Open Access Article
Open Access ArticleA smart use of biomass derivatives to template an ad hoc hierarchical SAPO-5 acid catalyst†
Francesco Mariattia,
Ivana Miletto b,
Geo Paul
b,
Geo Paul b,
Leonardo Marchese
b,
Leonardo Marchese b,
Silvia Tabasso
b,
Silvia Tabasso c,
Maela Manzoli
c,
Maela Manzoli a,
Giancarlo Cravotto
a,
Giancarlo Cravotto a and
Enrica Gianotti
a and
Enrica Gianotti *b
*b
aDepartment of Drug Science and Technology, Università degli Studi di Torino, V. Pietro Giuria 9, 10125 Torino, Italy
bDepartment of Science and Technological Innovation, Università del Piemonte Orientale, V. Teresa Michel 11, 15121 Alessandria, Italy. E-mail: enrica.gianotti@uniupo.it
cDepartment of Chemistry, Università degli Studi di Torino, V. Pietro Giuria 7, 10125 Torino, Italy
First published on 20th October 2020
Abstract
A smart design of hierarchical SAPO-5 acid catalyst using biomass derived monosaccharides as sustainable and low-cost mesoporogens has been developed. The hierarchical SAPO-5 was characterized by several physico-chemical techniques to elucidate structure–properties relationships and was tested as a catalyst in the MW-assisted glucose transformation in 5-HMF using γ-valerolactone (GVL) as green solvent.
Zeolites and related microporous solids are well known heterogeneous acid catalysts for oil refining, petrochemistry, pollution abatement and synthesis of fine chemicals.1,2 One recently emerging field of zeolite application is in biomass valorization processes to produce biofuels, platform molecules and bio-based chemicals.3–5 Due to the biomass chemical complexity, the development of novel approaches to synthesize zeolites or zeo-type materials with tailored properties towards clean, selective and efficient processes for biomass conversion is nowadays a great challenge for the scientific community.
The tunability of Brønsted/Lewis acidity in a zeolite framework is an important parameter that will affect significantly the catalytic activity and selectivity in biomass upgrading. Moreover, because biomass is made of different kinds of large molecules (mainly polysaccharides, disaccharides and lignin), the accessibility of the acid active sites is a key parameter to avoid mass transfer constraints. In fact, zeolites and zeo-type systems impose diffusion limitation due to the presence of micropores and expedite catalyst deactivation through coke deposition.6 To circumvent this drawback, hierarchical porous zeolites have been developed, following top-down or bottom-up synthetic approaches, to combine the microporous structure with an additional mesoporous network to improve mass transport to the internal active sites.7,8
Among zeolite-type materials, the silicoaluminophosphates (SAPOs) represent a viable alternative to aluminosilicates for applications requiring acid catalysis.9,10 In particular, SAPO-5 (AFI framework type) is a well known microporous acid catalyst with one-dimensional 12-ring channels (7.3 Å × 7.3 Å).10,11 In this communication, hierarchical SAPO-5 has been synthetized by a bottom-up approach using a mixture of monosaccharides obtained after the microwave (MW)-assisted conversion of a residual biomass (MsB). The aim was to design the pore architecture of the hierarchical SAPO-5 catalyst, which was tested in the MW-assisted conversion of a model sugar structure (glucose) into 5-(hydroxymethyl)furfural (5-HMF). Glucose was chosen as benchmark substrate because of its similarity with the employed biomass-derived sugar templates. According to this smart approach, MsB have been used as sustainable and low-cost mesoporogens for the preparation of hierarchical porous materials,12,13 avoiding the use of sophisticated and expensive templates. Indeed, the mixture of MsB used to template the inorganic structure was obtained through a flash MW-assisted conversion process (2 min) of a cost-effective feedstock such as post-harvest tomato plants.14
Hierarchical SAPO-5 (labelled HierSAPO-5) was synthesized from a gel of molar composition: 1.0 Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.93 P
0.93 P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.58 Si
0.58 Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.068 SDAmeso
0.068 SDAmeso![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.58 TEA
0.58 TEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44H2O under hydrothermal conditions at 473 K for 60 h, using triethylamine (TEA) as the structure-directing agent (SDA) for the micropores, and a mixture of monosaccharides (see ESI for details†) from biomass as the mesoporogens (SDAmeso). For comparison, microporous SAPO-5 (labelled MicroSAPO-5) was also prepared under identical conditions, excluding the addition of mesoporogen. All the catalysts were calcined in air flow at 873 K for 16 h to remove the organic templates.
44H2O under hydrothermal conditions at 473 K for 60 h, using triethylamine (TEA) as the structure-directing agent (SDA) for the micropores, and a mixture of monosaccharides (see ESI for details†) from biomass as the mesoporogens (SDAmeso). For comparison, microporous SAPO-5 (labelled MicroSAPO-5) was also prepared under identical conditions, excluding the addition of mesoporogen. All the catalysts were calcined in air flow at 873 K for 16 h to remove the organic templates.
To confirm the phase-purity of the HierSAPO-5, XRD analysis was performed on the calcined material (Fig. S1†) and compared to XRD pattern of MicroSAPO-5; all the observable peaks are attributed to the AFI framework type, confirming that no significant secondary phases were present.
The textural properties of HierSAPO-5 were assessed by N2 adsorption/desorption volumetric analysis at 77 K (Fig. 1). HierSAPO-5 exhibits type IV isotherm characteristic of a mesoporous material (Fig. 1) with mesopores of approximately 36 and 49 Å (Fig. 1, inset); in addition, micropores typical of AFI structure are also present. Significantly, with respect to MicroSAPO-5, the hierarchical catalyst exhibits substantial enhancements in mesopore volume (Vmeso), total pore volume (Vtot) and mesopore surface area (Smeso) (Table 1). Volumetric data strongly support the successful preparation of HierSAPO-5 when MsB are used as mesoporogen, since there is experimental evidence for the coexistence of multiple levels of porosity.
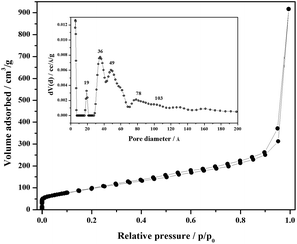 | ||
| Fig. 1 N2 adsorption/desorption isotherms at 77 K of hierarchical SAPO-5. Inset: the pore size distribution in the range of micropores and mesopores. | ||
| HierSAPO-5 | MicroSAPO-5 | |
|---|---|---|
| SBET/m2 g−1 | 370 | 270 |
| SDFT/m2 g−1 | 345 | 290 |
| Smicro/m2 g−1 | 97 | 143 |
| Smeso/m2 g−1 | 248 | 147 |
| Vtot DFT/cm3 g−1 | 0.54 | 0.43 |
| Vmicro/cm3 g−1 | 0.025 | 0.044 |
| Vmeso/cm3 g−1 | 0.51 | 0.39 |
Thermogravimetric and its derivative analyses (TGA/DTG) of the hierarchical and microporous SAPO-5 (Fig. S2†) revealed that, beside the weight loss due to physisorbed water (423 K), a weight loss at 673 K is present in both samples, attributed to the removal of TEA, i.e. the organic template for micropores. The amount of TEA in hierarchical catalyst is lower with respect to the microporous sample and in addition, a peak at ca. 453 K is visible due to the removal of the mesoporogen sugars.
The nature, strength and accessibility of the acid sites present in HierSAPO-5 was assessed by combined characterization using solid-state NMR and probe-FTIR15 spectroscopy. The chemical environment of the framework atoms in hierarchical and microporous SAPO-5 was evaluated by 27Al, 31P, and 29Si solid-state NMR spectroscopy (Fig. S3–S5†).
The 27Al and 31P MAS NMR spectra evidenced the presence of tetrahedrally-coordinated Al (at ∼37.8 ppm, Fig. S3†) and P atoms (at −30.3 ppm, Fig. S4†), confirming strict alternation of Al and P at the T-positions of the AlPO-5 framework. In the 31P MAS spectrum of the hierarchical material a shoulder down-field to the main peak is also present, due to P–OH defect sites.18 On the other hand, the 29Si NMR spectrum of MicroSAPO-5 exhibits signals at around −90, −95, −100, −105 and −110 ppm due to tetrahedral framework Si coordinated to four, three, two, one and zero Al atoms, respectively (Fig. S5†). 29Si NMR spectrum of hierarchical material is dominated by contributions from Si(4Al) and Si(3Al) units while the formation of silicon islands (Si(0Al)) cannot be excluded.
The nature of the acid sites in HierSAPO-5 was investigated using 1H MAS NMR and FTIR spectroscopy. 1H MAS NMR spectra (Fig. 2) recorded using a spin-echo technique (a pulse sequence specially designed to avoid probe background signals) reveal two resonance peaks at around 3.5 and 4.5 ppm, associated with Brønsted Si(OH)Al sites (BAS1 and BAS2). In addition, signals due to P–OH/Al–OH (centred at 2.4 ppm) and isolated Si–OH (1.6 ppm) are also present.
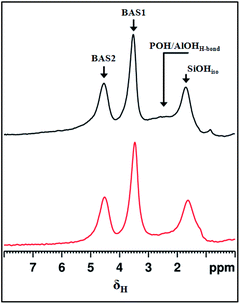 | ||
| Fig. 2 1H spin-echo MAS NMR spectra of microporous (top) and hierarchical (bottom) SAPO-5. BAS: Brønsted acid sites, SiOHiso: isolated silanols, POH/AlOHH-bond: hydrogen bonded POH and AlOH sites. | ||
In the FTIR spectra (Fig. S6†), SAPO-5 exhibits two well-separated bands at 3630 cm−1 and 3513 cm−1. Both bands are associated with the Al(OH)Si Brønsted acid sites in different positions inside the AFI framework. The high frequency band is due to sites located in the 12-ring channels, whereas the band at 3513 cm−1 can be attributed to bridging OH located in the 6-ring channels. Defects ascribed to Si–OH (3745 cm−1) or P–OH (3678 cm−1) sites are also visible in the SAPO-5 catalyst.16
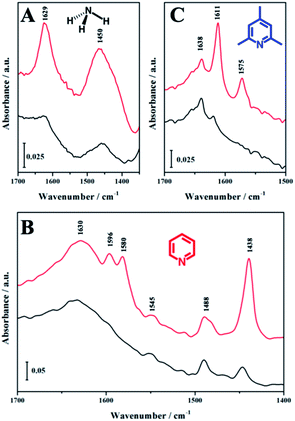
To discriminate the accessibility of the Brønsted acid sites, basic probe molecules with different kinetic diameters were adsorbed on HierSAPO-5 and followed by FTIR spectroscopy (Fig. 3). Firstly, to quantify the total amount of Brønsted acid sites, ammonia, that can enter both micro and mesopores due to its small dimension, was used.
Subsequently, pyridine and 2,4,6-trimethylpyridine (2,4,6-TMP or collidine) with larger kinetic diameters, were adsorbed.12,17,18 The FTIR difference spectra of NH3 (Fig. 3A), pyridine (Figure 3B) and 2,4,6-TMP (Fig. 3C) adsorbed on HierSAPO-5 at room temperature are reported in the low frequency region where the signals of protonated species are observed. Upon NH3 adsorption at room temperature (Fig. 3A), a band at 1450 cm−1, due to the asymmetric bending mode of NH4+ ions, indicates NH3 protonation by Brønsted acid sites of the hierarchical catalyst. Furthermore, the bending mode of ammonia H-bonded to Si–OH or P–OH groups is detected at 1629 cm−1. In the C–C ring-stretching region, pyridine adsorption (Fig. 3B) produces bands due to physisorbed pyridine (1580 ν8b and 1438 ν19b cm−1), pyridine hydrogen-bonded to Si–OH or P–OH or on Lewis acid sites (1596 ν8b and 1445 ν19b cm−1), and protonated species from interaction with strong Brønsted acid sites (1630 ν8a, 1545 ν19b, and 1488 ν19a cm−1). Finally, upon 2,4,6-TMP adsorption, bands at 1611 and 1575 cm−1 (owning to physisorbed 2,4,6-TMP) and a signal at 1638 cm−1 (owning to protonated 2,4,6-TMP) are formed.
The total number of accessible Brønsted acid sites (N) of HierSAPO-5 determined by using NH3, pyridine and 2,4,6-TMP is reported in Table 2, together with the nature and the IR position of the bands of their protonated species. The accessibility factor (AF) is also calculated for pyridine derivatives. These data highlighted that only a small fraction of the total Brønsted acid sites are accessible to the bulkier probe molecules.
This newly designed catalyst was then applied for the MW-assisted conversion of glucose through Brønsted acidic catalysis. A well-known example hereof is the dehydration of sugars towards platform molecules like 5-HMF.19
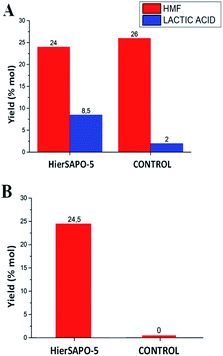
This compound has been identified as among the top biomass-derived value-added chemicals,20 since it is the starting material for biomass-based polymers, pharmaceuticals, agrochemicals, flavours and fragrances. It could be also a precursor for macro- and heterocycles, natural products and fuel components.21 The peculiar properties of MW as a source of volumetric and selective dielectric heating have recently been exploited in the catalytic conversion of biomass, as they provide higher product yields and selectivity in reduced times.22 Alternatively to aqueous media, non-aqueous systems have been proposed to prompt the MW-assisted dehydration to 5-HMF.23 The catalytic activity of hierarchical SAPO-5 was thus tested either in water (Fig. 4A) or in the biomass-based solvent γ-valerolactone (GVL, Fig. 4B), which can reduce the side reactions and thereby increases the selectivity toward 5-HMF. The reactions were performed at 443 K as this was the temperature providing the best yields in similar systems.24 The optimized conditions involved a reaction time of 20 minutes.
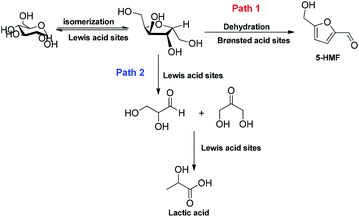
Indeed, a time decrease leads to a lower conversion, while longer times provide lower yields owing to a partial product degradation into unidentified soluble polymers and humines (data not shown). A reaction without the addition of HierSAPO-5 was also performed in the same conditions, as the control. As reported in Fig. 4A, using water as the solvent, the reaction proceeds with almost the same yields of 5-HMF either with or without the catalyst. However, the zeolite catalysed the formation of higher yields of lactic acid. It is now well established that glucose isomerization to fructose takes place on Lewis acid sites, and the dehydration step requires Brønsted acid sites.4 Basing on the catalytic results, i.e. on the obtained yields of 5-HMF and lactic acid, it can be hypothesized that in water only the small amount Lewis acid sites (detected by FTIR) play a role in the product formation, whilst the Brønsted sites may be reasonably involved in strong interaction with water. Indeed, there is no contribution by the HierSAPO-5 catalyst in the 5-HMF yield (being the same as in the control experiment), which is due only to the hydrothermal conversion under MW irradiation. On the contrary, lactic acid is formed by the conversion of fructose to glyceraldehyde and dihydroxyacetone by a retro-aldol reaction involving the C–C bond cleavage (Fig. 5, Path 2), which can be favoured by Lewis acid sites.25 When performing the reaction in GVL, no glucose conversion was observed in the absence of the catalyst (Fig. 4B). In addition, lactic acid was not produced under these conditions.
Therefore, it is very much worth noting that the solvent mediates glucose conversion to 5-HMF through a synergism with HierSAPO-5, prompting the action of both Lewis and Brønsted acid sites according to Path 1 (Fig. 5).
These preliminary results pointed out the synergism between the solvent and the HierSAPO-5 catalyst in the selectivity of glucose conversion, despite the yields to 5-HMF still need to be improved. In addition, MicroSAPO-5 was also tested in the same condition and the yield to 5-HMF achieved was almost comparable to HierSAPO-5, due to the small dimension of glucose substrate. However, XRD measurements on both MicroSAPO-5 and HierSAPO-5 samples before and after reaction in GVL (Fig. S7†) revealed that the introduction of mesopores guaranteed an improved structural stability of the HierSAPO-5 catalyst with respect to MicroSAPO-5 and a better diffusion of reactants, opening the door to the design of robust heterogeneous catalysts for biomass conversion.
Indeed, other SAPOs systems24,26 gave good results, however the catalytic test were performed using different reaction media.
In summary, a new hierarchical SAPO-5 was synthesised using a mixture of MsB coming from the flash conversion of waste biomass. This new catalyst was fully characterized and tested in the MW-assisted glucose conversion. Full selectivity toward 5-HMF was achieved using GVL as the solvent. This is a sustainable solvent, as it is biomass-derived, nontoxic and biodegradable. Furthermore, the use of MW further prompts the sustainability of the process by reducing the reaction times, compared to literature experiments.
In perspective, the sugars obtained from biomass conversion can be used both as templates and as substrates for the synthesis of high value added products through the sustainable MW-assisted processes.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The project leading to these results has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement no. 720783 – MULTI2HYCAT and from the University of Turin, Italy (Ricerca locale 2020).References
- J. Cejka, G. Centi, J. Perez-Pariente and W. J. Roth, Catal. Today, 2012, 179, 2–15 CrossRef CAS.
- Zeolites and Catalysis: Synthesis, Reactions and Applications, ed. J. Cejka, A. Corma and S. Zones, Wiley-VCH, Weinheim, 2010, vol. 2 Search PubMed.
- D. P. Serrano, J. A. Melero, G. Morales, J. Iglesias and P. Pizarro, Catal. Rev.: Sci. Eng., 2018, 60, 1–70 CrossRef CAS.
- T. Ennaert, J. Van Aelst, J. Dijkmans, R. De Clercq, W. Schutyser, M. Dusselier, D. Verboekend and B. F. Sels, Chem. Soc. Rev., 2016, 45(3), 584–611 RSC.
- P. Sudarsanam, E. Peeters, E. V. Makshina, V. I. Parvulescu and B. F. Sels, Chem. Soc. Rev., 2019, 48, 2366–2421 RSC.
- X. Zhu, J. P. Hofmann, B. Mezari, N. Kosinov, L. Wu, Q. Qian, B. M. Weckhuysen, S. Asahina, J. Ruiz-Martinez and E. J. M. Hensen, ACS Catal., 2016, 6, 2163–2177 CrossRef CAS.
- W. Schwieger, A. G. Machoke, T. Weissenberger, A. Inayat, T. Selvam, M. Klumpp and A. Inayat, Chem. Soc. Rev., 2016, 45, 3353–3376 RSC.
- M. Moliner, C. Martínez and A. Corma, Angew. Chem., Int. Ed., 2015, 54, 3560–3579 CrossRef CAS.
- M. Hartmanna and L. Kevan, Chem. Rev., 1999, 99, 635–664 CrossRef.
- E. Gianotti, M. Manzoli, M. E. Potter, V. N. Shetti, D. Sun, J. Paterson, T. M. Mezza, A. Levy and R. Raja, Chem. Sci., 2014, 5, 1810–1819 RSC.
- E. M. Flanigen, B. M. Lok, R. L. Patton and S. T. Wilson, Stud. Surf. Sci. Catal., 1986, 28, 103–112 CrossRef CAS.
- I. Miletto, C. Ivaldi, G. Paul, S. Chapman, L. Marchese, R. Raja and E. Gianotti, ChemistryOpen, 2018, 7, 297–301 CrossRef CAS.
- D. Nandan, S. K. Saxena and N. Viswanadham, J. Mater. Chem. A, 2014, 2, 1054–1059 RSC.
- S. Tabasso, E. Montoneri, D. Carnaroglio, M. Caporaso and G. Cravotto, Green Chem., 2014, 16(1), 73–76 RSC.
- G. Paul, C. Bisio, I. Braschi, M. Cossi, G. Gatti, E. Gianotti and L. Marchese, Chem. Soc. Rev., 2018, 47, 5684–5739 RSC.
- M. E. Potter, M. E. Cholerton, J. Kezina, R. Bounds, M. Carravetta, M. Manzoli, E. Gianotti, M. Lefenfeld and R. Raja, ACS Catal., 2014, 4, 4161–4169 CrossRef CAS.
- F. Thibault-Starzyk, I. Stan, S. Abellu, A. Bonilla, K. Thomas, C. Fernandez, J.-P. Gilson and J. Perez-Ramirez, J. Catal., 2009, 264, 11–14 CrossRef CAS.
- I. Miletto, G. Paul, S. Chapman, G. Gatti, L. Marchese, R. Raja and E. Gianotti, Chem.–Eur. J., 2017, 23, 9952–9961 CrossRef CAS.
- T. Ennaert, J. Van Aelst, J. Dijkmans, R. De Clercq, W. Schutyser, M. Dusselier, D. Verboekend and B. F. Sels, Chem. Soc. Rev., 2016, 45, 584–611 RSC.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–554 RSC.
- L. T. Mika, E. Cséfalvay and Á. Németh, Chem. Rev., 2018, 118, 505–613 CrossRef CAS.
- E. Calcio Gaudino, G. Cravotto, M. Manzoli and S. Tabasso, Green Chem., 2019, 21, 1202–1245 RSC.
- X. Jia, I. K. M. Yu, D. C. W. Tsang and A. C. K. Yip, Microporous Mesoporous Mater., 2019, 284, 43–52 CrossRef CAS.
- L. Zhang, G. Xi, Z. Chen, Z. Qi and X. Wang, Chem. Eng. J., 2017, 307, 877–883 CrossRef CAS.
- L. Yang, X. Yang, E. Tian, V. Vattipalli, W. Fan and H. Lin, J. Catal., 2016, 333, 207–216 CrossRef CAS.
- P. Bhaumik and P. L. Dhepe, RSC Adv., 2013, 3, 17156 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra06353c |
| This journal is © The Royal Society of Chemistry 2020 |