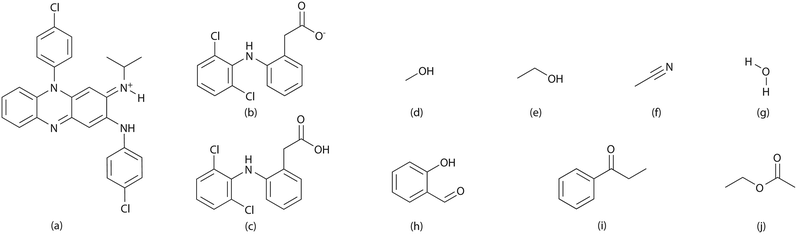
Taking advantage of solvate formation to modulate drug–drug ratio in clofaziminium diclofenac salts†
Laurie
Bodart
 *a,
Maria
Prinzo
b,
Amélie
Derlet
a,
Nikolay
Tumanov
*a,
Maria
Prinzo
b,
Amélie
Derlet
a,
Nikolay
Tumanov
 a and
Johan
Wouters
a and
Johan
Wouters
 *a
*a
aUniversity of Namur (UNamur)Namur Medicine and Drug Innovation Center – Namur Research Institute for LIfe Science (NAMEDIC-NARILIS), Namur Institute of Structured Matter (NISM), Department of Chemistry, University of Namur (UNamur), 61 Rue de Bruxelles, 5000 Namur, Belgium. E-mail: laurie.bodart@unamur.be; johan.wouters@unamur.be
bDrug Science Department, University of Catania, Viale Andrea Doria 6, 95125 Catania, Italy
First published on 17th November 2020
Abstract
Non-steroidal anti-inflammatory drugs, such as diclofenac, are gaining attention as repurposed compounds for the treatment of multi-drug resistant tuberculosis. In this study, salts combining diclofenac with clofazimine, are prepared by solvent crystallization and by liquid-assisted grinding. Diclofenac anion possesses an H-bond acceptor which can strongly interact with protic solvent molecules. In this context, selected solvents (protic, aprotic and solvents with increasing molecular volume) are screened in order to investigate solvent impact on crystallization of solvated or unsolvated salt of clofazimine with diclofenac in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Five solvated salts and one unsolvated salt were successfully crystallized. The ability of the diclofenac anion to interact with a protic molecule is also exploited in order to crystallize a cocrystal of salt with drug
1 ratio. Five solvated salts and one unsolvated salt were successfully crystallized. The ability of the diclofenac anion to interact with a protic molecule is also exploited in order to crystallize a cocrystal of salt with drug![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) drug ratio different from 1
drug ratio different from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Structures of two solvated cocrystal of salts (with acetonitrile and ethylacetate) and two polymorphs of an unsolvated cocrystal of salt combining clofazimine with diclofenac in 1
1. Structures of two solvated cocrystal of salts (with acetonitrile and ethylacetate) and two polymorphs of an unsolvated cocrystal of salt combining clofazimine with diclofenac in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratios are determined.
2 ratios are determined.
1 Introduction
Pharmaceutical compounds are often exposed to solvents (or their vapors) during manufacturing (i.e. at the steps of crystallization, wet granulation, spray drying or lyophilization).1,2 Organic compounds including pharmaceuticals are often found in the form of solvates.3,4 However, crystalline changes induced by solvate formation or solvent evaporation from the structure can considerably affect the physico-chemical properties of the compounds. Although, in the pharmaceutical industry, solvate formation can sometimes be beneficial to the properties of drugs (e.g., indinavir shows better bioavailability when in the form of a sulfate ethanol solvated form1,5,6), in many other cases it is a nuisance: for instance, if the solvent is toxic or the solvated form exhibits undesired physicochemical and/or mechanical properties. Thus, selecting a proper solvent which would present a reduced probability of solvate formation is of crucial importance for drug manufacturing.7Several factors have been investigated for their impact on solvate formation. The two most important ones have been identified as solvent–solute affinity and molecular size and shape of the solvent.8,9 The first parameter, among other factors, depends on hydrogen bonding and aromatic interaction abilities of the molecule. The second parameter is in correlation with the concept of packing efficiency, since solvent presenting low affinity for a compound can still be incorporated into its structure if it allows a better packing.8,10 While various solvents, depending on their molecular volume, size and shape, may lead to different crystal packings, it is also possible that bulky molecules of the compound itself fail to form efficient packing and thus may incorporate solvent molecules in order to improve it. The presence of hydrogen bonding groups tends to promote inclusion of polar solvents by strong and specific interactions.11 Despite identification of certain factors contributing to solvate formation, theoretical solvate prediction is challenging and research in this area is still ongoing.9,10
In this study, we focus on the solvated salts of a drug–drug system comprising clofazimine and diclofenac. Clofazimine (CFZ), exhibits antimycobacterial and anti-inflammatory properties and has been recently reevaluated as a potential treatment for multidrug-resistant tuberculosis.12–17 Diclofenac (DCF) belongs to the class of non-steroidal anti-inflammatory drugs (NSAIDs) that has been recently proposed as a host-directed therapy in the treatment of tuberculosis owing to its antitubercular properties.18–22 Several studies showing the ability of clofazimine (pKa: 9.29 (ref. 23)) to form salts with organic and inorganic acids24–28 point that combining CFZ with DCF in a drug–drug salt should be achievable. Several solvates were previously reported for clofaziminium as well as diclofenac salts26,28 and strong H-bond interaction between DCF and solvent molecules have been reported.29 Solvate formation is thus also expected with protic solvents during the preparation the drug–drug CFZ–DCF salts.
In our experiments, as expected, diclofenac tended to bind protic solvents and incorporate them into structure, thereby resulting in the solvated form of the CFZ–DCF 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 salt. Our further hypothesis was that diclofenac molecule, being also protic, should be able to compete with the solvent molecule for that binding site and thus instead of a solvated 1
1 salt. Our further hypothesis was that diclofenac molecule, being also protic, should be able to compete with the solvent molecule for that binding site and thus instead of a solvated 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 form lead to a 1
1 form lead to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CFZ–DCF cocrystal of salt, with diclofenac molecule being connected to diclofenac anion. To verify this and also to see whether an unsolvated 1
2 CFZ–DCF cocrystal of salt, with diclofenac molecule being connected to diclofenac anion. To verify this and also to see whether an unsolvated 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 salt can also be obtained, we have selected a number of aprotic solvents with increasing molecular volume, which due to their chemical nature should have a lower probability of binding to diclofenac. Experimenting with various solvents, we obtained in total five solvated 1
1 salt can also be obtained, we have selected a number of aprotic solvents with increasing molecular volume, which due to their chemical nature should have a lower probability of binding to diclofenac. Experimenting with various solvents, we obtained in total five solvated 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 forms, two solvated 1
1 forms, two solvated 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 forms, one unsolvated 1
2 forms, one unsolvated 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CFZ–DCF salt and two unsolvated 1
1 CFZ–DCF salt and two unsolvated 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
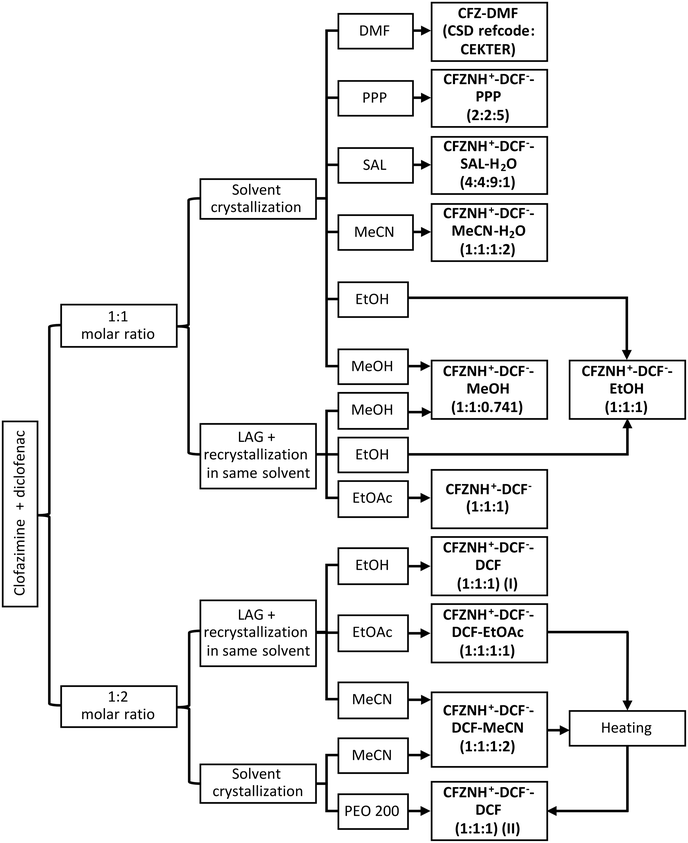
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 cocrystal of salt (Fig. 1), thus proving that selecting a proper solvent is essential in crystallization processes and might be key to obtaining desired structures.
2 cocrystal of salt (Fig. 1), thus proving that selecting a proper solvent is essential in crystallization processes and might be key to obtaining desired structures.
2 Materials and methods
2.1 Materials
Clofazimine and diclofenac were respectively purchased from TCI Europe N.V. (Zwijndrecht, Belgium) and Sigma Aldrich (Schnelldorf, Germany). Propiophenone, salicylaldehyde and ethylacetate (Sigma Aldrich, Schnelldorf, Germany), acetonitrile (Thermo Fisher Scientific, Geel, Belgium), methanol (ChemLab, Zedelgem, Belgium), ethanol (Merck, Overijse, Belgium), N,N′-dimethylformamide and poly(ethyleneglycol) of average MW 200 (Acros Organics, Geel, Belgium) were used without further purification as crystallization solvents.2.2 (Solvated/hydrated) clofaziminium diclofenac salts preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0.74), CFZNH+–DCF−–EtOH (1
0.74), CFZNH+–DCF−–EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) and CFZNH+–DCF−(1
1) and CFZNH+–DCF−(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) can be obtained by grinding 75.0 mg, 0.158 mmol of CFZ with 46.9 mg, 0.158 mmol of DCF in presence of 30 μL of MeOH, EtOH and EtOAc respectively (liquid-assisted grinding). CFZNH+–DCF−–DCF (1
1) can be obtained by grinding 75.0 mg, 0.158 mmol of CFZ with 46.9 mg, 0.158 mmol of DCF in presence of 30 μL of MeOH, EtOH and EtOAc respectively (liquid-assisted grinding). CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (polymorph I), CFZNH+–DCF−–DCF-MeCN (1
1) (polymorph I), CFZNH+–DCF−–DCF-MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) and CFZNH+–DCF−–DCF–EtOAc (1
2) and CFZNH+–DCF−–DCF–EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) are prepared by grinding 75.0 mg (0.158 mmol) of CFZ with 93.8 mg (0.316 mmol) of DCF in presence of 30 μL of EtOH, MeCN and EtOAc respectively. Crystals of CFZNH+–DCF−–DCF (1
1) are prepared by grinding 75.0 mg (0.158 mmol) of CFZ with 93.8 mg (0.316 mmol) of DCF in presence of 30 μL of EtOH, MeCN and EtOAc respectively. Crystals of CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (polymorph I), CFZNH+–DCF−–DCF–MeCN (1
1) (polymorph I), CFZNH+–DCF−–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) and CFZNH+–DCF−–DCF–EtOAc (1
2) and CFZNH+–DCF−–DCF–EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) were obtained by recrystallization of the corresponding powder (CFZ–DCF 1
1) were obtained by recrystallization of the corresponding powder (CFZ–DCF 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 LAG EtOH, LAG MeCN and LAG EtOAc) in ethanol, acetonitrile and ethylacetate respectively (Fig. 2). All ball milling experiments were performed at 30 Hz, during 90 minutes (samples were homogenized each 30 minutes) with a Retsch MM 400 Mixer Mill apparatus. Powders were inserted in 2 mL Eppendorf tubes with eight stainless steel balls (7 balls of 2 mm diameter and 1 ball of 3 mm diameter) and were then placed in two grinding jars in which up to 5 Eppendorf tubes can be installed. Powder of CFZNH+–DCF−–DCF (1
2 LAG EtOH, LAG MeCN and LAG EtOAc) in ethanol, acetonitrile and ethylacetate respectively (Fig. 2). All ball milling experiments were performed at 30 Hz, during 90 minutes (samples were homogenized each 30 minutes) with a Retsch MM 400 Mixer Mill apparatus. Powders were inserted in 2 mL Eppendorf tubes with eight stainless steel balls (7 balls of 2 mm diameter and 1 ball of 3 mm diameter) and were then placed in two grinding jars in which up to 5 Eppendorf tubes can be installed. Powder of CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (polymorph II) cocrystal of salt is obtained by desolvation (heating at 130–140 °C) of CFZNH+–DCF−–DCF–MeCN (1
1) (polymorph II) cocrystal of salt is obtained by desolvation (heating at 130–140 °C) of CFZNH+–DCF−–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) or of CFZNH+–DCF−–DCF–EtOAc (1
2) or of CFZNH+–DCF−–DCF–EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (obtained by liquid-assisted grinding, Fig. 2).
1) (obtained by liquid-assisted grinding, Fig. 2).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0.74), CFZNH+–DCF−–EtOH (1
0.74), CFZNH+–DCF−–EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) and CFZNH+–DCF−–MeCN–H2O (1
1) and CFZNH+–DCF−–MeCN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) were obtained in MeOH, EtOH and MeCN respectively. Crystals of CFZNH+–DCF−–DCF–MeCN (1
2) were obtained in MeOH, EtOH and MeCN respectively. Crystals of CFZNH+–DCF−–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) and of CFZNH+–DCF−–DCF (1
2) and of CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (polymorph II) were obtained in MeCN and PEG 200 respectively, by the same method but from a powder consisting of clofazimine (37.4 mg, 0.0790 mmol) and diclofenac acid (46.8 mg, 0.158 mmol) in 1
1) (polymorph II) were obtained in MeCN and PEG 200 respectively, by the same method but from a powder consisting of clofazimine (37.4 mg, 0.0790 mmol) and diclofenac acid (46.8 mg, 0.158 mmol) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio.
2 molar ratio.
Given the low vapor pressure at room temperature (20–25 °C) of propiophenone and salicylaldehyde and the quite good solubility of CFZ and DCF in these solvents, crystallization by solvent evaporation is quite difficult. For this reason, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 powder mixture of CFZ and DCF was not fully dissolved in these solvent (i.e. an excess powder was placed in the solvent and left at room temperature for crystallization) to give CFZNH+–DCF−–PPP (2
1 powder mixture of CFZ and DCF was not fully dissolved in these solvent (i.e. an excess powder was placed in the solvent and left at room temperature for crystallization) to give CFZNH+–DCF−–PPP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5) and CFZNH+–DCF−–SAL–H2O (4
5) and CFZNH+–DCF−–SAL–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1).
1).
2.3 Single-crystal X-ray diffraction (SCXRD)
Data collection was performed with an Oxford Diffraction Gemini Ultra R system equipped with a four-circle kappa platform and a Ruby CCD detector, using Cu Kα (λ = 1.54184 Å) radiation. Full data sets were collected either at 295 K (CFZNH+–DCF−–MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0.74), CFZNH+–DCF−(1
0.74), CFZNH+–DCF−(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1), CFZNH+–DCF−–DCF–MeCN (1
1), CFZNH+–DCF−–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2), CFZNH+–DCF−–DCF–EtOAc (1
2), CFZNH+–DCF−–DCF–EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) and CFZNH+–DCF−–DCF (1
1) and CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (polymorphs I and II)), at 100 K (CFZNH+–DCF−–MeCN–H2O (1
1) (polymorphs I and II)), at 100 K (CFZNH+–DCF−–MeCN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) and CFZNH+–DCF−–SAL–H2O (4
2) and CFZNH+–DCF−–SAL–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1)) or at both temperatures (CFZNH+–DCF−–EtOH (1
1)) or at both temperatures (CFZNH+–DCF−–EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) and CFZNH+–DCF−–PPP (2
1) and CFZNH+–DCF−–PPP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5)). Low-temperature data collection was necessary for CFZNH+–DCF−–MeCN–H2O (1
5)). Low-temperature data collection was necessary for CFZNH+–DCF−–MeCN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) because crystals are unstable at room temperature. Crystals of CFZNH+–DCF−–SAL–H2O (4
2) because crystals are unstable at room temperature. Crystals of CFZNH+–DCF−–SAL–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) were stable at room temperature but disorder in the structure could not be resolved without low-temperature data collection. First, analytical numerical absorption correction implemented using a multifaceted crystal model based on expressions derived by Clark and Reid30 was performed using CrysAlis PRO.31 Then, empirical absorption correction was applied, within CrysAlis PRO, using spherical harmonics32 as implemented in the SCALE3 ABSPACK scaling algorithm. Dual-space method implemented within SHELXT33 was used for structure solution. Refinement was performed within Olex2 (ref. 34) and ShelXle35 using the least-square method (SHELXL-2016/6 (ref. 36)). Anisotropic refinement was performed for non hydrogen atoms. If not involved in strong H-bonds, hydrogen atoms were refined as riding atoms with displacement spheres fixed to 1.2 times that of the parent atom (1.5 for methyl groups). Positions of H atoms involved in strong H-bond were located in Fourier map and refined (except for disordered MeOH molecule in the structure of CFZNH+–DCF−–MeOH (1
1) were stable at room temperature but disorder in the structure could not be resolved without low-temperature data collection. First, analytical numerical absorption correction implemented using a multifaceted crystal model based on expressions derived by Clark and Reid30 was performed using CrysAlis PRO.31 Then, empirical absorption correction was applied, within CrysAlis PRO, using spherical harmonics32 as implemented in the SCALE3 ABSPACK scaling algorithm. Dual-space method implemented within SHELXT33 was used for structure solution. Refinement was performed within Olex2 (ref. 34) and ShelXle35 using the least-square method (SHELXL-2016/6 (ref. 36)). Anisotropic refinement was performed for non hydrogen atoms. If not involved in strong H-bonds, hydrogen atoms were refined as riding atoms with displacement spheres fixed to 1.2 times that of the parent atom (1.5 for methyl groups). Positions of H atoms involved in strong H-bond were located in Fourier map and refined (except for disordered MeOH molecule in the structure of CFZNH+–DCF−–MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0.74), disordered EtOH molecule in the structure of CFZNH+–DCF−–EtOH (1
0.74), disordered EtOH molecule in the structure of CFZNH+–DCF−–EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (data collected at 295 K) and for disordered salicylaldehyde molecules and the water molecule in CFZNH+–DCF−–SAL–H2O (4
1) (data collected at 295 K) and for disordered salicylaldehyde molecules and the water molecule in CFZNH+–DCF−–SAL–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1)). The solvent molecules are disordered in several structures. More particularly, the methanol molecule in CFZNH+–DCF−–MeOH (1
1)). The solvent molecules are disordered in several structures. More particularly, the methanol molecule in CFZNH+–DCF−–MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0.74); all solvent molecules in CFZNH+–DCF−–PPP (2
0.74); all solvent molecules in CFZNH+–DCF−–PPP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5) (data collected at 295 K); one molecule of propiophenone (which is located close to an inversion center) in CFZNH+–DCF−–PPP (2
5) (data collected at 295 K); one molecule of propiophenone (which is located close to an inversion center) in CFZNH+–DCF−–PPP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5) (data collection at 100 K); five salicylaldehyde molecules (which are not involved in intermolecular H-bonds) in the structure of CFZNH+–DCF−–SAL–H2O (4
5) (data collection at 100 K); five salicylaldehyde molecules (which are not involved in intermolecular H-bonds) in the structure of CFZNH+–DCF−–SAL–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1); the ethanol molecule in the structure of CFZNH+–DCF−–EtOH (1
1); the ethanol molecule in the structure of CFZNH+–DCF−–EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) for which data were collected at 295 K and one MeCN molecule in CFZNH+–DCF−–DCF–MeCN (1
1) for which data were collected at 295 K and one MeCN molecule in CFZNH+–DCF−–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) are disordered.
2) are disordered.
2.4 Powder X-ray diffraction (PXRD)
Powder diffraction data were collected with Cu Kα radiation (λ = 1.54184 Å) from 4 to 40° 2θ angle (step size of 0.0167°) on a PANalytical X'PERT PRO Bragg–Brentano diffractometer with an X'Celerator linear detector. Tension and current of the generator were set to 45 kV and 30 mA for data collection.Variable-temperature PXRD (VT-PXRD) experiments were conducted on the same diffractometer equipped with an Anton-Paar TTK 450 system. Data were collected at 25 °C and then from 30 °C to 140 °C with data collection every 10 °C.
2.5 Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC)
Thermal properties of powders obtained by liquid-assisted grinding and corresponding to determined structures were analysed using a Mettler Toledo TGA/DSC 3+ apparatus. Around 5–10 mg of solid samples were placed in 100 μL aluminium pans. The analysis was performed from 25 °C to 250 °C with a heating rate of 10 °C min−1 and using nitrogen (60 mL min−1) as purge gas. TGA/DSC results were analysed using the STARe software (version 16.20).2.6 Structure visualization, crystal packing comparison and solvent molecular volume calculations
Structures and crystal packings were visualized and compared using Mercury.37 Voids were calculated with the ‘Voids’ option within Mercury37 with a probe radius of 1.2 Å and an approximated grid spacing of 0.1 Å. Images were generated with de same program. Crystal packings were more specifically compared using the ‘Crystal packing similarity’ tool in Mercury.37 Packing shell size, distance and angle tolerance parameters were set to 15 molecules, 30% and 30° respectively. Moreover, molecular differences and structure inversion were allowed while bond types, H-atom position, atoms' hydrogen counts and atoms bond counts were ignored. As structures contain different solvents, smallest molecular component was ignored for crystal packing comparison. Solvent molecular volumes were determined with the free Molinspiration molecular property calculation service.383 Results and discussion
The current work includes two parts. In the first part, we have investigated solvated and unsolvated forms of CFZ–DCF 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 salt. The second part is focused on the ability of diclofenac anion to bind diclofenac molecule instead of a solvent molecule (a phenomenon often observed in 1
1 salt. The second part is focused on the ability of diclofenac anion to bind diclofenac molecule instead of a solvent molecule (a phenomenon often observed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 salts) to obtain a 1
1 salts) to obtain a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
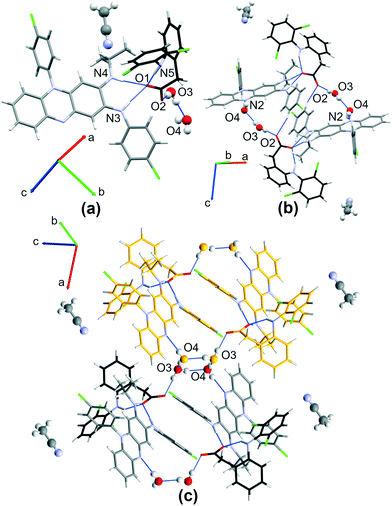
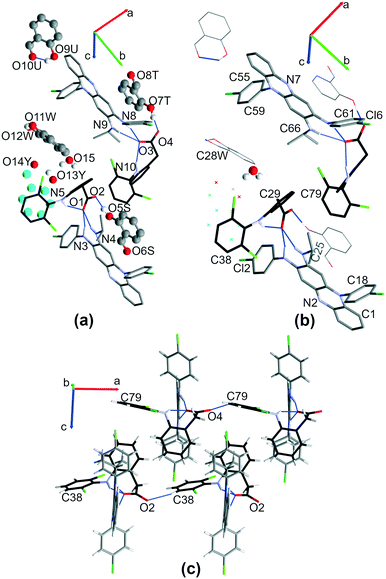
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CFZ–DCF cocrystal of salt. To obtain drug–drug salts, clofazimine and diclofenac were subjected to solvent evaporation crystallization and liquid-assisted grinding (Fig. 2). Below and for each part of the work, we will first present structural analysis of the new crystal structures combining clofazimine with diclofenac (Fig. 1 and S1 and S2† (labelling)†) and discuss the impact of solvent selection on the crystallization outcome.
2 CFZ–DCF cocrystal of salt. To obtain drug–drug salts, clofazimine and diclofenac were subjected to solvent evaporation crystallization and liquid-assisted grinding (Fig. 2). Below and for each part of the work, we will first present structural analysis of the new crystal structures combining clofazimine with diclofenac (Fig. 1 and S1 and S2† (labelling)†) and discuss the impact of solvent selection on the crystallization outcome.
3.1 Investigation of CFZ–DCF 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 1 salts
1 salts
The solvents used in the first part of this work were selected based on their ability to interact with the solute (CFZ cations and DCF anions) via hydrogen bonding or other weak interactions and on their molecular size and shape – two main parameters influencing the formation of solvates. First, we selected a series of protic solvents of increasing molecular volume (MeOH, EtOH, salicylaldehyde) in order to investigate the impact on solvate formation of those solvents that are capable of forming H-bond with diclofenac anion. In addition to H-bonds, the aromatic system of salicylaldehyde could also be involved in stacking interactions with clofaziminium cation and/or diclofenac anion. Second, we took aprotic solvents of variable molecular volume (MeCN, DMF, EtOAc, propiophenone) in order to study the effect of solvents that do not have the ability to interact with diclofenac anion through H-bond. Propiophenone, that could potentially interact with CFZNH+ and DCF− through stacking has also been included among the selected aprotic solvents. Below we present structural analysis of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solvated and unsolvated CFZ–DCF salts that were obtained using aforementioned solvents followed by the discussion of the solvent impact on crystallization outcome.
1 solvated and unsolvated CFZ–DCF salts that were obtained using aforementioned solvents followed by the discussion of the solvent impact on crystallization outcome.
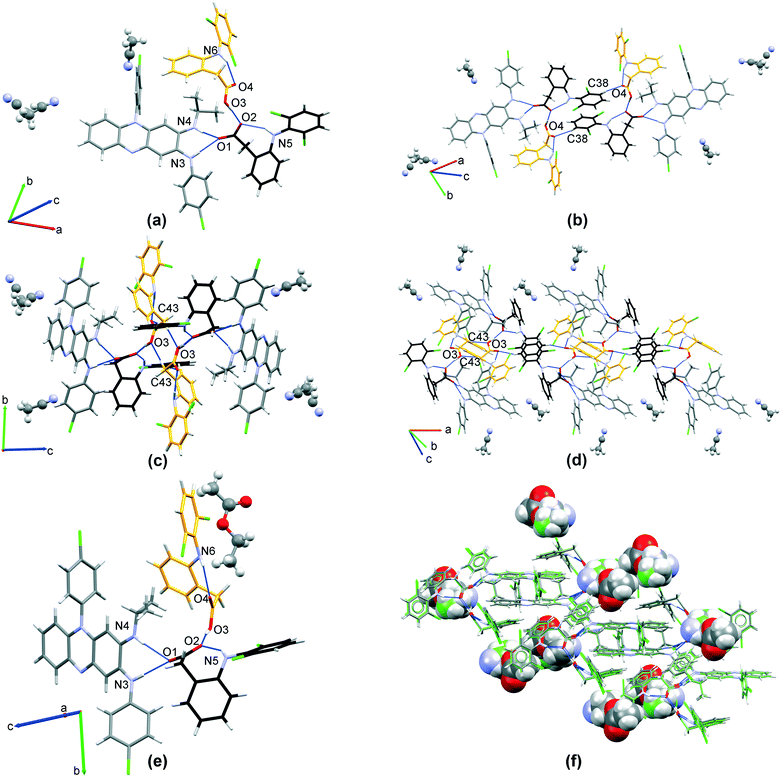
3.1.1.1 CFZNH + –DCF − –MeOH (1 : 1 : 0.74) solvated salt. CFZNH + –DCF − –MeOH (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
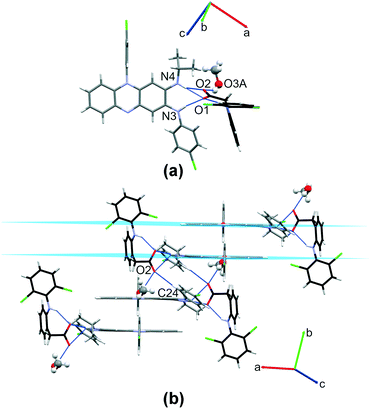
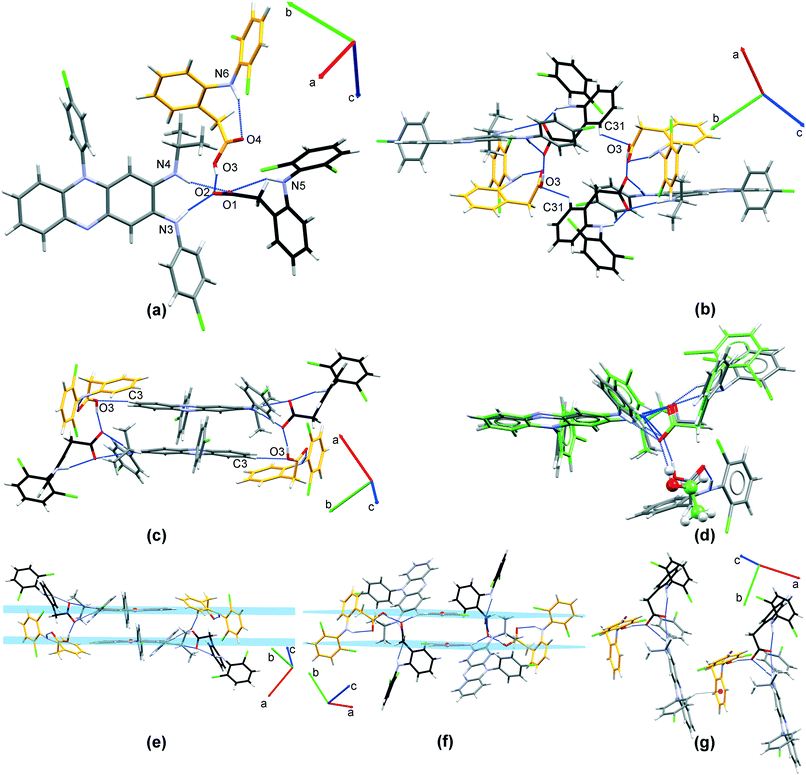
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0.74) solvated salt crystallizes in C2/c space group (Table 1). The asymmetric unit contains one clofaziminium cation, one diclofenac anion and methanol molecule, which is disordered over two positions (one position is located around C2 axis). A R12(7) motif is observed between clofazimine and diclofenac (N4–H4⋯O1 and N3–H3⋯O1 charge-assisted H-bonds, Fig. 3(a) and Table S2†). A weaker H-bond is also observed between CFZNH+ and DCF− (N4–H4⋯O2, Fig. 3(a) and Table S2†). Methanol (position with an occupancy of 0.5) interacts with diclofenac through H-bond (O3A–H3OA⋯O2, Fig. 3(a) and Table S2†). An intramolecular N5–H5⋯O1 H-bond is also present within the diclofenac anion. A weak C24–H24⋯O2 H-bond stabilizes dimers of CFZNH+–DCF−–MeOH trimolecular assemblies (Fig. 3(b)).
0.74) solvated salt crystallizes in C2/c space group (Table 1). The asymmetric unit contains one clofaziminium cation, one diclofenac anion and methanol molecule, which is disordered over two positions (one position is located around C2 axis). A R12(7) motif is observed between clofazimine and diclofenac (N4–H4⋯O1 and N3–H3⋯O1 charge-assisted H-bonds, Fig. 3(a) and Table S2†). A weaker H-bond is also observed between CFZNH+ and DCF− (N4–H4⋯O2, Fig. 3(a) and Table S2†). Methanol (position with an occupancy of 0.5) interacts with diclofenac through H-bond (O3A–H3OA⋯O2, Fig. 3(a) and Table S2†). An intramolecular N5–H5⋯O1 H-bond is also present within the diclofenac anion. A weak C24–H24⋯O2 H-bond stabilizes dimers of CFZNH+–DCF−–MeOH trimolecular assemblies (Fig. 3(b)).
CFZNH
+
–DCF
−
–MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0.74) 0.74)
|
CFZNH
+
–DCF
−
–EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
CFZNH
+
–DCF
−
–EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
CFZNH
+
–DCF
−
–MeCN–H
2
O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) 2)
|
|
|---|---|---|---|---|
| Chemical formula | C27H23Cl2N4·C14H10Cl2NO2·0.741 (CH4O) | C27H23Cl2N4·C14H10Cl2NO2·C2H6O | C27H23Cl2N4·C14H10Cl2NO2·C2H6O | C27H23Cl2N4·C14H10Cl2NO2·C2H3N·2(H2O) |
| Mr | 793.42 | 815.59 | 815.59 | 846.61 |
| Crystal system, space group | Monoclinic, C2/c | Triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| Temperature (K) | 295(2) | 295(2) | 100(2) | 100(2) |
| a, b, c (Å) | 16.7209(3), 17.2504(5), 27.8319(7) | 12.5818(4), 13.4486(3), 13.5318(3) | 12.5325(5), 13.1139(3), 13.1773(4) | 11.6515(3), 11.7149(3), 16.0793(4) |
| α, β, γ (°) | 90, 93.438(2), 90 | 80.291(2), 71.638(2), 71.710(2) | 82.032(2), 71.833(3), 74.538(3) | 103.558(2), 90.461(2), 105.250(2) |
| V (Å3) | 8013.4(3) | 2057.12(10) | 1979.61(12) | 2052.78(9) |
| Z | 8 | 2 | 2 | 2 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 679, 7080, 4722 679, 7080, 4722 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 818, 7236, 6397 818, 7236, 6397 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 176, 6977, 6386 176, 6977, 6386 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 621, 7196, 6969 621, 7196, 6969 |
| R int | 0.040 | 0.024 | 0.046 | 0.019 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.059, 0.193, 1.02 | 0.050, 0.146, 1.06 | 0.057, 0.166, 1.07 | 0.031, 0.080, 1.03 |
| CCDC number | 2032488 | 2032489 | 2032490 | 2032491 |
CFZNH
+
–DCF
−
–SAL–H
2
O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 9 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
CFZNH
+
–DCF
−
–PPP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5) 5)
|
CFZNH
+
–DCF
−
–PPP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5) 5)
|
CFZNH
+
–DCF
−
(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
|
|---|---|---|---|---|
| Chemical formula | 4(C27H23Cl2N4)·4(C14H10Cl2NO2)·9(C7H6O2)·H2O | 2(C27H23Cl2N4)·2(C14H10Cl2NO2)·5(C9H10O) | 2(C27H23Cl2N4)·2(C14H10Cl2NO2)·5(C9H10O) | (C27H23Cl2N4)·(C14H10Cl2NO2) |
| Mr | 4195.16 | 2209.89 | 2209.89 | 769.52 |
| Crystal system, space group | Triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| Temperature (K) | 100(2) | 295(2) | 100(2) | 295(2) |
| a, b, c (Å) | 10.4132(5), 21.2266(7), 25.0657(7) | 10.7319(4), 13.6005(6), 21.5496(9) | 10.5086(4), 13.4783(5), 21.4224(8) | 12.4461(6), 13.2751(5), 13.3667(6) |
| α, β, γ (°) | 66.238(3), 89.969(3), 86.658(3) | 73.502(4), 77.404(4), 73.540(4) | 73.629(3), 76.913(3), 73.271(3) | 84.423(3), 67.963(4), 66.662(4) |
| V (Å3) | 5060.5(3) | 2859.7(2) | 2752.92(19) | 1876.21(16) |
| Z | 1 | 1 | 1 | 2 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 166, 17 166, 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 765, 13 765, 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 730 730 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 672, 10 672, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 049, 6767 049, 6767 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 107, 9723, 7857 107, 9723, 7857 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 078, 6630, 5192 078, 6630, 5192 |
| R int | 0.069 | 0.051 | 0.038 | 0.037 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.060, 0.171, 1.03 | 0.052, 0.160, 1.02 | 0.035, 0.090, 1.03 | 0.048, 0.141, 1.03 |
| CCDC number | 2032492 | 2032493 | 2032494 | 2032495 |
CFZNH
+
–DCF
−
–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (I) 1) (I)
|
CFZNH
+
–DCF
−
–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) 2)
|
CFZNH
+
–DCF
−
–DCF–EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
CFZNH
+
–DCF
−
–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (II) 1) (II)
|
|
|---|---|---|---|---|
| Chemical formula | C27H23Cl2N4·C14H10Cl2NO2·C14H11Cl2NO2 | C27H23Cl2N4·C14H10Cl2NO2·C14H11Cl2NO2·2(C2H3N) | C27H23Cl2N4·C14H10Cl2NO2·C14H11Cl2NO2·C4H8O2 | C27H23Cl2N4·C14H10Cl2NO2·C14H11Cl2NO2 |
| Mr | 1065.66 | 1147.77 | 1153.76 | 1065.66 |
| Crystal system, space group | Triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| Temperature (K) | 295(2) | 295(2) | 295(2) | 295(2) |
| a, b, c (Å) | 10.8837(2), 14.4737(3), 17.7519(4) | 15.0694(11), 15.2735(10), 15.5991(13) | 15.0569(4), 15.3773(4), 15.4247(4) | 11.9326(3), 15.2549(4), 16.0042(4) |
| α, β, γ (°) | 101.568(2), 105.173(2), 94.286(2) | 74.882(7), 61.685(8), 64.227(7) | 76.043(2), 61.975(3), 63.541(3) | 89.161(2), 70.570(2), 69.693(2) |
| V (Å3) | 2620.06(10) | 2839.6(4) | 2820.07(16) | 2559.79(12) |
| Z | 2 | 2 | 2 | 2 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 706, 9218, 8043 706, 9218, 8043 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 894, 10 894, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 010, 7543 010, 7543 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 162, 9942, 8898 162, 9942, 8898 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 042, 9036, 7679 042, 9036, 7679 |
| R int | 0.022 | 0.032 | 0.022 | 0.025 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.050, 0.141, 1.05 | 0.050, 0.159, 1.08 | 0.042, 0.122, 1.05 | 0.045, 0.129, 1.04 |
| CCDC number | 2032496 | 2032497 | 2032498 | 2032499 |
These dimers are further stacked in a head-to-tail fashion (Fig. 3(b), centroid–centroid distance of 3.623(2) Å, orthogonal projection distance of 3.378(1) Å and horizontal displacement of 1.311 Å). This salt can also be prepared by liquid-assisted grinding of CFZ with DCF (in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) and with MeOH as solvent (Fig. S3(a)†). TG/DSC analysis of the powder indicates a weight loss of 2.6% between 30 and 150 °C (calculated MeOH content: 2.99%) (Fig. S4†). The powder of CFZNH+–DCF−–MeOH (1
1 molar ratio) and with MeOH as solvent (Fig. S3(a)†). TG/DSC analysis of the powder indicates a weight loss of 2.6% between 30 and 150 °C (calculated MeOH content: 2.99%) (Fig. S4†). The powder of CFZNH+–DCF−–MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0.74) melts at 118 °C (as confirmed by an analysis performed on a Koffler apparatus). Complete desolvation is difficult to achieve before melting of the powder (at 100 °C, the solvate is still present) and the crystalline phase obtained upon heating of CFZNH+–DCF−–MeOH (1
0.74) melts at 118 °C (as confirmed by an analysis performed on a Koffler apparatus). Complete desolvation is difficult to achieve before melting of the powder (at 100 °C, the solvate is still present) and the crystalline phase obtained upon heating of CFZNH+–DCF−–MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0.74) at 110 °C could not be identified (Fig. S5†).
0.74) at 110 °C could not be identified (Fig. S5†).
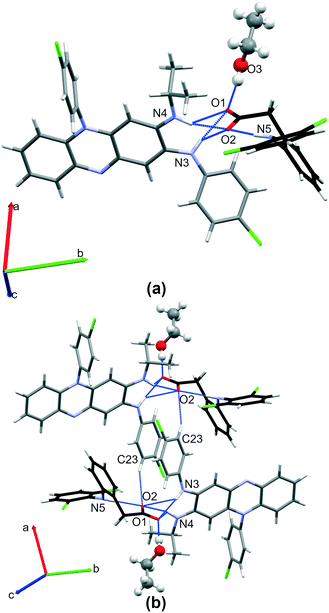
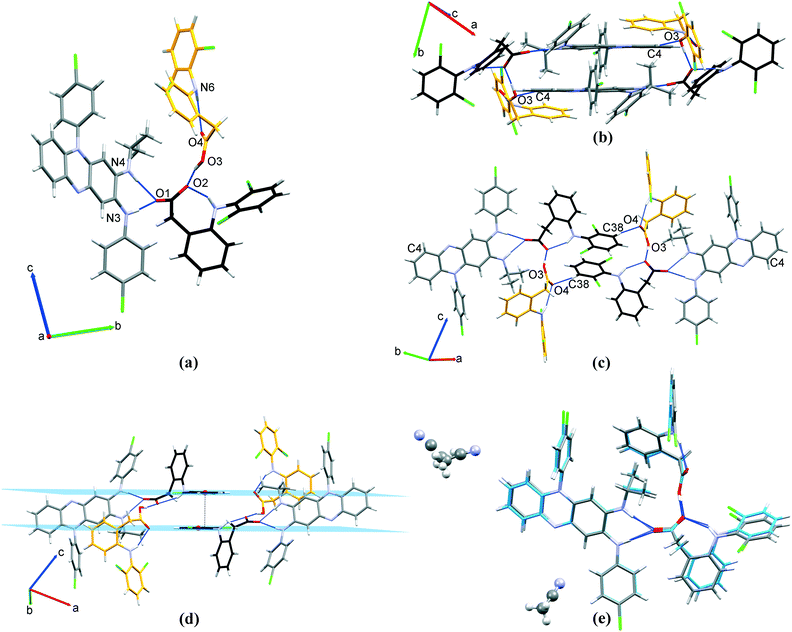
3.1.1.2 CFZNH + –DCF − –EtOH (1 : 1 : 1) solvated salt. CFZNH + –DCF − –EtOH (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) solvated salt crystallizes in P
1) solvated salt crystallizes in P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) triclinic space group (Table 1). The asymmetric unit consists of the clofaziminium cation, diclofenac anion and one solvent molecule (EtOH). This structure was determined at 295 and 100 K. At 295 K, EtOH molecule is disordered over two positions, both of which allow O3–H⋯O1 interaction between EtOH and diclofenac anion while at 100 K the same interaction is observed but EtOH is not disordered. At both temperatures, diclofenac anion and clofaziminium interact through bifurcated charge-assisted H-bonds (N4–H4⋯O2, N3–H3⋯O2, N4–H4⋯O1 and N3–H3⋯O1, Table S2† and Fig. 4(a)). A D11(2) motif between ethanol and diclofenac anion as well as an intramolecular H-bond (S11(7) motif) in diclofenac anion are also observed (O3–H3OB⋯O1 and N5–H5⋯O2 respectively, Table S2† and Fig. 4(a)). Dimers of CFZNH+–DCF−–EtOH three-component assemblies are stabilized through weak H-bonds (C23–H23⋯O2, Table S2† and Fig. 4(b)). PXRD pattern of the powder obtained by liquid-assisted grinding of CFZ with DCF (in 1
triclinic space group (Table 1). The asymmetric unit consists of the clofaziminium cation, diclofenac anion and one solvent molecule (EtOH). This structure was determined at 295 and 100 K. At 295 K, EtOH molecule is disordered over two positions, both of which allow O3–H⋯O1 interaction between EtOH and diclofenac anion while at 100 K the same interaction is observed but EtOH is not disordered. At both temperatures, diclofenac anion and clofaziminium interact through bifurcated charge-assisted H-bonds (N4–H4⋯O2, N3–H3⋯O2, N4–H4⋯O1 and N3–H3⋯O1, Table S2† and Fig. 4(a)). A D11(2) motif between ethanol and diclofenac anion as well as an intramolecular H-bond (S11(7) motif) in diclofenac anion are also observed (O3–H3OB⋯O1 and N5–H5⋯O2 respectively, Table S2† and Fig. 4(a)). Dimers of CFZNH+–DCF−–EtOH three-component assemblies are stabilized through weak H-bonds (C23–H23⋯O2, Table S2† and Fig. 4(b)). PXRD pattern of the powder obtained by liquid-assisted grinding of CFZ with DCF (in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) and with EtOH as solvent corresponds to the calculated pattern of CFZNH+–DCF−–EtOH (1
1 molar ratio) and with EtOH as solvent corresponds to the calculated pattern of CFZNH+–DCF−–EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (Fig. S3(b)†). This powder was analyzed by TG/DSC (Fig. S4†) that revealed a weight loss of 5.6% which is consistent with calculated EtOH content (5.6%). Only one endothermic event, associated with salt melting and solvent evaporation was observed at 113 °C. Such a high temperature of desolvation suggests that EtOH is strongly bound to diclofenac anion (Fig. S4† and Table 2). This is in accordance with the O3⋯O1 distance (between 2.672(3) and 2.82(1) Å) observed in the structure, which is characteristic of strong H-bond, as defined by Desiraju and Steiner.39
1) (Fig. S3(b)†). This powder was analyzed by TG/DSC (Fig. S4†) that revealed a weight loss of 5.6% which is consistent with calculated EtOH content (5.6%). Only one endothermic event, associated with salt melting and solvent evaporation was observed at 113 °C. Such a high temperature of desolvation suggests that EtOH is strongly bound to diclofenac anion (Fig. S4† and Table 2). This is in accordance with the O3⋯O1 distance (between 2.672(3) and 2.82(1) Å) observed in the structure, which is characteristic of strong H-bond, as defined by Desiraju and Steiner.39
| Compound | Transition onset (°C) | Melting onset (°C) |
|---|---|---|
a Value obtained from desolvated powder of CFZNH+–DCF−–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2). 2).
|
||
| CFZ | — | 218 |
| DCF | — | 177 |
CFZNH
+
–DCF
−
–EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
Desolvation at melting | 113 |
CFZNH
+
–DCF
−
(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
— | 186 |
CFZNH
+
–DCF
−
–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (polymorph I) 1) (polymorph I) |
— | 171 |
CFZNH
+
–DCF
−
–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) 2)
|
93 | 157 |
CFZNH
+
–DCF
−
–DCF–EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
93 | 157 |
CFZNH
+
–DCF
−
–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (polymorph II) 1) (polymorph II) |
— | 157a |
3.1.1.3 CFZNH + –DCF − –MeCN–H 2 O (1 : 1 : 1 : 2) solvated dihydrated salt. CFZNH + –DCF − –MeCN–H 2 O (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) solvated dihydrated salt crystallizes in space group P
2) solvated dihydrated salt crystallizes in space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Tables 1 and S1†). One clofaziminium cation, one diclofenac anion, two water and one acetonitrile molecules constitute the asymmetric unit. A R12(7) motif is observed between clofaziminium and diclofenac (N4–H4⋯O1 and N3–H3⋯O1 charge-assisted H-bonds, Table S2† and Fig. 5(a)). An intramolecular N5–H5⋯O1 H-bond is also present within the diclofenac anion. Water molecules bridge diclofenac anion and clofaziminium through O3–H3OA⋯O2, O4–H4OB⋯O3 and O4–H4OA⋯N2 H-bonds (Table S2†), forming 8-component clusters (2 clofaziminium cations, 2 diclofenac anions and 4 water molecules, Fig. 5(b) and (c)). These 8-component clusters are extended along a-axis thanks to water tetramers (R44(8) motif, O3–H3OB⋯O4 and O4–H4OB⋯O3, Table S2† and Fig. 5(c)).
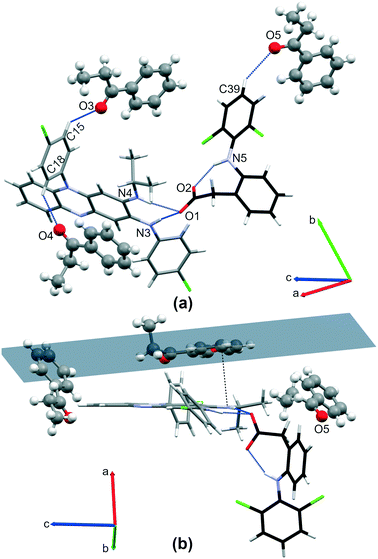
(Tables 1 and S1†). One clofaziminium cation, one diclofenac anion, two water and one acetonitrile molecules constitute the asymmetric unit. A R12(7) motif is observed between clofaziminium and diclofenac (N4–H4⋯O1 and N3–H3⋯O1 charge-assisted H-bonds, Table S2† and Fig. 5(a)). An intramolecular N5–H5⋯O1 H-bond is also present within the diclofenac anion. Water molecules bridge diclofenac anion and clofaziminium through O3–H3OA⋯O2, O4–H4OB⋯O3 and O4–H4OA⋯N2 H-bonds (Table S2†), forming 8-component clusters (2 clofaziminium cations, 2 diclofenac anions and 4 water molecules, Fig. 5(b) and (c)). These 8-component clusters are extended along a-axis thanks to water tetramers (R44(8) motif, O3–H3OB⋯O4 and O4–H4OB⋯O3, Table S2† and Fig. 5(c)).
3.1.1.4 CFZNH + –DCF − –SAL–H 2 O (4 : 4 : 9 : 1) solvated hydrated salt. CFZNH + –DCF − –SAL–H 2 O (4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) solvated hydrated salt crystallizes in P
1) solvated hydrated salt crystallizes in P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (Tables 1 and S1†). The asymmetric unit contains two clofaziminium cations, two diclofenac anions, 4.5 salicylaldehyde and a half water molecules (one salicylaldehyde and the water are located on an inversion center). Clofaziminium cations interact with diclofenac anions through charge-assisted H-bonds (R12(7) motif, N4–H4⋯O1 and N3–H3⋯O1, as well as N8–H8⋯O3 and N9–H9⋯O3, Table S2† and Fig. 6(a)). Intramolecular H-bonds are observed within diclofenac anions (N5–H5⋯O1 and N10–H10⋯O3, Table S2†). Two salicylaldehyde molecules are H-bonded, through their phenol moiety, to diclofenac anion (O5S–H5OS⋯O2 as well as O7T–H7OT⋯O4, Table S2† and Fig. 6(a)). Other solvent molecules are disordered and have their phenol moiety oriented to allow intramolecular H-bonds (O9U–H9OU⋯O10U, O9V–H9OV⋯O10V; O11W–H11W⋯O12W, O11X–H11X⋯O12X and O13Y–H13Y⋯O14Y, Table S2† and Fig. 6(b)). The water molecule interacts with the salicylaldehyde molecule located on an inversion center through O15–H15O⋯O13Y (Table S2†), which is H-bonded to diclofenac anion (C29–H29B⋯O13Y, Table S2†). Chains along a-axis are stabilized by weak H-bonds between diclofenac anions (C38–H38⋯O2 and C79–H79⋯O4, Table S2† and Fig. 6(c)). Several weak H-bonds are also observed between clofazimine and salicylaldehyde molecules (C1–H1⋯O12W, C18–H18⋯O6S, C55–H55⋯O8T, C61–H61A⋯O7T and C28W–H28W⋯O5S, Table S2†). Weak H-bonds are also observed between two CFZNH+ cations (C14–H14⋯N2, C25–H25⋯Cl6, C59–H59⋯N7 and C66–H66⋯Cl2, Table S2†).
space group (Tables 1 and S1†). The asymmetric unit contains two clofaziminium cations, two diclofenac anions, 4.5 salicylaldehyde and a half water molecules (one salicylaldehyde and the water are located on an inversion center). Clofaziminium cations interact with diclofenac anions through charge-assisted H-bonds (R12(7) motif, N4–H4⋯O1 and N3–H3⋯O1, as well as N8–H8⋯O3 and N9–H9⋯O3, Table S2† and Fig. 6(a)). Intramolecular H-bonds are observed within diclofenac anions (N5–H5⋯O1 and N10–H10⋯O3, Table S2†). Two salicylaldehyde molecules are H-bonded, through their phenol moiety, to diclofenac anion (O5S–H5OS⋯O2 as well as O7T–H7OT⋯O4, Table S2† and Fig. 6(a)). Other solvent molecules are disordered and have their phenol moiety oriented to allow intramolecular H-bonds (O9U–H9OU⋯O10U, O9V–H9OV⋯O10V; O11W–H11W⋯O12W, O11X–H11X⋯O12X and O13Y–H13Y⋯O14Y, Table S2† and Fig. 6(b)). The water molecule interacts with the salicylaldehyde molecule located on an inversion center through O15–H15O⋯O13Y (Table S2†), which is H-bonded to diclofenac anion (C29–H29B⋯O13Y, Table S2†). Chains along a-axis are stabilized by weak H-bonds between diclofenac anions (C38–H38⋯O2 and C79–H79⋯O4, Table S2† and Fig. 6(c)). Several weak H-bonds are also observed between clofazimine and salicylaldehyde molecules (C1–H1⋯O12W, C18–H18⋯O6S, C55–H55⋯O8T, C61–H61A⋯O7T and C28W–H28W⋯O5S, Table S2†). Weak H-bonds are also observed between two CFZNH+ cations (C14–H14⋯N2, C25–H25⋯Cl6, C59–H59⋯N7 and C66–H66⋯Cl2, Table S2†).
3.1.1.5 CFZNH + –DCF − –PPP (2 : 2 : 5) solvated salt. CFZNH + –DCF − –PPP (2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5) solvated salt crystallizes in P
5) solvated salt crystallizes in P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (Tables 1 and S1†). The asymmetric unit contains one clofaziminium cation, one diclofenac anion, and 2.5 propiophenone molecules (one PPP is located on an inversion center). The structure of this solvated salt was determined at 295 K and at 100 K. Same interactions are observed at both temperatures, but PPP molecules are disordered at 295 K. Clofaziminium cation and diclofenac anion interact through N3–H3⋯O1 and N4–H4⋯O1 charge-assisted H-bonds (Table S2† and Fig. 7(a)). Two propiophenone molecules interact with clofaziminium through weak H-bonds (C15–H15⋯O3 and C18–H18⋯O4, Table S2† and Fig. 7(a)). Clofaziminium cations are stacked in a head-to-tail fashion (centroid–centroid distance of 3.519(1) Å, interplanar distance of 3.430(1) Å and horizontal displacement of 0.788 Å (planes and centroids were calculated using atoms C5 C6 C11 C12)). Moreover, a cation–π interaction is observed between iminium moiety of CFZNH+ and one propiophenone molecule (vertical distance: 3.719 Å (between N4+ and the plane passing through C54 C55 C56 C57 C58 and C59) and horizontal displacement of 0.837 Å, Fig. 7(b)).
space group (Tables 1 and S1†). The asymmetric unit contains one clofaziminium cation, one diclofenac anion, and 2.5 propiophenone molecules (one PPP is located on an inversion center). The structure of this solvated salt was determined at 295 K and at 100 K. Same interactions are observed at both temperatures, but PPP molecules are disordered at 295 K. Clofaziminium cation and diclofenac anion interact through N3–H3⋯O1 and N4–H4⋯O1 charge-assisted H-bonds (Table S2† and Fig. 7(a)). Two propiophenone molecules interact with clofaziminium through weak H-bonds (C15–H15⋯O3 and C18–H18⋯O4, Table S2† and Fig. 7(a)). Clofaziminium cations are stacked in a head-to-tail fashion (centroid–centroid distance of 3.519(1) Å, interplanar distance of 3.430(1) Å and horizontal displacement of 0.788 Å (planes and centroids were calculated using atoms C5 C6 C11 C12)). Moreover, a cation–π interaction is observed between iminium moiety of CFZNH+ and one propiophenone molecule (vertical distance: 3.719 Å (between N4+ and the plane passing through C54 C55 C56 C57 C58 and C59) and horizontal displacement of 0.837 Å, Fig. 7(b)).
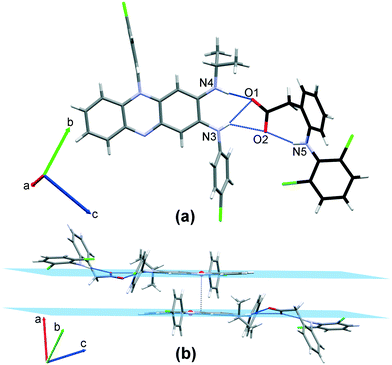
3.1.1.6 CFZNH + –DCF − (1 : 1) salt. CFZNH + –DCF − (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) salt crystallizes in space group P
1) salt crystallizes in space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Tables 1 and S1†) with one clofaziminium cation and one diclofenac anion in the asymmetric unit. Clofaziminium interacts with diclofenac anion through charge-assisted H-bond (R12(7) motif, N4–H4⋯O1 and N3–H3⋯O1, Table S2† and Fig. 8). An intramolecular H-bond is observed N5–H5⋯O2, Table S2† within diclofenac anion. Interestingly, in this structure, the carboxylate of diclofenac anion is almost coplanar to the iminophenazine moiety of clofazimine (angle between planes passing through N4–C9–C8–N3 and O1–C28–O2–C29 is only 14.3°, Table 3) which is not the case in solvated structures (angles between the same planes are comprised between 83.2° and 62.5°, Table 3). This orientation results in a supplementary H-bond between clofaziminium and diclofenac (N3–H3⋯O2 Table S2† and Fig. 8). Clofaziminium cations are stacked in a head-to-tail fashion (Fig. 8, centroid–centroid distance: 3.592(2) Å, orthogonal projection distance: 3.443(1) Å and horizontal offset of 1.026 Å, with centroids and planes calculated from C5 C6 C11 and C12 atoms). This crystalline phase can also be obtained by liquid-assisted grinding of CFZ with DCF (in 1
(Tables 1 and S1†) with one clofaziminium cation and one diclofenac anion in the asymmetric unit. Clofaziminium interacts with diclofenac anion through charge-assisted H-bond (R12(7) motif, N4–H4⋯O1 and N3–H3⋯O1, Table S2† and Fig. 8). An intramolecular H-bond is observed N5–H5⋯O2, Table S2† within diclofenac anion. Interestingly, in this structure, the carboxylate of diclofenac anion is almost coplanar to the iminophenazine moiety of clofazimine (angle between planes passing through N4–C9–C8–N3 and O1–C28–O2–C29 is only 14.3°, Table 3) which is not the case in solvated structures (angles between the same planes are comprised between 83.2° and 62.5°, Table 3). This orientation results in a supplementary H-bond between clofaziminium and diclofenac (N3–H3⋯O2 Table S2† and Fig. 8). Clofaziminium cations are stacked in a head-to-tail fashion (Fig. 8, centroid–centroid distance: 3.592(2) Å, orthogonal projection distance: 3.443(1) Å and horizontal offset of 1.026 Å, with centroids and planes calculated from C5 C6 C11 and C12 atoms). This crystalline phase can also be obtained by liquid-assisted grinding of CFZ with DCF (in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) with EtOAc as solvent (Fig. S3(c)†).
1 molar ratio) with EtOAc as solvent (Fig. S3(c)†).
| Structure | Packing coefficient | Angle between N4–C9–C8–N3 and O1–C28–O2–C29 planes (°) |
|---|---|---|
| a Angle between planes passing through N8–C49–C50–N9 atoms of second clofaziminium cation and O3–C69–O4–C70 atoms of second diclofenac anion. | ||
CFZNH
+
–DCF
−
–MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0.74) 0.74)
|
0.661 | 83.0 |
CFZNH
+
–DCF
−
–EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
0.658 | 73.3 |
CFZNH
+
–DCF
−
–EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (100 K) 1) (100 K) |
0.675 | 72.2 |
CFZNH
+
–DCF
−
–MeCN–H
2
O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) (100 K) 2) (100 K) |
0.677 | 64.9 |
CFZNH
+
–DCF
−
–SAL–H
2
O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 9 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (100 K) 1) (100 K) |
0.700 | 74.5; 83.2a |
CFZNH
+
–DCF
−
–PPP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5) 5)
|
0.698 | 81.3 |
CFZNH
+
–DCF
−
–PPP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5) (100 K) 5) (100 K) |
0.696 | 80.9 |
CFZNH
+
–DCF
−
(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
0.653 | 14.3 |
CFZNH
+
–DCF
−
–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (polymorph I) 1) (polymorph I) |
0.642 | 66.8 |
CFZNH
+
–DCF
−
–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) 2)
|
0.665 | 63.4 |
CFZNH
+
–DCF
−
–DCF–EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
0.657 | 62.5 |
CFZNH
+
–DCF
−
–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (polymorph II) 1) (polymorph II) |
0.660 | 46.4 |
Melting point of this unsolvated salt was determined as 186 °C by DSC analysis (Fig. S4† and Table 2). Interestingly, this unsolvated salt has a smaller packing coefficient than the solvated 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 salts (Table 3). This is in accordance with the presence of voids (15.41 Å3, 0.8% of the unit cell volume). This lower packing efficiency could explain solvent insertion (and so solvate crystallization) when diclofenac anion–solvent or clofaziminium cation–solvent interactions are possible.
1 salts (Table 3). This is in accordance with the presence of voids (15.41 Å3, 0.8% of the unit cell volume). This lower packing efficiency could explain solvent insertion (and so solvate crystallization) when diclofenac anion–solvent or clofaziminium cation–solvent interactions are possible.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 salts.
Salts combining clofazimine with diclofenac are, as initially expected, prone to solvate formation as indicated by the majority of the structures described in this work. First determined structure (CFZNH+–DCF−–MeOH (1
1 salts.
Salts combining clofazimine with diclofenac are, as initially expected, prone to solvate formation as indicated by the majority of the structures described in this work. First determined structure (CFZNH+–DCF−–MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0.74)) contains toxic solvent (MeOH), which is H-bonded to diclofenac anion.
0.74)) contains toxic solvent (MeOH), which is H-bonded to diclofenac anion.
To avoid presence of toxic solvent, several options can be considered. The most evident ones, would be salt preparation without solvent (neat grinding) or with non-toxic/pharmaceutically accepted solvents (water, ethanol40) or desolvation of solvated salts. Grinding clofazimine with diclofenac in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in absence of solvent as well as in presence of water (LAG H2O) resulted in a physical mixture of clofazimine with diclofenac (Fig. S3(h)†), thus not yielding any reaction. Moreover, crystallization experiment in only water as solvent was unsuccessful because of the low aqueous solubility of clofazimine. Since water as a solvent turned out to be unsuccessful and complete desolvation of CFZNH+–DCF−–MeOH (1
1 molar ratio in absence of solvent as well as in presence of water (LAG H2O) resulted in a physical mixture of clofazimine with diclofenac (Fig. S3(h)†), thus not yielding any reaction. Moreover, crystallization experiment in only water as solvent was unsuccessful because of the low aqueous solubility of clofazimine. Since water as a solvent turned out to be unsuccessful and complete desolvation of CFZNH+–DCF−–MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0.74) is difficult to achieve before melting of the powder, another pharmaceutically accepted solvent, namely ethanol, was chosen. In this case, solvent crystallization experiment as well as liquid-assisted ball milling, led to the same solvated salt: CFZNH+–DCF−–EtOH (1
0.74) is difficult to achieve before melting of the powder, another pharmaceutically accepted solvent, namely ethanol, was chosen. In this case, solvent crystallization experiment as well as liquid-assisted ball milling, led to the same solvated salt: CFZNH+–DCF−–EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1), in which, as expected, ethanol interacted with DCF− through H-bond.
1), in which, as expected, ethanol interacted with DCF− through H-bond.
To further investigate the parameters influencing the crystallization of (un)solvated salts, another protic solvent of higher molecular volume, salicylaldehyde (111 Å3 (ref. 38)) was selected. Despite their high molecular volume, salicylaldehyde molecules are included in the structure of CFZNH+–DCF−–SAL–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1). Interestingly more than one salicylaldehyde molecule as well as one water molecule have been accommodated in the structure probably to allow a good packing (this structure turned to have the best packing coefficient, Table 3). The existence of this solvate proves that the ability of solvent to interact with solute via weak interactions may be one of the factors explaining why even bulky solvents can still be trapped in the crystal.
1). Interestingly more than one salicylaldehyde molecule as well as one water molecule have been accommodated in the structure probably to allow a good packing (this structure turned to have the best packing coefficient, Table 3). The existence of this solvate proves that the ability of solvent to interact with solute via weak interactions may be one of the factors explaining why even bulky solvents can still be trapped in the crystal.
High molecular volume did not hamper solvent insertion in the structure, at least for protic solvents that are able to interact with the solute through H-bond. We then focused on aprotic solvents. First, we selected acetonitrile (molecular volume of 46 Å3 (ref. 38)). However crystals obtained in these conditions corresponded to CFZNH+–DCF−–MeCN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2), a solvated hydrated salt, unstable at room temperature. Three other aprotic solvents (N,N-dimethylformamide, 78 Å3, ethylacetate, 91 Å3 and propiophenone, 136 Å3 (ref. 38)) with larger molecular volumes were then selected to perform crystallization experiments of CFZ and DCF in 1
2), a solvated hydrated salt, unstable at room temperature. Three other aprotic solvents (N,N-dimethylformamide, 78 Å3, ethylacetate, 91 Å3 and propiophenone, 136 Å3 (ref. 38)) with larger molecular volumes were then selected to perform crystallization experiments of CFZ and DCF in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. A larger molecular volume could potentially hamper solvent inclusion in the structure. However, we still observed solvate formation: crystals obtained from DMF corresponded to the known clofazimine solvate CFZ–DMF (CSD refcode: CEKTER41); crystallization experiments performed in propiophenone led to a solvated salt, CFZNH+–DCF−–PPP (2
1 molar ratio. A larger molecular volume could potentially hamper solvent inclusion in the structure. However, we still observed solvate formation: crystals obtained from DMF corresponded to the known clofazimine solvate CFZ–DMF (CSD refcode: CEKTER41); crystallization experiments performed in propiophenone led to a solvated salt, CFZNH+–DCF−–PPP (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5), despite the absence of H-bond donor on propiophenone. The formation of the propiophenone solvate highlights again the fact that the molecular volume is not the only parameter affecting solvate formation, and that the interactions between the solvent and the solute are of crucial importance as well. In this case, propiophenone interacts with clofaziminium through π–cation interaction. Very interestingly, crystals of CFZNH+–DCF−(1
5), despite the absence of H-bond donor on propiophenone. The formation of the propiophenone solvate highlights again the fact that the molecular volume is not the only parameter affecting solvate formation, and that the interactions between the solvent and the solute are of crucial importance as well. In this case, propiophenone interacts with clofaziminium through π–cation interaction. Very interestingly, crystals of CFZNH+–DCF−(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) unsolvated salt could however be obtained in ethylacetate, an aprotic molecule with a smaller molecular volume than propiophenone. The absence of the aromatic ring in ethylacetate, in contrast to propiophenone, results in a smaller number of potential interactions that it can form with clofazimine and thus may explain why ethylacetate is not incorporated into the structure. This unsolvated salt does not correspond to the crystalline phase appearing upon desolvation of CFZNH+–DCF−–MeOH (1
1) unsolvated salt could however be obtained in ethylacetate, an aprotic molecule with a smaller molecular volume than propiophenone. The absence of the aromatic ring in ethylacetate, in contrast to propiophenone, results in a smaller number of potential interactions that it can form with clofazimine and thus may explain why ethylacetate is not incorporated into the structure. This unsolvated salt does not correspond to the crystalline phase appearing upon desolvation of CFZNH+–DCF−–MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0.74), which could indicate the existence of another polymorph of CFZNH+–DCF−(1
0.74), which could indicate the existence of another polymorph of CFZNH+–DCF−(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1).
1).
3.2 Investigation of CFZ–DCF 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 2 cocrystal of salts
2 cocrystal of salts
The second part of this work is dedicated to studying whether diclofenac molecule can bind diclofenac anion instead of the solvent molecule via O–H⋯O bond. We followed the same solvent selection criteria as in the first part and chose the solvents that would be less likely to be incorporated into the structure. The addition of a second diclofenac molecule in the system is expected to generate other types of crystal packings than those obtained for salts in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. Therefore, solvents leading to unsolvated form(s) in the case of 1
1 molar ratio. Therefore, solvents leading to unsolvated form(s) in the case of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 salts could potentially lead to solvated salts in a 1
1 salts could potentially lead to solvated salts in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio and vice versa. The solvents selected for this second part of the study were therefore restricted to low-boiling aprotic (EtOAc and MeCN) and non-toxic protic (EtOH) solvents. Indeed, MeCN and EtOAc could potentially be included in the system if they allow a more efficient packing, however these two solvents should not normally interact with the solute (CFZNH+, DCF− and DCF) through H-bond or stacking. These solvents should therefore not be strongly bound within the crystal structure and, due to their low boiling point, may easily evaporate from the solvated structure if the latter is formed. When a diclofenac molecule is available in the solution, it should compete with protic solvents for the diclofenac anion binding site, potentially leading to 1
2 molar ratio and vice versa. The solvents selected for this second part of the study were therefore restricted to low-boiling aprotic (EtOAc and MeCN) and non-toxic protic (EtOH) solvents. Indeed, MeCN and EtOAc could potentially be included in the system if they allow a more efficient packing, however these two solvents should not normally interact with the solute (CFZNH+, DCF− and DCF) through H-bond or stacking. These solvents should therefore not be strongly bound within the crystal structure and, due to their low boiling point, may easily evaporate from the solvated structure if the latter is formed. When a diclofenac molecule is available in the solution, it should compete with protic solvents for the diclofenac anion binding site, potentially leading to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CFZ–DCF forms. However, inclusion of a protic solvent could still be possible (if its shape and size can be accommodated in the crystal packing) because DCF molecule also possesses H-bond acceptor. Although, the potential O–H⋯O interaction between DCF molecule and the solvent would be expected to be less strong with diclofenac molecule than with the anion.
2 CFZ–DCF forms. However, inclusion of a protic solvent could still be possible (if its shape and size can be accommodated in the crystal packing) because DCF molecule also possesses H-bond acceptor. Although, the potential O–H⋯O interaction between DCF molecule and the solvent would be expected to be less strong with diclofenac molecule than with the anion.
3.2.1.1 CFZNH + –DCF − –DCF (1 : 1 : 1) cocrystal of salt (polymorph I). CFZNH + –DCF − –DCF (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) cocrystal of salt (polymorph I) crystallizes in P
1) cocrystal of salt (polymorph I) crystallizes in P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (Tables 1 and S1†). The asymmetric unit comprises one clofaziminium cation, one diclofenac anion and one diclofenac molecule. Clofaziminium interacts with diclofenac anion through charge-assisted H-bond (R22(9) motif, N4–H4⋯O1 and N3–H3⋯O2, Table S2† and Fig. 9(a)). Intramolecular H-bonds are observed within diclofenac anion and molecule (N5–H5⋯O1 and N6–H6⋯O4, Table S2† and Fig. 9(a)). Diclofenac anion and molecule interact together through O3–H3O⋯O2 H-bond (Table S2†). This interaction is very similar to the one between diclofenac anion and ethanol in CFZNH+–DCF−–EtOH (1
space group (Tables 1 and S1†). The asymmetric unit comprises one clofaziminium cation, one diclofenac anion and one diclofenac molecule. Clofaziminium interacts with diclofenac anion through charge-assisted H-bond (R22(9) motif, N4–H4⋯O1 and N3–H3⋯O2, Table S2† and Fig. 9(a)). Intramolecular H-bonds are observed within diclofenac anion and molecule (N5–H5⋯O1 and N6–H6⋯O4, Table S2† and Fig. 9(a)). Diclofenac anion and molecule interact together through O3–H3O⋯O2 H-bond (Table S2†). This interaction is very similar to the one between diclofenac anion and ethanol in CFZNH+–DCF−–EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1). Indeed, even if these two structures are not isostructural, when clofaziminium and diclofenac anion are overlaid it can be observed that EtOH has a conformation similar to the first atoms (H3O, O3, C42 and C43) of diclofenac molecule (Fig. 9(d)).
1). Indeed, even if these two structures are not isostructural, when clofaziminium and diclofenac anion are overlaid it can be observed that EtOH has a conformation similar to the first atoms (H3O, O3, C42 and C43) of diclofenac molecule (Fig. 9(d)).
All previously described interactions stabilize CFZNH+–DCF−–DCF assemblies. Two types of dimers of these assemblies, which are respectively stabilized by C31–H31⋯O3 and C3–H3A⋯O3 weak H-bonds (Table S2 † and Fig. 9(b) and (c)), are also observed in the structure of CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) cocrystal of salt. π–π stacking interactions are also observed between clofaziminium cations. The first one occurs between the phenazine core of two CFZNH+, with a centroid–centroid distance of 3.669(1) Å, an orthogonal projection distance of 3.413(1) Å and an horizontal offset of 1.345 Å (planes and centroid calculated using C5 C6 C11 and C12 atoms, Fig. 9(e)). This stacking is further stabilized by C3–H3A⋯O3 (Fig. 9(c)) weak H-bonds. A second π–π stacking interaction is also observed between two C19 C20 C21 C22 C23 C24 aromatic rings of clofaziminium with a centroid–centroid distance of 3.801(1) Å, an orthogonal projection distance of 3.357(1) Å and an horizontal offset of 1.781 Å (Fig. 9(f)). Finally a C–H⋯π interaction is observed between C18–H18 of clofaziminium and diclofenac molecule (C44 C45 C46 C47 C48 C49) with a H⋯centroid distance of 2.59 Å, a C⋯centroid distance of 3.443(3) Å and a C–H⋯centroid angle of 153° (Fig. 9(g)).
1) cocrystal of salt. π–π stacking interactions are also observed between clofaziminium cations. The first one occurs between the phenazine core of two CFZNH+, with a centroid–centroid distance of 3.669(1) Å, an orthogonal projection distance of 3.413(1) Å and an horizontal offset of 1.345 Å (planes and centroid calculated using C5 C6 C11 and C12 atoms, Fig. 9(e)). This stacking is further stabilized by C3–H3A⋯O3 (Fig. 9(c)) weak H-bonds. A second π–π stacking interaction is also observed between two C19 C20 C21 C22 C23 C24 aromatic rings of clofaziminium with a centroid–centroid distance of 3.801(1) Å, an orthogonal projection distance of 3.357(1) Å and an horizontal offset of 1.781 Å (Fig. 9(f)). Finally a C–H⋯π interaction is observed between C18–H18 of clofaziminium and diclofenac molecule (C44 C45 C46 C47 C48 C49) with a H⋯centroid distance of 2.59 Å, a C⋯centroid distance of 3.443(3) Å and a C–H⋯centroid angle of 153° (Fig. 9(g)).
The powder pattern calculated from SCXRD data corresponds to the one of the batch powder prepared by LAG EtOH (Fig. S3(d)†). This cocrystal of salt melts at 171 °C (Fig. S4† and Table 2).
3.2.1.2 CFZNH + –DCF − –DCF–MeCN (1 : 1 : 1 : 2) solvated cocrystal of salt. CFZNH + –DCF − –DCF–MeCN (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) solvated cocrystal of salt also crystallizes in space group P
2) solvated cocrystal of salt also crystallizes in space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Tables 1 and S1†). The asymmetric unit contains one clofaziminium cation, one diclofenac anion, one diclofenac molecule and two acetonitrile molecules (one MeCN molecule is disordered). Clofaziminium and diclofenac anion interact through charge-assisted H-bonds (R12(7) motif, N4–H4⋯O1 and N3–H3⋯O1, Table S2† and Fig. 10(a)). In both diclofenac (anion and molecule) an intramolecular H-bond is observed (N5–H5⋯O2 and N6–H6⋯O4, Table S2†). Molecular diclofenac and diclofenac anion interact through O3–H3B⋯O2 H-bond (Table S2† and Fig. 10(a)). Such H-bonds (N4–H4⋯O1, N3–H3⋯O1 and O3–H3B⋯O2) stabilize CFZNH+–DCF−–DCF assemblies, which further form dimers through C38–H38⋯O4 (Fig. 10(b)). A weak H-bond, stabilizing chains along a-axis, is also observed between diclofenac molecules (C43–H43A⋯O3, Fig. 10(c) and (d)). This crystalline phase can also be obtained from liquid-assisted grinding of CFZ and DCF in 1
(Tables 1 and S1†). The asymmetric unit contains one clofaziminium cation, one diclofenac anion, one diclofenac molecule and two acetonitrile molecules (one MeCN molecule is disordered). Clofaziminium and diclofenac anion interact through charge-assisted H-bonds (R12(7) motif, N4–H4⋯O1 and N3–H3⋯O1, Table S2† and Fig. 10(a)). In both diclofenac (anion and molecule) an intramolecular H-bond is observed (N5–H5⋯O2 and N6–H6⋯O4, Table S2†). Molecular diclofenac and diclofenac anion interact through O3–H3B⋯O2 H-bond (Table S2† and Fig. 10(a)). Such H-bonds (N4–H4⋯O1, N3–H3⋯O1 and O3–H3B⋯O2) stabilize CFZNH+–DCF−–DCF assemblies, which further form dimers through C38–H38⋯O4 (Fig. 10(b)). A weak H-bond, stabilizing chains along a-axis, is also observed between diclofenac molecules (C43–H43A⋯O3, Fig. 10(c) and (d)). This crystalline phase can also be obtained from liquid-assisted grinding of CFZ and DCF in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio and with MeCN as solvent (LAG) (Fig. S3(e)†).
2 molar ratio and with MeCN as solvent (LAG) (Fig. S3(e)†).
TG/DSC analysis of CFZNH+–DCF−–DCF–MeCN (1 : 1 : 1 : 2) (powder obtained by liquid-assisted grinding experiment) reveals a 6.2% weight loss between 50 and 140 °C on the TG curve (Fig. S4†) which is associated with an endothermic event on the DSC curve (onset: 93 °C) (Fig. S4† and Table 2). These events can be attributed to desolvation (calculated MeCN content of 7.2% in CFZNH+–DCF−–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2)). The endothermic event observed at 157 °C corresponds to the melting of the desolvated phase and suggests the formation of either an eutectic mixture or of an unsolvated crystalline phase combining CFZ and DCF (Fig. S4† and Table 2). A variable-temperature PXRD experiment performed on CFZNH+–DCF−–DCF–MeCN (1
2)). The endothermic event observed at 157 °C corresponds to the melting of the desolvated phase and suggests the formation of either an eutectic mixture or of an unsolvated crystalline phase combining CFZ and DCF (Fig. S4† and Table 2). A variable-temperature PXRD experiment performed on CFZNH+–DCF−–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) confirmed the formation of a new crystalline phase upon heating which does not correspond to CFZNH+–DCF−–DCF (1
2) confirmed the formation of a new crystalline phase upon heating which does not correspond to CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) polymorph I (Fig. S6†). Results from variable-temperature PXRD experiment and DSC/TG analyses suggest the formation of a second polymorph of CFZNH+–DCF−–DCF (1
1) polymorph I (Fig. S6†). Results from variable-temperature PXRD experiment and DSC/TG analyses suggest the formation of a second polymorph of CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1).
1).
3.2.1.3 CFZNH + –DCF − –DCF–EtOAc (1 : 1 : 1 : 1) solvated cocrystal of salt. CFZNH + –DCF − –DCF–EtOAc (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) solvated cocrystal of salt crystallizes in P
1) solvated cocrystal of salt crystallizes in P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (Tables 1 and S1†). The asymmetric unit contains one clofaziminium cation, one diclofenac anion, one diclofenac molecule and one ethylacetate molecule. CFZNH+–DCF−–DCF–EtOAc (1
space group (Tables 1 and S1†). The asymmetric unit contains one clofaziminium cation, one diclofenac anion, one diclofenac molecule and one ethylacetate molecule. CFZNH+–DCF−–DCF–EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) is isostructural to CFZNH+–DCF−–DCF–MeCN (1
1) is isostructural to CFZNH+–DCF−–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) and the same interactions are observed in both structures (Fig. 10(e) and (f)). CFZNH+–DCF−–DCF–EtOAc (1
2) and the same interactions are observed in both structures (Fig. 10(e) and (f)). CFZNH+–DCF−–DCF–EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) is prepared by ball-milling CFZ and DCF in 1
1) is prepared by ball-milling CFZ and DCF in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio in presence of few drops of EtOAc (Fig. S3(f)†). TG/DSC analysis of the CFZ–DCF 1
2 molar ratio in presence of few drops of EtOAc (Fig. S3(f)†). TG/DSC analysis of the CFZ–DCF 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 LAG EtOAc powder reveals a 5.6% weight loss between 50 and 140 °C, associated to an endothermic event on the DSC curve (onset: 73 °C), indicating desolvation of CFZNH+–DCF−–DCF–EtOAc (1
2 LAG EtOAc powder reveals a 5.6% weight loss between 50 and 140 °C, associated to an endothermic event on the DSC curve (onset: 73 °C), indicating desolvation of CFZNH+–DCF−–DCF–EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (expected solvent content from SCXRD data: 7.6%). The single endothermic event observed at 157 °C corresponds to the melting of the desolvated phase (Fig. S4† and Table 2). A PXRD experiment performed on heated powder (130 °C) of CFZNH+–DCF−–DCF–EtOAc (1
1) (expected solvent content from SCXRD data: 7.6%). The single endothermic event observed at 157 °C corresponds to the melting of the desolvated phase (Fig. S4† and Table 2). A PXRD experiment performed on heated powder (130 °C) of CFZNH+–DCF−–DCF–EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) confirmed the formation of a new crystalline phase, identical to the one obtained by heating CFZNH+–DCF−–DCF–MeCN (1
1) confirmed the formation of a new crystalline phase, identical to the one obtained by heating CFZNH+–DCF−–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) (Fig. S3(g)†).
2) (Fig. S3(g)†).
3.2.1.4 CFZNH + –DCF − –DCF (1 : 1 : 1) cocrystal of salt (polymorph II). CFZNH + –DCF − –DCF (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) cocrystal of salt (polymorph II) crystallizes in triclinic P
1) cocrystal of salt (polymorph II) crystallizes in triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (Tables 1 and S1†). Single crystals of this unsolvated cocrystal of salt were obtained by recrystallization of CFZ–DCF 1
space group (Tables 1 and S1†). Single crystals of this unsolvated cocrystal of salt were obtained by recrystallization of CFZ–DCF 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 powder (physical mixture) in PEG 200. Charge-assisted H-bonds (R12(7), N3–H3⋯O1 and N4–H4⋯O1, Table S2†) are observed between clofaziminium and diclofenac anion. The second oxygen atom of DCF− is involved in a D11(2) H-bond with diclofenac molecule (O3–H3O⋯O2, Table S2†).These H-bonds (R12(7) and D11(2)) stabilize CFZNH+–DCF−–DCF assemblies (Fig. 11(a)). Weak H-bonds between CFZNH+ and DCF molecule (C4–H4⋯O3, Table S2†) stabilize dimers of CFZNH+–DCF−–DCF assemblies (Fig. 11(b)). A second type of such dimers is stabilized by a weak H-bond between DCF− and DCF molecule (C38–H38⋯O4, Table S2† and Fig. 11(c)). Intramolecular H-bonds in DCF− and DCF are also observed (N5–H5⋯O2 and N6–H6⋯O4, Table S2† and Fig. 11(a)). In comparison with CFZNH+–DCF−–DCF (1
2 powder (physical mixture) in PEG 200. Charge-assisted H-bonds (R12(7), N3–H3⋯O1 and N4–H4⋯O1, Table S2†) are observed between clofaziminium and diclofenac anion. The second oxygen atom of DCF− is involved in a D11(2) H-bond with diclofenac molecule (O3–H3O⋯O2, Table S2†).These H-bonds (R12(7) and D11(2)) stabilize CFZNH+–DCF−–DCF assemblies (Fig. 11(a)). Weak H-bonds between CFZNH+ and DCF molecule (C4–H4⋯O3, Table S2†) stabilize dimers of CFZNH+–DCF−–DCF assemblies (Fig. 11(b)). A second type of such dimers is stabilized by a weak H-bond between DCF− and DCF molecule (C38–H38⋯O4, Table S2† and Fig. 11(c)). Intramolecular H-bonds in DCF− and DCF are also observed (N5–H5⋯O2 and N6–H6⋯O4, Table S2† and Fig. 11(a)). In comparison with CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) polymorph I, π–π stacking contribution to crystal packing stabilization is less important as there is no stacking between clofaziminium nor C–H⋯π interactions between clofaziminium and diclofenac molecule. There is however one π–π stacking interaction between two diclofenac anions with a centroid–centroid distance of 3.506(1) Å, an orthogonal projection distance of 3.355(1) Å and an horizontal offset of 1.017 Å (Fig. 11(d), planes and centroids calculated with C36 C37 C38 C39 C40 and C41).
1) polymorph I, π–π stacking contribution to crystal packing stabilization is less important as there is no stacking between clofaziminium nor C–H⋯π interactions between clofaziminium and diclofenac molecule. There is however one π–π stacking interaction between two diclofenac anions with a centroid–centroid distance of 3.506(1) Å, an orthogonal projection distance of 3.355(1) Å and an horizontal offset of 1.017 Å (Fig. 11(d), planes and centroids calculated with C36 C37 C38 C39 C40 and C41).
Despite a quite good overlay of the molecules constituting their asymmetric unit (Fig. 11(e)), CFZNH+–DCF−–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) and CFZNH+–DCF−–DCF (1
2) and CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) are not isostructural as revealed by the crystal packing comparison performed with Mercury (only 9 molecules over 15 overlay despite distance and angle tolerance of 30% and 30°). Diclofenac anion orientation is slightly modified in the unsolvated cocrystal of salt in comparison to the one observed in CFZNH+–DCF−–DCF–MeCN (1
1) are not isostructural as revealed by the crystal packing comparison performed with Mercury (only 9 molecules over 15 overlay despite distance and angle tolerance of 30% and 30°). Diclofenac anion orientation is slightly modified in the unsolvated cocrystal of salt in comparison to the one observed in CFZNH+–DCF−–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2). Indeed, the angle between the planes passing through N4–C9–C8–N3 atoms of CFZNH+ and through O1–C28–O2–C29 of DCF− is different in the two structures (63.44° in CFZNH+–DCF−–DCF–MeCN (1
2). Indeed, the angle between the planes passing through N4–C9–C8–N3 atoms of CFZNH+ and through O1–C28–O2–C29 of DCF− is different in the two structures (63.44° in CFZNH+–DCF−–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2)vs. 46.41° in CFZNH+–DCF−–DCF (1
2)vs. 46.41° in CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1)).
1)).
VT-PXRD and PXRD data measured after heating CFZNH+–DCF−–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) and CFZNH+–DCF−–DCF–EtOAc (1
2) and CFZNH+–DCF−–DCF–EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) indicate a phase transformation from the solvated cocrystal of salts to the CFZNH+–DCF−–DCF (1
1) indicate a phase transformation from the solvated cocrystal of salts to the CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) unsolvated cocrystal of salt (polymorph II) (Fig. S3(g) and S6†). TG/DSC data obtained from CFZNH+–DCF−–DCF–MeCN (1
1) unsolvated cocrystal of salt (polymorph II) (Fig. S3(g) and S6†). TG/DSC data obtained from CFZNH+–DCF−–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) indicate that CFZNH+–DCF−–DCF (1
2) indicate that CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) polymorph II melts at 157 °C (Fig. S4† and Table 2). The difference in melting point observed between polymorph I (171 °C) and polymorph II (157 °C), can be partially explained by the fact that there are more π interactions in polymorph I while intermolecular H-bonds are of comparable strength in both polymorphs. It is interesting to notice that polymorph II has however a better packing (higher density) than polymorph I (packing coefficient of 0.660 vs. 0.642, density of 1.383 g cm−3vs. 1.351 g cm−3 (data collected at 295 K for both structures)) (Table 3).
1) polymorph II melts at 157 °C (Fig. S4† and Table 2). The difference in melting point observed between polymorph I (171 °C) and polymorph II (157 °C), can be partially explained by the fact that there are more π interactions in polymorph I while intermolecular H-bonds are of comparable strength in both polymorphs. It is interesting to notice that polymorph II has however a better packing (higher density) than polymorph I (packing coefficient of 0.660 vs. 0.642, density of 1.383 g cm−3vs. 1.351 g cm−3 (data collected at 295 K for both structures)) (Table 3).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2 cocrystal of salts.
Changing the clofazimine–diclofenac molecular ratio during crystallization experiment allowed the crystallization of a 1
2 cocrystal of salts.
Changing the clofazimine–diclofenac molecular ratio during crystallization experiment allowed the crystallization of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 unsolvated cocrystal of salt in EtOH, while this solvent previously led to a solvated 1
2 unsolvated cocrystal of salt in EtOH, while this solvent previously led to a solvated 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 salt. This result indicates that diclofenac molecule can successfully compete with the solvent for the interaction site with diclofenac anion. However, preparation of 1
1 salt. This result indicates that diclofenac molecule can successfully compete with the solvent for the interaction site with diclofenac anion. However, preparation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 cocrystal of salts did not completely rule out crystallization of solvated forms, as two solvated phases were obtained in acetonitrile and ethylacetate. Interestingly, crystallization of a 1
2 cocrystal of salts did not completely rule out crystallization of solvated forms, as two solvated phases were obtained in acetonitrile and ethylacetate. Interestingly, crystallization of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 cocrystal of salt in EtOAc leads to a solvated form while the same solvent previously lead to an unsolvated CFZNH+–DCF−(1
2 cocrystal of salt in EtOAc leads to a solvated form while the same solvent previously lead to an unsolvated CFZNH+–DCF−(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) salt. EtOAc and MeCN both have a small molecular volume that can be accommodated in the structure if packing allows. However, as expected no strong interactions exist between EtOAc or MeCN and the solutes (CFZNH+, DCF− and DCF).
1) salt. EtOAc and MeCN both have a small molecular volume that can be accommodated in the structure if packing allows. However, as expected no strong interactions exist between EtOAc or MeCN and the solutes (CFZNH+, DCF− and DCF).
TG/DSC analysis of these two solvated cocrystal of salts (CFZNH+–DCF−–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2), CFZNH+–DCF−–DCF–EtOAc (1
2), CFZNH+–DCF−–DCF–EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1)) revealed a weight loss between 50 and 100 °C and a melting point around 157 °C (Fig. S4†). A phase transformation, probably associated with desolvation (and formation of an unsolvated cocrystal of salt combining CFZ and DCF), was identified by PXRD experiments (Fig. S6 and S3(g)†). The powder diffraction pattern obtained after desolvation of CFZNH+–DCF−–DCF–MeCN (1
1)) revealed a weight loss between 50 and 100 °C and a melting point around 157 °C (Fig. S4†). A phase transformation, probably associated with desolvation (and formation of an unsolvated cocrystal of salt combining CFZ and DCF), was identified by PXRD experiments (Fig. S6 and S3(g)†). The powder diffraction pattern obtained after desolvation of CFZNH+–DCF−–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) or CFZNH+–DCF−–DCF–EtOAc (1
2) or CFZNH+–DCF−–DCF–EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) does not correspond to the one of CFZNH+–DCF−–DCF (1
1) does not correspond to the one of CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (polymorph I) (Fig. S6 and S3(g)†), which could be the indication of the existence of a second polymorph of CFZNH+–DCF−–DCF (1
1) (polymorph I) (Fig. S6 and S3(g)†), which could be the indication of the existence of a second polymorph of CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1). To verify this hypothesis, we have tried to obtain single crystals of this new unsolvated crystalline phase using other solvents that cannot be incorporated into the structure. We selected short chain polymers such as PEG (average MW: 200 g mol−1 and 400 g mol−1) and polycaprolactone triol (average MW: 300 g mol−1) as recrystallization solvents because of their higher molecular weight and also because of their molecular ‘flexibility’ in comparison to salicylaldehyde and propiophenone which previously led to solvated salts. Few single crystals of CFZNH+–DCF−–DCF (1
1). To verify this hypothesis, we have tried to obtain single crystals of this new unsolvated crystalline phase using other solvents that cannot be incorporated into the structure. We selected short chain polymers such as PEG (average MW: 200 g mol−1 and 400 g mol−1) and polycaprolactone triol (average MW: 300 g mol−1) as recrystallization solvents because of their higher molecular weight and also because of their molecular ‘flexibility’ in comparison to salicylaldehyde and propiophenone which previously led to solvated salts. Few single crystals of CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (polymorph II) cocrystal of salt were obtained in PEG (average MW: 200 g mol−1). Comparison of the calculated powder pattern of CFZNH+–DCF−–DCF (1
1) (polymorph II) cocrystal of salt were obtained in PEG (average MW: 200 g mol−1). Comparison of the calculated powder pattern of CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (polymorph II) with the one obtained after variable-temperature PXRD experiment performed on CFZNH+–DCF−–DCF–MeCN (1
1) (polymorph II) with the one obtained after variable-temperature PXRD experiment performed on CFZNH+–DCF−–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) confirmed desolvation and phase transformation that were inferred from VT-PXRD and TG/DSC experiments.
2) confirmed desolvation and phase transformation that were inferred from VT-PXRD and TG/DSC experiments.
4 Conclusions
Clofaziminium![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : diclofenac system is a great example of structural variety that can be reached when a clever solvent selection is applied to explore various crystalline forms. As expected, diclofenac anion is able to interact with protic solvent molecules through H-bonds, leading to the crystallization of solvated drug–drug salts of clofazimine and diclofenac. Protic and aprotic solvents of increasing molecular volumes were taken in order to understand how solvent impacts crystallisation of clofazimine and diclofenac.
: diclofenac system is a great example of structural variety that can be reached when a clever solvent selection is applied to explore various crystalline forms. As expected, diclofenac anion is able to interact with protic solvent molecules through H-bonds, leading to the crystallization of solvated drug–drug salts of clofazimine and diclofenac. Protic and aprotic solvents of increasing molecular volumes were taken in order to understand how solvent impacts crystallisation of clofazimine and diclofenac.
When facing probable solvate formation, the potential solute–solvent interactions can be more relevant than the size of the solvent. For example, the unsolvated CFZNH+–DCF−(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) salt crystallized only in ethylacetate, an aprotic solvent having a lower molecular volume than another selected solvent, propiophenone. Despite its high molecular volume, propiophenone was incorporated into the structure owing to π interactions, resulting in the corresponding solvated salt. A particular feature of the CFZNH+–DCF−(1
1) salt crystallized only in ethylacetate, an aprotic solvent having a lower molecular volume than another selected solvent, propiophenone. Despite its high molecular volume, propiophenone was incorporated into the structure owing to π interactions, resulting in the corresponding solvated salt. A particular feature of the CFZNH+–DCF−(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) unsolvated structure is that the carboxylate of DCF− is almost coplanar to CFZNH+. It is, to the best of our knowledge, the first time that this specific carboxylate orientation is reported in a clofaziminium salt. These results highlight the importance of considering potential solute–solvent interactions not only in terms of H-bond interactions, but also other weak interactions such as probable π interactions.
1) unsolvated structure is that the carboxylate of DCF− is almost coplanar to CFZNH+. It is, to the best of our knowledge, the first time that this specific carboxylate orientation is reported in a clofaziminium salt. These results highlight the importance of considering potential solute–solvent interactions not only in terms of H-bond interactions, but also other weak interactions such as probable π interactions.
Changing the clofazimine to diclofenac ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 allowed an extra diclofenac molecule to compete for the binding site with the protic solvent molecules. More particularly, diclofenac anion successfully beated EtOH for the H-bond interaction on diclofenac anion resulting in an unsolvated cocrystal of salt (CFZNH+–DCF−–DCF (1
2 allowed an extra diclofenac molecule to compete for the binding site with the protic solvent molecules. More particularly, diclofenac anion successfully beated EtOH for the H-bond interaction on diclofenac anion resulting in an unsolvated cocrystal of salt (CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) polymorph I) with the 1
1) polymorph I) with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 clofazimine to diclofenac molar ratio. The second polymorph of CFZNH+–DCF−–DCF (1
2 clofazimine to diclofenac molar ratio. The second polymorph of CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) emerged from desolvation of CFZNH+–DCF−–DCF–MeCN (1
1) emerged from desolvation of CFZNH+–DCF−–DCF–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) and CFZNH+–DCF−–DCF–EtOAc (1
2) and CFZNH+–DCF−–DCF–EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1). The latter underlines that undesired solvated forms can potentially give access to other desired structures. Recrystallization using unconventional solvents such as short chain polymers exhibiting good fluidity, high molecular flexibility and high molecular weight can also be an option to avoid solvent inclusion into the structure, as illustrated by the successful growth of CFZNH+–DCF−–DCF (1
1). The latter underlines that undesired solvated forms can potentially give access to other desired structures. Recrystallization using unconventional solvents such as short chain polymers exhibiting good fluidity, high molecular flexibility and high molecular weight can also be an option to avoid solvent inclusion into the structure, as illustrated by the successful growth of CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (polymorph II) in PEG 200.
1) (polymorph II) in PEG 200.
Changing the drug![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) drug ratio in the structure expectedly affects the physico-chemical properties of the corresponding solid forms, for instance, the melting point of CFZNH+–DCF−(1
drug ratio in the structure expectedly affects the physico-chemical properties of the corresponding solid forms, for instance, the melting point of CFZNH+–DCF−(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) is 186 °C whereas those of CFZNH+–DCF−–DCF (1
1) is 186 °C whereas those of CFZNH+–DCF−–DCF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) polymorphs I and II are 171 °C and 157 °C respectively.
1) polymorphs I and II are 171 °C and 157 °C respectively.
Conflicts of interest
There are no conflicts to declare. The European Commission's support for the production of this publication does not constitute an endorsement of the contents, which reflect the views only of the authors, and the commission cannot be held responsible for any use which may be made of the information contained therein.Acknowledgements
This work was performed on XRD and TA equipments from the PC2 platform at the University of Namur. This research used resources of the “Plateforme Technologique de Calcul Intensif (PTCI)” (http://www.ptci.unamur.be) located at the University of Namur, Belgium, which is supported by the FNRS-FRFC, the Walloon Region, and the University of Namur (Conventions No. 2.5020.11, GEQ U.G006.15, 1610468, and RW/GEQ2016). The PTCI is member of the “Consortium des Équipements de Calcul Intensif (CÉCI)” (http://www.cecihpc.be). M. P. thanks the ERASMUS mobility program for the financial support and Prof S. Guccione. L. B. thanks the FRS-FNRS for the funding (research fellow grant). The authors would like to thank Natalia Tumanova for her invaluable advice on editing and formulation of the article.Notes and references
- U. J. Griesser, in Polymorphism: in the Pharmaceutical Industry, ed. R. Hilfiker, Wiley Online Library, 2006, ch. 8, pp. 211–2332 Search PubMed.
- Ö. Almarsson and M. J. Zaworotko, Chem. Commun., 2004, 1889–1896 RSC.
- H. D. Clarke, K. K. Arora, H. Bass, P. Kavuru, T. T. Ong, T. Pujari, L. Wojtas and M. J. Zaworotko, Cryst. Growth Des., 2010, 10, 2152–2167 CrossRef CAS.
- E. Grothe, H. Meekes, E. Vlieg, J. Ter Horst and R. D. de Gelder, Cryst. Growth Des., 2016, 16, 3237–3243 CrossRef CAS.
- B. D. Johnson, A. Howard, R. Varsolona, J. McCauley and D. K. Ellison, in Analytical profiles of drug substances and excipients, Elsevier, 1999, vol. 26, pp. 319–357 Search PubMed.
- M. L. Peterson, M. B. Hickey, M. J. Zaworotko and Ö. Almarsson, J. Pharm. Pharm. Sci., 2006, 9, 317–326 Search PubMed.
- C. Loschen and A. Klamt, Pharm. Res., 2016, 33, 2794–2804 CrossRef CAS.
- S. Boothroyd, A. Kerridge, A. Broo, D. Buttar and J. Anwar, Cryst. Growth Des., 2018, 18, 1903–1908 CrossRef CAS.
- A. Bērzinš, A. Kons, K. Saršūns, S. Belyakov and A. Actinš, Cryst. Growth Des., 2020, 20, 5767–5784 CrossRef.
- K. Smokrović and V. Stilinović, CrystEngComm, 2020, 22, 1822–1833 RSC.
- K. Takieddin, Y. Z. Khimyak and L. Fabian, Cryst. Growth Des., 2016, 16, 70–81 CrossRef CAS.
- M. C. Cholo, H. C. Steel, P. B. Fourie, W. A. Germishuizen and R. Anderson, J. Antimicrob. Chemother., 2012, 67, 290–298 CrossRef CAS.
- S. Tyagi, N. C. Ammerman, S.-Y. Li, J. Adamson, P. J. Converse, R. V. Swanson, D. V. Almeida and J. H. Grosset, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 869–874 CrossRef CAS.
- D. R. Silva, M. Dalcolmo, S. Tiberi, M. A. Arbex, M. Munoz-Torrico, R. Duarte, L. D'Ambrosio, D. Visca, A. Rendon and M. Gaga, et al. , J. Bras. Pneumol., 2018, 44, 153–160 CrossRef.
- S. Zhang, W. Shi, J. Feng, W. Zhang and Y. Zhang, Emerging Microbes Infect., 2017, 6, e28 Search PubMed.
- G. Sotgiu, S. Tiberi, R. Centis, L. D. Ambrosio, Z. Fuentes, A. Zumla and G. Battista, Int. J. Infect. Dis., 2017, 56, 190–193 CrossRef.
- C. Lange, D. Chesov and J. Heyckendorf, Clin. Microbiol. Infect., 2019, 25, 128–130 CrossRef CAS.
- N. K. Dutta, S. G. Dastidar, A. Kumar, K. Mazumdar, R. Ray and A. N. Chakrabarty, Braz. J. Microbiol., 2004, 35, 316–323 CrossRef CAS.
- K. Mazumdar, S. Dastidar, J. Park and N. Dutta, Eur. J. Clin. Microbiol. Infect. Dis., 2009, 28, 881 CrossRef CAS.
- A. Maitra, S. Bates, M. Shaik, D. Evangelopoulos and I. Abubakar, Br. Med. Bull., 2016, 118, 145–155 CAS.
- V. M. Kroesen, M. I. Gröschel, N. Martinson, A. Zumla, M. Maeurer, T. S. van der Werf and C. Vilaplana, Front. Microbiol., 2017, 8, 772 CrossRef.
- J. Ivanyi and A. Zumla, J. Infect. Dis., 2013, 208, 185–188 CrossRef.
- R. K. Keswani, J. Baik, L. Yeomans, C. Hitzman, A. M. Johnson, A. S. Pawate, P. J. Kenis, N. Rodriguez-Hornedo, K. A. Stringer and G. R. Rosania, Mol. Pharmaceutics, 2015, 12, 2528–2536 CrossRef CAS.
- G. Bolla and A. Nangia, Cryst. Growth Des., 2012, 12, 6250–6259 CrossRef CAS.
- P. Bannigan, E. Durack, C. Madden, M. Lusi and S. P. Hudson, ACS Omega, 2017, 2, 8969–8981 CrossRef CAS.
- L. Bodart, N. Tumanov and J. Wouters, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2019, 75, 674–686 CrossRef CAS.
- M. L. Sousa, M. C. Sarraguça, A. O. dos Santos, J. M. Sarraguça, J. Lopes and P. R. S. Ribeiro, J. Mol. Struct., 2020, 1214, 128226 CrossRef CAS.
- L. Bodart, A. Derlet, X. Buol, T. Leyssens, N. Tumanov and J. Wouters, J. Pharm. Sci., 2020, 109, 3645–3652 CrossRef CAS.
- W. Sun, L. Zuo, T. Zhao, Z. Zhu and G. Shan, Acta Crystallogr., Sect. C: Struct. Chem., 2019, 75, 1644–1651 CrossRef CAS.
- R. C. Clark and J. S. Reid, Acta Crystallogr., Sect. A: Found. Crystallogr., 1995, 51, 887–897 CrossRef.
- Rigaku Oxford Diffraction, CrysAlis PRO, Rigaku Oxford Diffraction Ltd, Yarnton, England, 2019 Search PubMed.
- R. H. Blessing, Acta Crystallogr., Sect. A: Found. Crystallogr., 1995, 51, 33–38 CrossRef.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3–8 CrossRef.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- C. B. Hübschle, G. M. Sheldrick and B. Dittrich, J. Appl. Crystallogr., 2011, 44, 1281–1284 CrossRef.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- C. F. Macrae, I. Sovago, S. J. Cottrell, P. T. Galek, P. McCabe, E. Pidcock, M. Platings, G. P. Shields, J. S. Stevens and M. Towler, et al. , J. Appl. Crystallogr., 2020, 53, 226–235 CrossRef CAS.
- M. Cheminformatics, Molinspiration [Internet], accessed on 21–02- 2020, https://www.molinspiration.com/ Search PubMed.
- G. R. Desiraju and T. Steiner, The weak hydrogen bond: in structural chemistry and biology, International Union of Crystal, 2001, vol. 9 Search PubMed.
- European Medicinal Agency (EMA), Information for the package leaflet regarding ethanol used as an excipient in medicinal products for human use, European Medicinal Agency (EMA), 2019 Search PubMed.
- D. S. Eggleston, W. E. Marsh and D. J. Hodgson, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1984, 40, 288–292 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Extended table of experimental details for the described structures, table of H-bond parameters, ellipsoids plots of the described structures, powder diffraction patterns of the salts prepared by liquid-assisted grinding, TG/DSC data and variable-temperature powder X-ray diffraction data. CCDC 2032488–2032499. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ce01400a |
| This journal is © The Royal Society of Chemistry 2021 |