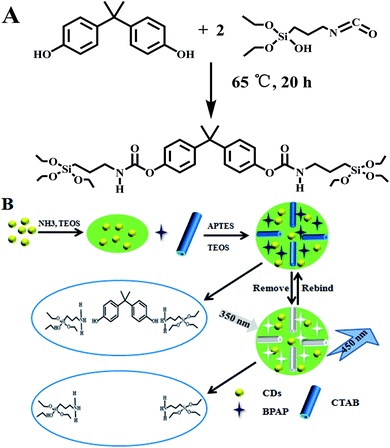
A semi-covalent molecularly imprinted fluorescent sensor for highly specific recognition and optosensing of bisphenol A†
Jinna
Zhang
,
Haiyang
Wang
,
Longhua
Xu
* and
Zhixiang
Xu
 *
*
Key Laboratory of Food Processing Technology and Quality Control in Shandong Province, College of Food Science and Engineering, Shandong Agricultural University, Tai'an, Shandong 271018, P. R. China. E-mail: xulonghua6@163.com; zhixiangxu@sina.com; Fax: +86 538 8242482; Tel: +86 538 8242263
First published on 23rd November 2020
Abstract
A novel mesoporous fluorescent molecularly imprinted sensor for selective detection of bisphenol A (BPA) in food materials was fabricated via a semi-covalent imprinting method. The imprinting precursor that served as an alternative template molecule for BPA was prepared via thermally reversible isocyanate bonding, which effectively improved the imprinting efficiency for the molecularly imprinted sensor. Carbon dots (CDs) were embedded in mesoporous silica as signal recognition elements that exhibited quenching upon BPA binding. Subsequently, through the sol–gel process, the molecularly imprinted layer was coated on the CDs silica layer and provided specific recognition sites for BPA. The composite of CDs embedded in the mesoporous molecularly imprinted polymer (CDs@MIP) was characterized with scanning electron microscopy, transmission electron microscopy, Fourier transform infrared spectroscopy, Brunauer–Emmett–Teller measurements and thermogravimetric analysis. The mechanism of carbon dots quenching and the high selectivity of CDs@MIP towards BPA were explored. The linear response range of the sensor was from 0.025 mg L−1 to 2 mg L−1 with a limit of detection of 0.016 mg L−1. The method was successfully applied for the determination of food samples and recoveries ranged from 92.5% to 101.1%. The BPA contents in actual samples were determined using high performance liquid chromatography and the proposed sensor, showing no significant difference between the two methods.
Introduction
As a food packaging material, bisphenol A (BPA) is widely found in the inner coatings of baby bottles, lunch boxes, and beverages and cans.1 It is released into the environment by direct discharge, which affects the safety of water resources.2 The human endocrine system is affected by BPA residues, which even cause tumors and threaten lives.3 At present, the detection methods of BPA include chromatography,4 capillary electrophoresis5 and immunochromatographic column methods.6 However, these methods require high detection costs7 and long analysis times8 and thus are not suitable for rapid, simple detection. Therefore, fluorescent sensors with rapid response time and high sensitivity are used to detect BPA in actual food samples.9Fluorescent materials such as semiconductor quantum dots and fluorescent organic dyes are widely used in optical sensors for highly sensitive detection of analytes.10 However, due to the legacy of toxicity to the environment of traditional semiconductor quantum dots11 and the poor light stability of fluorescent organic dyes,12 the development of non-toxic quantum dots with stable optical properties is urgently required.13 In particular, carbon dots (CDs) with unique optical properties including photo-stability,14 low toxicity,15 highly adjustable photoluminescence16 and broad excitation spectra17 have attracted great attention as fluorescent sensors.18 During the preparation of fluorescent sensors, the carbon dots are usually embedded in a suitable solid matrix in order to maintain the fluorescence characteristics of the carbon dots and prevent the carbon dots from oozing out.19 And silica substrates are gradually being applied due to the good stability in the medium.20 Furthermore, a mesoporous silica matrix with a large surface area not only provides more binding sites for the reaction of the substance, but also needs less time for molecule interactions.21
Matrix interference is an important factor affecting the accuracy of analytical methods. To improve the selectivity of the method,22 molecular imprinting technology can be combined with a fluorescent sensor to specifically detect the target.23 Molecular imprinting technology means that the imprintingmolecules and functional monomers are cross-linked and polymerized in a covalent or non-covalent manner,24 creating specific imprinted adsorption cavities for the target. The imprinted cavities contain special functional groups25 that covalently or non-covalently interact specifically with the target molecules.26 The synthesis methods for molecularly imprinted polymers (MIPs) include bulk polymerization,27 precipitation polymerization,28 suspension polymerization,29in situ polymerization30 and the sol–gel method.31 The molecular imprinting sol–gel method is widely used due to the fast mass transfer rate32 and high affinity.33 At present, most of the MIPs prepared by sol–gel molecular imprinting use BPA as a template molecule directly,34,35 which will cause incomplete elution and affect the adsorption. Jennifer E. Lofgreen et al.37 prepared BPAP as an alternative template to BPA, and the imprinted cavities surrounded by silica are formed by imprinting elution, and they have high adsorption selectivity.
In this work, we introduce a novel methodology based on the semi-covalent imprinting technique which is combined with fluorescent CDs directly encapsulated in a silica MIP. To achieve rapid identification, a semi-covalently synthesized imprinting template, BPAP, was used instead of BPA. The high sensitivity of fluorescent CDs and the high selectivity of molecular imprinting are combined to provide a rapid, sensitive and selective sensor. The mechanisms of fluorescence quenching and selectivity are discussed; meanwhile the CDs@MIP sensor was used to analyze the content of BPA in actual samples.
Experimental section
Reagents and materials
Bisphenol A (BPA, 99%) and tetraethoxysilane (TEOS, 99%) were purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). 3-Aminopropyltriethoxysilane (APTES, 98%), (3-isocyanatopropyl) triethoxysilane (ICPTES, 95%), bisphenol F (BPF, 99%), bisphenol B (BPB, 99.5%), bisphenol AF (BPAF, 98%), bisphenol S (BPS, 99%), 4,4′-bisphenol (DOD, 97%), resorcinol (99%), hydroquinone (HQ), and phenol (99.5%) were obtained from Aladdin Industrial Corporation (Shanghai, China). Cetyltrimethylammonium bromide (CTAB, 99%) was purchased from Beijing Boland Technology Co., Ltd. (Beijing, China). N-(β-Aminoethyl)-γ-aminopropyl methyldimethoxysilane (AEAPMS, 97%) and citric acid anhydrous (AC, 99.5%) were obtained from TCI Co., Ltd. (Shanghai, China).Canned fruits, canned meat and drinks were purchased randomly from a supermarket in Taian (Shandong, China). Ultra-pure water was prepared with an Aike ultrapure water system (Chengdu, China).
Instruments and measurements
Fourier transform infrared (FT-IR) spectroscopy was performed on a Nicolet 10 Fourier transform infrared spectrometer (Thermo, America). Fluorescence measurements were performed with an F97 pro fluorescence spectrophotometer (Lengguang Tech., China). Transmission electron microscopy (TEM) images were obtained with a Jem-2100F electron microscope (Jeol, Japan), while scanning electron microscopy (SEM) images were obtained with a Model S4008 electron microscope (Hitachi, Japan). Surface areas were analyzed by the Brunauer–Emmett–Teller (BET) method (Quantachrome, USA). The thermal stability of the material was measured with an STA449F5 thermal gravimetric analysis (TGA) instrument (Netzsch, Germany).The high performance liquid chromatography (HPLC) system consisted of two LC-10ATVP pumps and a fluorescence detector (Shimadzu, Japan). All separations were achieved on a C18 reversed-phase column (4.6 × 250 mm, 5 μm) at a mobile flow rate of 1 mL min−1. The mobile phase was methanol/water (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, v/v).
70, v/v).
Synthesis of CDs
Silane functionalized CDs were synthesized as described previously.38 In brief, 10 mL of AEAPMS was degassed with N2 for 5 min in a 100 mL three-necked flask and then heated to 240 °C, after which 0.5 g anhydrous citric acid was quickly added and stirred vigorously for 1 min. After cooling to room temperature, the CD products were collected and washed with petroleum ether three times. Finally the CDs were dissolved in ethanol and stored at 4 °C.Synthesis of the imprinting molecule, BPAP
The imprinting molecule, BPAP, was synthesized according to previous methods.37 ICPTES (5.97 mL, 24 mmol) and BPA (2.751 g, 12 mmol) were added to a round-bottom flask containing 25 mL of anhydrous tetrahydrofuran and were stirred continuously for 20 h under N2 at 65 °C. The solvent was removed at 66 °C using a rotary evaporator, and then an oily liquid was obtained. The synthesis process of BPAP is shown in Scheme 1A.Synthesis of mesoporous CDs@MIP and CDs@NIP
In a 250 mL round bottom flask, 10 mL of ethanol and 25 mL of ultrapure water were added, and then 100 μL of CDs and 100 μL of ammonia (25%) were injected with vigorous stirring. Subsequently, 100 μL of TEOS and 20 mL of ethanol were added dropwise and the solution mixture was stirred for another 8 h. Then 66 mg of BPAP dispersed in 30 mL of methanol and 60 μL of APTES were injected into the reaction system and stirred for 20 min. Afterwards, 1.5 mL of CTAB (0.2 M), 0.2 mL of NaOH (0.2 M) and 150 μL of TEOS were added, and the solution was stirred at room temperature for 12 h in the dark. The resultant CDs@MIP was collected by centrifugation (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 × g, 10 min) and ultrasonically washed with water and methanol several times to remove the unreacted BPA and CTAB, until they were no longer detected with an ultraviolet spectrophotometer. Finally, CDs@MIP was dried in a vacuum oven at 40 °C for 12 h and stored at 4 °C. The CDs@NIP was prepared in the same manner as CDs@MIP without the addition of template molecules (BPAP).
180 × g, 10 min) and ultrasonically washed with water and methanol several times to remove the unreacted BPA and CTAB, until they were no longer detected with an ultraviolet spectrophotometer. Finally, CDs@MIP was dried in a vacuum oven at 40 °C for 12 h and stored at 4 °C. The CDs@NIP was prepared in the same manner as CDs@MIP without the addition of template molecules (BPAP).
Fluorescence measurement procedure
We proposed a strategy to combine semi-covalent imprinting with fluorescence detection to prepare fluorescent molecularly imprinted polymers and then establish a fluorescent sensor (Scheme 1B). Fluorescence measurements were acquired at the emission wavelength of 360 nm at 650 ν, and with 10 nm excitation and an emission slit. In a 4 mL cuvette, 1 mL of the CDs@MIP ethanol solution and 1 mL of a solution with different concentrations of BPA were combined, and the quenched fluorescence intensity F under these conditions was measured. All solutions were shaken sufficiently to completely disperse before using.Sample preparation
To evaluate the accuracy of the CDs@MIP sensor, canned fish and orange juice samples were used to perform recovery tests. The content of BPA in these samples was determined by HPLC before spiking. 5 g samples were placed in 25 mL centrifuge tubes. After being spiked with 2 mL of a BPA standard solution (0.4 mg kg−1, 0.8 mg kg−1, and 1.2 mg kg−1), the samples were incubated for 24 h. The spiked samples were pre-treated according to the standard methods (GB 31660.2-2019 and GB 31604.10-2016). For orange juice, 10 mL of acetonitrile was added into the solution and the mixture was vortexed for 3 min and then centrifuged at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 × g. Then, the acetonitrile layer was transferred to a 100 mL round bottom flask, and the mixture was extracted twice using the same process. The above solutions were mixed and rotary evaporated to near dryness. Subsequently, 2 mL of ethanol was added and filtered through a 0.22 μm filter membrane for extraction. For canned fish, 10 mL of acetonitrile and 10 mL of n-hexane were added, vortexed for 2 min, and centrifuged at 11
180 × g. Then, the acetonitrile layer was transferred to a 100 mL round bottom flask, and the mixture was extracted twice using the same process. The above solutions were mixed and rotary evaporated to near dryness. Subsequently, 2 mL of ethanol was added and filtered through a 0.22 μm filter membrane for extraction. For canned fish, 10 mL of acetonitrile and 10 mL of n-hexane were added, vortexed for 2 min, and centrifuged at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 × g, the acetonitrile layer was transferred to a round bottom flask. The residue was extracted again and collected. It was then rotary evaporated at 50 °C to near dryness and re-dissolved in 2 mL of ethanol. Finally, the resulting extract was filtered with a 0.22 μm membrane and then analyzed with the CDs@MIP sensor; the recovery was calculated.
180 × g, the acetonitrile layer was transferred to a round bottom flask. The residue was extracted again and collected. It was then rotary evaporated at 50 °C to near dryness and re-dissolved in 2 mL of ethanol. Finally, the resulting extract was filtered with a 0.22 μm membrane and then analyzed with the CDs@MIP sensor; the recovery was calculated.
Canned hawthorn, canned croaker, sardine and canned (glass) hawthorn were pretreated with the same procedure as that for canned fish, except without adding the BPA standard solution. The resulting extract was analyzed with the CDs@MIP sensor and HPLC method, and the BPA level was obtained.
Data analysis
SPSS software was used to analyze the significance of the data and verify the accuracy.Results and discussion
Synthesis condition optimization for CDs@MIP
In this method, BPAP was used as an alternative template, APTES was used as a functional monomer, and TEOS was used as a cross-linker. Ammonia water was used as a precursor of the silicon layer to modify the surface of the carbon dots. The silica layer was used to improve the interaction between the CDs and the imprinting molecules. After elution, specific recognition cavities for BPA were generated and the imprinted layer outside the silica layer provided selectivity for BPA. For good selectivity and adsorptivity, CDs@MIP synthesis was optimized, including the ratio of the template molecule, cross-linker and functional monomer and the amount of carbon dots.The ratio of the fluorescence intensity before and after the adsorption of CDs@MIP towards BPA was used to evaluate the adsorption performance of CDs@MIP.
As shown in Fig. S1,† the ratios of the template molecule, cross-linker and functional monomer of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2:3 to 1
2:3 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 were optimized with respect to the relative fluorescence intensity (F0/F) of CDs@MIP. When the ratio was 1
7 were optimized with respect to the relative fluorescence intensity (F0/F) of CDs@MIP. When the ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, the value of F0/F was at the maximum, and thus 1
7, the value of F0/F was at the maximum, and thus 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 was used as the best synthesis condition in subsequent experiments.
7 was used as the best synthesis condition in subsequent experiments.
The amount of CDs played a vital role in the whole performance of CDs@MIP. The effect of different amounts of CDs added (50 μL to 200 μL) on the CDs@MIP fluorescence intensity was investigated (Fig. S2†). The fluorescence intensity started to decrease after the addition amount exceeded 100 μL. Therefore, the optimal amount of added CDs was 100 μL.
Characterization of CDs@MIP
The morphology of CDs@MIP was characterized with scanning electron microscopy and transmission electron microscopy (Fig. S3†). Scanning electron microscope images and transmission electron microscopy showed that CDs@MIP was approximately spherical.The FT-IR spectra of BPAP and ICPTES are compared in Fig. 1(A). The isocyanate stretching vibration of ICPTES was found at 2270 cm−1, while that of BPAP was significantly reduced. The characteristic peak of BPAP was derived from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration at 1720 cm−1, which proved that the template precursor BPAP was successfully synthesized. Fig. 1(B) shows that for the imprinted polymer before elution, a peak derived from C
O stretching vibration at 1720 cm−1, which proved that the template precursor BPAP was successfully synthesized. Fig. 1(B) shows that for the imprinted polymer before elution, a peak derived from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration at 1720 cm−1 was observed.37 However, the C
O stretching vibration at 1720 cm−1 was observed.37 However, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration was not observed for CDs@MIP after elution and in CDs@NIP, which indicates that the C
O stretching vibration was not observed for CDs@MIP after elution and in CDs@NIP, which indicates that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond was broken and the template molecule was completely eluted.
O bond was broken and the template molecule was completely eluted.
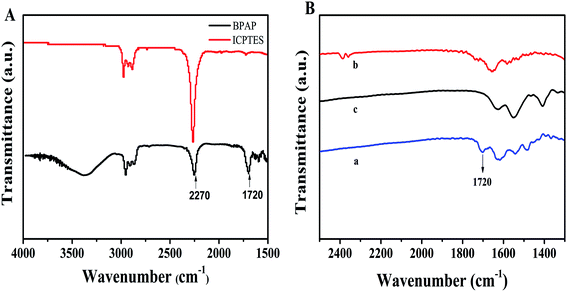 | ||
| Fig. 1 (A) Fourier-transform infrared spectra of BPAP and ICPTES. (B) Fourier-transform infrared spectra of CDs@MIP before (a) and after elution (b) and CDs@NIP (c). | ||
The mesoporous nature of the CDs@MIP was compared with that of CDs@NIP and non-mesoporous structured CDs@MIP (prepared without adding CTAB). Brunauer–Emmett–Teller analysis (Fig. S4†) indicated that the CDs@MIP had a surface area of 166 m2 g−1, which was higher than that of the corresponding non-mesoporous structured CDs@MIP (140 m2 g−1) and CDs@NIP (123 m2 g−1).
The thermal stability of CDs@MIP was obtained through thermal gravimetric analysis. As shown in Fig. S5,† the weight loss rate was 6% over 0 to 100 °C, probably due to evaporation of water molecules. However, the weight loss rate was 22% over the range 300–700 °C, which may be attributed to the collapse of the mesoporous silica framework. Over 100–300 °C, the curve trend was stable, indicating that the polymer has good thermal stability in this range.
Optimization of the experimental conditions
Due to the physical and chemical properties of the reaction solvent, the properties of the CDs@MIP dissolved in the system or the interaction between CDs@MIP and the template molecule will be affected. Fig. S6† shows the fluorescence spectra in methanol, ethanol, acetonitrile and water. The CDs@MIP was dispersed well in ethanol and had the best relative fluorescence intensity. Thus, ethanol was selected as the best solvent for reactions.Different concentrations of CDs@MIP will affect its dispersion and then affect the fluorescence intensity. As shown in Fig. S7,† as the concentration increased, the fluorescence intensity gradually decreased. However, taking into account the fluorescence intensity and dispersibility, 1 mg mL−1 was selected for subsequent experiments.
The response time of the proposed sensor to BPA was then studied. The F0/F value remained constant after 20 min (Fig. S8†). And the CDs@MIP sensor had a faster response speed compared with other methods.37 Therefore, 20 minutes was chosen as the optical response time in the following experiment.
The F0/F of CDs@MIP at different pH values was measured in the range of 5.0–10.0 (Fig S9†). Good fluorescence quenching was observed at 6.0–8.0, and thus pH 7.0 was selected for further experiments.
Fluorescence quenching mechanism
As shown in Fig. 2(A), the fluorescence intensity of CDs@MIP was comparable to that of CDs@NIP. However, in the presence of BPA, the CDs@MIP fluorescence intensity decreased sharply. Since the ultraviolet absorption band of BPA and the fluorescence emission spectrum of CDs@MIP do not overlap (Fig. 2(B)), energy transfer can be eliminated. The effect of electron transfer on fluorescence quenching can be considered by noting that the CDs@MIP emission band is close enough to the BPA ultraviolet absorption band such that electrons could be transferred from CD donors to BPA acceptors. Therefore, electron transfer was considered to be the most likely mechanism for fluorescence quenching.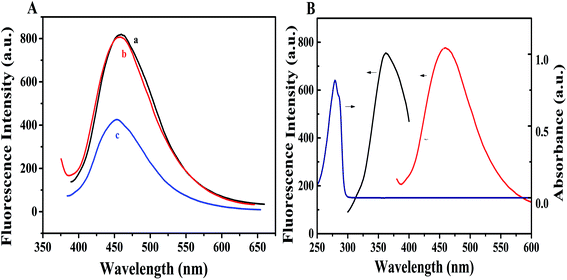 | ||
| Fig. 2 (A) Fluorescence emission spectra of CDs@NIP (a) and CDs@MIP after elution (b) and CDs@MIP in BPA (c). (B) UV absorption spectrum of BPA and PL emission spectra of CDs. | ||
Fluorescence measurement and calibration curve for BPA
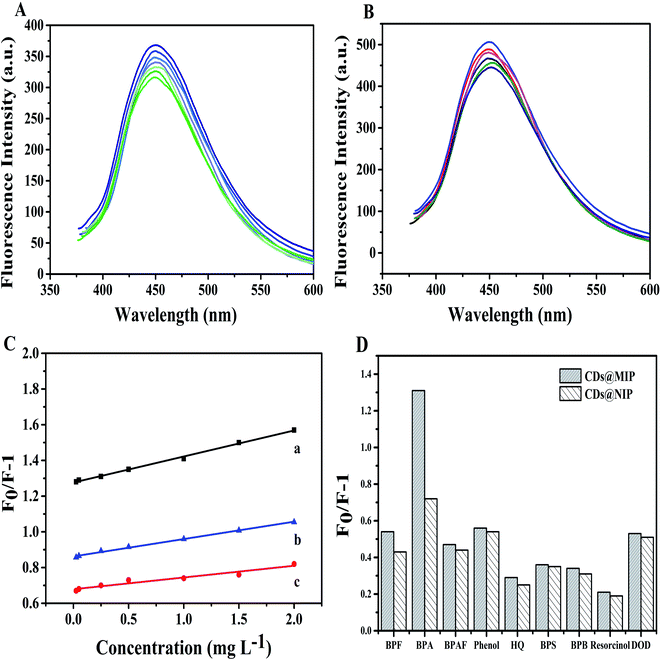
Under optimal conditions, the fluorescence spectra of CDs@MIP (Fig. 3(A)) and CDs@NIP (Fig. 3(B)) were acquired in the presence of different concentrations of BPA (0.025–2 mg L−1). When the BPA concentration increased, the fluorescence intensity decreased. The fluorescence quenching performance can be described using the Stern–Volmer equation of F0/F − 1 = KSV[C], where [C] is the concentration of BPA, KSV is the quenching constant of the target, and F0 and F are the fluorescence intensity in the presence and absence of BPA. As shown in Fig. 3(C), although CDs@MIP and CDs@NIP both exhibited fluorescence quenching for BPA, KSVNIP was lower than KSVMIP. Therefore, CDs@MIP has a higher quenching efficiency. The imprinting factor (IF) of KsvMIP and KsvNIP was 2.21, which might result from the effective imprinting effect, and imprinted cavities were generated during the polymerization. However CDs@NIP does not have a spatial structure and binding site that can match BPA. And CDs@MIP (BPAP as the template molecule) has a better fluorescence quenching effect than CDs@MIP (by using BPA as the template molecule). The results show the superiority of BPAP as an alternative template to synthesize fluorescent molecularly imprinted sensors.Selectivity and repeatability experiments
The selectivity of CDs@MIP to BPA was an important parameter, and the mechanism of selectivity and the ability to selectively identify BPA have been explored. Two groups of structural analogs of BPA were selected for comparison (Fig. S10†). The first group contained five targets (BPA, BPF, BPB, BPS, and BPAF) that have similar benzene ring sizes and arrangements, but hydrophobicity was increased due to bridged carbon atom substitution. The second group contained five targets (phenol, hydroquinone, resorcinol, 4,4′-biphenol, and BPA) that have phenols and aromatic rings in different quantities and arrangements. The fluorescence response of CDs@MIP and CDs@NIP towards BPA and its structural analogues is shown in Fig. 3(D). In the first group, the quenching efficiency of fluorescence from high to low was in the order BPA, BPAF, BPF, BPS, BPB. BPA had the best fluorescence quenching effect, which confirmed the specific selectivity of CDs@MIP for BPA due to the formation of imprinted cavities for BPA. BPAF had better solubility and fluorescence quenching in ethanol than BPF, BPS and BPB, because BPAF contained the more hydrophobic –CF3 group. The fluorescence quenching effect of BPF was better than that of BPS and BPB, because it is a smaller molecule that binds more easily at the imprinted cavities. The hydrophobicity of thioamide in BPS was weaker than that of –CF3 in BPAF; thus the fluorescence response was worse than of that of BPAF. BPB contains only two methyl groups without hydrophobic properties, and thus the fluorescence response was lower. In the second group, BPA exhibited a better fluorescence quenching effect than the others. Phenol more easily diffused to the imprinted sites due to the smaller molecule than those of hydroquinone, resorcinol and 4,4′-biphenol. Hydroquinone and resorcinol exhibited similar fluorescence quenching efficiency due to the similar molecular structure. Overall, we can conclude that the CDs@MIP sensor has a specific selectivity for BPA. Meanwhile, hydrophobic functional groups, as well as molecular structures, can affect target adsorption on CDs@MIP and fluorescence quenching.Fig. S11† summarizes the binding capacity of CDs@MIP in the presence of different concentrations of BPA and other structural analogues, which also suggested the fluorescence quenching of CDs@MIP had high selectivity to BPA.
Repeatability is another important feature of sensors. CDs@MIP sensors of different batches were measured. As shown in Fig. S12,† there was no difference in the fluorescence intensity for several different batches of CDs@MIP sensors. The influence of interfering ions on the fluorescence effect was studied (Fig. S13†). The results showed that the CDs@MIP fluorescent sensor was stable enough in a complex matrix. The thermal stability of CDs@MIP at different temperatures was explored (Fig. S14†), and the fluorescence intensity of CDs@MIP was stable at different temperatures. These results indicated that this method has good repeatability and can be applied to complicated actual sample detection.
Application to real sample analysis
To assess the accuracy of the sensor, the recovery was studied by adding different amounts of BPA to the samples as shown in Table 1. The recovery of the sample was between 92.5% and 101.1%. Therefore, CDs@MIP fluorescent sensors were suitable for fast and accurate detection of BPA in complex samples.| Samples | Original level (mg kg−1) | Added level (mg kg−1) | Found level (mg kg−1) | Recovery (%, ±RSD) |
|---|---|---|---|---|
| Canned fish | 0.63 | 0.4 | 1.01 | 94.6 ± 1.1 |
| 0.65 | 0.8 | 1.45 | 101.1 ± 0.8 | |
| 0.66 | 1.2 | 1.83 | 97.3 ± 2.3 | |
| Orange juice | 0.46 | 0.4 | 0.85 | 98.6 ± 4.1 |
| 0.49 | 0.8 | 1.28 | 99.3 ± 3.5 | |
| 0.48 | 1.2 | 1.59 | 92.5 ± 2.5 |
The content of BPA in canned hawthorn, canned (glass) hawthorn, canned croaker, and sardine was detected using the CDs@MIP sensor and high performance liquid chromatography. The results in Table 2 show no significant difference (P > 0.05) between the two detection methods, indicating that the CDs@MIP sensor was accurate and reliable and can be applied to detect BPA in actual samples.
| Samples | HPLC (mg kg−1, ±SD) | Sensor (mg kg−1, ±SD) |
|---|---|---|
| a Mean values in the same column with different letters (a–f) are significantly different (P < 0.05). | ||
| Canned hawthorn | 3.54 ± 0.09a | 3.48 ± 0.02ab |
| Canned hawthorn (glass) | 0.96 ± 0.02d | 0.85 ± 0.06de |
| Canned croaker | 0.87 ± 0.08de | 0.89 ± 0.04e |
| Sardine | 0.65 ± 0.08f | 0.63 ± 0.04f |
Advantages of the CDs@MIP sensor
Compared with reported methods38–41 the CDs@MIP sensor had a faster response time and wider linear and pH ranges (Table S1†). The reaction time of the CDs@MIP sensor was 20 min shorter than that of MMIPs.39 Furthermore the limit of detection of the CDs@MIP sensor was lower than that of MMIPs.38 Therefore, this sensor has enough sensitivity to be used for fast detection of BPA in samples.Conclusion
A new type of fluorescent molecularly imprinted sensor was presented using silica-modified CDs as the fluorescent probe. The sensor was successfully applied to detect BPA with high selectivity and sensitivity. This paper provides a new analysis method for BPA.Conflicts of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31701684), the Natural Science Foundation of Shandong Province (No. ZR2017BC014), and the Incubation Program for Youth Innovation Team in Shandong Province.References
- A. U. R. Bacha, I. Nabi, H. Cheng, K. Li, S. Ajmal, T. Wang and L. Zhang, Chem. Eng. J., 2020, 389, 124482 CrossRef CAS.
- J. Du, J. Bao, Y. Liu, S. H. Kim and D. D. Dionysiou, Chem. Eng. J., 2019, 376, 119193 CrossRef CAS.
- G. Zhou, Y. Cao, Y. Jin, C. Wang, Y. Wang, C. Hua and S. Wu, J. Cleaner Prod., 2020, 274, 122929 CrossRef CAS.
- J. Im and F. E. Löffler, Environ. Sci. Technol., 2016, 50, 8403–8416 CrossRef CAS PubMed.
- H. Mirzajani, C. Cheng, J. Wu, J. Chen, S. Eda, E. Najafi Aghdam and H. Badri Ghavifekr, Biosens. Bioelectron., 2017, 89, 1059–1067 CrossRef CAS.
- W. Sheng, Y. Liu, S. Li, Y. Lu, Q. Chang, Y. Zhang and S. Wang, Food Anal. Methods, 2017, 11, 675–685 CrossRef.
- R. Z. Wang, D. L. Huang, Y. G. Liu, Z. W. Peng, G. M. Zeng, C. Lai, P. Xu, C. Huang, C. Zhang and X. M. Gong, RSC Adv., 2016, 6, 106201–106210 RSC.
- W. Sheng, W. Duan, Y. Shi, Q. Chang, Y. Zhang, Y. Lu and S. Wang, Anal. Methods, 2018, 10, 5313–5320 RSC.
- Y. Wang, Y. Yang, L. Xu and J. Zhang, Electrochim. Acta, 2011, 56, 2105–2109 CrossRef CAS.
- L. Zhang and L. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 16248–16256 CrossRef CAS PubMed.
- S. Li, J. Luo, G. Yin, Z. Xu, Y. Le, X. Wu, N. Wu and Q. Zhang, Sens. Actuators, B, 2015, 206, 14–21 CrossRef CAS.
- X. Zhou, A. Wang, C. Yu, S. Wu and J. Shen, ACS Appl. Mater. Interfaces, 2015, 7, 11741–11747 CrossRef CAS.
- Z. Zhou, T. Li, W. Xu, W. Huang, N. Wang and W. Yang, Sens. Actuators, B, 2017, 240, 1114–1122 CrossRef CAS.
- F. Wang, Z. Xie, H. Zhang, C. Y. Liu and Y. G. Zhang, Adv. Funct. Mater., 2011, 21, 1027–1031 CrossRef CAS.
- M. Wang, Q. Fu, K. Zhang, Y. Wan, L. Wang, M. Gao, Z. Xia and D. Gao, Microchim. Acta, 2019, 186, 86–96 CrossRef PubMed.
- J. Dai, X. Dong and M. Fidalgo de Cortalezzi, Microchim. Acta, 2017, 184, 1369–1377 CrossRef CAS.
- T. Hao, X. Wei, Y. Nie, Y. Xu, Y. Yan and Z. Zhou, Microchim. Acta, 2016, 183, 2197–2203 CrossRef CAS.
- Z. Wang, C. Xu, Y. Lu, X. Chen, H. Yuan, G. Wei, G. Ye and J. Chen, Sens. Actuators, B, 2017, 241, 1324–1330 CrossRef CAS.
- S. Carrasco, E. Benito-Peña, F. Navarro-Villoslada, J. Langer, M. N. Sanz-Ortiz, J. Reguera, L. M. Liz-Marzán and M. C. Moreno-Bondi, Chem. Mater., 2016, 28, 7947–7954 CrossRef CAS.
- J. Gauczinski, Z. Liu, X. Zhang and M. Schonhoff, Langmuir, 2012, 28, 4267–4273 CrossRef CAS PubMed.
- T. Cai, H. Zhang, A. F. M. M. Rahman, Y. P. Shi and H. Qiu, Microchim. Acta, 2017, 184, 2629–2636 CrossRef CAS.
- L. Pan, Y. Ding, X. Ni, C. Z. Wang, B. Jiang, Y. Zhang, N. Jiang, Y. Tang, L. Chen and C. S. Yuan, RSC Adv., 2020, 10, 7671–7681 RSC.
- K. Puzio, R. Delepee, R. Vidal and L. A. Agrofoglio, Anal. Chim. Acta, 2013, 790, 47–55 CrossRef CAS.
- Z. Lu, G. Zhou, M. Song, X. Liu, H. Tang, H. Dong, P. Huo, F. Yan, P. Du and G. Xing, Appl. Catal., B, 2020, 268, 118433 CrossRef CAS.
- Z. Li, H. Xu, D. Wu, J. Zhang, X. Liu, S. Gao and Y. Kong, ACS Appl. Mater. Interfaces, 2019, 11, 2840–2848 CrossRef CAS PubMed.
- Y. Yuan, Y. Yang and G. Zhu, ACS Cent. Sci., 2020, 6, 1082–1094 CrossRef CAS PubMed.
- J. J. BelBruno, Chem. Rev., 2019, 119, 94–119 CrossRef CAS PubMed.
- Y. Lu, Y. Zhu, Y. Zhang and K. Wang, J. Chem. Eng. Data, 2019, 64, 1045–1050 CrossRef CAS.
- X. Liu, F. Wu, C. Au, Q. Tao, M. Pi and W. Zhang, J. Appl. Polym. Sci., 2019, 136, 46984 CrossRef.
- X. Yang, T. Muhammad, J. Yang, A. Yasen and L. Chen, Sens. Actuators, A, 2020, 313, 112190 CrossRef CAS.
- C. Schirmer and H. Meisel, Anal. Bioanal. Chem., 2008, 392, 223–229 CrossRef CAS PubMed.
- S. Xu, H. Lu, L. Chen and X. Wang, RSC Adv., 2014, 4, 45266–45274 RSC.
- Z. Lian and J. Wang, Mar. Pollut. Bull., 2017, 122, 500–504 CrossRef CAS PubMed.
- W. L. Guo, W. Hu, J. M. Pan, H. C. Zhou, W. Guan, X. Wang, J. D. Dai and L. C. Xu, Chem. Eng. J., 2011, 171, 603–611 CrossRef CAS.
- Y. M. Ren, W. Q. Ma, J. Ma, Q. Wen, J. Wang and F. B. Zhao, J. Colloid Interface Sci., 2012, 367, 355–361 CrossRef CAS PubMed.
- I. L. Moudrakovski, J. E. Lofgreen and G. A. Ozin, ACS Nano, 2011, 5, 2277–2287 CrossRef PubMed.
- L. Xu, G. Fang, J. Liu, M. Pan, R. Wang and S. Wang, J. Mater. Chem. A, 2016, 4, 15880–15887 RSC.
- L. Zhu, Y. Cao and G. Cao, Biosens. Bioelectron., 2014, 54, 258–261 CrossRef CAS PubMed.
- R. Z. Wang, D. L. Huang, Y. G. Liu, Z. W. Peng, G. M. Zeng, L. Cui, P. Xu, C. Huang, Z. Chen, C. Zhang and X. M. Gong, RSC Adv., 2016, 6, 106201–106210 RSC.
- H. Z. Lu and S. Xu, Biosens. Bioelectron., 2017, 92, 147–153 CrossRef CAS.
- Q. Kong, Y. Wang, L. Zhang, S. Ge and J. Yu, Sens. Actuators, B, 2017, 243, 130–136 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ay01822h |
| This journal is © The Royal Society of Chemistry 2021 |