) for their cooperation and support in the preparation of this paper.References
- R. Belibel, T. Avramoglou, A. Garcia, C. Barbaud and L. Mora, Mater Sci Eng C, 2016, 59, 998 CrossRef CAS.
- R. Belibel, C. Barbaud and L. Mora, Mater Sci Eng C, 2016, 69, 1192 CrossRef CAS.
- R. Belibel, N. Marinval, H. Hlawaty and C. Barbaud, Polym. Degrad. Stab., 2016, 130, 288 CrossRef CAS.
- R. Belibel, S. Sali, N. Marinval, A. Garcia-Sanchez, C. Barbaud and H. Hlawaty, Mater. Sci. Eng. C, 2020, 117, 111284 CrossRef CAS.
- F. Ouhib, S. Randriamahefa, P. Guérin and C. Barbaud, Des. Monomers Polym., 2005, 8, 25 CrossRef CAS.
- R. Belibel, I. Azzouz and C. Barbaud, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 1495 CrossRef CAS.
- R. K. Mann and P. A. Beachy, Biochim. Biophys. Acta Mol. Cell Biol. Lipids, 2000, 1529, 188 CrossRef CAS.
- F. Schmidt, M. Spoerner, H. R. Kalbitzer and B. König, Synth. Commun., 2011, 41, 2876 CrossRef CAS.
- V. Gilles, M. A. Vieira and V. Lacerda, et al., J. Braz. Chem. Soc., 2015, 26, 74 CAS.
- B. Neises and W. Steglich, Angew. Chem., Int. Ed. Engl., 1978, 17, 522 CrossRef.
- A. B. Lutjen, M. A. Quirk, A. M. Barbera and E. M. Kolonko, Bioorg. Med. Chem., 2018, 26, 5291 CrossRef CAS.
- S. El-Sayed, K. H. Mahmoud, A. A. Fatah and A. Hassen, Phys. B Condens. Matter, 2011, 406, 4068 CrossRef CAS.
- N. A. Grunina, T. V. Belopolskaya and G. I. Tsereteli, J. Phys. Conf., 2006, 40, 105 CrossRef CAS.
- B. Gornicka and L. Gorecki, J. Therm. Anal. Calorim., 2010, 101, 647 CrossRef CAS.
- F. E. Kohn, J. W. A. Van Den Berg, G. Van De Ridder and J. Feijen, J. Appl. Polym. Sci., 1984, 29, 4265 CrossRef CAS.
- C. Barbaud, M. Guerrouache and P. Guérin, Tetrahedron Lett., 2002, 43, 9513 CrossRef CAS.
- C. Barbaud, F. Abdillah, F. Fabienne, M. Guerrouache and P. Guérin, Des. Monomers Polym., 2003, 6, 353 CrossRef CAS.
- R. Belibel and C. Barbaud, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 2586 CrossRef CAS.
- C. Barbaud, F. Faÿ, F. Abdillah, S. Randriamahefa and P. Guérin, Macromol. Chem. Phys., 2004, 205, 199 CrossRef CAS.
Footnote |
| † Co-first authorship. |
| This journal is © The Royal Society of Chemistry 2020 |