Open Access Article
Open Access ArticleGlycerine-based synthesis of a highly efficient Fe2O3 electrocatalyst for N2 fixation†
Meng Wang,
Feifei Li * and
Juan Liu
* and
Juan Liu
Kangda College of Nanjing Medical University, Lianyungang, 222000, China. E-mail: ffl@foxmail.com
First published on 11th August 2020
Abstract
The electrochemical nitrogen reduction reaction (NRR) is a promising approach to convert N2 into high value-added NH3. However, it is still a challenge to achieve an efficient electrocatalyst for the NRR. Herein, it is demonstrated that the Fe2O3 nanoparticles (NPs) generated from a glycerine-based synthesis can be applied as highly efficient catalysts for the NRR. The Fe2O3 NPs show good performance with a high NH3 yield (22 μg mgcat−1 h−1) and a favorable Faradaic efficiency (FE) (3.5%) at −0.5 V vs. reversible hydrogen electrode (RHE). The facile synthesis strategy and satisfactory electrochemical properties demonstrate the potential application of the as-synthesized Fe2O3 NPs for NRR.
1. Introduction
Ammonia (NH3) is a highly important chemical in many aspects of industrial production and daily life.1,2 Fixation of N2 to NH3 is an important step for the natural N2 cycle.3–5 The current synthesis of NH3 mainly depends on the industrial Haber–Bosch process, which involves serious energy consumption and leads to large amounts of greenhouse gas emissions.6,7 To realize a green and sustainable strategy for N2 fixation, electrochemical reduction of N2 has recently attracted much attention, being an environmentally friendly route involving mild conditions.8–10To date, a number of catalysts have been developed for the NRR, including noble metals,11–13 transition metals,14,15 metal-free materials,16–18 metal–C composite materials19–21 and Au–Fe3O4.22 These catalysts have demonstrated potential applications in the NRR with improved FE and NH3 yield. Most of the catalysts were synthesized with the assistance of surfactants (structure-directing agents) through solution methods.23 However, the surfactants could passivate the catalyst surface, which decreases the activity of the catalyst since the reactions take place on the catalyst surface. Therefore, the catalyst with a clean surface could be very important for achieving the high activity.
Herein, we successfully synthesized the clean-surface Fe2O3 electrocatalyst for NRR by using glycerine as solvent with a subsequent calcination process. There was no need of surfactants in the synthesis process and the particles were further calcinated, guaranteeing the clean nature of the Fe2O3 surface. The as-prepared Fe2O3 NPs demonstrated a good electrocatalytic performance for NRR, with a high NH3 yield (22 μg mgcat−1 h−1) and a favorable FE (3.5%) at −0.5 V vs. reversible hydrogen electrode (RHE).
2. Materials and reagents
Glycerine (C3H8O3) (purity, 99.5%), iron(III) nitrate nonahydrate (Fe(NO3)3·9H2O) (purity, 98.5%) and ethanol (CH3CH2OH) (purity ≥ 98.5%) were purchased from Sinopharm Chemical Reagent Co. Ltd.2.1. Synthesis of Fe2O3 nanoparticles (NPs)
121.2 mg of iron(III) nitrate nonahydrate was added into 5 mL glycerin. Then the solution was ultrasonicated for 10 min. The uniform solution was transferred into the Teflon-lined stainless-steel autoclave and heated at 180 °C for 20 h. The obtained product washed with ethanol and water for three times and dried at room temperature for 12 h. Then the product was put into a tubular furnace, heated to 450 °C for 2 h at the heating rate of 10 °C min−1 under air. Finally, Fe2O3 NPs was obtained.2.2. Characterization
A Rigaku Dmax-rc X-ray diffractometer was used to perform X-ray diffraction (XRD) characterization. The transmission electron microscopy (TEM) images were obtained on a JEM 1400 TEM instrument. The X-ray photoelectron spectroscopy (XPS) characterization was conducted with ESCALAB 250.2.3. Electrode preparation
5 mg as-obtained Fe2O3, 40 μL Nafion (5% wt) and 960 μL ethanol were mixed by ultrasound for forming suspension. 50 μL suspension was modified on 1 × 1 cm carbon cloth (CC). The Fe2O3–CC was used as working electrode.2.4. Electrochemical reduction of N2
Electrochemical reduction of N2 was carried out in a typical three-electrode gastight two-compartment reaction vessel separated by an anion exchange membrane (Nafion 211) on a CHI760 electrochemical workstation (Chenhua, Shanghai). The experiment was performed in 0.1 M Na2SO4 solution (50 mL each compartment). A Pt wire was used as the counter electrode and an Ag/AgCl (4.0 M KCl) was used as reference. All potentials were converted to RHE. The electrolyte was then purged with N2 for at least 30 min. N2 was delivered into the cathodic compartment at a constant rate of 20 mL min−1. The potentiostatic tests were performed in 0.1 M Na2SO4 aqueous solution at different potentials such as −0.3, −0.4, −0.5, −0.6, and −0.7 V vs. RHE. The NH3 yields and FEs of products were calculated as follows:| NH3 yields (μg mgcat−1 h−1) = m/(t × mcat) | (1) |
| FE (%) = αmF/MQ | (2) |
2.5. Determination of NH3 and N2H2
The detections of NH3 and N2H2 were made by indophenol blue and Watt–Chrisp methods, respectively, according to the reported literature.243. Results and discussion
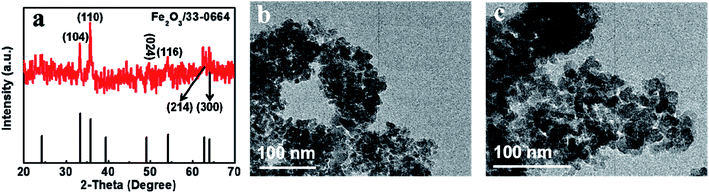
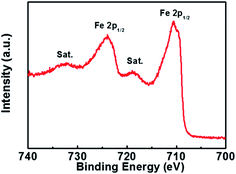
The Fe2O3 electrocatalysts were synthesized by using glycerine as the solvent with a subsequent calcination process. In the synthesis system, there was no surfactant and the particles were further calcinated. Therefore, the Fe2O3 particles with clean surface could be obtained by our present synthetic route. Fig. 1(a) shows the X-ray diffraction (XRD) pattern of the as-synthesized Fe2O3, which agrees well with the JCPDS No. 33-0664. This demonstrates the successful formation of Fe2O3. Low peak intensity may be caused by small size of Fe2O3 particles. Transmission electron microscopy (TEM) images of the Fe2O3 suggests that the diameters of as-synthesized Fe2O3 nanoparticles are in the range of 4–6 nm (Fig. 1(b) and (c)).To investigate the surface elemental state of Fe2O3 NPs, we analyzed the chemical states of the Fe2O3 NPs by XPS analysis. There are two Fe3+ peaks located at 711.8 eV and 725.3 eV, corresponding to Fe 2p1/2 and Fe 2p3/2,25 agreeing well with Fe2O3 NPs (Fig. 2). The XPS spectrum result further demonstrated the successful synthesis of Fe2O3 NPs.
The synthesized Fe2O3 NPs were then used as the electrocatalysts for the electrochemical nitrogen reduction reaction (NRR). The produced ammonia was analyzed and quantified based on the indophenol blue method. Before NRR experiment, the corresponding calibration curve for ammonia by indophenol blue method was first determined and shown in Fig. 3.
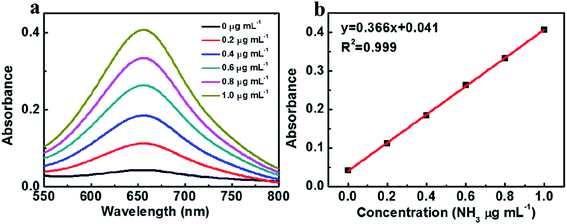 | ||
| Fig. 3 (a) UV-vis absorption spectra of different concentration ammonia in 0.1 M Na2SO4 solution, (b) standard curve of ammonia in 0.1 M Na2SO4 solution. | ||
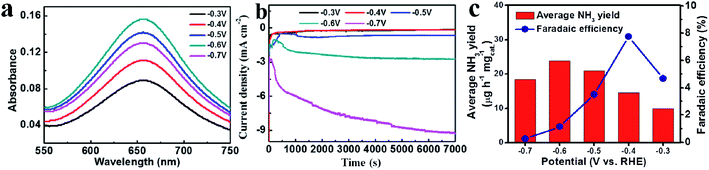
The N2-fed electrolytes in 0.1 M Na2SO4 electrolytes at different potentials for 2 h were mixed with the indophenol reagent, and their absorbance changed at 660 nm were depicted in Fig. 4(a). It indicated the substantial electroreduction of inert N2 into valuable NH3 was achieved using the as-synthesized Fe2O3 electrocatalyst. Fig. 4(b) presented the chronoamperometric curves as a function of reaction time at varying applied potentials in 0.1 M Na2SO4, demonstrating the Fe2O3 nanoparticles have good stability for NRR from −0.3 V to −0.6 V. The average NH3 yield and the corresponding Faradaic efficiency (FE) was given in Fig. 4(c), in which the favorable NH3 yield was 22.0 μg mgcat−1 h−1 with FE value of 3.5% at −0.5 V. The NRR performance of the as-synthesized Fe2O3 is comparable to lots of the NRR electrocatalysts (Table S1†). Therefore, the present synthesized Fe2O3 with clean surface might be as a potential catalyst for electrochemical NRR in consideration that there are abundant Fe element in the earth. TEM image of catalyzed Fe2O3 was tested after nitrogen fixation at −0.5 V. It was found that the morphology did not changed significantly, which indicated the good stability of the as-synthesized Fe2O3 (Fig. S1†).
The NNR performance is highly related to the electrochemically active surface area (ECSA). Therefore, the ECSA of the as-prepared Fe2O3 was further studied. Here, the ECSA was reflected by double layer capacitance (Cdl) since there was a linear proportional relationship between ECSA and Cdl, which could be obtained by cyclic voltammetry curves in the range of 0.1–0.2 V (Fig. 5(a)). Based on Fig. 5(b), the high Cdl of 1.6 mF cm−2 (Fig. 5(b)) further demonstrated that Fe2O3 had high ECSA for NRR.
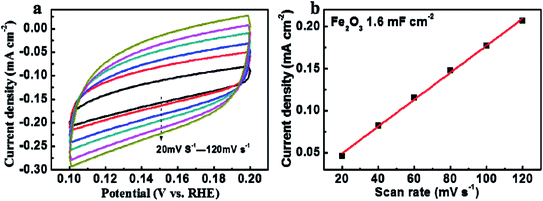 | ||
| Fig. 5 (a) Cyclic voltammetry curves of Fe2O3 at different scanning rate of potential in 0.1 M Na2SO4 solution, (b) the double-layer capacitance of Fe2O3. | ||
The hydrazine byproduct was further determined through a Watt–Chrisp method. Fig. 6(a) showed the corresponding calibration curve based on UV-vis absorption at 455 nm. As shown in Fig. 6(b), the by-product of hydrazine was not detected, implying the high selectivity of Fe2O3 for NH3 production (Fig. 6). Hence, the as-prepared Fe2O3 catalyst could be used as a high-selective catalyst for producing NH3 by electrochemical NRR.
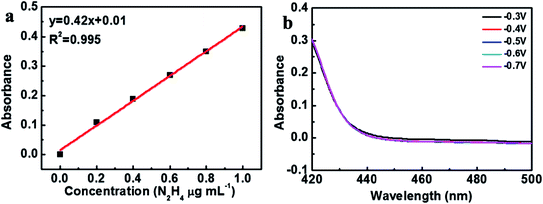 | ||
| Fig. 6 (a) Standard curve of hydrazine in 0.1 M Na2SO4 solution, (b) UV-vis absorption spectra of hydrazine in 0.1 M Na2SO4 electrolytes after NRR electrolysis at a series of potentials for 2 h. | ||
We also explored the NRR performance of Fe2O3 in 0.1 M KOH. The selected potential was −0.5 V. Unfortunately, the current density declined rapidly within 2 h (Fig. S2†).
4. Conclusions
In summary, Fe2O3 synthesized by glycerine-based route with subsequent calcination process can be adopted as highly efficient NRR catalysts. Thanks to the clean surface of the catalyst, the Fe2O3 NPs exhibited good performance with a 22 μg mgcat−1 h−1 NH3 yield and a 3.5% Faraday efficiency at −0.5 V for NH3 production, which outperformed lots of the previous catalysts. The flexible strategy and the good electrochemical performance endow Fe2O3 with potential application in NRR.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The research was supported by Science and technology development foundation, Nanjing Medical University, China (No. NMUB2019273).References
- J. G. Chen, R. M. Crooks, L. C. Seefeldt, K. L. Bren, R. M. Bullock, M. Y. Darensbourg, P. L. Holland, B. Hoffman, M. J. Janik, A. K. Jones, M. G. Kanatzidis, P. King, K. M. Lancaster, S. V. Lymar, P. Pfromm, W. F. Schneider and R. R. Schrock, Science, 2018, 360, 6391–6398 Search PubMed.
- M. Van Damme, L. Clarisse, S. Whitburn, J. Hadji-Lazaro, D. Hurtmans, C. Clerbaux and P. F. Coheur, Nature, 2018, 564, 99–103 CrossRef CAS PubMed.
- Y. Song, D. Johnson, R. Peng, D. K. Hensley, P. V. Bonnesen, L. Liang, J. Huang, F. Yang, F. Zhang, R. Qiao, A. P. Baddorf, T. J. Tschaplinski, N. L. Engle, M. C. Hatzell, Z. Wu, D. A. Cullen, H. M. Meyer, B. G. Sumpter and A. J. Rondinone, Sci. Adv., 2018, 4, 1700336–1700344 CrossRef PubMed.
- J. S. Anderson, J. Rittle and J. C. Peters, Nature, 2013, 501, 84–87 CrossRef CAS PubMed.
- J. W. Erisman, M. A. Sutton, J. Galloway, Z. Klimont and W. Winiwarter, Nat. Geosci., 2008, 1, 636–639 CrossRef CAS.
- V. Smil, Nature, 1999, 400, 415 CrossRef CAS.
- B. M. Hoffman, D. Lukoyanov, Z. Y. Yang, D. R. Dean and L. C. Seefeldt, Chem. Rev., 2014, 114, 4041–4062 CrossRef CAS PubMed.
- X. Cui, C. Tang and Q. Zhang, Adv. Energy Mater., 2018, 8, 1800369–1800394 CrossRef.
- X. Wang, Q. Zhang, X. Zhang, C. Wang, Z. Xie and Z. Zhou, Small Methods, 2019, 3, 1800334–1800339 CrossRef.
- Q. Hao, C. W. Liu, G. H. Jia, Y. Wang, H. Arandiyan, W. Wei and B. J. Ni, Mater. Horiz., 2020, 7, 1014–1029 RSC.
- Z. Geng, Y. Liu, X. Kong, P. Li, K. Li, Z. Liu, J. Du, M. Shu, R. Si and J. Zeng, Adv. Mater., 2018, 30, 1803498–1803504 CrossRef PubMed.
- J. Wang, L. Yu, L. Hu, G. Chen, H. Xin and X. Feng, Nat. Commun., 2018, 9, 1795–1802 CrossRef PubMed.
- C. Y. Yang, B. L. Huang, S. X. Bai, Y. G. Feng, Q. Shao and X. Q. Huang, Adv. Mater., 2020, 32, 2001267–2001277 CrossRef CAS PubMed.
- X. M. Hu, S. Y. Guo, S. L. Zhang, X. Y. Guo, Y. F. Li, S. P. Huang, K. Zhang and J. W. Zhu, J. Mater. Chem. A, 2019, 7, 25887–25893 RSC.
- Y. T. Liu, X. X. Chen, J. Y. Yu and B. Ding, Angew. Chem., Int. Ed., 2019, 58, 18903–18907 CrossRef CAS PubMed.
- W. H. Kong, R. Zhang, X. X. Zhang, L. Ji, G. S. Yu, T. Wang, Y. L. Luo, X. F. Shi, Y. H. Xu and X. P. Sun, Nanoscale, 2019, 11, 19274–19277 RSC.
- H. Zou, W. Rong, B. Long, Y. Ji and L. Duan, ACS Catal., 2019, 9, 10649–10655 CrossRef CAS.
- Y. Liu, Q. Li, X. Guo, X. Kong, J. Ke, M. Chi, Q. Li, Z. Geng and J. Zeng, Adv. Mater., 2020, 32, 1907690–1907697 CrossRef CAS PubMed.
- H. Cheng, P. X. Cui, F. R. Wang, L. X. Ding and H. H. Wang, Angew. Chem., Int. Ed., 2019, 58, 15541–15547 CrossRef CAS PubMed.
- H. T. Xie, Q. Geng, X. J. Zhu, Y. L. Luo, L. Chang, X. B. Niu, X. F. Shi, A. M. Asire, S. Y. Gao, Z. M. Wang and X. P. Sun, J. Mater. Chem. A, 2019, 7, 24760–24764 RSC.
- B. Yu, H. Li, J. White, S. Donne, J. B. Yi, S. B. Xi, Y. Fu, G. Henkelman, H. Yu, Z. L. Chen and T. Y. Ma, Adv. Funct. Mater., 2019, 30, 1905665–1905676 CrossRef.
- J. Zhang, Y. J. Ji, P. T. Wang, Q. Shao, Y. Y. Li and X. Q. Huang, Adv. Funct. Mater., 2019, 30, 1906579–1906587 CrossRef.
- X. Yang, L. Chen, Y. Li, J. C. Rooke, C. Sanchez and B. Su, Chem. Soc. Rev., 2017, 46, 481–558 RSC.
- C. Zhang, S. Liu, T. Chen, Z. Li and J. Hao, Chem. Commun., 2019, 55, 7370–7373 RSC.
- X. Huang, M. Lu, X. Zhang, G. Wen, Y. Zhou and L. Fei, Scr. Mater., 2012, 67, 613–616 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra05831a |
| This journal is © The Royal Society of Chemistry 2020 |