Conversion of syngas into light olefins over bifunctional ZnCeZrO/SAPO-34 catalysts: regulation of the surface oxygen vacancy concentration and its relation to the catalytic performance†
Yaoya
Luo
ab,
Sen
Wang
 *a,
Shujia
Guo
ab,
Kai
Yuan
ab,
Hao
Wang
a,
Mei
Dong
a,
Zhangfeng
Qin
*a,
Shujia
Guo
ab,
Kai
Yuan
ab,
Hao
Wang
a,
Mei
Dong
a,
Zhangfeng
Qin
 *a,
Weibin
Fan
*a,
Weibin
Fan
 a and
Jianguo
Wang
a and
Jianguo
Wang
 *b
*b
aState Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, P. O. Box 165, Taiyuan, Shanxi 030001, PR China. E-mail: wangsen@sxicc.ac.cn; qzhf@sxicc.ac.cn; Fax: +86 351 4041153; Tel: +86 351 4046092
bUniversity of the Chinese Academy of Sciences, Beijing 100049, PR China. E-mail: iccjgw@sxicc.ac.cn
First published on 2nd November 2020
Abstract
The surface oxygen vacancy concentration on ZnCeZrO composite oxides was finely tuned through modulating the preparation method and altering the precursor calcination temperature; the effect of the surface oxygen vacancy concentration on the performance of bifunctional ZnCeZrO/SAPO-34 catalysts in the direct conversion of syngas into light olefins (STO) was then investigated. The results indicate that the surface oxygen vacancy concentration of Zn0.5CeZrOx composite oxides can be markedly elevated by preparation through a sol–gel method with glucose as the complexing agent and calcination at 500 °C; a higher surface oxygen vacancy concentration leads to a higher space–time yield of methanol for syngas conversion over the Zn0.5CeZrOx oxide. When combined with SAPO-34 molecular sieves, the bifunctional Zn0.5CeZrOx/SAPO-34 catalyst with high surface oxygen vacancy concentration also exhibits a high space–time yield of light olefins (ethene to butenes) in the synthesis of olefins directly from syngas. With the help of in situ DRIFTS, it can be concluded that the surface oxygen vacancies on the ZnCeZrO oxide play an important role in the catalytic conversion of syngas. The abundant surface oxygen vacancies on Zn0.5CeZrOx can improve the formation of methanol-related intermediates from the syngas over the Zn0.5CeZrOx moiety, promote the evolution of these intermediates into the olefin products over the SAPO-34 moiety, and then enhance the overall capacity of the bifunctional Zn0.5CeZrOx/SAPO-34 composite catalyst in STO.
1. Introduction
The conversion of syngas directly into light olefins (STO) has been considered as an efficient process to produce valuable chemicals from multiple carbon-containing resources such as coal, natural gas and biomass.1–5 The STO process can be operated via two alternative routes, viz., the modified Fischer–Tropsch synthesis (FTS) and methanol intermediated routes. Because of the limitation of the Anderson–Schulz–Flory (ASF) distribution on the FTS products, the selectivity to light olefins (C2=–C4=) via the modified FTS route was in general rather low, whereas the selectivity to methane could be as high as 25%.6,7 In contrast, via the methanol intermediated route over an oxide–zeolite (OX–ZEO) composite catalyst, where the formation of methanol or ketene intermediates from syngas on the metal oxide is coupled with the conversion of methanol into light olefins on the acidic zeolite, the selectivity to light olefins can then be greatly enhanced.8 For STO over a ZnCrOx/MSAPO-34 catalyst, as reported by Jiao and co-workers, a high selectivity of 80% to C2=–C4= olefins was achieved at 400 °C and 2.5 MPa, with a CO conversion of 17%. Cheng and co-workers prepared a bifunctional catalyst composed of ZnO–ZrO2 composite oxide and SAPO-34 molecular sieves,9 which offered a selectivity of around 70% to C2=–C4= olefins for STO at 400 °C and 1.0 MPa, with a CO conversion of 10%. Inspired by the concept of OX–ZEO, a series of bifunctional catalysts such as ZnCrOx/MOR, MnOx/SAPO-34 and Zr–In/SAPO-34 were successively designed for the direct conversion of syngas into light olefins.10–12 Recently, we also observed that a ZnxCe2−yZryO4/SAPO-34 catalyst prepared by a sol–gel method exhibited excellent performance in STO, with a high selectivity of 79.5% to C2=–C4= at 300 °C and 1.0 MPa.13For STO over bifunctional OX–ZEO catalysts, it is now widely accepted that CO molecules are first adsorbed and activated on the surface oxygen vacancies of metal oxides, which interact with the adjacent active H* species generated from the dissociation of H2, forming formaldehyde (HCO*), formate (HCOO*) and methoxy (H3CO*) intermediates as well as methanol through successive hydrogenation reactions.14 These methanol-related intermediates or methanol molecules migrate or diffuse quickly onto the acid sites of zeolites, producing light olefins through the well-known hydrocarbon pool (HCP) mechanism.15 Undoubtedly, an efficient STO process via the methanol intermediated route needs a bifunctional OX–ZEO catalyst that can effectively couple these two steps, where the surface oxygen vacancies of metal oxides may play a very important role in the formation of methanol-related intermediates and the subsequent evolution of these intermediates into olefins.13
In this work, the surface oxygen vacancy concentration on ZnCeZrO composite oxides was finely tuned through modulating the sol–gel preparation method by varying the complexing agents and altering the precursor calcination temperature; the effect of the surface oxygen vacancy concentration on the performance of bifunctional ZnCeZrO/SAPO-34 catalysts in the direct conversion of syngas into light olefins (STO) was then investigated.
2. Experimental
2.1. Catalyst preparation
A series of ZnCeZrO composite oxides with the composition Zn0.5CeZrOx were prepared by a sol–gel synthesis method.13 Briefly, desired amounts of zinc nitrate (Zn(NO3)2·6H2O), zirconium nitrate (Zr(NO3)4·5H2O) and cerium nitrate (Ce(NO3)3·6H2O), with the molar ratio of Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce
Ce![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zr of 0.5
Zr of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0, were first dissolved in deionized water and stirred at room temperature for 2 h. After that, various complexing agents including glucose, citric acid, tartaric acid, adipic acid and L-alanine were slowly added into the above solution at 80 °C and the mixture was then vigorously stirred for 8 h. The obtained colloid was dried at 100 °C for 12 h and then calcined at 300 °C for 1 h and 500 °C for 3 h in air. Depending on the complexing agent, the obtained ZnCeZrO composite oxides are labeled Zn0.5CeZrOx-glucose, Zn0.5CeZrOx-citric, Zn0.5CeZrOx-tartaric, Zn0.5CeZrOx-adipic and Zn0.5CeZrOx-alanine.
1.0, were first dissolved in deionized water and stirred at room temperature for 2 h. After that, various complexing agents including glucose, citric acid, tartaric acid, adipic acid and L-alanine were slowly added into the above solution at 80 °C and the mixture was then vigorously stirred for 8 h. The obtained colloid was dried at 100 °C for 12 h and then calcined at 300 °C for 1 h and 500 °C for 3 h in air. Depending on the complexing agent, the obtained ZnCeZrO composite oxides are labeled Zn0.5CeZrOx-glucose, Zn0.5CeZrOx-citric, Zn0.5CeZrOx-tartaric, Zn0.5CeZrOx-adipic and Zn0.5CeZrOx-alanine.
To evaluate the influence of calcination temperature on the surface properties of ZnCeZrO composite oxides, the ZnCeZrO oxide precursor (prepared with glucose as the complexing agent and pre-calcined at 300 °C for 1 h) was subjected to calcination at different temperatures (400, 500, 600 and 700 °C) for 3 h in air; the resultant products are denoted as Zn0.5CeZrOx-glucose-T, where T is the calcination temperature in °C.
SAPO-34 molecular sieves were synthesized by a hydrothermal method with silica sol (JN-40), phosphoric acid (H3PO4), pseudo-boehmite (Al2O3) and tetraethyl ammonium hydroxide (TEAOH). The synthesis gel with the composition 2.0TEAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05SiO2
0.05SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0Al2O3
1.0Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0P2O5
1.0P2O5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70H2O was sealed into a Teflon-lined stainless steel autoclave and then crystallized under rotation conditions (15 rpm) at 200 °C for 20 h. H-SAPO-34 molecular sieves were attained by directly calcining the as-synthesized SAPO-34 sample at 550 °C for 10 h in air.
70H2O was sealed into a Teflon-lined stainless steel autoclave and then crystallized under rotation conditions (15 rpm) at 200 °C for 20 h. H-SAPO-34 molecular sieves were attained by directly calcining the as-synthesized SAPO-34 sample at 550 °C for 10 h in air.
The ZnCeZrO composite oxide and H-SAPO-34 powder with the same mass were then mixed, compressed into tablets and crushed and sieved to 20–40 mesh, to obtain the bifunctional ZnCeZrO/SAPO-34 catalysts for evaluation in the conversion of syngas into light olefins (STO). It should be noted that the composite catalyst here was prepared by uniformly mixing the powdered ZnCeZrO oxide and the powdered SAPO-34 molecular sieves with a mass ratio of 1 (unless specifically indicated). Although the bifunctional catalysts could be realized through various ways for the assembly of two catalyst components such as integrated synthesis, powder mixing, granule stacking and dual bed filling, it was demonstrated recently that the powder mixing of metal oxide and zeolite moieties in general could achieve an intimate contact between the two components, facilitating the transfer of the methanol-related intermediates formed on the metal oxide moiety to the zeolite moiety, leading to a high yield of light olefins.16,17
2.2. Catalyst characterization
The X-ray diffraction (XRD) patterns were collected on a Bruker D8 Advance X-ray diffractometer (CuKα radiation, λ = 1.5418 Å, 40 kV and 40 mA), in the 2θ range of 5–85° and with a scanning rate of 2 degrees per min. The Rietveld refinement was performed by using the HighScore plus software and the average particle size was estimated by using the Scherrer equation.The field emission-scanning electron microscopy (FE-SEM) images were taken on a JEOL JSM-7001F microscope. High-resolution transmission electron microscopy (HR-TEM) and TEM images were obtained on a field emission-transmission electron microscope (JEM-2100F, JEOL). The average size of catalyst particles was estimated by counting more than 100 particles shown in the TEM images. Scanning transmission electron microscopy (STEM) images and elemental distributions were acquired at 200 kV using a JEOL JEM-2100F microscope equipped with an energy-dispersive X-ray spectroscopy (EDX) detector.
The textural properties were measured by N2 sorption on a Micromeritics TriStar II 3020 instrument at −196 °C. Prior to the measurement, the catalyst samples were degassed at 250 °C for 8 h. The total surface area was obtained from the adsorption branch isotherm in the relative pressure range of 0.05–0.25 by the BET method, the pore volume was calculated from the desorption isotherm by the t-plot method, whilst the pore size distribution was determined by the BJH method.
The Raman spectra were collected on a LabRAM HR Evolution Raman spectrometer equipped with a 532 nm Ar+ laser.
The X-ray photoelectron spectra (XPS) of O (1s) and Ce (3d) were measured at 5 × 10−7 Pa on an AXIS ULTRA DLD instrument with an Al Kα monochromator X-ray source (hν = 1486.6 eV) and calibrated with the binding energy of carbonaceous deposit (C 1s, 284.6 eV).
The temperature-programmed desorption of CO or NH3 (CO-TPD or NH3-TPD) was carried out on a Micromeritics AutoChem II 2920 apparatus. First, about 0.1 g catalyst was loaded into the sample tube and pretreated at 300 °C for 1 h in a He flow; it was then cooled to room temperature to allow saturated adsorption of CO or NH3. After that, the catalyst sample was swept with a He flow and the CO-TPD or NH3-TPD profiles were then recorded at 50–600 °C with a heating rate of 10 °C min−1, through measuring the quantity of desorbed CO or NH3 with a TCD.
The time-dependent diffuse reflectance infrared Fourier transform (DRIFT) spectra were recorded on a Bruker Vertex 80 infrared spectrometer equipped with a liquid nitrogen-cooled MCT detector and an in situ high-temperature chamber. First, about 50 mg of catalyst sample was placed in the in situ chamber and pretreated at 400 °C for 2 h in a H2 flow (30 mL min−1). Next, it was purged with an Ar flow (30 mL min−1) for 0.5 h and cooled to the reaction temperature (300 °C) to collect the background spectrum. After that, the catalyst sample was exposed to a syngas flow (CO/H2 = 1/2, 45 mL min−1) at 300 °C; the IR spectra in the range of 4000–1000 cm−1 were then collected every 5 min up to 90 min at a resolution of 4 cm−1 by accumulating 128 scans.
2.3. Catalytic tests
The catalytic tests for the conversion of syngas to olefins (STO) were performed in a stainless steel tubular fixed-bed reactor with an inner diameter of 10 mm, into which 0.5 g of Zn0.5CeZrOx/SAPO-34 composite catalyst (20–40 mesh) was loaded. Prior to the reaction, the catalyst was reduced at 400 °C and atmospheric pressure for 2 h in a pure H2 flow (30 mL min−1); it was then cooled to 300 °C in a N2 flow (30 mL min−1). After that, a syngas flow with a CO/H2 molar ratio of 1/2 was introduced and the STO reaction was then carried out at 300 °C and 1.0 MPa, with a gas hourly space velocity (GHSV) of 5400 mL g−1 h−1, where 3 vol% N2 was added to the feed as an internal standard.The effluent products were periodically analyzed online by using an Agilent 7890A gas chromatograph (GC) equipped with one TCD and two FIDs and two capillary columns (J&W 127-7031, 30 m × 530 μm × 0.25 μm; Agilent 19095P-S25, 50 m × 530 μm × 15 μm). The conversion of CO (x(CO)) and the selectivity to CO2 (s(CO2)) are calculated as
| x(CO) = (n(CO,in) − n(CO,out))/n(CO,in) × 100% |
| s(CO2) = n(CO2,out)/(n(CO,in) − n(CO,out)) × 100% |
| s(CiHix) = i·n(CiHix)/(∑j·n(CjHjx) + n(CH3OH) + 2n(DME)) × 100% |
| s(CH3OH) = n(CH3OH)/(∑j·n(CjHix) + n(CH3OH) + 2n(DME)) × 100% |
| s(DME) = 2n(DME)/(∑j·n(CjHjx) + n(CH3OH) + 2n(DME)) × 100% |
3. Results and discussion
3.1. Structural characterization of the Zn0.5CeZrOx composite oxide and SAPO-34 molecular sieves
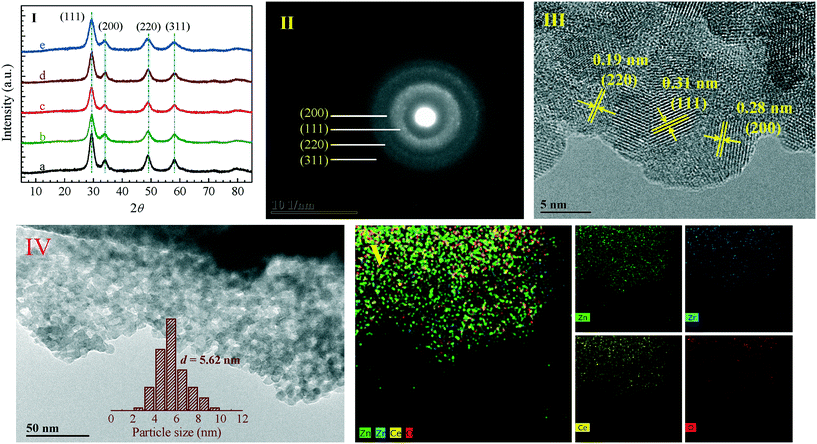
Fig. 1(I) shows the XRD patterns of the Zn0.5CeZrOx composite oxides prepared with different complexing agents; four diffraction peaks at 29.1°, 33.9°, 48.7° and 57.9° should correspond to the (111), (200), (220) and (311) crystal facets of cubic CeZrO4 solid solution, respectively (JCPDS no. 70-4048).18,19 These crystal facets can be further confirmed by the selected-area electron diffraction (SAED) pattern, as shown in Fig. 1(II); interplanar spacings of 0.31, 0.28 and 0.19 nm, representing the (111), (200) and (220) facets, respectively, were also observed in the HR-TEM image, as shown in Fig. 1(III) as well as Fig. S1, ESI.†The particle sizes estimated from the diffraction peak at 29.1° by using the Scherrer equation as well as the cell parameters determined by the Rietveld refinement of the XRD patterns referenced to the cubic CeZrO4 solid solution are given in Table 1. No diffraction peaks belonging to the ZnO phase are detected in the XRD patterns, suggesting that the Zn species are highly dispersed in the Zn0.5CeZrOx composite oxides. Meanwhile, as shown in Fig. 1(IV) and S2, ESI,† the Zn0.5CeZrOx composite oxides appear as aggregates of small spherical nanoparticles (NPs) with a mean size of around 3–7 nm, in line with the XRD results (Table 1). The STEM-EDX elemental mapping results shown in Fig. 1(V) further illustrate that the Zn, Ce and Zr elements are uniformly dispersed in the Zn0.5CeZrOx composite oxides.
| Entry | Sample | Particle size (nm) | Cell parameter (Å) | S BET (m2 g−1) | V micro (cm3 g−1) |
|---|---|---|---|---|---|
| Note: (a) the particle sizes reported here were estimated from the diffraction peak at 29.1° in the XRD patterns by using the Scherrer equation. (b) The cell parameters were determined by the Rietveld refinement of the XRD patterns referenced to the cubic CeZrO4 solid solution. (c) The surface areas (SBET) and micropore volumes (Vmicro) were derived from the nitrogen sorption isotherms by the BET and t-plot methods, respectively. | |||||
| A | Zn0.5CeZrOx-glucose-500 | 4.5 | 10.5476 | 72 | 0.116 |
| B | Zn0.5CeZrOx-citric-500 | 4.0 | 10.5518 | 34 | 0.044 |
| C | Zn0.5CeZrOx-tartaric-500 | 4.1 | 10.5393 | 37 | 0.044 |
| D | Zn0.5CeZrOx-adipic-500 | 4.3 | 10.5343 | 25 | 0.053 |
| E | Zn0.5CeZrOx-alanine-500 | 3.4 | 10.5866 | 15 | 0.018 |
| A1 | Zn0.5CeZrOx-glucose-400 | 4.0 | 10.5524 | 115 | 0.131 |
| A2(A) | Zn0.5CeZrOx-glucose-500 | 4.5 | 10.5476 | 72 | 0.116 |
| A3 | Zn0.5CeZrOx-glucose-600 | 5.0 | 10.5474 | 47 | 0.089 |
| A4 | Zn0.5CeZrOx-glucose-700 | 6.5 | 10.5437 | 39 | 0.092 |
The textural properties given in Table 1 (as determined from the nitrogen sorption isotherms shown in Fig. S3, ESI†) show that the Zn0.5CeZrOx-glucose composite oxide prepared with glucose as the complexing agent has a much larger surface area (72 m2 g−1) and micropore volume (0.116 cm3 g−1) than the Zn0.5CeZrOx samples synthesized with other complexing agents (14–37 m2 g−1 and 0.018–0.053 cm3 g−1). Meanwhile, for the Zn0.5CeZrOx-glucose composite oxide, as given in Table 1 (Fig. S4, ESI†), with an increase in the calcination temperature from 400 to 700 °C, the surface area decreases from 115 to 39 m2 g−1, whereas the average particle size increases slightly from 4.0 to 6.5 nm (estimated by using the Scherrer equation from the XRD patterns shown in Fig. S5, ESI†); in particular, weak diffraction lines for the ZnO phase are also detected in the XRD patterns of Zn0.5CeZrOx-glucose calcined at high temperature (>600 °C).
As the second component of the bifunctional ZnCeZrO/SAPO-34 catalysts, as shown in Fig. 2, the as-synthesized SAPO-34 molecular sieves show a typical CHA crystal structure (Fig. 2(I)), with a cubic morphology and an average crystal size of about 300 nm (Fig. 2(II)). The surface area and pore volume determined by nitrogen sorption are 442 m2 g−1 and 0.26 cm3 g−1, respectively. Fig. 2(III) shows the NH3-TPD profile of SAPO-34; it was estimated that the densities of weak (peaking at 170 °C) and strong (at 350 °C) acid sites are 0.28 and 0.45 mmol g−1, respectively. After the powder mixing of the Zn0.5CeZrOx composite oxide with the H-SAPO-34 molecular sieve moieties, as shown in Fig. S6 and S7, ESI,† the crystal structure of the two components is well maintained, whereas a moderate decrease in the surface area, pore volume and quantity of acid sites (in particular the strong acid sites) is observed, originating from the intimate interaction between the metal oxide and molecular sieve moieties.
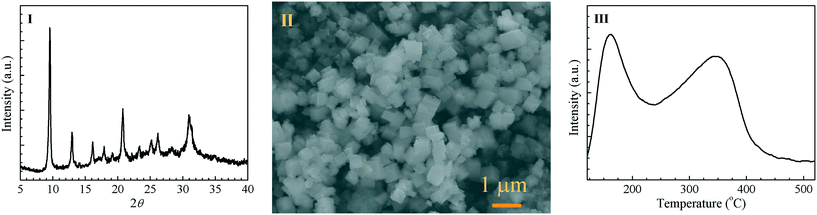 | ||
| Fig. 2 Structural characterization of SAPO-34. (I) XRD pattern of SAPO-34. (II) SEM image of SAPO-34. (III) NH3-TPD profile of SAPO-34. | ||
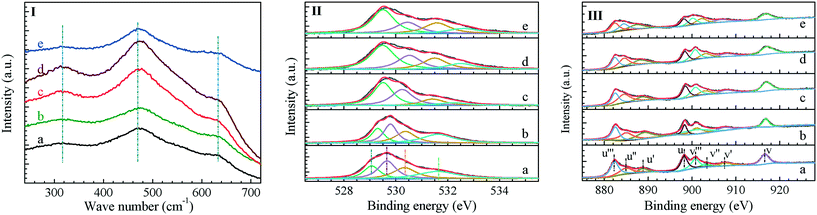
The surface oxygen vacancies of the Zn0.5CeZrOx composite oxides were characterized by Raman spectroscopy and XPS, as shown in Fig. 3 and S8, ESI.† In the Raman spectra (Fig. 3(I)), the peak at 475 cm−1 corresponds to the F2g symmetrical stretching vibration in the cubic fluorite structure of CeO2.20 Another two peaks at around 320 and 630 cm−1 are ascribed to the displacement of O atoms from their ideal fluorite lattice position, as the introduction of Zr in the Zn0.5CeZrOx composite oxide may distort the Ce–O bonds.21 The surface oxygen vacancy concentration of the Zn0.5CeZrOx composite oxides can then be estimated from the intensity of the peak at 630 cm−1,22 as given in Table 2. Obviously, the Zn0.5CeZrOx-glucose composite oxide shows a much higher surface oxygen vacancy concentration than the other samples, suggesting that high concentration of surface oxygen vacancies or defect sites was created in Zn0.5CeZrOx when glucose was used as the complexing agent in the sol–gel preparation.
Moreover, the concentration of surface oxygen vacancies in Zn0.5CeZrOx-glucose is also greatly influenced by the calcination temperature; the Zn0.5CeZrOx-glucose composite calcined at 500 °C has the highest concentration of surface oxygen vacancies (Table 2). An adequate calcination temperature (500 °C) is probably necessary to promote the mutual isomorphous substitution of Zr4+ and Ce4+ as well as the formation of abundant defect sites in the composite oxide; however, a higher calcination temperature (>500 °C) may lead to a decrease in the oxygen storage capacity and the surface oxygen vacancy concentration, due to the sintering and maturation of the Zn0.5CeZrOx composite oxides at high temperature.
Fig. 3(II) shows the O 1s XPS spectra of the Zn0.5CeZrOx composite oxides. The peaks at 529.0, 530.5, 531.6 and 532.0 eV, representing the lattice O species (Olattice), O atoms around vacancies (Ov), O atoms in the surface hydroxyl (–OH) and O atoms in the absorbed water (H2O), respectively,23 are clearly observed. On the basis of the relative intensity of these peaks, the concentration of oxygen at defect sites was then estimated, as given in Table 2.
| Entry | Sample | C Ov (%) | C O-defect (%) | C Ce3+ (%) | CH3OH STY (mmol h−1 g−1) | C2=–C4= STY (mmol h−1 g−1) |
|---|---|---|---|---|---|---|
| Note: (1) the concentration of surface oxygen vacancies (COv) was obtained from the Raman spectra as COv = Ov/Ototal = I630/(I475 + I630) × 100%,22 where I630 and I475 correspond to the intensity of the peaks at 600 and 475 cm−1, respectively. (2) The concentration of oxygen at defect sites (CO-defect) was calculated from the O 1s XPS spectra as CO-defect = IO-defect/(IO-defect + IO-lattice) × 100%,23 where IO-defect and IO-lattice are the intensity of the peaks at 530.5 and 529.5 eV, respectively. (3) The concentration of Ce3+ on the surface (CCe3+) was calculated from the Ce 3d XPS spectra as CCe3+ = (I(u1) + I(v1))/∑(I(ui) + I(vi)) × 100%,25–27 where I(x) represents the signal intensity of u0 (900.9 eV) and v0 (882.5 eV), u1 (903.2 eV) and v1 (884.7 eV), u2 (907.3 eV) and v2 (888.8 eV), and u3 (916.6 eV) and v3 (898.4 eV) in the Ce 3d XPS spectra. (4) The CH3OH STY refers to the space–time yield of methanol for the syngas conversion over the Zn0.5CeZrOx composite oxides, at 300 °C, 1 MPa, and CO/H2 = 1/2, with a GHSV of 5400 mL g−1 h−1, reported at a time on stream (TOS) of 30 h. (5) The C2=–C4= STY is the space–time yield of C2=–C4= olefins on the basis of carbon, for the syngas conversion over the corresponding bifunctional Zn0.5CeZrOx/SAPO-34 catalyst with an oxide/zeolite mass ratio of 1, at 300 °C, 1 MPa and CO/H2 = 1/2, with a GHSV of 5400 mL g−1 h−1, reported at a TOS of 30 h. (6) The CH3OH STYs for syngas conversion over the Zn0.5CeZrOx-glucose oxides calcined at 400, 600 and 700 °C were not determined. | ||||||
| A | Zn0.5CeZrOx-glucose-500 | 23.8 | 29.7 | 30.0 | 0.24 | 0.40 |
| B | Zn0.5CeZrOx-citric-500 | 22.2 | 26.9 | 28.9 | 0.19 | 0.25 |
| C | Zn0.5CeZrOx-tartaric-500 | 20.9 | 25.1 | 27.0 | 0.18 | 0.20 |
| D | Zn0.5CeZrOx-adipic-500 | 19.3 | 24.4 | 25.0 | 0.15 | 0.17 |
| E | Zn0.5CeZrOx-alanine-500 | 18.9 | 24.2 | 24.3 | 0.13 | 0.13 |
| A1 | Zn0.5CeZrOx-glucose-400 | 21.6 | 21.6 | 21.7 | n.d. | 0.37 |
| A2(A) | Zn0.5CeZrOx-glucose-500 | 23.8 | 29.7 | 30.0 | 0.24 | 0.40 |
| A3 | Zn0.5CeZrOx-glucose-600 | 20.5 | 20.4 | 20.9 | n.d. | 0.28 |
| A4 | Zn0.5CeZrOx-glucose-700 | 20.2 | 20.2 | 20.0 | n.d. | 0.17 |
In addition, Fig. 3(III) shows the Ce 3d XPS spectra of the Zn0.5CeZrOx composite oxides. As Ce3+ was associated with the coordination-unsaturated O-substances on the ceria surface, the surface oxygen vacancy concentration could also be estimated using the ratio of Ce3+/(Ce3+ + Ce4+) from the surface concentration of Ce3+ and Ce4+ species determined from the Ce 3d XPS spectra.24–27 As given in Table 2, the surface O-defect concentration determined from the O 1s XPS spectra and the Ce3+ concentration determined from the Ce 3d XPS spectra display exactly the same trend as the surface oxygen vacancy concentration estimated from the Raman spectra. That is, the surface oxygen vacancies on the Zn0.5CeZrOx composite can be regulated by altering the complexing agent and calcination temperature; the Zn0.5CeZrOx-glucose-500 composite oxide, prepared with glucose as the complexing agent and calcined at 500 °C, has the most abundant surface oxygen vacancies.
During the sol–gel preparation process, various complexing agents may interact differently with the metal cations and hydrates of Zn, Ce and Zr, forming coordinate complexes different in structure and thereby composite oxides different in surface properties. It was reported that glucose as the complexing agent, owing to the abundant hydroxyl groups, could serve as the linkage to adsorb metal ions, forming nanospindles, fix these metal ions on the surface of glucose and thereby create abundant surface oxygen vacancies upon later calcination treatment,28,29 which may contribute to the high surface oxygen vacancy concentration of the Zn0.5CeZrOx-glucose composite oxide.
Moreover, the CO-TPD profiles shown in Fig. S9, ESI† also show that the Zn0.5CeZrOx-glucose composite oxide exhibits an intense desorption peak between 150 and 350 °C, in good accordance with the observation of Zhang, Zhou, Gao and co-workers that abundant surface oxygen vacancies could promote the adsorption and activation of CO on the composite oxides.30–32
3.2. Performance of the Zn0.5CeZrOx/SAPO-34 composite catalysts in the conversion of syngas to olefins
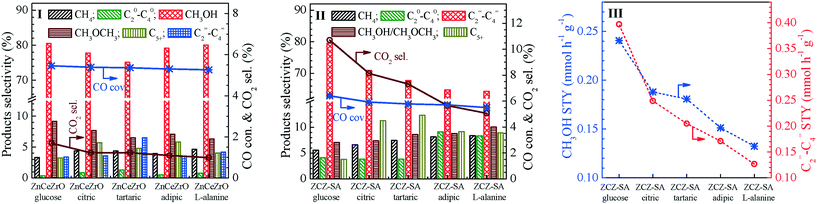
For the syngas conversion over the Zn0.5CeZrOx composite oxides, as shown in Fig. 4(I), methanol appears as the dominant product with a selectivity ranging from 75.1% to 80.5% at 300 °C and 1.0 MPa. Undoubtedly, the complexing agent used to prepare the Zn0.5CeZrOx composite oxides has a significant influence on the catalytic capacity of Zn0.5CeZrOx to form methanol from syngas. As shown in Table 2 and Fig. 4(III), among the various Zn0.5CeZrOx composite oxides prepared with different complexing agents, Zn0.5CeZrOx-glucose exhibits the highest methanol space–time yield (STY, 0.24 mmol h−1 g−1), followed by Zn0.5CeZrOx-citric, Zn0.5CeZrOx-tartaric, Zn0.5CeZrOx-adipic, and Zn0.5CeZrOx-alanine.When the Zn0.5CeZrOx composite oxides are combined with the SAPO-34 molecular sieves, as shown in Fig. 4(II), the bifunctional ZnCeZrO/SAPO-34 catalysts exhibit excellent performance in the conversion of syngas to olefins. Interestingly, the catalytic capacity of Zn0.5CeZrOx/SAPO-34 prepared with different complexing agents for producing light olefins from syngas follows the same trend as the capacity of Zn0.5CeZrOx for forming methanol (Fig. 4(III) and Table 2); that is, the bifunctional Zn0.5CeZrOx-glucose/SAPO-34 composite catalyst also gives a much higher selectivity to C2=–C4= (79.5%) and a larger space–time yield of C2=–C4= (0.4 mmol h−1 g−1) than the other composite catalysts.
Besides the complexing agent, the calcination temperature used in preparing the Zn0.5CeZrOx composite oxides also has a significant influence on the catalytic performance of Zn0.5CeZrOx/SAPO-34 in the STO reactions, as shown in Table 2 and Fig. S10, ESI.† In good accordance with the concentration of surface oxygen vacancies, the bifunctional Zn0.5CeZrOx-glucose-500/SAPO-34 catalyst, where Zn0.5CeZrOx-glucose-500 was prepared with glucose as the complexing agent and calcined at 500 °C, exhibits a much higher selectivity to C2=–C4= and a larger space–time yield of C2=–C4= than the other Zn0.5CeZrOx-glucose/SAPO-34 catalysts prepared by using a lower (400 °C) or a higher calcination temperature (600 and 700 °C).
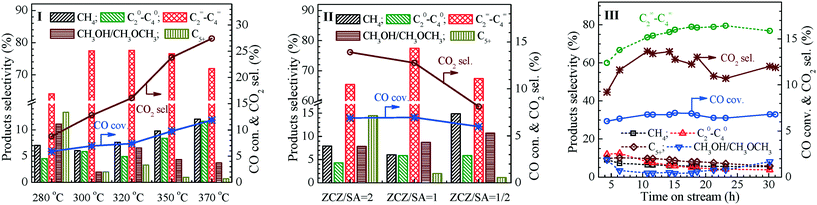
The influence of the reaction temperature and ZnCeZrO/SAPO-34 mass ratio on the conversion of syngas to olefins over the bifunctional Zn0.5CeZrOx-glucose-500/SAPO-34 composite catalyst was also considered. As shown in Fig. 5(I), the selectivity to C2=–C4= is 64.1% at 280 °C, with a CO conversion of 5.9%, 7.0% for CH4, 4.5% for C20–C40 alkanes and 8.8% for CO2. With an increase in the reaction temperature, the conversion of CO increases gradually, but is accompanied with an increase in the selectivity to CO2 emission, due to the enhanced water-gas shift (WGS) reaction at higher temperature. The selectivity to C2=–C4= olefins presents a volcanic curve relationship with the reaction temperature and a maximum selectivity of 79.5% to C2=–C4= is achieved at 300 °C. Meanwhile, the ZnCeZrO/SAPO-34 mass ratio in the composite catalysts also has a significant influence on the product selectivity for STO, as shown in Fig. 5(II); under the current reaction conditions, the ZnCeZrO/SAPO-34 composite with an equal mass of Zn0.5CeZrOx and SAPO-34 is appropriate to give a high yield of light olefins for the conversion of syngas. A decrease in the content of the metal oxide moiety may lead to the decrease of CO conversion, whereas a decrease in the content of the molecular sieve moiety may not be able to realize complete evolution of the methanol intermediates into the olefin products.
Similarly, the reaction pressure, H2/CO ratio and space velocity of the syngas feed in the STO reaction were also optimized to maximize the formation of C2=–C4= olefins, as shown in Fig. S11, ESI.† It was demonstrated that for the STO reactions over the Zn0.5CeZrOx-glucose-500/SAPO-34 catalyst with an oxide/molecular sieve mass ratio of 1, a high space–time yield of C2=–C4= olefins can be achieved at 300 °C and 1.0 MPa, with a CO/H2 ratio of 1/2 and a syngas feed velocity of 5400 mL g−1 h−1. As shown in Fig. 5(III), for STO over the Zn0.5CeZrOx-glucose-500/SAPO-34 catalyst, the conversion of CO is kept at 6.5% during the 30 h reaction and the selectivities to CO2, CH4 and C20–C40 alkanes are lower than 10.7%, 5.5% and 4.1%, respectively, whereas the selectivity to C2=–C4= olefins increases gradually with the time on stream (TOS) from the initial 60.0% to 79.5% at a TOS of about 15 h and remains at this level in the later reaction stage, demonstrating the excellent performance of the bifunctional Zn0.5CeZrOx/SAPO-34 composite catalyst in the conversion of syngas to olefins.
After conducting the STO reactions, the crystal structure of the spent Zn0.5CeZrOx/SAPO-34 composite catalyst remains almost intact, as shown in Fig. S6, ESI,† although the surface area and pore volume decrease moderately in comparison with the fresh catalysts, due to the carbonaceous deposition on the acid sites of H-SAPO-34. Although SAPO-34 as a catalyst in MTO is somewhat notorious for its susceptibility to deactivation by the carbonaceous deposition, the Zn0.5CeZrOx/SAPO-34 composite catalyst exhibits relatively much better stability in STO (Fig. 5(III)). This is probably related to the low methanol formation rate over Zn0.5CeZrOx for syngas conversion (about 0.13–0.24 mmol h−1 g−1), which leads to a much lower concentration of methanol-related intermediate species on SAPO-34 in STO compared to that in a typical MTO process.33 Moreover, the presence of high concentration hydrogen in the feed is also beneficial for suppressing the formation of polycyclic aromatics and thereby elevating the catalytic lifetime of SAPO-34 zeolite in STO.34
3.3. Relationship of the catalytic performance of the Zn0.5CeZrOx/SAPO-34 composite in the conversion of syngas to olefins (STO) with the surface oxygen vacancy concentration
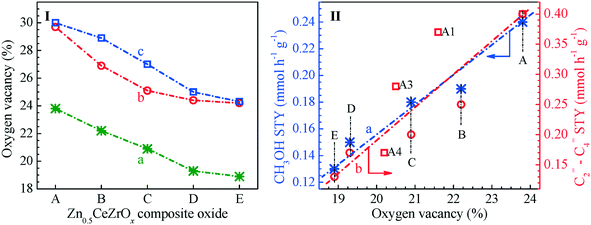
As mentioned above, the surface oxygen vacancies of the Zn0.5CeZrOx composite oxides prepared by the sol–gel method can be regulated by altering the complexing agent as well as the calcination temperature. The concentration of surface oxygen vacancies can be estimated from the Raman spectra, O 1s XPS spectra and Ce 3d XPS spectra; in fact, three manners give exactly the same trend on the oxygen vacancy concentrations, as shown in Fig. 6(I), although there are some differences in the absolute values. Meanwhile, the catalytic test results indicate that the Zn0.5CeZrOx composite oxides as well as the bifunctional Zn0.5CeZrOx/SAPO-34 catalysts derived whereafter are rather different in their catalytic capacity in the conversion of syngas to olefins.However, when the space–time yields (STYs) of methanol over Zn0.5CeZrOx and those of C2=–C4= olefins over Zn0.5CeZrOx/SAPO-34 composites are associated with the concentration of surface oxygen vacancies on Zn0.5CeZrOx, as shown in Fig. 6(II), a good linear relationship is observed. That is, the surface oxygen vacancies play an important role in the catalytic conversion of syngas; an increase in the surface oxygen vacancy concentration of Zn0.5CeZrOx can almost proportionally improve the formation of methanol-related intermediates from the syngas over the Zn0.5CeZrOx moiety, promote the evolution of these intermediates into olefin products over the SAPO-34 moiety, and then enhance the overall capacity of the Zn0.5CeZrOx/SAPO-34 bifunctional catalysts in the conversion of syngas to light olefins, agreeing well with previous reports.30–32 The Zn0.5CeZrOx-glucose-500 composite oxide, prepared with glucose as the complexing agent and calcined at 500 °C, is provided with the most abundant surface oxygen vacancies; thereafter, the Zn0.5CeZrOx-glucose-500/SAPO-34 composite also exhibits excellent catalytic performance in the conversion of syngas to light olefins.
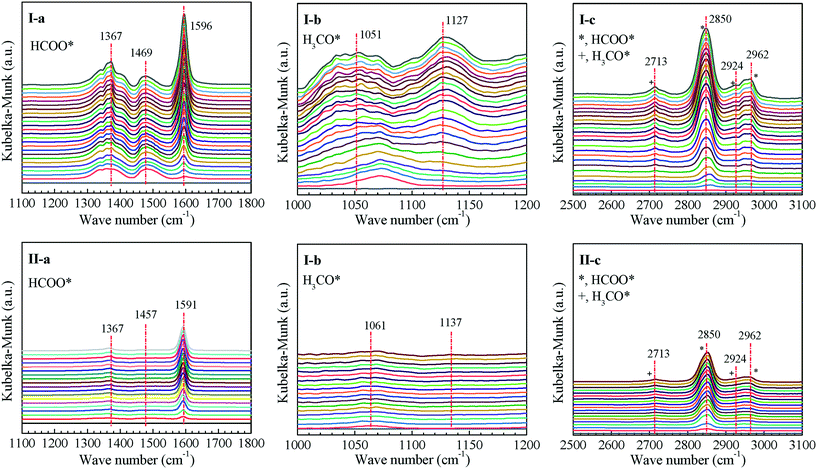
The promoting effect of surface oxygen vacancies on the formation of methanol intermediates over the ZnCeZrO composite oxide was further validated by in situ diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy, as shown in Fig. 7. After the exposure of ZnCeZrO composite oxides to syngas, the CO adsorbed on ZnCeZrO can quickly interact with the active H* species, forming formate and methoxy intermediates through successive hydrogenations. The peaks in the DRIFT spectra centered at 1367, 1469 and 1596 cm−1 are attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching and CH2 bending vibrations of surface formate species.35–37 The formate species are quickly generated after the reaction for 1 min and their peak intensity increases gradually with the reaction time. Another two peaks at 1051 and 1127 cm−1 representing the methoxy species are also clearly observed, accompanied by the characteristic peaks of C–H asymmetric and symmetric stretching vibrations of surface formate and methoxy species at 2962, 2850, 2924 and 2713 cm−1. Obviously, the peak intensity of formate and methoxy species on the Zn0.5CeZrOx-glucose composite is much stronger than that on Zn0.5CeZrOx-citric, as the former Zn0.5CeZrOx-glucose is provided with much more surface oxygen vacancies that can promote the conversion of syngas.
O stretching and CH2 bending vibrations of surface formate species.35–37 The formate species are quickly generated after the reaction for 1 min and their peak intensity increases gradually with the reaction time. Another two peaks at 1051 and 1127 cm−1 representing the methoxy species are also clearly observed, accompanied by the characteristic peaks of C–H asymmetric and symmetric stretching vibrations of surface formate and methoxy species at 2962, 2850, 2924 and 2713 cm−1. Obviously, the peak intensity of formate and methoxy species on the Zn0.5CeZrOx-glucose composite is much stronger than that on Zn0.5CeZrOx-citric, as the former Zn0.5CeZrOx-glucose is provided with much more surface oxygen vacancies that can promote the conversion of syngas.
4. Conclusions
A series of ZnCeZrO composite oxides were prepared by using a sol–gel method with different complexing agents and calcined at different temperatures. The ZnCeZrO composite oxides were then combined with SAPO-34 molecular sieves to obtain bifunctional ZnCeZrO/SAPO-34 catalysts for the direct conversion of syngas to olefins.The results indicate that the surface oxygen vacancies on the ZnCeZrO composite oxide play an important role in the catalytic conversion of syngas, whose concentration can be finely tuned through altering the complexing agent and calcination temperature. An increase in the concentration of surface oxygen vacancies on Zn0.5CeZrOx can almost proportionally improve the formation of methanol-related intermediates from the syngas over the Zn0.5CeZrOx moiety, promote the evolution of these intermediates into the olefin products over the SAPO-34 moiety, and then enhance the overall capacity of the bifunctional Zn0.5CeZrOx/SAPO-34 composite catalyst in the conversion of syngas into light olefins.
The surface oxygen vacancy concentration of Zn0.5CeZrOx composite oxide can be markedly elevated by preparation through a sol–gel method with glucose as the complexing agent and calcination at 500 °C. When combined with the SAPO-34 molecular sieves, the bifunctional Zn0.5CeZrOx-glucose-500/SAPO-34 catalyst with abundant surface oxygen vacancies exhibits excellent performance in the synthesis of light olefins directly from syngas under mild reaction conditions (300 °C and 1.0 MPa), with a space–time yield of 0.40 mmol h−1 g−1 and a high selectivity of 79.5% to C2=–C4= olefins, whereas the selectivities to CH4, C20–C40 alkanes and CO2 are below 5.5%, 4.1% and 10.7%, respectively.
The current results depict a clear relationship between the concentration of surface oxygen vacancies on the ZnCeZrO moiety and the performance of the bifunctional ZnCeZrO/SAPO-34 catalyst in STO and provide an effective measure to elevate the abundance of surface oxygen vacancies, which should be beneficial to the design of efficient catalysts in the direct synthesis of light olefins from syngas.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (21991092, U1910203, 21802157, U1862101, 21875275, 21773281), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA21020500), the Natural Science Foundation of Shanxi Province of China (201901D211581), the East-West Cooperation Project of Ningxia Key R & D Plan in China (2017BY063), the Innovative Talent Program of Shanxi Province in China (201605D211001), the CAS/SAFEA International Partnership Program for Creative Research Teams in China, Youth Innovation Promotion Association, CAS (2016161), the Excellent Doctoral Student Award and Subsidy Program of Shanxi Province (BK2018001) and the Independent Research Project of State Key Laboratory of Coal Conversion, Institute of Coal Chemistry, CAS (2020BWZ004).References
- H. M. Torres Galvis and K. P. de Jong, Catalysts for Production of Lower Olefins from Synthesis Gas: A Review, ACS Catal., 2013, 3, 2130–2149 CrossRef CAS.
- W. Wang, S. Wang, X. Ma and J. Gong, Recent advances in catalytic hydrogenation of carbon dioxide, Chem. Soc. Rev., 2011, 40, 3703–3727 RSC.
- M. Aresta, A. Dibenedetto and A. Angelini, Catalysis for the Valorization of Exhaust Carbon: from CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2, Chem. Rev., 2014, 114, 1709–1742 CrossRef CAS.
- H. Yang, C. Zhang, P. Gao, H. Wang, X. Li, L. Zhong, W. Wei and Y. Sun, A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons, Catal. Sci. Technol., 2017, 7, 4580–4598 RSC.
- G. Raveendra, C. M. Li, B. Liu, Y. Cheng, F. Meng and Z. Li, Synthesis of lower olefins from syngas over Zn/Al2O3–SAPO-34 hybrid catalysts: role of doped Zr and influence of the Zn/Al2O3 ratio, Catal. Sci. Technol., 2018, 8, 3527–3538 RSC.
- H. M. Torres Galvis, J. H. Bitter, T. Davidian, M. Ruitenbeek, A. I. Dugulan and K. P. de Jong, Iron Particle Size Effects for Direct Production of Lower Olefins from Synthesis Gas, J. Am. Chem. Soc., 2012, 134, 16207–16215 CrossRef CAS.
- K. Cheng, J. Kang, D. Ding, V. Subramanian, C. Zhou, Q. Zhang and Y. Wang, Chapter Three - Advances in Catalysis for Syngas Conversion to Hydrocarbons, Adv. Catal., 2017, 60, 125–208 CAS.
- F. Jiao, J. Li, X. Pan, J. Xiao, H. Li, H. Ma, M. Wei, Y. Pan, Z. Zhou, M. Li, S. Miao, J. Li, Y. Zhu, D. Xiao, T. He, J. Yang, F. Qi, Q. Fu and X. Bao, Selective conversion of syngas to light olefins, Science, 2016, 351, 1065–1068 CrossRef CAS.
- K. Cheng, B. Gu, X. Liu, J. Kang, Q. Zhang and Y. Wang, Direct and Highly Selective Conversion of Synthesis Gas into Lower Olefins: Design of a Bifunctional Catalyst Combining Methanol Synthesis and Carbon–Carbon Coupling, Angew. Chem., Int. Ed., 2016, 55, 4725–4728 CrossRef CAS.
- Y. Zhu, X. Pan, F. Jiao, J. Li, J. Yang, M. Ding, Y. Han, Z. Liu and X. Bao, Role of Manganese Oxide in Syngas Conversion to Light Olefins, ACS Catal., 2017, 7, 2800–2804 CrossRef CAS.
- X. Liu, W. Zhou, Y. Yang, K. Cheng, J. Kang, L. Zhang, Q. Zhang, X. Min, Q. Zhang and Y. Wang, Design of efficient bifunctional catalysts for direct conversion of syngas into lower olefins via methanol/dimethyl ether intermediate, Chem. Sci., 2018, 9, 4708–4718 RSC.
- J. Su, D. Wang, Y. Wang, H. Zhou, C. Liu, S. Liu, C. Wang, W. Yang, Z. Xie and M. He, Direct Conversion of Syngas into Light Olefins over Zirconium-Doped Indium(III) Oxide and SAPO-34 Bifunctional Catalysts: Design of Oxide Component and Construction of Reaction Network, ChemCatChem, 2018, 10, 1536–1541 CrossRef CAS.
- Y. Luo, S. Wang, S. Guo, K. Yuan, H. Wang, M. Dong, Z. Qin, W. Fan and J. Wang, Study on different synthesis methods of ZnxCe2-yZryO4/SAPO-34 catalyst and its catalytic performance in syngas to low-carbon olefins, Ranliao Huaxue Xuebao, 2020, 48, 594–600 Search PubMed.
- W. Zhou, K. Cheng, J. Kang, C. Zhou, V. Subramanian, Q. Zhang and Y. Wang, New horizon in C1 chemistry: breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels, Chem. Soc. Rev., 2019, 48, 3193–3228 RSC.
- U. Olsbye, S. Svelle, K. Lillerud, Z. Wei, Y. Chen, J. Li, J. Wang and W. Fan, The formation and degradation of active species during methanol conversion over protonated zeotype catalysts, Chem. Soc. Rev., 2015, 44, 7155–7176 RSC.
- Z. Li, J. Wang, Y. Qu, H. Liu, C. Tang, S. Miao, Z. Feng, H. An and C. Li, Highly selective conversion of carbon dioxide to lower olefins, ACS Catal., 2017, 7, 8544–8548 CrossRef CAS.
- S. Wang, P. Wang, D. Shi, S. He, L. Zhang, W. Yan, Z. Qin, J. Li, M. Dong, J. Wang, U. Olsbye and W. Fan, Direct conversion of syngas into light olefins with low CO2 emission, ACS Catal., 2020, 10, 2046–2059 CrossRef CAS.
- B. M. Reddy, A. Khan, P. Lakshmanan, M. Aouine, S. Loridant and J. C. Volta, Structural Characterization of Nanosized CeO2-SiO2, CeO2-TiO2, and CeO2-ZrO2 Catalysts by XRD, Raman, and HREM Techniques, J. Phys. Chem. B, 2005, 109, 3355–3363 CrossRef CAS.
- A. Chen, Y. Zhou, N. Ta, Y. Li and W. Shen, Redox properties and catalytic performance of ceria–zirconia nanorods, Catal. Sci. Technol., 2015, 5, 4184–4192 RSC.
- Z. Zhang, Y. Wang, J. Lu, J. Zhang, M. Li, X. Liu and F. Wang, Pr-Doped CeO2 Catalyst in the Prins Condensation–Hydrolysis Reaction: Are All of the Defect Sites Catalytically Active?, ACS Catal., 2018, 8, 2635–2644 CrossRef CAS.
- C. M. Kalamaras, D. D. Dionysiou and A. M. Efstathiou, Mechanistic Studies of the Water–Gas Shift Reaction over Pt/CexZr1–xO2 Catalysts: The Effect of Pt Particle Size and Zr Dopant, ACS Catal., 2012, 2, 2729–2742 CrossRef CAS.
- M. Piumetti, S. Bensaid, D. Fino and N. Russo, Nanostructured ceria-zirconia catalysts for CO oxidation: Study on surface properties and reactivity, Appl. Catal., B, 2016, 197, 35–46 CrossRef CAS.
- F. Liang, Y. Yu, W. Zhou, X. Xu and Z. Zhu, Highly defective CeO2 as a promoter for efficient and stable water oxidation, J. Mater. Chem. A, 2015, 3, 634–640 RSC.
- M. Piumetti, S. Bensaid, N. Russo and D. Fino, Nanostructured ceria-based catalysts for soot combustion: Investigations on the surface sensitivity, Appl. Catal., B, 2015, 165, 742–751 CrossRef CAS.
- R. Leppelt, B. Schumacher, V. Plzak, M. Kinne and R. J. Behm, Kinetics and mechanism of the low-temperature water-gas shift reaction on Au/CeO2 catalysts in an idealized reaction atmosphere, J. Catal., 2006, 244, 137–152 CrossRef CAS.
- W. Lei, T. Zhang, L. Gu, P. Liu, J. A. Rodriguez, G. Liu and M. Liu, Surface-Structure Sensitivity of CeO2 Nanocrystals in Photocatalysis and Enhancing the Reactivity with Nanogold, ACS Catal., 2015, 5, 4385–4393 CrossRef CAS.
- C. Li, Y. Sun, I. Djerdj, P. Voepel, C. C. Sack, T. Weller, R. Ellinghaus, J. Sann, Y. Guo, B. Smarsly and H. Over, Shape-Controlled CeO2 Nanoparticles: Stability and Activity in the Catalyzed HCl Oxidation Reaction, ACS Catal., 2017, 7, 6453–6463 CrossRef CAS.
- D. Zhang, F. Niu, T. Yan, L. Shi, X. Du and J. Fang, Ceria nanospindles: Template-free solvothermal synthesis and shape-dependent catalytic activity, Appl. Surf. Sci., 2011, 257, 10161–10167 CrossRef CAS.
- M. Xu, X. Hu, S. Wang, J. Yu, D. Zhu and J. Wang, Photothermal effect promoting CO2 conversion over composite photocatalyst with high graphene content, J. Catal., 2019, 377, 652–661 CrossRef CAS.
- P. Zhang, L. Tan, G. Yang and N. Tsubaki, One-pass selective conversion of syngas to para-xylene, Chem. Sci., 2017, 8, 7941–7946 RSC.
- C. Zhou, J. Shi, W. Zhou, K. Cheng, Q. Zhang, J. Kang and Y. Wang, Highly Active ZnO-ZrO2 Aerogels Integrated with H-ZSM-5 for Aromatics Synthesis from Carbon Dioxide, ACS Catal., 2020, 10, 302–310 CrossRef CAS.
- P. Gao, S. Dang, S. Li, X. Bu, Z. Liu, M. Qiu, C. Yang, H. Wang, L. Zhong, Y. Han, Q. Liu, W. Wei and Y. Sun, Direct Production of Lower Olefins from CO2 Conversion via Bifunctional Catalysis, ACS Catal., 2018, 8, 571–578 CrossRef CAS.
- D. L. S. Nieskens, J. D. Lunn and A. Malek, Understanding the enhanced lifetime of SAPO-34 in a direct syngas-to-hydrocarbons process, ACS Catal., 2019, 9, 691–700 CrossRef CAS.
- G. Li, F. Jiao, X. Pan, N. Li, D. Miao, L. Li and X. Bao, Role of SAPO-18 acidity in direct syngas conversion to light olefins, ACS Catal., 2020, 10, 12370–12375 CrossRef CAS.
- Y. Yang, C. Mims, D. Mei, C. H. F. Peden and C. T. Campbell, Mechanistic studies of methanol synthesis over Cu from CO/CO2/H2/H2O mixtures: The source of C in methanol and the role of water, J. Catal., 2013, 298, 10–17 CrossRef CAS.
- B. Yan, B. Zhao, S. Kattel, Q. Wu, S. Yao, D. Su and J. Chen, Tuning CO2 hydrogenation selectivity via metal-oxide interfacial sites, J. Catal., 2019, 374, 60–71 CrossRef CAS.
- X. Liu, M. Wang, C. Zhou, W. Zhou, K. Cheng, J. Kang, Q. Zhang, W. Deng and Y. Wang, Selective transformation of carbon dioxide into lower olefins with a bifunctional catalyst composed of ZnGa2O4 and SAPO-34, Chem. Commun., 2018, 54, 140–143 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: More characterization results for the Zn0.5CeZrOx composite oxides prepared with different complexing agents and calcined at different temperatures; optimization of the reaction conditions for the conversion of syngas to olefins over the Zn0.5CeZrOx–glucose-500/SAPO-34 bifunctional catalyst. See DOI: 10.1039/d0cy01759k |
| This journal is © The Royal Society of Chemistry 2021 |