Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
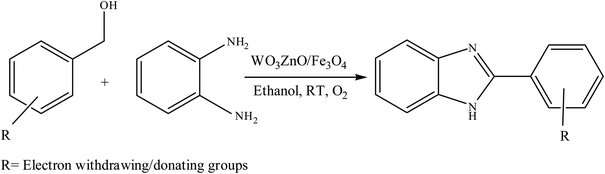
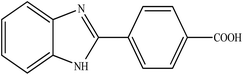
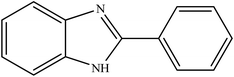
Selective photocatalytic oxidation of aromatic alcohols to aldehydes with air by magnetic WO3ZnO/Fe3O4. In situ photochemical synthesis of 2-substituted benzimidazoles†
Bozhi Lia,
Reza Tayebee *b,
Effat Esmaeilic,
Mina S. Namaghib and
Behrooz Malekib
*b,
Effat Esmaeilic,
Mina S. Namaghib and
Behrooz Malekib
aDepartment of Food Science and Engineering, Jinzhou Medical University, Jinzhou, China. E-mail: rtayebee@hsu.ac.ir
bDepartment of Chemistry, School of Sciences, Hakim Sabzevari University, Sabzevar, 96179-76487, Iran
cDepartment of Chemistry, Payame Noor University (PNU), Tehran, 19395-4697, Iran
First published on 9th November 2020
Abstract
Recently, visible light-driven organic photochemical synthesis has been a pioneering field of interest from academic and industrial associations due to its unique features of green and sustainable chemistry. Herein, WO3ZnO/Fe3O4 was synthesized, characterized, and used as an efficient magnetic photocatalyst in the preparation of a range of 2-substituted benzimidazoles via the condensation of benzyl alcohol and o-phenylenediamine in ethanol at room temperature for the first time. The key feature of this work is focused on the in situ photocatalytic oxidation of benzyl alcohols to benzaldehydes under atmospheric air and in the absence of any further oxidant. This new heterogeneous nanophotocatalyst was characterized via XRD, FT-IR, VSM and SEM. Short reaction time, cost-effectiveness, broad substrate scope, easy work-up by an external magnet, and excellent product yield are the major advantages of the present methodology. A number of effective experimental parameters were also fully investigated to clear broadness and generality of the protocol.
1. Introduction
Recently, green energy issues have attracted considerable attention to develop newer effective sustainable energy resources and environmentally benign routes to achieve important synthetic pharmaceuticals broadly used in the human life. The visible region of the solar energy (∼43%) is the most useful alternative to perform many green organic photochemical reactions under catalytic conditions due to its sustainable and abundant nature.1 Therefore, a great deal of concern is devoted to the photocatalytic degradation of organic wastes in water resources, efficient photocatalytic oxyfunctionalization of organic compounds and selective organic transformations under ambient environmentally friendly conditions, in particular with atmospheric air as a natural oxidant, under solar light.2–6 Among various organic transformations, the selective photochemical oxidation of alcohols to carbonyl compounds is of prime importance due to its significant role in the synthesis of a variety of fine chemicals, confectionaries, fragrances, and pharmaceutical industries.7–17A photocatalyst is capable of absorbing light and producing electron–hole pairs to force chemical reactions.18 Among the various photocatalysts, a great deal of attention has been paid to semiconducting metal oxides because of their compatible band gap in the visible region. Usually, the absorption of a photon by a semiconductor with an energy equal to or greater than the band gap energy can transfer an electron from the valence band to the conduction band, leaving a hole in the conduction band with a high oxidation capacity. The generated hole can absorb electrons from the hydroxyl ions in water to generate highly unstable and reactive ·OH. Therefore, the excited electron can also react with the oxygen gas to produce an O2− radical-anion. The created electron–hole pair can follow different routes to react with the organic materials adsorbed on the surface of the photocatalyst. Notable features such as optimum band gap, wide surface area, good stability and reusability and suitable morphology are intended from an effective photocatalyst. In addition, the performed photocatalyst should be fabricated under environmentally friendly and cost-effective conditions.18–20 Furthermore, the photocatalyst concentration, reactant concentration, type of the chemical transformation, temperature, kind and intensity of the light source can directly affect the performance and effectiveness of a photochemical reaction.21,22 Semiconducting metal oxides such as zinc, titanium, tungsten, vanadium, and cerium oxides are the most familiar photocatalysts, which have been used extensively in electronic and chemical technology, hydrogen production, storage, and environmental remediation because of their suitable electronic structure, charge transfer characteristics, and light absorption properties.18,22–24
Among nitrogen-containing heteroaromatics, benzimidazoles are vital core materials to generate different and significant drugs. They exhibit various biological activities such as antiviral,25 anti-inflammatory,26 antibacterial,27 antiparasitic,28 analgesic,29 antihypertensive,30 antituberculosis,31 and antiprotozoal effects.32 Considering such a wide range of biological functionalities, extensive efforts have been made to the progress of efficient strategies for the synthesis of various 2-substituted benzimidazoles. Condensation of 2-aminothiophenol, o-phenylenediamine, and 2-aminophenol with aromatic aldehydes under acidic conditions is the well-known method to prepare these drugs33 by the mediation of various catalysts such as magnetic catalysts,34 porous catalysts,35 metal–organic framework catalysts,36 ionic liquid catalysts,37 heavy metal catalysts,38 graphene oxides39 and so on. Moreover, quinoxalines are also important heteroaromatic compounds that have drawn extensive attention for use in many pharmaceuticals,40,41 various biofunctional molecules,42 semiconductor materials43 and organic synthons.44
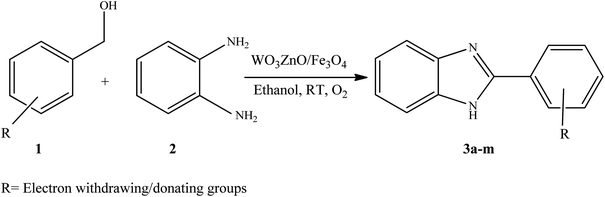
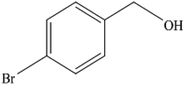
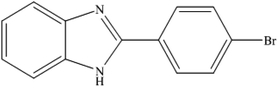
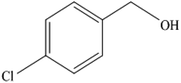
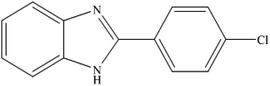
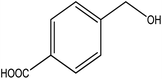
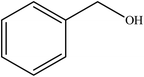
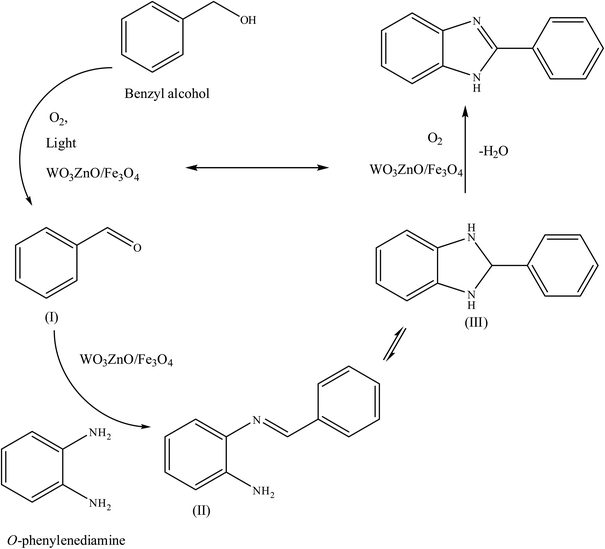
To the best of our knowledge, WO3ZnO/Fe3O4 was prepared and investigated for the first time as an ideal magnetic nanophotocatalyst in the synthesis of 2-substituted benzimidazoles via the reaction of ortho-substituted anilines with various substituted benzyl alcohols in the presence of air as a natural oxidant under the irradiation of a high-pressure Hg lamp (Scheme 1). The impacts of various experimental conditions such as solvent type, reactant concentration, temperature, contact time, and source of illumination on the efficacy of the photocatalytic synthesis were also monitored. Furthermore, we have used different benzyl alcohols with different electron-rich and electron-deficient groups in the condensation with anilines.
2. Experimental section
2.1. Materials and methods
Zinc chloride, sodium hydroxide, zinc acetate, sodium tungstate, benzyl alcohols, and other starting materials and solvents were purchased from Merck and used without further treatment. A high-pressure mercury vapor lamp (NAVIFLUX, 400 W, Berlin, NARVA) with a lamp operating current of 3.25 A and a nominal voltage of 230 V was used for the photocatalytic experiments. The light intensity of the UVC lamp was equal to 1020 μW cm−2 as measured using a Spectroline model DRC-100X digital radiometer combined with a DIX-365 radiation sensor (Shokofan Tosee, Iran). 1H and 13C-NMR spectra were recorded using a 300 MHz Bruker AVANCE III spectrometer in DMSO-d6 using TMS as the internal reference. Melting points were determined in open capillary tubes using a Stuart BI Branstead Electrothermal cat. no. IA9200 apparatus and uncorrected. The morphology of the synthesized nanomaterials was studied using a Tescan 3Mira 3-XMU field emission scanning electron microscope (FESEM). Fourier transform infrared (FT-IR) spectroscopy was performed using a Shimadzu 8700 Fourier transform spectrophotometer in the range of 400 to 4000 cm−1 with KBr pellets. UV-visible spectra were recorded using a Photonix UV-visible array spectrophotometer. X-ray diffraction (XRD) patterns were acquired using an Xpert MPD diffractometer with Cu Kα radiation at 30 mA and 40 keV and a scanning rate of 3° min−1 in the 2θ domain from 5° to 80°. The room-temperature magnetic measurements were carried out using a Vibrating Sample Magnetometer model of Magnetic Daghigh Kavir, MDKB.2.2. Preparation of Fe3O4 nanoparticles by a chemical co-precipitation method
Fe3O4 magnetic nanoparticles were prepared according to the previously reported procedure.45 FeCl3·6H2O (10.3 g) and FeCl2·4H2O (3.9 g) were dissolved in 100 mL deionized water, purged with N2 for 15 min, and heated to 85 °C. Thereafter, 15 mL of NH4OH solution (32%) was added drop-wise as well. The precipitated solid material was separated using an external magnet after 15 min and washed thoroughly with a NaCl solution (0.1 M, 3 × 10 mL). Finally, the obtained dark powder was dried overnight in a vacuum oven at 80 °C for 3 h.2.3. Preparation of Fe3O4 nanoparticles via bioreduction of S. cumini seed extract (Fe3O4 SMNT)
Fe3O4 SMNT was prepared by an easy and environmentally friendly method. First, 2.20 g of FeCl3·6H2O and 6.60 g of sodium acetate were dissolved in 50 mL of freshly prepared S. cumini seed extract. The resulting solution contained polysaccharides and other biomolecules, and the mixture was agitated at 67 °C for 2 h. After 2 h, the resulting solution became a black homogeneous dispersion, indicating generation of Fe3O4 SMNT. The colloidal solution was cooled to 25 °C and the black precipitate was separated using an external magnetic field, washed three times thoroughly with ethanol and dried overnight in vacuum at 90 °C overnight.2.4. Preparation of zinc oxide nanoparticles
In a typical route, 12.6 g of hydrated zinc acetate was dissolved in 450 mL of double-distilled water under stirring until it was completely dissolved. Thereafter, the solution was heated to 50 °C, and 500 mL of absolute alcohol was slowly added under stirring. Then, 6 mL of H2O2 (47%) was added drop-wise and mixed using a magnetic stirrer until the solution becomes clear. This solution was treated for 24 h and, then, dried at 80 °C for several hours to obtain the white nanopowder of zinc oxide. The attained zinc oxide was washed with double-distilled water to completely remove contaminants. After that, ZnO nanoparticles were dried at 80 °C to attain complete conversion and growth of the crystals.462.5. Preparation of WO3ZnO and WO3ZnO/Fe3O4 nanoparticles
First, 30 mL of NaOH (4 M) was added drop-wise into a 20 mL solution of zinc acetate (1 M) and Na2WO4·2H2O (2 mL, 0.5 M). Then, the obtained suspension was maintained at room temperature under mild continuous stirring for 3 h to form the precursor. After that, the obtained suspension was subsequently transferred into a 250 mL ground-glass-stopped conical flask and aged at 95 °C for 10 h. Finally, the generated precipitate was collected, washed with distilled water, and air-dried at room temperature. To fabricate WO3ZnO/Fe3O4, the preparation method for WO3ZnO was repeated as described, but, in the presence of the as-synthesized Fe3O4 nanoparticles (0.3 g).2.6. Preparation of melamine/H3PW12O40 nanoparticles
First, 0.1 g of melamine was added to 10 mL of chloroform and left on the stirrer for 1 h to dissolve. During this process, pure concentrated sulfuric acid was added drop-wise to the solution to obtain an oily solution. Thereafter, 0.1 g of H3PW12O40 was dissolved in 5 mL of deionized water and slowly added to the above oily mixture. The final mixture was stirred at room temperature to yield a white powder. After stirring for 2 h, the white solid powdered material was filtered, washed several times with water and dried under an open-air autoclave.2.7. General experimental procedure for the synthesis of 2-substituted benzimidazoles
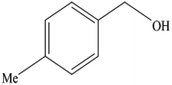
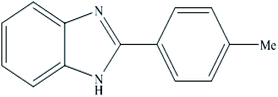
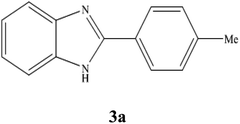
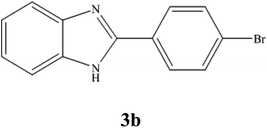
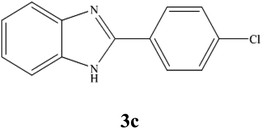
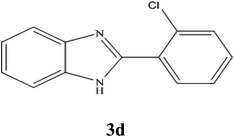
A mixture of benzyl alcohol (1 mmol), o-phenylenediamine (1 mmol), and WO3ZnO/Fe3O4 nanophotocatalyst (0.01 g) was taken in 10 mL ethanol at room temperature. Then, the stirring reaction mixture was exposed to the HP mercury light irradiation under mild air bubbling (1 mL min−1), and progress of the reaction was monitored by TLC. After completion of the reaction, the magnetic nanophotocatalyst was separated easily from the homogeneous reaction mixture using an external magnet. Finally, the yellow product appeared by adding ice-water to this solution. The precipitated product was filtered and re-crystallized in ethanol to bring further purified products. For the sake of completeness, the NMR characterization data for 2-substituted benzimidazoles (3a–m) are provided in Table 3.3. Results and discussion
3.1. Characterization and physicochemical properties of WO3ZnO/Fe3O4 nanoparticles
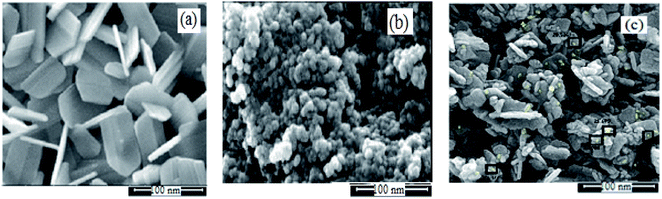
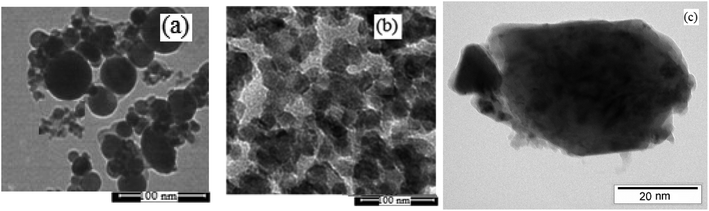
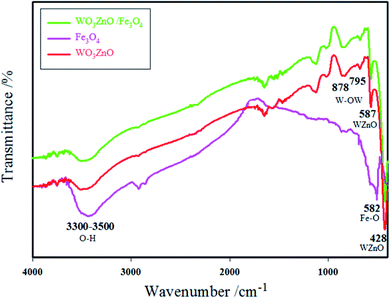
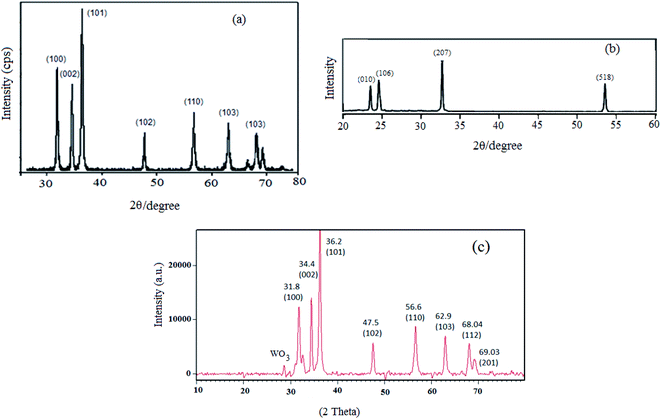
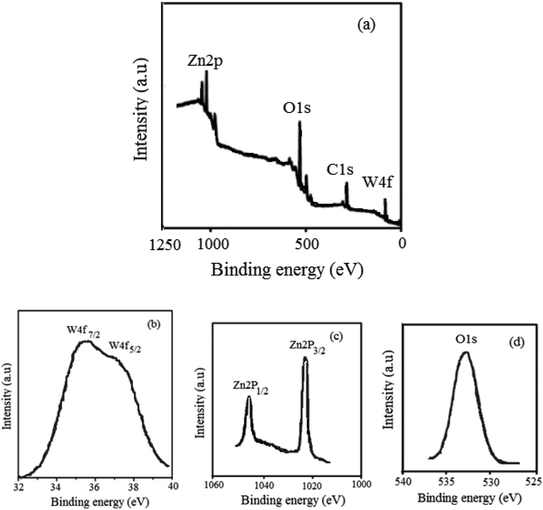
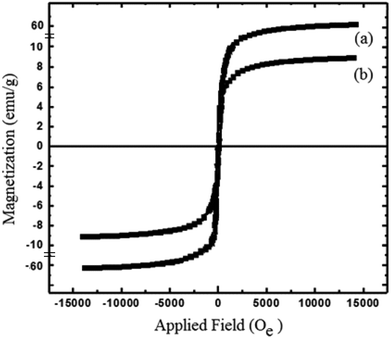
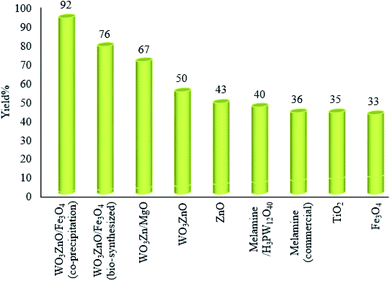
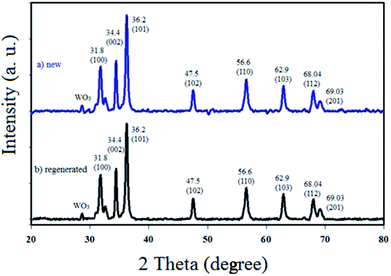
The physicochemical and morphological properties of the supported WO3ZnO/Fe3O4 nanophotocatalyst were explored using the most informative techniques such as FESEM, FT-IR, VSM, and XRD.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times and with a resolution of about 1–20 nm. Field-emission scanning electron microscopy was used to monitor the morphology and particle distribution in WO3 (Fig. 1a), ZnO (Fig. 1b), and WO3ZnO/Fe3O4 (Fig. 1c). From FE-SEM images, it can be seen that the prepared nanocomposite had a uniform surface morphology with a concomitant irregular distribution. The FESEM image of WO3ZnO/Fe3O4 nanoparticles showed random and irregular distribution of nanoparticles. The surface of the material also showed layered and plate-like nanoparticles. In addition, the TEM images of WO3 (Fig. 2a), ZnO (Fig. 2b), and WO3ZnO/Fe3O4 (Fig. 2c) are also provided. It can be found that the surface of the WO3–ZnO nanocomposite constructed revealed layered-shape particles with an average thickness of about 27 nm and with effectively enhanced surface area, providing superior photocatalytic performance.
000 times and with a resolution of about 1–20 nm. Field-emission scanning electron microscopy was used to monitor the morphology and particle distribution in WO3 (Fig. 1a), ZnO (Fig. 1b), and WO3ZnO/Fe3O4 (Fig. 1c). From FE-SEM images, it can be seen that the prepared nanocomposite had a uniform surface morphology with a concomitant irregular distribution. The FESEM image of WO3ZnO/Fe3O4 nanoparticles showed random and irregular distribution of nanoparticles. The surface of the material also showed layered and plate-like nanoparticles. In addition, the TEM images of WO3 (Fig. 2a), ZnO (Fig. 2b), and WO3ZnO/Fe3O4 (Fig. 2c) are also provided. It can be found that the surface of the WO3–ZnO nanocomposite constructed revealed layered-shape particles with an average thickness of about 27 nm and with effectively enhanced surface area, providing superior photocatalytic performance.
3.2. Photocatalytic preparation of 2-substituted benzimidazoles and studying different reaction parameters
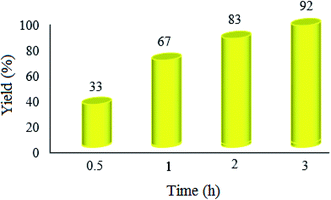 | ||
| Fig. 8 Effect of the reaction time on the condensation reaction. The reaction conditions are the same as described for Fig. 7; 20 mg of WO3ZnO/Fe3O4 was used in all cases. | ||
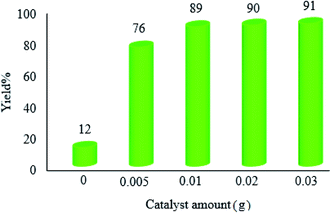 | ||
| Fig. 9 Effect of the catalyst amount on the photocatalytic efficacy of WO3ZnO/Fe3O4. The reaction conditions are the same as described for Fig. 7. Reactions were performed for 2.5 h. Yield% was increased to 18 and 31% in the absence of photocatalyst after 4 and 24 h, respectively. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
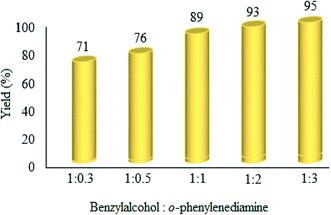
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) o-phenylenediamine molar ratio. The effect of changes in the ratio of raw materials was investigated, and it was found that the product yield increases with the increase in ratio of benzyl alcohol
o-phenylenediamine molar ratio. The effect of changes in the ratio of raw materials was investigated, and it was found that the product yield increases with the increase in ratio of benzyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) o-phenylenediamine (Fig. 10). Clearly, the yield% was enhanced with the mentioned ratio. However, the 1
o-phenylenediamine (Fig. 10). Clearly, the yield% was enhanced with the mentioned ratio. However, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio was chosen as the optimum, because no significant improvement was attained at higher ratios.
1 ratio was chosen as the optimum, because no significant improvement was attained at higher ratios.
| Catalyst | Light source | Yield% |
|---|---|---|
| WO3ZnO/Fe3O4 chemical | High pressure Hg lamp | 89 |
| WO3ZnO/Fe3O4 chemical | Xenon lamp | 38 |
| WO3ZnO/Fe3O4 chemical | White LED | 17 |
| WO3ZnO/Fe3O4 chemical | No light | 4 |
| WO3ZnO/Fe3O4 chemical | Sunlight | 45 (8 h) |
A mixture of benzyl alcohol (1 mmol), o-phenylenediamine (1 mmol), and WO3ZnO/Fe3O4 nanophotocatalyst (0.01 g) was taken in 10 mL ethanol at room temperature for 2.5 h. The stirring reaction mixture was exposed to the light source under mild air bubbling (1 mL min−1) and progress of the reaction was monitored by TLC.
A mixture of aromatic alcohol (1 mmol), o-phenylenediamine (1 mmol), and WO3ZnO/Fe3O4 nanophotocatalyst (0.01 g) was reacted in 10 mL ethanol at room temperature for 2.5 h. The stirring reaction mixture was exposed to the light source under mild air bubbling (1 mL min−1) and progress of the reaction was monitored by TLC. Yield% refers to the isolated product.
4. Conclusions
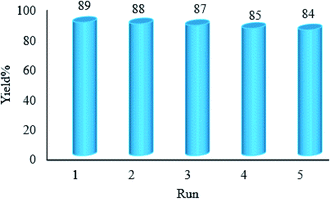
We have developed a straightforward photochemical synthesis strategy of 2-substituted benzimidazoles from the condensation of benzyl alcohols with o-phenylenediamine in the presence of WO3ZnO/Fe3O4 in ethanol. This new heterogeneous nanophotocatalyst was characterized by XRD, FT-IR, VSM and SEM. It is noteworthy that all produced benzimidazoles were obtained in good to excellent yields. The used photocatalyst shows excellent recovery and reusability without any significant loss of the photocatalytic activity. The key feature of this work is the in situ photocatalytic oxidation of a range of benzyl alcohols to benzaldehydes under atmospheric air and in the absence of any further oxidant. In addition, the present protocol is also viable to convert secondary aromatic alcohols to quinoxalines. Moreover, ethanol is used as a green solvent instead of the common harmful solvents for the synthesis of 2-substituted benzimidazoles. This methodology has several benefits such as high photocatalytic activity, simple operation, high yield, and use of a minimum amount of the photocatalyst. To the best of our knowledge, this report is the first on the preparation of 2-substituted benzimidazoles under the photocatalytic conditions by means of a commercially available Hg lamp. More importantly, this photochemical reaction can be achieved under the irradiation of sunlight as a plentiful free source of solar light.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work has been partially supported by Hakim Sabzevari University and Youth Fund Project of Education Department of Liaoning Province (Grant No. JYTQN201723).References
- Y. Chen, L. Q. Lu, D. G. Yu, C. J. Zhu and W. J. Xiao, Sci. China: Chem., 2019, 62(1), 24–57 CrossRef CAS
.
- S. Xu, H. Lu, L. Chen and X. Wang, RSC Adv., 2014, 4(85), 45266–45274 RSC
.
- R. M. Kakhki, R. Tayebee and F. Ahsani, J. Mater. Sci.: Mater. Electron., 2017, 28(8), 5941–5952 CrossRef
.
- R. Mohammadzadeh Kakhki, R. Tayebee and S. Hedayat, Appl. Organomet. Chem., 2018, 32(2), e4033 CrossRef
.
- R. Tayebee, R. Mohammadzadeh Kakhki, P. Audebert, M. M. Amini, M. Salehi, N. Mahdizadeh Ghohe, V. Mandanipour and G. R. Karimipour, Appl. Organomet. Chem., 2018, 32(7), e4391 CrossRef
.
- E. Parvizi, R. Tayebee, E. Koushki, M. F. Abdizadeh, B. Maleki, P. Audebert and L. Galmiche, RSC Adv., 2019, 9(41), 23818–23831 RSC
.
- N. Zhang, S. Liu, X. Fu and Y. J. Xu, J. Mater. Chem., 2012, 22(11), 5042–5052 RSC
.
- S. Shen, J. Chen, R. T. Koodali, Y. Hu, Q. Xiao, J. Zhou and L. Guo, Appl. Catal., B, 2014, 150, 138–146 CrossRef
.
- Y. Dai, P. Ren, Y. Li, D. Lv, Y. Shen, Y. Li, H. Niemantsverdriet, F. Besenbacher, H. Xiang, W. Hao and N. Lock, Angew. Chem., Int. Ed., 2019, 58(19), 6265–6270 CrossRef CAS
.
- X. Ye, Y. Chen, Y. Wu, X. Zhang, X. Wang and S. Chen, Appl. Catal., B, 2019, 242, 302–311 CrossRef CAS
.
- S. Zhang, W. Huang, X. Fu, G. Chen, S. Meng and S. Chen, J. Hazard. Mater., 2018, 360, 182–192 CrossRef CAS
.
- C. Xu, F. Yang, B. Deng, Y. Zhuang, D. Li, B. Liu, W. Yang and Y. Li, J. Catal., 2020, 383, 1–12 CrossRef CAS
.
- S. Hosseini, A. Amoozadeh and Y. Akbarzadeh, Photochem. Photobiol., 2019, 95(6), 1320–1330 CrossRef CAS
.
- M. Shuangyan, Y. Hongju, Z. Nan, Y. Jiao, Y. Ruirui and Y. Zhiwang, Acta Chim. Sin. (Engl. Ed.), 2019, 77(5), 461–468 Search PubMed
.
- A. R. Niaraki, M. R. Saraee, F. Kazemi and B. Kaboudin, Org. Biomol. Chem., 2020, 18(12), 2326–2330 RSC
.
- R. Tayebee, J. Korean Chem. Soc., 2008, 52(1), 23–29 CrossRef CAS
.
- E. Parvizi, R. Tayebee and E. Koushki, Semiconductors, 2019, 53(13), 1769–1783 CrossRef CAS
.
- T. Hisatomi, J. Kubota and K. Domen, Chem. Soc. Rev., 2014, 43(22), 7520–7535 RSC
.
- J. Yu, J. Low, W. Xiao, P. Zhou and M. Jaroniec, J. Am. Chem. Soc., 2014, 136(25), 8839–8842 CrossRef CAS
.
- B. Wang, G. Zhang, X. Leng, Z. Sun and S. Zheng, J.
Hazard. Mater., 2015, 285, 212–220 CrossRef CAS
.
- H. Wang, L. Zhang, Z. Chen, J. Hu, S. Li and Z. Wang, Chem. Soc. Rev., 2014, 43(15), 5234–5244 RSC
.
- H. Chen, C. E. Nanayakkara and V. H. Grassian, Chem. Rev., 2012, 112(11), 5919–5948 CrossRef CAS
.
- S. Kalathil, M. M. Khan, S. A. Ansari, J. Lee and M. H. Cho, Nanoscale, 2013, 5(14), 6323–6326 RSC
.
- M. M. Khan, S. A. Ansari, D. Pradhan, D. H. Han, J. Lee and M. H. Cho, Ind. Eng. Chem. Res., 2014, 53(23), 9754–9763 CrossRef CAS
.
- B. Bouchal, F. Abrigach, A. Takfaoui, M. E. Errahhali, M. E. Errahhali, P. H. Dixneuf and M. Bellaoui, BMC Chem., 2019, 13(1), 1–12 CrossRef CAS
.
- N. Mateeva, M. Gangapuram, E. Mazzio, S. Eyunni, K. F. Soliman and K. K. Redda, Med. Chem. Res., 2015, 24(4), 1672–1680 CrossRef CAS
.
- Y. Li, X. Zhou, H. Wu, Z. Yu, H. Li and S. Yang, Ind. Crops Prod., 2020, 150, 112406 CrossRef CAS
.
- R. Cedillo-Rivera and O. Munoz, J. Med. Microbiol., 1992, 37(3), 221–224 CrossRef CAS
.
- A. Eswayah, S. Khaliel, S. Saad, N. Shebani, O. Fhid, A. Belaid and E. Baga, Am. J. Chem. Appl., 2017, 4(5), 30–35 CAS
.
- M. C. Sharma, S. Sharma, N. K. Sahu and D. V. Kohli, J. Saudi Chem. Soc., 2013, 17(2), 167–176 CrossRef CAS
.
- K. Jagannath, Curr. Chem. Lett., 2020, 9(1), 1–8 Search PubMed
.
- P. Flores-Carrillo, J. M. Velázquez-López, R. Aguayo-Ortiz, A. Hernandez-Campos, P. J. Trejo-Soto, L. Yepez-Mulia and R. Castillo, Eur. J. Med. Chem., 2017, 137, 211–220 CrossRef CAS
.
- J. Sharma, P. K. Soni, R. Bansal and A. K. Halve, Curr. Org. Chem., 2018, 22(23), 2280–2299 CrossRef CAS
.
- A. Sajjadi and R. Mohammadi, J. Med. Chem. Sci., 2019, 2(2), 55–58 CAS
.
- M. Bharathi, S. Indira, G. Vinoth and K. S. Bharathi, J. Porous Mater., 2019, 26(5), 1377–1390 CrossRef CAS
.
- A. Nozarie, Chem. Methodol., 2019, 3(6), 704–712 Search PubMed
.
- S. Sajjadifar, I. Amini, H. Jabbari, O. Pouralimardan, M. H. Fekri and K. Pal, Eurasian Chem. Commun., 2019, 1(2), 191–199 CrossRef CAS
.
- K. Wada, H. Yu and Q. Feng, Chin. Chem. Lett., 2020, 31(3), 605–608 CrossRef CAS
.
- K. B. Dhopte, R. S. Zambare, A. V. Patwardhan and P. R. Nemade, RSC Adv., 2016, 6(10), 8164–8172 RSC
.
- L. A. Lebel and P. A. Seymour, J. Med. Chem., 1990, 33, 2240 CrossRef
.
- L. E. Seitz, W. J. Suling and R. C. Reynolds, J. Med. Chem., 2002, 45, 5605 CrossRef
.
- A. Gazit, H. App, G. McMahon, J. Chen, A. Levitzki and F. D. Bohmer, J. Med. Chem., 1996, 39, 2170 CrossRef CAS
.
- S. Dailey, J. W. Feast, R. J. Peace, I. C. Sage, S. Till and E. L. Wood, J. Mater. Chem., 2001, 11, 2238 RSC
.
- L. S. Jonathan, M. Hiromitsu, M. Toshihisa, M. L. Vincent and F. Hiroyuki, J. Am. Chem. Soc., 2002, 124, 13474 CrossRef
.
- D. Habibi, S. Kaamyabi and M. M. Amini, Appl. Surf. Sci., 2014, 320, 301–308 CrossRef CAS
.
- R. M. Alwan, Q. A. Kadhim, K. M. Sahan, R. A. Ali, R. J. Mahdi, N. A. Kassim and A. N. Jassim, Nanosci. Nanotechnol., 2015, 5(1), 1–6 CAS
.
- A. S. Hoseininasr and R. Tayebee, Appl. Organomet. Chem., 2008, 32(12), e4565 CrossRef
.
- X. Liu, J. Xu, Z. Ni, R. Wang, J. You and R. Guo, Chem. Eng. J., 2019, 356, 22–33 CrossRef CAS
.
- Y. M. Hunge, A. A. Yadav and V. L. Mathe, Ultrason. Sonochem., 2018, 45, 116–122 CrossRef CAS
.
- A. M. Awwad, M. W. Amer, N. M. Salem and A. O. Abdeen, Chem. Int., 2020, 6(3), 151–159 CAS
.
- H. Iida, K. Takayanagi, T. Nakanishi and T. Osaka, J. Colloid Interface Sci., 2007, 314(1), 274–280 CrossRef CAS
.
- R. Tayebee, E. Esmaeili, B. Maleki, A. Khoshniat, M. Chahkandi and N. Mollania, J. Mol. Liq., 2020, 317, 113928 CrossRef CAS
.
- J. Xie, Z. Zhou, Y. Lian, Y. Hao, X. Liu, M. Li and Y. Wei, Ceram. Int., 2014, 40(8), 12519–12524 CrossRef CAS
.
- Y. C. Liang, C. Y. Hua and Y. C. Liang, CrystEngComm, 2012, 14, 5579–5584 RSC
.
- Y. C. Liang, N. C. Xu, C. C. Wang and D. W. Wei, Materials, 2017, 10, 778 CrossRef
.
- U. Narang, K. K. Yadav, S. Bhattacharya and S. M. Chauhan, ChemistrySelect, 2017, 24, 7135–7140 CrossRef
.
- D. Q. Dong, W. J. Chen, Y. Yang, X. Gao and Z. L. Wang, ChemistrySelect, 2019, 4(8), 2480–2483 CrossRef CAS
.
- P. L. Reddy, R. Arundhathi, M. Tripathi, P. Chauhan, N. Yan and D. S. Rawat, ChemistrySelect, 2017, 2(13), 3889–3895 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra08403d |
| This journal is © The Royal Society of Chemistry 2020 |