Open Access Article
Open Access ArticleTMTHSI, a superior 7-membered ring alkyne containing reagent for strain-promoted azide–alkyne cycloaddition reactions†
Jimmy
Weterings
a,
Cristianne J. F.
Rijcken
a,
Harald
Veldhuis
b,
Tommi
Meulemans
b,
Darya
Hadavi
c,
Matt
Timmers
a,
Maarten
Honing
c,
Hans
Ippel
d and
Rob M. J.
Liskamp
 *adef
*adef
aCristal Therapeutics, Oxfordlaan 55, 6229 EV Maastricht, The Netherlands. E-mail: robert.liskamp@cristaltherapeutics.com
bMercachem, Kerkenbosch 1013, 6546 BB Nijmegen, The Netherlands
cThe Maastricht Multimodal Molecular Imaging Institute (M4I), Maastricht University, Universiteitssingel 50, 6229 ER Maastricht, The Netherlands
dDepartment of Biochemistry, CARIM, Maastricht University, Universiteitssingel 50, 6229 ER Maastricht, The Netherlands
eSchool of Chemistry, Joseph Black Building, University of Glasgow, University Avenue, Glasgow G12 8QQ, UK. E-mail: robert.liskamp@glasgow.ac.uk
fChemical Biology and Drug Discovery, Department of Pharmaceutics, Utrecht University, Universiteitsweg 99, 3584 CG Utrecht, The Netherlands
First published on 10th August 2020
Abstract
We describe the development of TMTH-SulfoxImine (TMTHSI) as a superior click reagent. This reagent combines a great reactivity, with small size and low hydrophobicity and compares outstandingly with existing click reagents. TMTHSI can be conveniently functionalized with a variety of linkers allowing attachment of a diversity of small molecules and (peptide, nucleic acid) biologics.
Introduction
The development of “click chemistry” initiated by the introduction of the copper-catalyzed azide alkyne cycloaddition reaction by Meldal et al.1 and Sharpless et al.2 had led to extremely important developments and ensuing applications in the chemical, biological, medical and materials sciences.3,4 Nevertheless, the requirement for catalysis by Cu(I) in the click reaction has been an obstacle for applications in the biological sciences.5 Apart from methods using limited amounts of copper or sequestering of the copper catalyst,6 powerful, alternative ‘click’ reagents have been developed containing strained triple bonds not requiring copper catalysis. However, a common feature of all these, often structurally very different, compounds is that their reactivity is considerably less than any simple triple bond in the presence of a copper catalyst (Fig. 1). In addition, several of the newly developed triple bond containing click reagents DIBO,7 DIBAC/DBCO8,9 have a significantly larger size. This increased steric bulk and hydrophobicity may induce a biological response of its own, thereby impeding with the structural features and biological response of the reagent to be clicked.10 For example the often used DBCO 1 has a sizable hydrophobic structure,8,9 which not only leads to a poor water solubility but also will engage in (undesired) hydrophobic interactions with proteins. These disadvantages led to the development of alternative smaller strain-promoted triple bond containing click reagents of which BCN 3 is probably the best representative.11,12a,b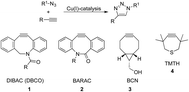 | ||
| Fig. 1 The classic Cu(I) catalysed ‘click’ reaction and the most important examples of reagents for strain-promoted azide–alkyne cycloaddition reactions. | ||
We were interested in a click reagent capable of a faster reaction with a variety of small to large azide containing compounds. This would be beneficial for yields of the clicked molecular constructs and reduction of side-products. In addition, a satisfactory aqueous solubility of the click-reagent is desired to allow reactions with relatively polar ligands as well as a small size, similar to that of BCN, in order to reduce the observer effect, that is a biological response induced by the steric bulk and lipophilicity of the click reagent.10
We were inspired by the reactivity of TMTH 4, a highly strained triple bond containing 7-membered ring system, which was already described by Krebs and Kimling in 1970.14 It was evident that this more strained 7-membered ring in TMTH, compared to a 8-membered ring system as is present in the BCN (3) and the earlier developed DIBO, DIBAC (1) and BARAC (2) compounds, should be more reactive in strain-promoted azide–alkyne cycloaddition.‡ Indeed, it was shown that TMTH was ca. 29–36 times more reactive than BCN‡,11,15 and ca. 4 times more reactive than the large BARAC-system.15,16 As was concluded by Bertozzi and co-workers already in 2012,15 thiacycloheptynes are a promising new class of reagents for bioorthogonal Cu-free click chemistry, but their low stability may impede with practical purposes.
We were also attracted by the small size of the TMTH ring system, which is expected to have minimal impact on the physicochemical properties of a compound to react with TMTH having other favourable properties (see above). The major challenge was the requirement of a convenient point of attachment to which different types of ligands could be connected. Attempts in this direction by Wagner et al. underlined this requirement.18 Thus we set out to develop a practical synthesis of a new and stable TMTH click reagent, in which unwanted side reactions can be mitigated by optimal reaction conditions and which above all contains a functional group for convenient introduction of a variety of linkers for ligand attachment.
Results and discussion
The first five steps toward the basic skeleton of the sulfur containing 7-membered (TMTH) ring system 4 have been described in the literature. Previously, the triple bond was introduced by heating using silveroxide or lead tetracetate (Scheme 1).13,14,17 We found that this was also possible using the greener alternative phenyliodine(III)diacetate (PIDA).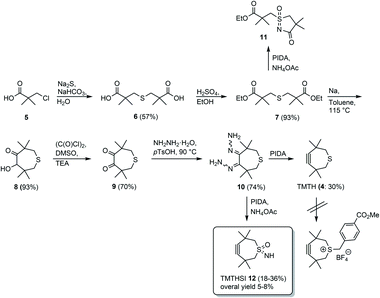 | ||
| Scheme 1 Synthesis of TMTH 4,13–15,17 attempted alkylation of TMTH, preparation of sulfoximine and its subsequent cyclization to 11 using PIDA and synthesis of TMTHSI 12. | ||
The introduction of a linker for ligand attachment turned out to be a major obstacle. Unfortunately, we were unable to reproduce the alkylation of bivalent sulfur in TMTH by benzyl bromide derivatives described by Wagner and Baati et al.18 (Scheme 1). In addition, the volatility and sensitivity to oxidation hampered handling of TMTH.
Thus, we considered functionalization of the sulfur-atom but then differently from the above alkylation. Therefore, it was decided to attempt to functionalize the sulfur atom before construction of the 7-membered ring. Sulfoximines seemed very attractive candidates to achieve this.19–22 Indeed, a test reaction on starting material 7 with PIDA in the presence of ammonium acetate,23 gave cyclic sulfoximine 11 showing that the bivalent sulfur atom could be functionalized (Scheme 1), but perhaps more importantly that the resulting (cyclic) sulfoximine could be subsequently derivatized as was apparent from its ammonolysis of the ethyl ester (Scheme 1).24 The crucial insight obtained from this reaction was the possibility of simultaneous functionalization of the bivalent sulfur atom and construction of the strained 7-membered ring using PIDA for both reactions in the presence of ammonium acetate. Realization of this approach led to the successful preparation of TMTHSI 12 (Scheme 1), which is highly attractive because of its potential reactivity of the triple bond in click reactions as well as the convenient functionalization (see below) of the sulfoximine-nitrogen.
Thus, as was shown in Scheme 1, TMTH bishydrazone 10 was prepared via diester 7 (see ESI†). After acyloin condensation and subsequent Swern oxidation diketone 9 was obtained. The last two steps leading to TMTHSI were only moderately yielding (range: 18–36%), which in view of the well-known instability and reactivity of the strained 7-membered was not unexpected. Nevertheless, the starting bishydrazone 10 was conveniently accessible in good overall (5-steps) yield (24%).
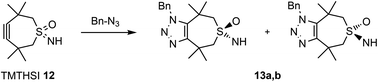
Since we were able to develop a relatively stable TMTH-system, it was now important to determine its reactivity in a copper free click reaction. Moreover, it was important to compare its reactivity to the presently most often used BCN (3). Benzyl azide was used as a reference to compare reactivities (Scheme 2).§
In view of the described attractive reactivity of TMTH,15 it was no surprise that the strain promoted azide–alkyne cycloaddition reaction of TMTHSI 12 and benzyl azide was too fast for determination of the reaction-rate by NMR. According to NMR near-complete conversion was already achieved in approximately 5–6 minutes (Fig. 2 and ESI†). The lack of sufficient time points from NMR prevented quantification of its reaction kinetics. Thus, there was great interest to use mass spectrometry (MS) for this purpose. This technique enabled monitoring the entire mass spectrum of the reaction on the microsecond scale and detection of any transient intermediates. Thus, ion mobility spectrometry-mass spectrometry (IMS-MS) in the millisecond scale was used for real-time monitoring of the click reaction,25 and cyclo-adduct product 13a,b formation was monitored (Fig. 2 and ESI†). By plotting the second-order reaction rate graph, the k value of the reaction was determined to be 0.8 M−1 s−1 (ESI†). The same method was applied to monitor the reaction of BCN–OH with benzylazide. The obtained k value was in agreement with k = 0.14 M−1 s−1 that has been previously determined by IR-based measurements.12a This observed reaction rate by MS showed that TMTHSI is a most reactive click reagent. It is >5 fold more reactive than BCN. It has a similar reactivity as BARAC but is a far less sterically bulky alkyne containing reagent for strain-promoted azide–alkyne cycloaddition reactions (Table 1). Together with the low, experimentally determined log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, facilitating a better water solubility, these characteristics contribute to the attractiveness of TMTHSI as a new click reagent.
P, facilitating a better water solubility, these characteristics contribute to the attractiveness of TMTHSI as a new click reagent.
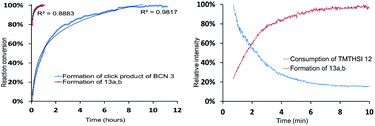 | ||
Fig. 2 Determination of the reaction rate of the strain-promoted azide–alkyne cycloaddition reaction of TMTHSI 12 and benzyl azide by NMR (left) and IMS-MS (right). Reaction of 5 mM TMTHSI and 6.38 mM benzyl azide (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3) in CDCl3 at 25 °C as compared to the reaction of endo-BCN–OH 3 (4.75 mM) and benzyl azide (6.02 mM) determined by NMR in CDCl3 (left). Reaction rate of 5 mM TMTHSI and benzyl azide (1 1.3) in CDCl3 at 25 °C as compared to the reaction of endo-BCN–OH 3 (4.75 mM) and benzyl azide (6.02 mM) determined by NMR in CDCl3 (left). Reaction rate of 5 mM TMTHSI and benzyl azide (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in water/acetonitrile (1 1) in water/acetonitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and 0.01% acetic acid at 25 °C by MS (right). The relative intensity of TMTHSI 12 consumption and triazole product 13a,b formation is shown (for more details see ESI†). 3) and 0.01% acetic acid at 25 °C by MS (right). The relative intensity of TMTHSI 12 consumption and triazole product 13a,b formation is shown (for more details see ESI†). | ||
| Click reagent | k [× 10−3 (M−1 s−1)] | Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pa,b Pa,b |
|
|---|---|---|---|
a These calculated values were taken from the review by Debets et al.16
b The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4. value was experimentally determined by Sygnature Discovery Ltd (Nottingham, UK). D7.4. value was experimentally determined by Sygnature Discovery Ltd (Nottingham, UK).
|
|||
| DBCO, DIBAC 1 | 310 | 3.5a | Ref. 8 |
| BARAC 2 | 960 | 4.2a | Ref. 16 |
| endo-BCN 326 | 140 | 2.0a | Ref. 12 |
| TMTHSI 12 | 800 | 0.72b | This work |
In contrast to difficulties we have experienced above in attempts to functionalize TMTH 4, the sulfoximine could be conveniently functionalized using a variety of reactions including N-alkylation, sulfonylation, acylation and carbamoylation (Scheme 3). Several of these transformations have been explored further to attach a diversity of linking-spacer containing-moieties with functional groups, which are useful for attachment to Active Pharmaceutical Ingredients (API's), immobilization onto surfaces, for polymer or nano particle attachment and in conjugation reactions with biologics. Of particular interest are biodegradable linkers allowing a reversible attachment of an API. One example is a linker containing a disulfide functionality, which can be released under reductive conditions(Scheme 3, 16). In addition, the stable, succinimide esters allow convenient ligand attachment and/or conjugation reactions.
 | ||
| Scheme 3 Functionalization of TMTHSI 12 followed introduction by linkage moieties for introduction of for example API's. | ||
To illustrate the versatility of ligand attachment to these linking moieties, we have reacted the resulting TMTHSI-derivatives with “small” molecules exemplified by the click-reaction with a dye and a folic acid derivative.
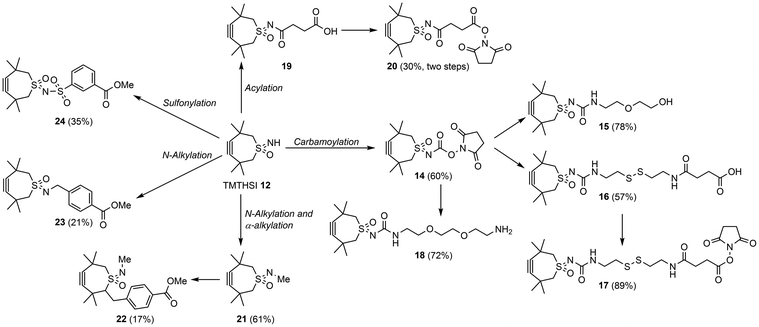
The generation of a near infrared (NIR) labelled Cy7–TMTHSI adduct and subsequent conjugation to core cross-linked polymeric micelles27 may allow tracking of these nanoparticles in an in vitro or in vivo setting. Thus TMSTHSI hydroxy–succinimide derivative 14 was furnished with a spacer affording 18 followed by reaction with Cy7 succinic ester derivative leading to Cy7–TMTHSI 25. Attachment of Cy7 onto the surface of nanoparticles was achieved by the strain promoted click reaction using 15N-azide functionalized nanoparticles 26 and Cy7–TMTHSI 25 yielding nanoparticles containing the dye (27) (Scheme 4).¶ HMBC 15N–1H NMR spectroscopy, showed a 100% conversion of the azides into the triazoles after 8 h of reaction. Cy7 labelling of these nanoparticles allowed visualisation of their cellular uptake by FACS (ESI, Fig. S2†). After 24 h, A431 cells had taken up more than 90% of the labelled nanoparticles.
 | ||
| Scheme 4 Preparation of Cy7 functionalized nanoparticles 27 from TMTHSI carbamoyl derivative 14, via18 and Cy7–TMTHSI (25) and core cross-linked polymeric micelles 26 in DMSO and phosphate buffer pH 7.4. The reaction was monitored by HMBC 15N–1H NMR (for details see ESI†). | ||
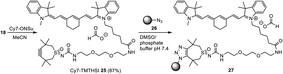
Coupling of a folic acid derivative to TMTHSI derivative 14 led to folic acid TMTHSI adduct 29. This compound was successfully click-conjugated to azide bearing core cross-linked polymeric micellar nanoparticles (Scheme 5).||
 | ||
| Scheme 5 Preparation of folic acid functionalized core cross-linked polymeric micellar nanoparticles 30 in DMSO/H2O starting with TMTHSI carbamoyl derivative 14. | ||
Next to “small” molecules, increasingly larger molecules, “biologics” are promising for drug delivery purposes. As examples of two important categories the HER2 peptide and an oligonucleotide were chosen for attachment to the click reagents, followed by attachment onto nanoparticles or attachment to a polymerizable moiety. For attachment of the HER2 targeting peptide,28 TMTHSI succinimide NHS ester 20 was used (Scheme 3).
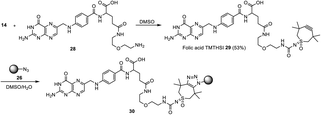
Reacting HER2 targeting peptide via its N-terminal amine (phenylalanine) with ester 20 (Scheme 6) afforded the TMTHSI-peptide adduct 32. Subsequently, this construct was attached to azide functionalized nanoparticles by strain-promoted click reaction, carried out in an aqueous environment (Scheme 6).
 | ||
| Scheme 6 Preparation of core crosslinked polymeric micellar nanoparticles in MeCN/H2O or aqueous buffer pH 5 incorporating the HER2-targeting peptide starting with TMTHSI carbamoyl derivative 20. | ||
Finally, a peptide–oligonucleotide conjugate was generated using TMTHSI for potential entrapment and release from core crosslinked polymeric micellar nanoparticles (Fig. 3 and ESI†).
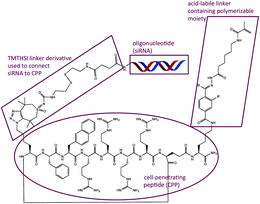 | ||
| Fig. 3 Strain promoted click was used to link an oligonucleotide (siRNA) to a peptide (CPP) having a polymerizable moiety through an acid labile hydrazone containing linker. Summary of the synthesis: step 1: siRNA + 17 in aqueous borate buffer pH 8.4/DMSO. Step 2: coupling of azido-CPP to TMTHSI-linker-siRNA in phosphate buffer pH 7.4. Step 3: coupling of the polymerizable moiety: aqueous borate buffer pH 8.4/DMSO. Full experimental details see ESI†). | ||
In general TMTHSI containing compounds are stable to both basic and acidic purification conditions and can conveniently be used in further synthesis. TMTHSI was stable on the bench for over a year and NMR and LCMS confirmed that TMTHSI derivatives have proven shelf lives of over a year. It was possible to use these derivatives where other click reagents have failed.|| With respect to this, future applications towards attachment of the TMTHSI click moiety in peptide and other highly functionalized compounds will be facilitated by the notion that this click moiety is stable towards peptide cleavage/deprotection conditions that is TFA/H2O or TFA/H2O/TIS. Further investigations of the stability profile of TMTHSI will likely increase the scope of potential applications.
Conclusions
We have developed TMTHSI as a superior cyclic alkyne containing click reagent for strain-promoted azide–alkyne cyclo addition reactions as was demonstrated by monitoring the click reaction by mass spectrometry. Its reaction rate is similar to the BARAC click reagent. However, TMTHSI is considerably smaller and much less hydrophobic than BARAC as is evident from its experimentally determined logP value. This will facilitate use in more aqueous environments as we have shown already in the preparation of 27–33 and of the siRNA molecular construct (Fig. 3). Additional applications in biochemical, aqueous environments, such as the construction of antibody drug conjugates (ADC's) are expected. This will be expanded to biological applications in the nearby future for example in cell lysates and in tissue culture. The smaller hydrophobic size, will likely lead to a smaller “observer effect” with less influence on the activity of the clicked ligand in addition to causing less protein binding.10 Although BARAC is the most reactive click reagent, it is inherently unstable and rapidly decomposes,12b which makes TMTHSI an attractive alternative. Adding to its attractiveness is the convenient functionalization of TMTHSI with a wide variety of linkers allowing attachment of a diversity of small molecules and (peptide, nucleic acid) biologics. In our opinion, the very attractive functionalization possibilities, combined with its great reactivity and small size offer opportunities for TMHSI to become the standard for non-copper catalyzed click reactions in a multitude of applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Cristal Therapeutics financially supported this work. J. Walther, E. Hebels and B. Mesquita from Utrecht University are gratefully acknowledged for their assistance with the cellular uptake experiment of Cy7 functionalized nanoparticles.Notes and references
- C. W. Tornøe, C. Christensen and M. J. Meldal, J. Org. Chem., 2002, 67, 3057–3064 CrossRef PubMed.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and B. K. Sharpless, Angew. Chem., 2002, 114, 2708–2711 ( Angew. Chem., Int. Ed. , 2002 , 41 , 2596–2599 ) CrossRef.
- (a) M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952–3015 CrossRef CAS PubMed; (b) L. Li and Z. Zhang, Molecules, 2016, 21, 1393 CrossRef PubMed.
- H. Li, R. Aneja and I. Chaiken, Molecules, 2013, 18, 9797–9817 CrossRef CAS PubMed.
- See for example: (a) E. M. Sletten and C. R. Bertozzi, Angew. Chem., 2009, 121, 7108–7133 ( Angew. Chem. Int. Ed. , 2009 , 48 , 6974–6998 ) CrossRef; (b) D. S. Del Amo, W. Wang, H. Jiang, C. Besanceney, A. C. Yan, M. Levy, Y. Liu, F. L. Marlow and P. Wu, J. Am. Chem. Soc., 2010, 132, 16893–16899 CrossRef PubMed.
- J. Clavadetscher, S. Hoffmann, A. Lilienkampf, L. Mackay, R. M. Yusop, S. A. Rider, J. J. Mullins and M. Bradley, Angew. Chem., 2016, 128, 15891–15895 ( Angew. Chem. Int. Ed. , 2016 , 55 , 15662–15666 ) CrossRef.
- X. Ning, J. Guo, M. A. Wolfert and G. J. Boons, Angew. Chem., 2008, 120, 2285–2287 ( Angew. Chem. Int. Ed. , 2008 , 47 , 2253–2255 ) CrossRef.
- M. F. Debets, S. S. van Berkel, S. Schoffelen, F. P. J. T. Rutjes, J. C. M. van Hest and F. L. van Delft, Chem. Commun., 2010, 46, 97–99 RSC.
- A. Kuzmin, A. Poloukhtine, M. A. Wolfert and V. V. Popik, Bioconjugate Chem., 2010, 21, 2076–2085 CrossRef CAS PubMed.
- The biological response induced by the steric bulk and lipophilicity is known as the "observer effect" see S. B. Engelsma, L. I. Williams, C. E. van Paaschen, S. van Kasteren, G. A. van der Marel and H. S. O. D. V. Filippov, Org. Lett., 2014, 16, 2744–2747 CrossRef CAS PubMed.
- J. Dommerholt, S. Schmidt, R. Temming, L. J. A. Hendriks, F. P. J. T. Rutjes, J. C. M. van Hest, D. J. Lefeber, P. Friendl and F. L. van Delft, Angew. Chem., 2010, 122, 9612–9615 ( Angew. Chem. Int. Ed. , 2010 , 49 , 9422–9425 ) CrossRef.
- (a) J. Dommerholt, O. van Rooijen, A. Borrmann, C. Fonseca Guerra, F. M. Bickelhaupt and F. L van Delft, Nat. Commun., 2014, 5, 5378–5385 CrossRef CAS PubMed; (b) J. Dommerholt, F. P. J. T. Rutjes and F. L. van Delft, Top. Curr. Chem., 2016, 374, 16 CrossRef PubMed; (c) For additional computational data on strain promoted click reactions see e.g. B. Gold, N. E. Shevchenko, N. Bonus, G. B. Dudley and I. V. Alubin, J. Org. Chem., 2012, 77, 75–89 CrossRef CAS PubMedT. Harris, G. dos Passos Gomes, S. Ayad, R. J. Clark, V. V. Lobodin, M. Tuscan, K. Hanson and I. V. Alabugin, Chem, 2017, 3, 629–640 CAS.
- Ae. de Groot and H. Wijnberg, J. Org. Chem., 1966, 31, 3954–3958 CrossRef CAS.
- A. Krebs and H. Kimling, Tetrahedron Lett., 1970, 761–764 CrossRef CAS.
- G. de Almeida, E. M. Sletten, H. Nakamura, K. K. Palaniappan and C. R. Bertozzi, Angew. Chem., 2012, 124, 2493–2497 ( Angew. Chem. Int. Ed. , 2012 , 51 , 2443–2447 ) CrossRef.
- M. F. Debets, J. C. M. van Hest and F. P. J. T. Rutjes, Org. Biomol. Chem., 2013, 11, 6439–6455 RSC.
- J. C. Jewett, E. M. Sletten and C. R. Bertozzi, J. Am. Chem. Soc., 2010, 132, 3688–3690 CrossRef CAS PubMed.
- M. King, R. Baati and A. Wagner, Chem. Commun., 2012, 48, 9308–9309 RSC.
- M. Frings, C. Bolm, A. Blum and C. Gnamm, Eur. J. Med. Chem., 2017, 126, 225–245 CrossRef CAS PubMed.
- T. L. Gilchrist and C. J. Moody, Chem. Rev., 1977, 77, 409–435 CrossRef CAS.
- T. Yoshimura, T. Omata, N. Furukawa and S. Oae, J. Org. Chem., 1976, 41, 1728–1733 CrossRef CAS.
- W. Frank and P. K. Claus, Monatsh. Chem., 1990, 121, 539–547 CrossRef.
- A. Tota, M. Zenzola, S. J. Chawner, S. St John-Campbell, C. Carlucci, G. Romanazzi, L. Degennaro, J. A. Bull and R. Luisi, Chem. Commun., 2017, 53, 348–351 RSC.
- For a precedent of a cyclic sulfoximine see: H. G. Bonacorso, R. P. Vezzosi, R. L. Drekener, N. Zanatta and M. A. P. Martins, Lett. Org. Chem., 2009, 6, 145–150 CrossRef CAS.
- A. B. Kanu, P. Dwivedi, M. Tam, L. Matz and H. H. Hill Jr, J. Mass Spectrom., 2008, 43, 1–22 CrossRef CAS PubMed.
- C. Gröst and T. Berg, described PYRROC: the first functionalized cycloalkyne that facilitates isomer-free generation of organic molecules by SPAAC, Org. Biomol. Chem., 2015, 13, 3866–3870 RSC . This is a functionalized pyrrolo “analog” of BCN with a rate constant, which was 60 × 10-3 M−1 s−1 and lower than BCN (Table 1). Its calculated (Chemdraw vs19.0) C
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P (3.4) was similar to DBCO.
P (3.4) was similar to DBCO. - (a) Q. Hu, C. J. Rijcken, R. Bansal, W. E. Hennink, G. Storm and J. Prakash, Biomaterials, 2015, 53, 370–378 CrossRef CAS PubMed; (b) Q. Hu, m C. F. Rijcken, E. van Gaal, P. Brundel, H. Kostkova, T. Etrych, B. weber, M. Barz, F. Kiessling, J. Prakash, G. Storm, W. E. Hennink and T. Lammers, J. Contr. Release, 2016, 244, 314–325 CrossRef CAS PubMed.
- S.-S. Guan, C.-T. Wu, C.-Y. Chiu, T.-Y. Luo, J.-Y. Wu, T.-Z. Liao and S.-H. Liu, J. Transl. Med., 2018, 16, 168 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, syntheses and analyses of compounds including NMR-spectra, LC-MS-traces, NMR and MS reactivity studies, as well as FACS analysis of cellular uptake of Cy7 nanoparticles. See DOI: 10.1039/d0sc03477k |
| ‡ An all carbon 7-membered ring system is likely to be too strained, therefore unstable and synthetically not accessible, but a 7-membered ring containing longer bonds due to the presence of carbon-hetero atom bonds and therefore less strained, was apparently synthetically feasible.14 |
| § Although aromatic azides especially containing electron withdrawing groups turned out to be very reactive in the strain promoted click reaction with BCN,12a benzylazide was chosen as a reference compound, since a majority of APIs used for preparing azide derivatives will be aliphatic ones. |
| ¶ Although the click reaction went smoothly, it seems – according to NMR – that slowly one indole part of the dye is reduced, possible by the presence of formate. |
| || Folic Acid BCN was capable of a strain promoted click reaction with an azide in DMSO and the click product remained stable upon further dilution with aqueous buffer. However, when folic acid BCN in DMSO was mixed with an azide containing compound in aqueous buffer no click reaction occurred. Possibly, due to an intramolecular reaction within folic acid the cyclopropane ring of BCN opened up, rendering the compound no longer capable to perform a click reaction. |
| This journal is © The Royal Society of Chemistry 2020 |