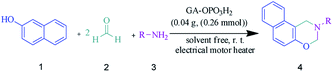Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Gum arabic-OPO3H2 as a new natural-based green catalyst for the one-pot pseudo-four-component synthesis of naphtho[1,2-e][1,3]oxazines†
Sahar Saadat Hosseinikhaha,
Bi Bi Fatemeh Mirjalili *a,
Naeimeh Salehia and
Abdolahamid Bamoniri
*a,
Naeimeh Salehia and
Abdolahamid Bamoniri b
b
aDepartment of Chemistry, College of Science, Yazd University, P. O. Box 89195-741, Yazd, Iran. E-mail: fmirjalili@yazd.ac.ir; Fax: +983538210644; Tel: +983531232672
bDepartment of Organic Chemistry, Faculty of Chemistry, University of Kashan, Kashan, Iran
First published on 6th November 2020
Abstract
Gum arabic-OPO3H2 (GA-OPO3H2) as a unique natural-based green catalyst was synthesized by the reaction of phosphorus pentoxide with gum arabic. The structure and properties of the catalyst were studied via several analysis methods such as FT-IR, MAPPING, EDS, SEM, XRD, and TGA. The efficiency of the above-mentioned catalyst was investigated for the synthesis of naphtho-1,3-oxazines via a pseudo-four-component reaction of primary amines, formaldehyde, and 2-naphthol under the solvent-free grinding condition at room temperature using an electrical mortar-heater. The obtained results indicated that GA-OPO3H2 is a highly efficient green catalyst for the synthesis of naphtho[1,2-e][1,3]oxazines with high yields, simple workup, and benign reaction condition.
Introduction
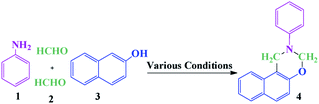
For the synthesis of organic compounds, the need for environmentally friendly conditions, green pathways, and low-cost with short reaction times is increasing. Therefore, it is preferable to use the natural-based catalysts and solvent-free methods. Gum Arabic (GA) is a biodegradable and renewable glucoprotein, which is an exudate collected from the stems and branches of Acacia trees.1,2 GA is also known to be a branched and complex acidic heteropolysaccharide whose main chain consists of (1→3)-β-D-galactopyranosyl units and side chains consists of L-arabinofuranosyl, L-rhamnopyranosyl, D-galactopyranosyl, and D-glucopyranosyluronic acid units.3,4 GA is generally safe, and in recent years, it has been widely used as a support,5–9 hydrogel10,11 and gold nanoparticle stabilizer.12 1,3-Oxazines as an important class of heterocycles have wide biological and pharmacological activities such as antitumor,13 antibacterial,14 anti-HIV,15 analgesic,16 antihypertensive,17 antithrombotic,18 and antiulcer activities.19 Moreover, naphthoxazine derivatives have exhibited therapeutic potential for the treatment of Parkinson's disease.20Based on the biological importance of benzo-fused 1,3-oxazines, numerous methods have been developed for the synthesis of pseudo-four-component including (1) Mannich-type condensation of 1- or 2-naphthol, formaldehyde, and a primary amine, (2) aza-acetalization of aromatic aldehydes with 2-(N-substituted aminomethyl) phenols in the presence of an acid as a catalyst and (3) electrooxidative cyclization of hydroxyamino compounds.21 The most common method for the synthesis of naphtho[1,2-e][1,3]oxazines is the multicomponent reaction of 2-naphthol, formaldehyde and a primary amine via a Mannich-type condensation. The synthesis of naphtho[1,2-e][1,3]oxazines using MCRs offers significant advantages including a reduction in the number of steps, energy consumption, and waste production.
Some of the catalysts that have previously been used for the synthesis of these products are KAl(SO4)2·12H2O (alum),22 ZrOCl2,23 1-benzyl- 3-methyl imidazolium hydrogen sulfate [bnmim] [HSO4],24 polyethylene glycol (PEG),25 and thiamine hydrochloride (VB1).26 Despite the remarkable achievements for the synthesis of naphtho[1,2-e][1,3]oxazine derivatives, some of these catalysts have limitations such as inefficient separation of the catalyst from reaction mixtures, unrecyclable and environmental limitations. Furthermore, this reaction was performed without a catalyst under harsh reaction conditions.27,28
In this study, we wish to report an efficient eco-friendly procedure for the synthesis of naphtho[1,2-e][1,3]oxazines using GA-OPO3H2 as a new natural-based green catalyst via the reaction of primary amines, formaldehyde, and 2-naphthol.
Results and discussion
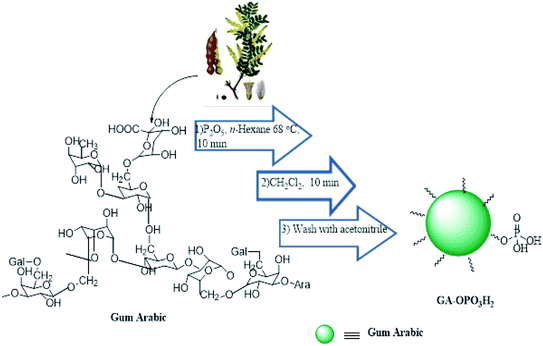
In this study, an efficient and environmentally benign protocol was developed for the synthesis of naphtho[1,2-e][1,3]oxazine derivatives using a pseudo-four-component reaction of primary amines, formaldehyde, and 2-naphthol in the presence of GA-OPO3H2. The steps for the synthesis of the GA-OPO3H2 catalyst are shown in Scheme 1. The as-synthesized catalyst was characterized via FT-IR, XRD, SEM, MAPPING, EDS, and TGA.The FT-IR (KBR) spectra of GA and GA-OPO3H2 are shown in Fig. 1. The characteristic absorption bands of GA at 1069 and 1148 cm−1 (C–O, stretch), 1423 (O–H, bending), 1616 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, aliphatic acid), 2928 cm−1 (C–H, stretch), and 3000–3600 cm−1 (O–H, stretch) can be observed (Fig. 1a). The strong peaks in the range of 900–1200 cm−1 are the fingerprints of carbohydrates. These peaks are observed in the case of GA and GA-OPO3H2. The bands at 1071 cm−1 (P–O–C) and 1228 cm−1 (P
O, aliphatic acid), 2928 cm−1 (C–H, stretch), and 3000–3600 cm−1 (O–H, stretch) can be observed (Fig. 1a). The strong peaks in the range of 900–1200 cm−1 are the fingerprints of carbohydrates. These peaks are observed in the case of GA and GA-OPO3H2. The bands at 1071 cm−1 (P–O–C) and 1228 cm−1 (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) are attributed to the stretching vibrations of the phosphoric acid section that overlaps with the absorption bands of GA in this region.
O) are attributed to the stretching vibrations of the phosphoric acid section that overlaps with the absorption bands of GA in this region.
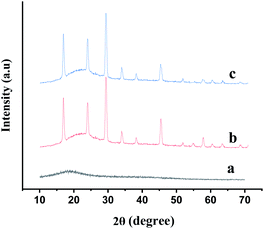
The particles size and morphology of GA-OPO3H2 were investigated via SEM. The exterior surface of GA-OPO3H2 appears as an irregular rocky surface in which the dimensions were found to be below 10 μm (Fig. 2). Fig. 3 illustrates the powder X-ray diffractograms obtained for natural GA, GA-OPO3H2, and reused GA-OPO3H2, respectively. The broad peak at 2θ = 19.895° (Fig. 3a) relates to the amorphous nature of GA. After the reaction of P2O5 with GA, the intensity of the corresponding peak decreases (Fig. 3b), verifying that P2O5 is bonded to GA. Other peaks in the GA-OPO3H2 spectrum may be due to the release of metals by the hydrolysis of calcium, magnesium, and potassium salts of GA, their binding to free hydroxyl groups, and the formation of metal complexes.
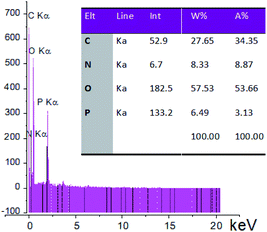
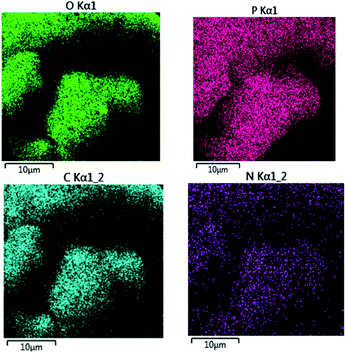
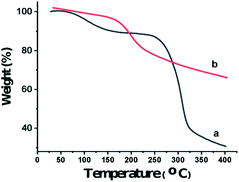
Also, the presence of C, O, N, and P in the catalyst was investigated via EDX analysis data (Fig. 4). The percentages of O, C, N, and P in the catalyst are 57.53, 27.65, 8.33, and 6.49, respectively. Fig. 5 shows the elemental mapping of GA-OPO3H2. The images and patterns recorded confirm the presence of carbon, nitrogen, oxygen, and phosphorus elements in the catalyst. In addition, it shows that catalyst functional groups are well-scattered throughout the catalyst. The thermal stability of gum arabic and GA-OPO3H2 were evaluated via thermo-gravimetric analysis (TGA) in the temperature range of 33–404 °C (Fig. 6). The TGA curve of gum arabic shows three steps of weight loss: (a) 10% at 70–150 °C, (b) 50% at 250–320 °C and (c) 10% at 320–400 °C. The char yield of gum arabic at 400 °C is 30%. Also, the TGA curve of GA-OPO3H2 shows three steps weight loss which contains (a) 5 % at 30–120 °C, (b) 10% at 150–220 °C and (c) 15% at 250–400 °C. The first weight loss is attributed to the evaporation of free water and the second and third weight loss steps corresponded to decomposition and burning of the GA section of the catalyst. The char yield of GA-OPO3H2 at 400 °C is 63.96%. These evidences show that thermal stability of GA-OPO3H2 is higher than that of gum arabic. The acidic capacity of the catalyst was measured via titrating it with 0.03 N of NaOH. The number of OPO3H2 groups on the gum GA-OPO3H2 is 6.6 mmol g−1. The catalytic performance of GA-OPO3H2 was investigated in a one-pot reaction of 2-naphthol (1 mmol), formalin (2 mmol), and primary amine (1 mmol) towards the synthesis of naphtho[1,2-e][1,3]oxazines. Initially, the optimization experiments were performed in the reaction of 2-naphthol (1 mmol), formalin (2 mmol), aniline (1 mmol)) as the model reaction. The reaction was optimized via various parameters such as temperature, solvent, and catalyst amounts, and the results are summarized in Table 1. To investigate the effect of reaction temperature, the reaction was performed at different temperatures under solvent-free conditions (Table 1, entries 8–11).
| Entry | Solvent | Catalysta (g [mmol]) | Condition | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a GA-OPO3H2.b Isolated yield.c Electrical mortar-heater. | |||||
| 1 | — | — | r. t.c | 60 | 15 |
| 2 | H2O | 0.05 [0.33] | r. t. | 25 | 56 |
| 3 | C2H5OH | 0.05 [0.33] | r. t. | 25 | 69 |
| 4 | CHCl3 | 0.05 [0.33] | r. t. | 25 | 49 |
| 5 | CH3CN | 0.05 [0.33] | r. t. | 25 | 62 |
| 6 | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C2H5OH (1 C2H5OH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.05 [0.33] | r. t. | 25 | 63 |
| 7 | CH3OH | 0.05 [0.33] | r. t.c | 25 | 55 |
| 8 | — | 0.05 [0.33] | r. t.c | 5 | 94 |
| 9 | — | 0.05 [0.33] | 50 °Cc | 60 | 25 |
| 10 | — | 0.05 [0.33] | 60 °Cc | 60 | 17 |
| 11 | — | 0.05 [0.33] | 80 °Cc | 60 | — |
| 12 | — | 0.04 [0.26] | r. t. c | 5 | 99 |
| 13 | — | 0.03 [0.2] | r. t. c | 5 | 92 |
| 14 | — | 0.02 [0.13] | r. t. c | 5 | 87 |
The highest yield was achieved at room temperature using an electrical mortar-heater (Table 1, entry 8). Different solvents including H2O, EtOH, CHCl3, CH3CN, H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH, MeOH were also screened (Table 1, entries 2–7). The model reaction was easier and gave the highest yield in a solvent-free condition. To optimize the catalyst amount, the model reaction was carried out in the presence of various amounts of the catalyst, and according to the obtained results, the optimum amount of the catalyst was 0.04 g (Table 1, entry 12). In a reaction, without catalyst, a low yield of the product was achieved after a long reaction time (Table 1, entry 1), and this indicates the high efficiency of catalyst for this reaction. According to the results, the best condition is using 0.04 g of the catalyst under the solvent-free condition at room temperature with an electrical mortar-heater (Table 1, entry 12).
EtOH, MeOH were also screened (Table 1, entries 2–7). The model reaction was easier and gave the highest yield in a solvent-free condition. To optimize the catalyst amount, the model reaction was carried out in the presence of various amounts of the catalyst, and according to the obtained results, the optimum amount of the catalyst was 0.04 g (Table 1, entry 12). In a reaction, without catalyst, a low yield of the product was achieved after a long reaction time (Table 1, entry 1), and this indicates the high efficiency of catalyst for this reaction. According to the results, the best condition is using 0.04 g of the catalyst under the solvent-free condition at room temperature with an electrical mortar-heater (Table 1, entry 12).
Due to remarkable results from the above experiments, we decided to synthesize naphtho[1,2-e][1,3]oxazines, and the results are summarized in Table 2. The products were obtained in good to excellent yields in short reaction times with aliphatic and aromatic amines with electron-withdrawing/donating substituents.
| Entry | R | Product | Time (min) | Yieldb (%) | MP (°C) |
|---|---|---|---|---|---|
a The amount ratio of 1 (mmol)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (mmol) 2 (mmol)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (mmol): GA-OPO3H2 (mmol) is equal to 1 3 (mmol): GA-OPO3H2 (mmol) is equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.26.b Isolated yield.c Decompose. 0.26.b Isolated yield.c Decompose. |
|||||
| 1 | C6H5– | 4a | 9 | 99 | 45–47 (ref. 22) |
| 2 | 4-Me–C6H4– | 4b | 10 | 94 | 86–88 (ref. 22) |
| 3 | 4-Et–C6H4– | 4c | 10 | 91 | 44–46 (ref. 21) |
| 4 | 4-Br–C6H4– | 4d | 9 | 97 | 116–118 (ref. 22) |
| 5 | 4-Cl–C6H4– | 4e | 9 | 97 | 100–104 (ref. 21) |
| 6 | 4-OMe–C6H4– | 4f | 9 | 95 | 76–78 (ref. 22) |
| 7 | C6H5–CH2- | 4g | 10 | 91 | 124–125 (ref. 21) |
| 8 | 2-Cl–C6H4–CH2- | 4h | 11 | 90 | 72–73 (ref. 21) |
| 9 | C6H5–CH2–CH2- | 4i | 9 | 97 | 233(d) (ref. 21) |
| 10 | 2-Furyl–CH2– | 4j | 9 | 94 | 98–100 (ref. 21) |
| 11 | Cyclohexyl- | 4k | 11 | 92 | 250(d)c (ref. 21) |
| 12 | n-Butyl– | 4l | 11 | 94 | 170(d)c (ref. 21) |
| 13 | n-Hexyl– | 4m | 11 | 95 | 178(d)c (ref. 21) |
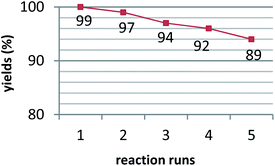
After the completion of the reaction, the reusability of the catalyst was also investigated in the model reaction. It was separated by filtration, washed 3 times with ethanol, and dried in an oven at 50 °C to provide an opportunity for recycling experiments. The separated catalyst was reused in the mentioned reaction five times without considerable loss of its catalytic activity (Fig. 7).
The separated catalyst was reused in the mentioned reaction five times without considerable loss of its catalytic activity (Fig. 7 and 3c).
The efficiency of the GA-OPO3H2 catalyst in comparison with some previously reported ones for the synthesis naphtho[1,2-e][1,3]oxazines are summarized in Table 3. As Table 3 indicates, the use of GA-OPO3H2 has remarkably improved the synthesis of naphtho[1,2-e][1,3]oxazines in different terms such as simplicity of protocol with compatibility with the environment.
| Entry | Conditions | Time (min) | Yieldd (%) |
|---|---|---|---|
| Solvent/temp(°C)/catalyst | |||
| a Room temperature.b 1-Benzyl-3-methyl imidazolium hydrogen sulphate.c Polyethylene glycol.d Isolated yield. | |||
| 1 | H2O/r.t.a/KAl(SO4)2·12H2O | 15 | 75 (ref. 22) |
| 2 | —/r.t/ZrOCl2 | 0.5 | 80 (ref. 23) |
| 3 | —/r.t/[bnmim][HSO4]b | 1 | 77 (ref. 24) |
| 4 | —/r.t/(PEG)c | 5 | 89 (ref. 27) |
| 5 | H2O/r.t/thiamine hydrochloride (VB1) | 60 | 65 (ref. 26) |
| 6 | H2O/r.t/nano-Al2O3/BF3/Fe3O4 | 20 | 92 (ref. 29) |
| 7 | —/r.t./GA-OPO3H2 | 9 | 99 (this work) |
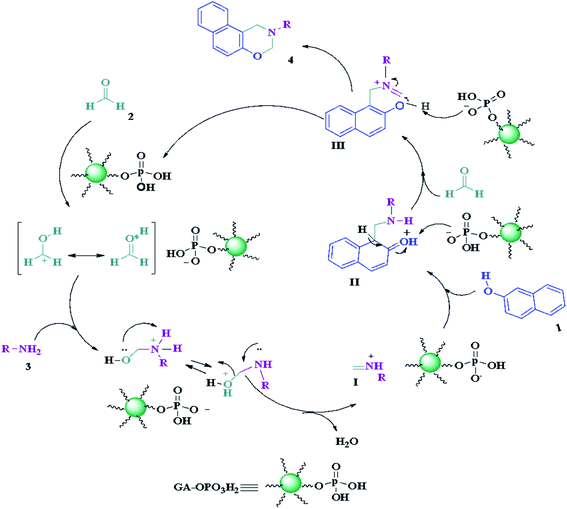
The suggested mechanism for the synthesis of naphtho[1,2-e][1,3]oxazines has been shown in Scheme 2. The GA-OPO3H2 catalyst operates as a Brønsted acid and at first, actuates the carbonyl group in formaldehyde. Then, the Mannich-type condensation of amine (3) with formaldehyde (2) gives imine I, which was attacked by the electron-rich center of 2-naphthol (1) to form intermediate II. Intermediate II, via a second Mannich-type condensation with a second molecule of formaldehyde, gives intermediate III, which through intramolecular cyclization afforded product (4).
Conclusions
In conclusion, we have introduced GA-OPO3H2 as a highly efficient, eco-friendly, inexpensive, and natural-based heterogeneous catalyst. The invented catalyst conveniently catalyzed the synthesis of naphtho[1,2-e][1,3]oxazine derivatives via a one-pot three-component reaction of 2-naphthol, formalin, and aniline using GA-OPO3H2 under the solvent-free condition at room temperature with an electrical mortar-heater. Short reaction time and excellent yields are among the advantages of this novel methodology.Experimental
Materials and methods
All compounds were purchased from Merck, Aldrich, and Fluka chemical companies and used without any additional purification. The electrical mortar-heater that was used for the grinding of the reaction mixture, was purchased from Borna-Kherad Co., Iran, Yazd. FT-IR spectra were recorded on a Bruker, Equinox 55 spectrometer. A Bruker (DRX-400 Avanes) NMR was used to record the 1H-NMR and 13C-NMR spectra. Melting points were determined by a Buchi melting point B-540 B. V. CHI apparatus and were uncorrected. X-ray diffraction (XRD) patterns were obtained by a Philips Xpert MPD diffractometer equipped with a Cu Kα anode (k = 1.54 Å) in the 2θ range from 10° to 80°. Quantitative elemental information and maps of GA-OPO3H2 were studied via energy-dispersive X-ray spectroscopy (EDS) by a Phenom pro X instrument. Thermal gravimetric analysis (TGA) was conducted using a “Universal V4.5A TA” instrument. The morphology was studied using a Philips XL30 scanning electron microscope (SEM).Preparation of GA-OPO3H2
GA is an exudate from the Acacia tree. Then, the catalyst (GA-OPO3H2) is simply prepared by adding P2O5 (3 g) in the n-hexane solution (5 ml) containing gum arabic (1 g). The mixture was stirred for 10 min at 68 °C. The mixture is sticky; hence, we have added dichloromethane (5 ml) to make it into a powder. The resulted suspension was filtered and washed 2 times with acetonitrile to remove unreacted P2O5 and dried at room temperature.General procedure the synthesis of naphtho[1,2-e][1,3]oxazines
A mixture of 2-naphthol (1 mmol), formalin (2 mmol), primary amine (1 mmol), and GA-OPO3H2 (0.04 g) was stirred under the solvent-free condition at room temperature with an electrical mortar-heater. After the completion of the reaction (monitored by TLC, n-hexane: EtOAc (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)), the reaction mixture was dissolved in hot ethanol (4 ml), and the catalyst was separated via filtration. After the evaporation of the solvent, the crude products were recrystallized from ethanol to give pure naphtho-1,3-oxazine derivatives. The recovered catalyst was washed 3 times with ethanol, dried in an oven at 50 °C, and then reused successfully.
1)), the reaction mixture was dissolved in hot ethanol (4 ml), and the catalyst was separated via filtration. After the evaporation of the solvent, the crude products were recrystallized from ethanol to give pure naphtho-1,3-oxazine derivatives. The recovered catalyst was washed 3 times with ethanol, dried in an oven at 50 °C, and then reused successfully.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Research Council of Yazd University gratefully acknowledged for the financial support for this work.References
- P. Mao, M. Zhao, F. Zhang, Y. Fang, G. O. Phillips, K. Nishinari and F. Jiang, Carbohydr. Polym., 2013, 98, 699 CrossRef CAS.
- S. A. Fazeli-Attar and B. F. Mirjalili, Environ. Chem. Lett., 2018, 16, 671 CrossRef CAS.
- P. S. Gils, D. Ray and P. K. Sahoo, Int. J. Biol. Macromol., 2010, 46, 237 CrossRef CAS.
- M. Ling, H. Zhao, X. Xiaoc, F. Shi, M. Wu, J. Qiu, S. Li, X. Song, G. Liu and S. Zhang, J. Mater. Chem. A, 2015, 3, 2036 RSC.
- A. Khazaei, S. Rahmati, A. Ghaderi and L. Roshani, J. Iran. Chem. Soc., 2014, 11, 263 CrossRef CAS.
- T. Baran and A. Menteş, Int. J. Biol. Macromol., 2020, 161, 1559 CrossRef CAS.
- B. Sreedhar, P. Surendra Reddy and D. Keerthi Devi, J. Org. Chem., 2009, 74, 8806 CrossRef CAS.
- C. Wu and D. Chen, Nanoscale Res. Lett., 2012, 7, 317 CrossRef.
- B. Sreedhar, P. S. Reddy and D. K. Devi, J. Org. Chem., 2009, 74, 8806 CrossRef CAS.
- S. L. Favaro, F. D. Oliveira, A. V. Reis, M. R. Guilherme, E. C. Muniz and E. B. Tambourgi, J. Appl. Polym. Sci., 2008, 107, 1500 CrossRef CAS.
- A. T. Paulino, M. R. Guilherme, L. H. C. Mattoso and E. B. Tambourgi, Macromol. Chem. Phys., 2010, 211, 1196 CrossRef CAS.
- V. Kattumuri, S. KattiK Bhaskaran, E. J. Boote, S. W. Casteel, G. M. Fent, D. J. Robertson, M. Chandrasekhar, R. Kannan and K. V. Katti, Nano.Micro.Small, 2007, 3, 333 CAS.
- J. Chylińska, T. Urbański and M. Mordarski, J. Med. Chem., 1963, 6, 484 CrossRef.
- B. P. Mathew, A. Kumar, S. Sharma, P. K. Shukla and M. Nath, Eur. J. Med. Chem., 2010, 45, 1502 CrossRef CAS.
- A. J. Cocuzza, D. R. Chidester, B. C. Cordova, S. Jeffrey, R. L. Parsons, L. T. Bacheler, S. Erickson-Viitanen, G. L. Trainor and S. S. Ko, Bioorg. Med. Chem. Lett., 2001, 11, 1177 CrossRef CAS.
- T. Kurz, Tetrahedron, 2005, 61, 3091 CrossRef CAS.
- M. Kajino, Y. Shibouta, K. Nishikawa and K. Meguro, Chem. Pharm. Bull., 1991, 39, 2896 CrossRef CAS.
- B. O. Buckman, R. Mohan, S. Koovakkat, A. Liang, L. Trinh and M. M. Morrissey, Bioorg. Med. Chem. Lett., 1998, 8, 2235 CrossRef CAS.
- Y. Katsura, S. Nishino and H. Takasugi, Chem. Pharm. Bull., 1991, 39, 2937 CrossRef CAS.
- J. N. Joyce, S. Presgraves, L. Renish, S. Borwege, T. Osredkar, D. Hagner, M. Replogle, M. PazSoldan and M. J. Millan, Exp. Neurol., 2003, 184, 393 CrossRef CAS.
- S. Azad and B. F. Mirjalili, Mol. Diversity, 2019, 23, 413 CrossRef CAS.
- S. A. Sadaphal, S. S. Sonar, B. B. Shingate and M. S. Shingare, Green Chem. Lett. Rev., 2010, 3, 213 CrossRef CAS.
- A. H. Kategaonkar, S. S. Sonar, R. U. Pokalwar, A. H. Kategaonkar, B. B. Shingate and M. S. Shingare, Bull. Korean Chem. Soc., 2010, 31, 1657 CrossRef CAS.
- A. H. Kategaonkar, S. S. Sonar, K. F. Shelke, B. B. Shingate and M. S. Shingare, Org. Commun., 2010, 3, 1 CAS.
- P. V. Shinde, A. H. Kategaonkar, B. B. Shingate and M. S. Shingare, Chin. Chem. Lett., 2011, 22, 915 CrossRef CAS.
- V. D. Dhakane, S. S. Gholap, U. P. Deshmukh, H. V. Chavan and B. P. Bandgar, C. R. Chim., 2014, 17, 431 CrossRef CAS.
- A. Y. Shen, C. T. Tsai and C. L. Chen, Eur. J. Med. Chem., 1999, 34, 877 CrossRef CAS.
- P. D. Woodgate, G. M. Horner, N. P. Maynard and C. E. Rickard, J. Organomet. Chem., 1999, 592, 180 CrossRef CAS.
- E. Babaei and B. F. Mirjalili, Polycyclic Aromat. Compd., 2019 DOI:10.1080/10406638.2019.1600561.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra07199d |
| This journal is © The Royal Society of Chemistry 2020 |