Open Access Article
Open Access ArticleTwo Ln-based metal–organic frameworks based on the 5-(1H-1,2,4-triazol-1-yl)-1,3-benzenedicarboxylic acid ligand: syntheses, structures, and photocatalytic properties†
Fei Yuan a,
Chunmei Yuana,
Baoyue Caoa,
Youying Dia,
Shumin Wang*a,
Mingbao Liua,
Abhinav Kumar
a,
Chunmei Yuana,
Baoyue Caoa,
Youying Dia,
Shumin Wang*a,
Mingbao Liua,
Abhinav Kumar *b,
Chuncheng Shi*c and
Mohd. Muddassir
*b,
Chuncheng Shi*c and
Mohd. Muddassir d
d
aShaanxi Key Laboratory of Comprehensive Utilization of Tailings Resources, College of Chemical Engineering and Modern Materials, Shangluo University, Shangluo 726000, China. E-mail: slyuanf@126.com; happywshm@126.com
bDepartment of Chemistry, Faculty of Science, University of Lucknow, Lucknow, 226007, India. E-mail: abhinavmarshal@gmail.com
cDepartment of Pharmacy, School of Medicine, Xi'an International University, Xi'an, 710077, Shaanxi, China. E-mail: shichch66xaiuedu@163.com
dDepartment of Chemistry, College of Sciences, King Saud University, Riyadh 11451, Saudi Arabia
First published on 30th October 2020
Abstract
Two new metal–organic frameworks (MOFs) having the formula [Ln2(H2O)3(L)3·3H2O]n (Ln = Sm for MOF-Sm and Tb for MOF-Tb) have been synthesized solvothermally by reacting LnCl3·6H2O with 5-(1H-1,2,4-triazol-1-yl)-1,3-benzenedicarboxylic acid (H2L) and characterized. Single crystal X-ray analyses for MOF-Sm and MOF-Tb revealed that both MOFs are isostructural and display a (6,8)-connected 3D structure with a point symbol of (35·44·66)(35·46·517). The natures of weak interactions existing in both MOFs have been assessed using Hirshfeld surface analyses and fingerprint plots. The utility of MOF-Sm as a photocatalyst for the safe photodegradation of the model aromatic dye methyl violet (MV) is also checked. The photocatalysis results showed that MOF-Sm offers reasonable photocatalytic degradation of this dye. The plausible photocatalytic mechanism of MOF-Sm aided photocatalysis has been explained with the help of band gap calculations using density of states (DOS) and partial DOS plots.
Introduction
The use of photocatalytic methods for degradation of aromatic pollutants have been explored extensively due to their simple operation, low energy consumption and high efficiency.1–3 Compared to traditional photocatalysts viz. traditional semiconductors (such as TiO2 and some metal sulphides), metal–organic frameworks (MOFs) have comprehensively been used in catalysis to treat organic pollutants in wastewater. This is because of their potential semiconducting properties which they exhibit on exposure to light.4,5 It has now been established that the structure of the resultant architecture in MOFs is usually affected by several factors viz. selection of a suitable ligand, metal cations, solvent, temperature, heating as well as cooling rate, etc. But amongst these pertinent factors, the apt selection of suitable organic linkers is considered as a sensible way to fabricate the desirable MOFs.6,7 The previous reports suggest that the imidazole-carboxylate bi-functional ligands offer some advantages for designing and fabrication of targeted MOFs. This is because, this ligand possess several coordination sites to meet the coordination requirements of metal ions.8,9The photocatalysis technology utilizing solar light has now been considered as one of the most effective technology for waste water treatment due to its various advantages, such as low cost, requiring low energy and better efficiency. The irradiation of UV-visible light can induce the excitation of electrons in dye molecules. The excited states of [dye]* can have stronger interactions with the active sites of MOF and hence results in more surface adsorption. Under UV light, the photo-excited [dye]* gets degraded by ˙OH radicals which is generated via direct excitation pathway by MOFs.10 Methyl violet (MV) is a typical cationic dye that is widely used in paper making, spraying and textile industries. Moreover, MV can affect human's physical health and can cause vomiting, shock, jaundice and cyanosis. This is because this dye can interact freely with the negatively charged membrane surface of cells, and then can reach cell's nucleus.10b Therefore, there is an utmost need to explore safe and sustainable treatment methods to degrade such aromatic dyes to convert them to relatively less harmful products.
Mostly transition metals mainly belonging to 3d transition series have been utilized to develop MOFs having varied architectures which eventually have been utilised as photocatalysts working under UV-vis irradiation.4–6 However, lanthanide based-MOFs which although possess enormous potential have not been explored exhaustively as photocatalyst to photodegrade pollutants especially the aromatic dyes present in the wastewater discharge.
Hence, in view of aforementioned points and in the pursuit of new lanthanide based MOFs bi-functional imidazole-carboxylate ligand a multifunctional ligand, 5-(1H-1,2,4-triazol-1-yl)-1,3-benzenedicarboxylic acid (H2L)11 have been selected in the presented study. The selection of this ligand is to increase the coordination function and possible synergism of the two functional groups, as the triangular linker. The H2L linker has been designed because of the following merits (Scheme 1). (1) The aromatic carboxylate and triazole ligands are two very important moieties utilized for the construction of MOFs due to their versatile coordinated modes.12 (2) The aromatic fragments can be regarded as antenna which increases light absorption and transfer energy to metal efficiently through “antenna effect”.13 With these view points, in the presented work two new isostructural MOFs having general formula [Ln2(H2O)3(L)3·3H2O]n (Ln = Sm for MOF-Sm and Tb for MOF-Tb), have been synthesized hydrothermally and characterized. In addition to molecular structure investigation of both MOFs their Hirshfeld surface analyses have been executed. The MOF-Sm has further been employed as possible photocatalyst for photodegradation of a model organic dye methyl violet. These results of all these studies are presented herewith.
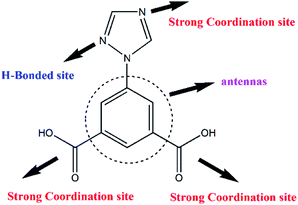 | ||
| Scheme 1 Chemical structure of 5-(1H-1,2,4-triazol-1-yl)-1,3-benzenedicarboxylic acid (H2L) showing different coordination and hydrogen bonding sites. | ||
Experimental
Materials and methods
These 5-(1H-1,2,4-triazol-1-yl)-1,3-benzenedicarboxylic acid (H2L) and LnCl3 materials were purchased from Jinan Henghua Sci and Tec Co., Ltd and used without further purification. The FTIR spectra were recorded in KBr discs on Nicolet Avatar 360 FTIR spectrometer in the 400–4000 cm−1 region. Powder X-ray diffraction (PXRD) data for the MOFs were recorded on Bruker D8 ADVANCE X-ray powder diffractometer using Cu-Kα radiation of 1.5418 Å.X-ray crystallography
The single crystal X-ray diffraction data were collected on Bruker SMART APEX diffractometer using graphite monochromated Mo-Kα radiation (λ = 0.71073 Å) by using an ω-scan technique. The structures were solved by direct method (SHLEXS-2014) and refined using the full-matrix least-square procedure based on F2 (Shelxl-2014).14 All the hydrogen atoms were generated geometrically and refined isotropically using a riding model. All non-hydrogen atoms were refined with anisotropic displacement parameters. Crystallographic details and selected bond dimensions for MOF-Sm and MOF-Tb are listed in Tables S1 and S2,† respectively. CCDC no.: 2014162 and 2014163.†Photocatalytic method
The finally divided sample of MOF-Sm (40 mg) was dispersed in 50 mL aqueous solution of methyl violet (MV) having concentration of 10 mg L−1. After adding the MOF, the mixture was stirred under dark for 30 min to ensure the establishment of adsorption–desorption equilibrium. After that, the photocatalytic degradation of MV was conducted using XPA-7 type photochemical reactor fitted with 100 W mercury lamp (mean wavelength 365 nm) having light intensity of 12.7 mW cm−2 at quartz tube positions. During the photocatalytic degradation, aliquots of 5.0 mL were isolated at specific time intervals and separated through centrifugation and then subsequently the intensity of characteristic electronic absorption band of MV recorded using UV-visible spectrophotometer. In addition, a control experiment was conducted under the similar reaction conditions but without the addition of MOF-Sm.Computational details
The possible photocatalytic mechanism by which the MOF-Sm performed the photodegradation of the MV have been explained with the aid of theoretical band gap calculations. The smallest unit of this MOF was geometry optimized using the B3LYP functional,15a,15b using 6-31G** basis set for all the atoms except Sm for which SDDALL basis set was employed. All the calculations were performed using Gaussian 09 program.15c The final results of the calculations were used to construct the density of states (DOS) and fragmented partial DOS plots using GaussSum 3.1 version of programs.15dHirshfeld surface analyses
Molecular Hirshfeld surfaces16–23 in the crystal structures of both MOFs were constructed by using the procedure mentioned previously.24Synthesis of [Sm2(H2O)3(L)3·3H2O]n (MOF-Sm)
To the 10 mL aqueous solution of SmCl3·6H2O (0.0382 g, 0.1 mmol), ligand H2L (0.0233 g, 0.1 mmol) was added and the mixture was stirred for 30 min. Thereafter the pH of this mixture was adjusted to 6.0 by adding aqueous solution of 0.5 M NaOH. The obtained mixture was transferred to a Teflon-lined stainless steel vessel (25 mL), sealed and heated to 160 °C for 72 h. After 72 h the vessel was cooled to room temperature at a rate of 5 °C h−1. The colourless block crystals of MOF-Sm were obtained which was washed with distilled water (5 mL), yield 28.8% based on H2L. IR (cm−1): 3486(vs); 3128(m); 2366(m); 1632(v); 1583(v); 1444(vs); 1388(v); 1219(w); 1134(m); 1076(m); 785(v); 729(v); 663(m).Synthesis of [Tb2(H2O)3(L)3·3H2O]n (MOF-Tb)
The solvothermal reaction adopted for the synthesis of MOF-Tb was similar to that used for MOF-Sm, except that TbCl3·6H2O (0.0373 g, 0.1 mmol) was used in the reaction. The faint yellow block crystals of MOF-Tb were obtained which was washed with distilled water (5 mL), yield 49.8% based on H2L. IR (cm−1): 3468(vs); 3129(m); 1632(v); 1583(v); 1448(vs); 1388(v); 1219(m); 1134(m); 1067(m); 785(v), 730(v), 672(v).Results and discussion
Molecular structure description
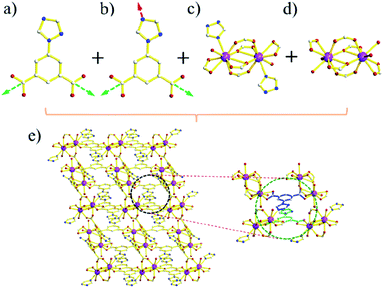
X-ray single crystal structural analysis reveals that MOF-Sm and MOF-Tb crystallize in the triclinic system with P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group and possess isostructural 3D network. Here, we select MOF-Sm as a representative example for a detailed discussion of the structure. As illustrated in Fig. S1,† the asymmetric unit of MOF-Sm contains two independent Sm(III) ions, three L2− ligands, three coordinated water molecules and three lattice water molecules.
space group and possess isostructural 3D network. Here, we select MOF-Sm as a representative example for a detailed discussion of the structure. As illustrated in Fig. S1,† the asymmetric unit of MOF-Sm contains two independent Sm(III) ions, three L2− ligands, three coordinated water molecules and three lattice water molecules.
Two kinds of L2− are existing in MOF-Sm. In addition to type-a L2− (Fig. 1a), type-b L2− is also present which through one of its triazole nitrogen and two carboxylates is participating in the formation of self-assembly framework (Fig. 1b).25 The MOF comprises of two different kinds of secondary building units (SBU). The first type of SBU consists of two nine-coordinated Sm(III) and surrounded by two oxygen atoms from two terminal H2O, two nitrogen atoms from two trizaole groups and twelve oxygen atoms from six carboxylate groups (Fig. 1c). While in second type of SBU, the nitrogen atoms are replaced by two oxygen atoms of two terminal H2O ligands (Fig. 1d and S2†). In other words, the carboxylate group exhibits bridging μ2-η2 mode in type-a L2− ligand, while two different coordination modes from the pair of carboxylate groups of type-b L2− ligand can be seen which coordinates to Sm(III) in μ1-η2 and μ2-η2:η1 modes.26
Each SBU connects adjacent SBU through two kinds of L2− bridges in four different directions which forms an unusual three-dimensional network with one-dimensional channels of ca. 5.2 Å × 6.3 Å (Fig. 1e and 2a). Topologically, each SBU can be simplified as nodes and the L2− ligands can be considered as 2-connected and 3-connected nodes, the complicated architecture of MOF-Sm is simplified to a new (6,8)-connected 3D network with a point symbol of (35·44·66)(35·46·517) as analysed by the TOPOS program (Fig. 2b).27 Moreover, the π⋯π stacking interactions between the trizaole rings of two kinds of L2− ligands (centroid-to-centroid distances of 3.86(3) Å) were observed in the channel (Fig. 1e). The water molecules and uncoordinated imidazole groups occupy the voids of three-dimensional network. The solvent accessible volume in MOF-Sm calculated excluding water molecules by PLATON analysis is 6.5% of the cell volume. Wang et al. have reported four water-stable lanthanide metal–organic frameworks (Ln(III)-MOFs) (1-Ln, Ln(III) = Tb, Eu, Dy, and Sm), [Ln(L)(HL)(H2O)2] (H2L = 4-(1H-1,2,4-triazol-1-yl)isophthalic acid), where the deprotonated H2L ligands have two different coordination modes. These adjacent SBUs were linked with L2− linkers to give a 2D layer along the c direction. Furthermore, these C8–H8A⋯N3 (1.935 Å) hydrogen bonds exist between the neighbouring layers, extending the 2D network into a 3D framework. Topologically, these structures could be viewed as a binodal (3,4)-connected topological net with a point symbol of (42·63·8)(42⋯6).13b The difference in structures is due to the isomerism of the functional groups of the ligands.
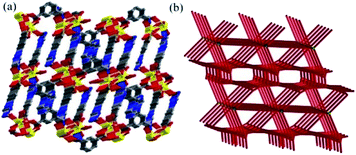 | ||
| Fig. 2 (a) Perspective view of 3D network in MOF-Sm; and (b) schematic representations of 6,8-connected dinuclear metal units coordinated with L2− ligands. | ||
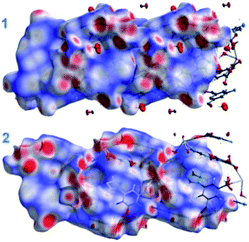
Hirshfeld surface analyses
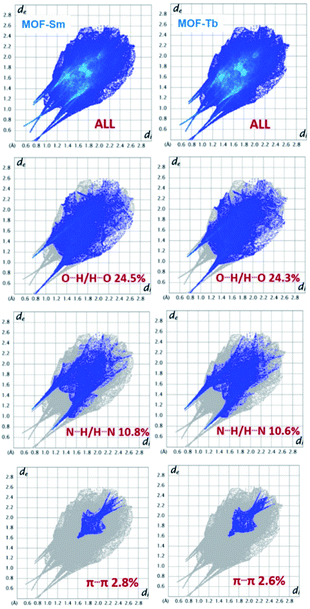
The Hirshfeld surfaces for both the MOFs are shown in Fig. 3. The dominant interactions are revealed by circular depressions in the dnorm plots which have been constructed between −0.7 to 1.4 Å. The weak interactions are shown effectively in dnorm surfaces with large circular depressions (deep red), suggesting the existence of strong interactions. While, the longer and weaker interactions are shown as small areas in the Hirshfeld surface plots.In fingerprint plots for both MOFs (Fig. 4), the complementary regions are visible where one molecule behaves as a donor (de > di) whilst another acts as an acceptor (de < di). In addition, the characteristic plots could be split apart to specify the atom pairs having close contacts. This enables us to evaluate the contributions by the different strong as well as weak interactions present in the structure. In the complex the O⋯H/H⋯O and N⋯H/H⋯N interactions appear as two distinct spikes of unequal lengths in the 2D fingerprint plots between the region 2.03 Å < (de + di) < 2.47 Å as light sky-blue pattern in full fingerprint 2D plots. While, the π⋯π non-covalent interactions are lying in the upper middle portion of the fingerprint plot in the region 1.6 Å < (de + di) < 2.4 Å. For MOF-Sm, the O⋯H/H⋯O, N⋯H/H⋯N and π⋯π interactions contribute 24.5%, 10.8% and 2.8%, respectively. While, in MOF-Tb the O⋯H/H⋯O, N⋯H/H⋯N and π⋯π interactions contribute 24.3%, 10.6% and 2.6%, respectively to its total Hirshfeld surface. The small variation in different contribution in the fingerprint plots could be ascribed to the isostructural nature of both the MOFs.
IR spectra and thermal properties
In the FTIR spectra of both MOFs (Fig. S3†), the main characteristic bands at ∼1384 cm−1 and ∼1631 cm−1 are mainly attributed to the asymmetric and symmetric stretching vibrations of the carboxylate groups, respectively. The characteristic absorption band was not observed at 1700 cm−1 which suggests the complete deprotonation of H2L ligand.28 This difference in the vibration magnitudes corresponding to symmetric and asymmetric stretch Δν[νas(COO)–νs(COO)] are ca. 139 cm−1 and 126 cm−1 for MOF-Sm and MOF-Tb, respectively. This indicate bridging bidentate coordination modes of carboxylate groups to the central metal atoms. The bands at 3486 and 3461 cm−1 for MOF-Sm and MOF-Tb respectively, are assigned to the stretching vibrations ν(OH) of water molecules. The bands at ca. 1583 cm−1 for both MOFs are assigned to the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) absorption in the imidazole ring of L2− ligand. To assess the thermal stabilities of both MOFs, thermogravimetric analyses (TGA) were performed under nitrogen atmosphere (Fig. S4†). The TGA analyses indicated that the weight loss of 9.8% (calcd 9.6%) in the first step from 57 to 255 °C corresponds to the removal of water molecules. The second weight loss occurred in a temperature range from 342 to 608 °C which corresponded to the decomposition of the L2− ligands which eventually ruptures the MOF framework.
N) absorption in the imidazole ring of L2− ligand. To assess the thermal stabilities of both MOFs, thermogravimetric analyses (TGA) were performed under nitrogen atmosphere (Fig. S4†). The TGA analyses indicated that the weight loss of 9.8% (calcd 9.6%) in the first step from 57 to 255 °C corresponds to the removal of water molecules. The second weight loss occurred in a temperature range from 342 to 608 °C which corresponded to the decomposition of the L2− ligands which eventually ruptures the MOF framework.
Photocatalytic property
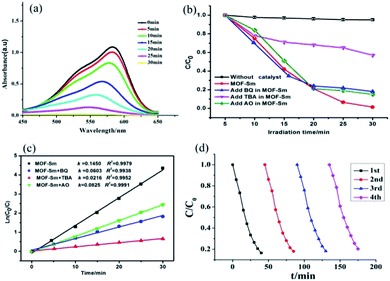
In this work, we employed the photodegradation of MV as a model to demonstrate the photocatalytic activities of MOF-Sm under UV light irradiation (Fig. 5). For MOF-Sm, about 98.7% degradation of MV was observed after 30 min. Apart for performing the photodegradation of MV in presence of MOF-Sm, for the sake of comparison, the control experiment (without the addition of MOF-Sm) was also executed. Under control experimental condition, the photodegradation of MV was only 13% within 40 min (Fig. S5†), which indicated that negligible decomposition of the dye without the photocatalyst MOF-Sm.29–31 Also, the change in the Ct/C0 plot of MV solutions vs. reaction time is presented in Fig. 5b. The distinct variation in photodegradation of MV compared with the control experiment indicates that MOF-Sm is an active photocatalyst for the decomposition of MV in the presence of UV irradiation. The photocatalytic performances of several Ln-based MOFs for aromatic dye putrefaction under UV radiation; visible radiation; or both UV and visible light irradiation are listed in Table S3.† This comparison indicates that the photocatalytic performances of MOFs are affected by several factors such as absorption of the light, charge separation efficiency, number as well as the nature of catalytic sites. This suggest that during Ln-based cation used to prepare MOFs, the resulting MOF frameworks, extent of conjugation, coordination environment and steric hindrance around the active metal sites play vital role in deciding the suitability of any MOF to be used as photocatalyst.In order to gain insight into the nature of active species involved in the dye degradation process, the photocatalytic reactions were performed under the similar conditions using catalyst MOF-Sm along with several radical scavengers such as tertiary butyl alcohol (TBA), benzoquinone (BQ) and ammonium oxalate (AO) (Fig. 5b and 6). The radical scavenging experiments demonstrated that the photocatalytic activity of MOF-Sm decreased to 47% after 30 min of irradiation and the degradation rate was 0.0216 min−1 when the TBA was added in the system (Fig. 5c and Table 1). However, the degradation of MV by MOF-Sm in the presence of other two radical scavengers viz. BQ and OA doesn't declined significantly (Fig. 5b, c and 6).
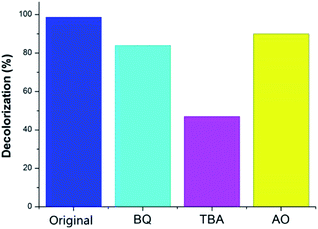 | ||
| Fig. 6 Comparison of the percentage photodegradation of MV solution with different scavenger in presence of MOF-Sm. | ||
| Material | MV | |
|---|---|---|
| k (min−1) | R 2 | |
| MOF-Sm | 0.1450 | 0.9979 |
| MOF-Sm + BQ | 0.0603 | 0.9938 |
| MOF-Sm + TBA | 0.0216 | 0.9952 |
| MOF-Sm + AO | 0.0825 | 0.9991 |
The degradation mechanism for MV could be explained as follows: the excited photo-electron moves from the valence band (VB) to the conduction band (CB), when MOF-Sm is under UV-vis light illumination. Meanwhile, the excited holes in the VB at the Sm(III) sites helps to produces hydroxyl radicals which decomposes the organic pollutants (Scheme 2). Thus, hydroxyl radicals play an important role in degrading different organic dyes because of its strong oxidising ability in aqueous solution. This mechanism is also found in other Ln-MOFs.40 Furthermore, when MOF-Sm was suspended into the aqueous solution of MV in the dark for two days, no significant decline in the characteristic absorption intensity of MV was observed, which ruled-out the possibility of adsorption of dye molecule into the framework of MOF-Sm.32–36 Hence, this experiment indicates that MOF-Sm showed excellent catalytic activity and good stability for the photodegradation of MV in water. To check the photocatalytic ability of the recycled MOF-Sm, after each photocatalytic cycle of MV, the photocatalyst MOF-Sm was filtered, washed with distilled water under stirring, and then filtered again. No significant reduction in percentage photodegradation of MV was observed even when the photocatalyst was used four cycles.37–39 The result indicated that MOF-Sm shows good reproducibility as photocatalyst. Moreover, the recovered sample of MOF-Sm was further characterized by powder X-ray diffraction (PXRD) which showed patterns nearly identical to that of the pristine MOF-Sm (Fig. S6†). This suggested that the MOF retained its framework integrity after performing photocatalysis of MV.
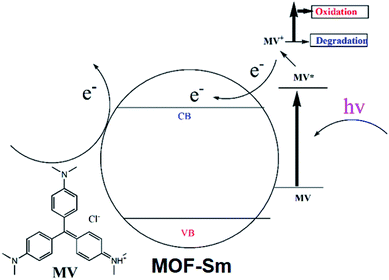 | ||
| Scheme 2 The possible mechanism involved in dye degradation and electron transfer in photocatalytic process. | ||
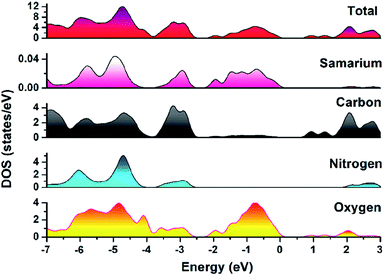
To explore the mechanism using which MOF-Sm performed photo-degradation of MV, the band structure calculations were performed which was based on density functional theory (vide supra). The obtained results of this calculation were used to construct DOS and partial DOS plots which are shown in Fig. 7. The plots display that the valence band (VB) in MOF-Sm is having major contributions from aromatic carbons and carboxylate oxygen atoms. While Sm(III) and nitogen centres of traizole offered almost negligible contribution in the VB. Likewise, the conduction band (CB) in MOF-Sm is again having contributions from aromatic carbon and oxygen centers. Hence, pDOS plots suggest that in MOF-Sm, the electronic transitions are of ligand-to-ligand type. During photo-excitation one L2− transfers electron to another L2− ligand which lead to generation of holes and these holes further assist in the production of hydroxyl radicals by reaction with water. These hydroxyl radicals then perform mineralization of MV.
Conclusion
In summary, we have successfully synthesized and characterized two isostructural Ln-MOFs based on a versatile bi-functional imidazole-carboxylate 5-(1H-1,2,4-triazol-1-yl)-1,3-benzenedicarboxylic acid ligand. They exhibit a unique (6,8)-connected network with a point symbol of (35·44·66)(35·46·517). The various interactions existing in the crystal structure of both the MOFs were investigated using Hirshfeld surface analyses and the fingerprint plots obtained during Hirshfeld surface calculations allowed scrutiny of all the important intermolecular interactions within the crystal structures. The photocatalytic results of MOF-Sm showed that this material can be suitable for performing photodegradation of MV and analogues aromatic dyes. This induced photocatalytic properties was ascribed to the formation of hydroxyl radicals generated during the ligand to ligand transitions. The presented work will generate interest amongst inorganic chemistry to design and synthesize newer Ln-based MOF for their use as photocatalyts for the safe and sustainable degradation of organic dyes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 21873063), the Nature Science Basic Research Program of Shanxi Province (Program No. 2018JQ2039), the Nature Science Foundation of Shanxi Province (No. 18JS034), the Natural Science Foundation of Shangluo University (No. 19SKY010 and 19HKY065) and the Youth Innovation Team of Shaanxi Universities. The authors also thank the Fund of Shangluo Universities Key Disciplines Project, Discipline name: Chemistry. This project is also supported by special fund of Shaanxi Key Laboratory of Special Fuel Chemistry and Material (No. SPCF-SKL-2020-0008). Authors are also grateful to Researchers Supporting Project (RSP-2020/141), King Saud University, Riyadh, Saudi Arabia for financial assistance.References
-
(a) M. Zalfani, Z. Y. Hu, W. B. Yu, M. Mahdouani, R. Bourguiga, M. Wu, Y. Li, G. V. Tendeloo, Y. Djaoued and B. L. Su, Appl. Catal., B, 2017, 205, 121–132 CrossRef CAS
; (b) Q. Liang, M. Zhang, Z. Zhang, C. Liu, S. Xu and Z. Li, J. Alloys Compd., 2017, 690, 123–130 CrossRef CAS
.
-
(a) W. Guan, X. Gao, G. Ji, Y. Xing, C. Du and Z. Liu, J. Solid State Chem., 2017, 255, 150–156 CrossRef CAS
; (b) V. A. Tran, A. N. Kadam and S.-W. Lee, J. Alloys Compd., 2020, 835, 155414 CrossRef CAS
; (c) K. G. Liu, F. Rouhani, X. M. Gao, M. Abbasi-Azad, J. Z. Li, X. D. Hu, W. Wang, M. L. Hu and A. Morsali, Catal. Sci. Technol., 2020, 10, 757–767 RSC
.
-
(a) M. L. Hu, V. Safarifard, E. Doustkhah, S. Rostamnia, A. Morsali, N. Nouruzi, S. Beheshti and K. Akhbari, Microporous Mesoporous Mater., 2018, 256, 111–127 CrossRef CAS
; (b) J.-L. Wang, C. Wang and W. Lin, ACS Catal., 2012, 2, 2630–2640 CrossRef CAS
; (c) A. Ma, J. Wu, Y. Han, F. Chen, B. Li, S. Cai, H. Huang, A. Singh, A. Kumar and J. Liu, Dalton Trans., 2018, 47, 9627–9633 RSC
.
-
(a) Y. Pan, Q. Ding, H. Xu, C. Shi, A. Singh, A. Kumar and J. Liu, CrystEngComm, 2019, 21, 4578–4585 RSC
; (b) Z. Zhang, J. Zhang, T. Wu, X. Bu and P. Feng, J. Am. Chem. Soc., 2008, 130, 15238–15239 CrossRef CAS
; (c) J. Cao, Z. Yang, W. Xiong, Y. Zhou, Y. Peng, X. Li, C. Zhou, R. Xu and Y. Zhang, Chem. Eng. J., 2018, 353, 126–137 CrossRef CAS
.
-
(a) M. Mon, R. Bruno, J. Ferrando-Soria, D. Armentano and E. Pardo, J. Mater. Chem. A, 2018, 6, 4912–4947 RSC
; (b) Q. Ding, Y. Pan, Y. Luo, M. Zhou, Y. Guan, B. Li, M. Trivedi, A. Kumar and J. Liu, ACS Omega, 2019, 4, 10775–10783 CrossRef CAS
; (c) J.-Z. Gu, Y. Cai, M. Wen, Z.-F. Shiand and A. M. Kirillov, Dalton Trans., 2018, 47, 14327–14339 RSC
.
-
(a) Y.-F. Peng, S. Zhao, K. Li, L. Liu, B.-L. Liand and B. Wu, CrystEngComm, 2015, 17, 2544–2552 RSC
; (b) M. S. Deenadayalan, N. Sharma, P. K. Verma and C. M. Nagaraja, Inorg. Chem., 2016, 55, 5320–5327 CrossRef CAS
; (c) M. L. Hu, Y. M. Mohammad and A. Morsali, Coord. Chem. Rev., 2019, 387, 415–435 CrossRef CAS
; (d) J. Liu, G. Liu, C. Gu, W. Liu, J. Xu, B. Li and W. Wang, J. Mater. Chem. A, 2016, 4, 11630–11634 RSC
.
-
(a) L. Qin, H.-Z. Chen, J. Lei, Y.-Q. Wang, T.-Q. Ye and H.-G. Zheng, Cryst. Growth Des., 2017, 17, 1293–1298 CrossRef CAS
; (b) L. L. Liu, C. X. Yu, Y. R. Li, J. J. Han, F. J. Ma and L. F. Ma, CrystEngComm, 2015, 17, 653–664 RSC
; (c) M. L. Hu, S. A. Razavi, M. Piroozzadeh and A. Morsali, Inorg. Chem. Front., 2020, 7, 1598–1632 RSC
; (d) H. He, L. Hashemi, M.-L. Hu and A. Morsali, Coord. Chem. Rev., 2018, 376, 319–347 CrossRef CAS
; (e) J. Liu, W. Wang, Z. Luo, B. Li and D. Yuan, Inorg. Chem., 2017, 56, 10215–10219 CrossRef CAS
.
- D. Zhao, D. J. Timmons, D. Yuan and C. Zhou, Acc. Chem. Res., 2011, 44, 123–133 CrossRef CAS
.
-
(a) J. P. Zhang, Y. B. Zhang, J. B. Lin and X. M. Chen, Chem. Rev., 2012, 112, 1001–1033 CrossRef CAS
; (b) H. Deng, S. Grunder, K. E. Cordova, C. Valente, H. Furukawa, M. Hmadeh, F. Gandara, A. C. Whalley, Z. Liu, S. Asahina, H. Kazumori, M. O'Keeffe, O. Terasaki, J. F. Stoddart and O. M. Yaghi, Science, 2012, 336, 1018–1023 CrossRef CAS
.
-
(a) V. A. Tran, A. N. Kadam and S. W. Lee, J. Alloys Compd., 2020, 835, 155414 CrossRef CAS
; (b) W. Liu, L. Liu, C. Lieu, Y. Hao, H. Yang, B. Yuan and J. Jiang, Biochem. Eng. J., 2016, 110, 115–124 CrossRef CAS
.
-
(a) M. Z. Wu, J. Y. Shi, P. Y. Chen, L. Tian and J. Chen, Inorg. Chem., 2019, 58, 3130–3136 CrossRef CAS
; (b) H. Wang, C. Huang, Y. Han, Z. Shao, H. Hou and Y. Fan, Dalton Trans., 2016, 45, 7776–7785 RSC
.
- G. Aromí, L. A. Barrios, O. Roubeau and P. Gamez, Coord. Chem. Rev., 2011, 255, 485–546 CrossRef
.
-
(a) L. J. Zhou, W. H. Deng, Y. L. Wang, G. Xu, S. G. Yin and Q. Y. Liu, Inorg. Chem., 2016, 55, 6271–6277 CrossRef CAS
; (b) F. Yang, G. P. Yang, Y. L. Wu, Y. T. Yan, J. Liu, R. C. Gao, W. Y. Zhang and Y. Y. Wang, J. Coord. Chem., 2018, 71, 2702–2713 CrossRef CAS
.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3–8 CrossRef
.
-
(a) A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS
; (b) C. T. Lee, W. T. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS
; (c) M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, T. Vreven Jr, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, W. M. Wong, C. Gonzalez and J. A. Pople, Gaussian 09 revision B.01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed
; (d) N. M. O'Boyle, A. L. Tenderholt and K. M. Langner, J. Comput. Chem., 2008, 29, 839–845 CrossRef
.
- M. A. Spackman and J. J. McKinnon, CrystEngComm, 2002, 4, 378–392 RSC
.
- M. A. Spackman and P. G. Byrom, Chem. Phys. Lett., 1997, 267, 309 CrossRef
.
- J. J. McKinnon, A. S. Mitchell and M. A. Spackman, Chem.–Eur. J., 1998, 4, 2136–2141 CrossRef CAS
.
- J. J. McKinnon, M. A. Spackman and A. S. Mitchell, Acta Crystallogr., Sect. B: Struct. Sci., 2004, 60, 627–668 CrossRef
.
- A. L. Rohl, M. Moret, W. Kaminsky, K. Claborn, J. J. McKinnon and B. Kahr, Cryst. Growth Des., 2008, 8, 4517–4525 CrossRef CAS
.
- A. Parkin, G. Barr, W. Dong, C. J. Gilmore, D. Jayatilaka, J. J. McKinnon, M. A. Spackman and C. C. Wilson, CrystEngComm, 2007, 9, 648–652 RSC
.
- S. K. Wolff, D. J. Greenwood, J. J. McKinnon, D. Jayatilaka and M. A. Spackman, Crystal Explorer 3.1, University of Western Australia, Perth, Australia, 2012 Search PubMed
.
- J. J. Koenderink and A. J. van Doorn, Image and Vision Computing, 1992, 10, 557–564 CrossRef
.
-
(a) P. Singh, A. Singh, A. Singh, A. K. Singh, G. Kociok-Köhn, A. Alowais, N. A. Y. Abduh, M. Muddassir and A. Kumar, CrystEngComm, 2020, 22, 2049–2059 RSC
; (b) R. Yadav, M. Trivedi, G. Kociok-Köhn, R. Prasad and A. Kumar, CrystEngComm, 2015, 17, 9175–9184 RSC
; (c) A. Kumar, V. Hüch and V. J. Ram, CrystEngComm, 2013, 15, 7019–7030 RSC
; (d) J. He, J. Wang, Q. Xu, X. Wu, A. Dutta, A. Kumar, M. Muddassir, A. Alowais and N. A. Y. Abduh, New J. Chem., 2019, 43, 13499–13508 RSC
.
-
(a) Z.-J. Li, X.-Y. Li, Y.-T. Yan, L. Hou, W.-Y. Zhang and Y.-Y. Wang, Cryst. Growth Des., 2018, 18, 2031–2039 CrossRef CAS
; (b) X. Feng, J. S. Zhao, B. Liu, L. Y. Wang, J. G. Wang, S. W. Ng, G. Zhang, X. G. Shi and Y. Y. Liu, Cryst. Growth Des., 2010, 10, 1399–1408 CrossRef CAS
.
-
(a) L. Jin, Q. Liu and W. Sun, CrystEngComm, 2014, 16, 3816–3928 RSC
; (b) X. Feng, B. Liu, L. Y. Wang, J. S. Zhao, J. G. Wang, S. W. Ng and X. G. Shi, Dalton Trans., 2010, 39, 8038–8049 RSC
.
- J. M. Zhou, W. Shi, N. Xu and P. Cheng, Inorg. Chem., 2013, 52, 8082–8090 CrossRef CAS
.
- A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7–13 CrossRef CAS
.
- S. Ou, J. P. Zheng, G. Q. Kong and C. D. Wu, Dalton Trans., 2015, 44, 7862 RSC
.
- J.-M. Hu, R. Guo, Y.-G. Liu and G.-H. Cui, Inorg. Chim. Acta, 2016, 450, 418–425 CrossRef CAS
.
- Y. Gong, P. G. Jiang, Y. X. Wang, T. Wu and J. H. Lin, Dalton Trans., 2013, 42, 7196–7203 RSC
.
- F. Wang, Z. S. Liu, H. Yang, Y. X. Tan and J. Zhang, Angew. Chem., Int. Ed., 2011, 50, 450–453 CrossRef CAS
.
- Z. L. Wu, C. H. Wang, B. Zhao, J. Dong, F. Lu, W. H. Wang, W. C. Wang, G. J. Wu, J. Z. Cui and P. Cheng, Angew. Chem., Int. Ed., 2016, 55, 4938–4942 CrossRef CAS
.
- F. Wang, Y. Pu, X. Zhang, F. Zhang, H. Cheng and Y. Zhao, J. Lumin., 2019, 206, 192–198 CrossRef CAS
.
- F. Q. Wang, C. M. Wang and Z. C. Yu, RSC Adv., 2015, 5, 70086–70093 RSC
.
-
(a) L. L. Wen, J. B. Zhao, K. L. Lv, Y. H. Wu, K. J. Deng, X. K. Leng and D. F. Li, Cryst. Growth Des., 2012, 12, 1603–1612 CrossRef CAS
; (b) X. Du, H. He, L. Du, W. Li, Y. Wang, Q. Jiang, L. Yang, J. Zhang and S. Guo, Polyhedron, 2019, 171, 221–227 CrossRef CAS
.
- L. Ding, J.-C. Zhong, X.-T. Qiu, Y.-Q. Sun and Y.-P. Chen, J. Solid State Chem., 2017, 246, 138–144 CrossRef CAS
.
- C. C. Wang, J.-R. Li, X. L. Lv, Y.-Q. Zhang and G. S. Guo, Energy Environ. Sci., 2014, 7, 2831–2867 RSC
.
-
(a) J.-J. Wang, P.-P. Si, J. Yang, S.-S. Zhao, P.-P. Li, B. Li, S.-Y. Wang, M. Lu and S.-X. Yu, Polyhedron, 2019, 162, 255–262 CrossRef CAS
; (b) J. J. Du, Y. P. Yuan, J. X. Sun, F. M. Peng, X. Jiang, L. G. Qiu, A. J. Xie, Y. H. Shen and J. F. Zhu, J. Hazard. Mater., 2011, 190, 945–951 CrossRef CAS
.
-
(a) W. Pan, C. H. Gong, X. H. Zeng, C. Y. Hu, Y. Zhang, D. R. Zhu, H. Xu, H. Y. Guo, J. Y. Zhang and J. L. Xie, Polyhedron, 2019, 169, 24–31 CrossRef CAS
; (b) T. Zhang and W. Lin, Chem. Soc. Rev., 2014, 43, 5982–5993 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2014162 and 2014163. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra07159e |
| This journal is © The Royal Society of Chemistry 2020 |