Open Access Article
Open Access ArticleMesoporous silica-coated gold nanorods loaded with tetrazolyl phthalocyanine as NIR light-activated nano-switches for synergistic photothermal and photodynamic inactivation of antibiotic-resistant Escherichia coli†
Qiuhao
Ye
a,
Shuanghuang
Xiao
a,
Ting
Lin
b,
Yufeng
Jiang
a,
Yiru
Peng
 *a and
Yide
Huang
*b
*a and
Yide
Huang
*b
aCollege of Chemistry & Material, Fujian Provincial Key Laboratory of Advanced Materials Oriented Chemical Engineering, Fujian Province Key Laboratory of Polymer Materials, Fujian Normal University, Fuzhou, China. E-mail: yirupeng@fjnu.edu.cn
bProvincial University Key Laboratory of Cellular Stress Response and Metabolic Regulation, College of Life Sciences, Fujian Normal University, Fuzhou, China. E-mail: ydhuang@fjnu.edu.cn
First published on 16th January 2021
Abstract
A light-controlled nano-switch was prepared by assembling mesoporous silica-coated gold nanorods with bis-(1-(4-hydroxyphenyl)-5-mercapto-tetrazolyl) silicon(IV) phthalocyanines, which exhibited excellent triple functions with controlled release of phthalocyanines and generation of reactive oxygen species (ROS) as well as temperature enhancement under laser irradiation. The nano-switch achieved an effective antimicrobial activity against a variety of antibiotic-resistant Escherichia coli strains through synergistic photodynamic therapy and photothermal therapy by damaging the genomic DNA and enzyme activity of bacteria.
1. Introduction
Overuse and misuse of antibiotics by humans for a long time has led to the emergence and prevalence of antibiotic-resistant bacteria, which has reduced the therapeutic efficacy of antibiotics for human and animal pathogens.1–3 It is estimated that, by 2050, approximately 10 million people will die directly or indirectly from a multidrug resistant infection.4 Despite enormous efforts in research, the development of new antimicrobial drugs cannot catch up with the emergence of antibiotic resistant pathogens.5 Pursuit of alternative strategies and drugs to overcome antibiotic resistance is thus highly desirable.In the past few decades, near-infrared (NIR) laser-induced photothermal therapy (PTT) has been used as a powerful strategy to combat cancers6 and bacterial infection7 because of its non-invasive manipulation, good controllability, and high tissue penetration. Various photothermal materials have been developed such as gold nanoparticles,8 gold nanorods (AuNRs),9 carbon nanotubes,10 two dimensional MoS211 and MnO212 as well as graphene nanoribbons and their supermolecules.13 Mesoporous silica-coated gold nanorods (AuNR-SiO2) have aroused great interest as photothermal materials because of their tenable surface plasmon resonance and excellent light-to-heat energy conversion efficiency14–17 as well as light controllable delivery of biomolecules into cytoplasm.18 Besides, the large specific surface area of mesoporous silica guarantees a high drug payload and optimizes the light-transparent window in the NIR region.19 Therefore, AuNR-SiO2 seems to be a desirable candidate for highly stable and NIR laser-induced antibacterial applications. Turcheniuk et al. reported that AuNR-SiO2 loaded with verteporfin could be used as an efficient near infrared nanostructure to eradicate Escherichia coli infection.20
Photodynamic therapy (PDT) against microbial cells is also considered to be an alternative high-efficiency strategy to eliminate bacteria both in vitro and in vivo.13,21,22 The antibacterial strategy uses a specific wavelength of light to activate photosensitizers (PSs), which react with oxygen to produce reactive oxygen species (ROS) to kill bacteria. Phthalocyanines (Pcs) and metal phthalocyanines (MPcs) have been used as promising photosensitisers for PDT of cancerous and noncancerous diseases. Recently, a series of new Pcs and their nano-formulations have exhibited excellent anticancer23–26 and antimicrobial27–31 activities. However, the properties of Pcs such as easy aggregation in water, lack of ability to target specific tissue, and a limited optimal wavelength for tissue penetration hinder their applications for PDT.32–34 It is desired to synthesize novel Pcs to address the drawbacks of traditional Pcs.
In this work, we synthesized a novel silicon Pc with mercapto-tetrazolyl functional groups, named bis-(1-(4-hydroxyphenyl)-5-mercapto-tetrazolyl)silicon(IV) phthalocyanine (Tet-SiPc), and used it as a photosensitizer for antibacterial research (Scheme 1). The mercapto-tetrazolyl group, a pharmaceutical synthetic intermediate, is a main pharmacophore of antibacterial and anti-inflammatory drugs.35,36 Introducing mercapto-tetrazolyl functional groups to the axial position of Pc rings is expected to reduce its aggregation and improve its photodynamic antibacterial performance. To achieve the synergistic PDT and PTT, a light-controlled nano-switch was assembled through the use of mesoporous silica-coated gold nanorods with bis-(1-(4-hydroxyphenyl)-5-mercapto-tetrazolyl) silicon(IV) phthalocyanine. The effect of the nano-switch against a variety of antibiotic-resistant E. coli strains was evaluated.
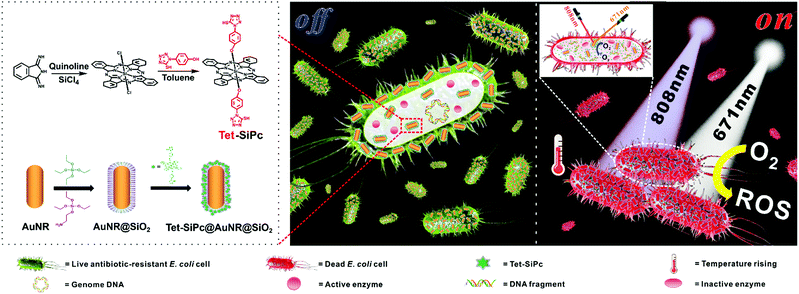 | ||
| Scheme 1 Schematic representation of the preparation of the Tet-SiPc@AuNR@SiO2 nano-switch and the antibacterial mechanism of Tet-SiPc@AuNR@SiO2. | ||
2. Materials and methods
2.1. Materials and instruments
The organic solvents used for the preparation of the nano-switch (Tet-SiPc@AuNR-SiO2) were of reagent grade. Sodium borohydride (NaBH4), silver nitrate (AgNO3), sodium hydroxide (NaOH) and hydrochloric acid (HCl) were purchased from Sinopharm Group Chemical Reagent Co., Ltd. Hydrogen tetra-chloroaurate(III) trihydrate (HAuCl4·3H2O) was purchased from Shanghai Bailingwei Chemical Technology Co., Ltd. Cetyltrimethylammonium bromide (CTAB) was obtained from Sigma-Aldrich (Mainland, China). 1-(4-Hydroxyphenyl)-5-mercapto-tetrazolium, tetraethyl orthosilicate (TEOS) and 3-aminopropyltriethoxysilane (APTES) were purchased from Energy Chemical. Ascorbic acid (AA) was purchased from the Xinning Chemical Plant in Shantou (Guangdong, China).The infrared spectra (KBr pellets) were recorded on a PE-983G spectrometer. 1H NMR spectra were recorded on a Bruker 400 MHz FT-NMR spectrometer using tetramethylsilane (TMS) as an internal standard. Mass spectra (MS) were measured on a Bruker MALDI-TOF mass spectrometer. UV/Vis spectra were recorded on a Cary 50 UV/Vis spectrophotometer. Fluorescence emission spectra were measured on an FL900/FS920 fluorescence spectrophotometer. Raman spectra were recorded on the XploRA Plus system. The particle size distribution was analyzed using a Mastersizer 3000E laser particle size analyser. Energy dispersive X-ray spectroscopy (EDX) was performed on an FESEM-7500F scanning electron microscope (SEM). Element analysis via Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) was performed on a PerkinElmer Optima 8000. A Mithras LB 940 multimode microplate reader was used to detect enzyme activity. Transmission electron microscopy (TEM) images were obtained using a JEM 1400 transmission electron microscope at an acceleration voltage of 100 kV.
2.2. Synthesis of bis-(1-(4-hydroxyphenyl)-5-mercapto-tetrazolyl)silicon(IV) phthalocyanine (Tet-SiPc)
A mixture of dichloro-phthalocyanine silicon (SiPcCl2) (0.061 g, 0.1 mmol),37 1-(4-hydroxyphenyl)-5-mercapto-tetrazole (Tet) (0.05826 g, 0.3 mmol) and potassium carbonate (0.028 g, 0.2 mmol) in toluene (30 mL) was heated at 110 °C for 48 h. The mixture was cooled to room temperature and the solvent was removed by filtration. After evaporation under reduced pressure, the crude product was purified twice via alumina column chromatography using acetone and hexane (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 1
v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) and methanol and methylene chloride (v
5) and methanol and methylene chloride (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 1
v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as eluents, respectively. The obtained product was further purified twice via chromatography on a silica gel column using acetone and methylene chloride (v
10) as eluents, respectively. The obtained product was further purified twice via chromatography on a silica gel column using acetone and methylene chloride (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 1
v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as eluents. Tet-SiPc was obtained as a dark blue-green solid in a yield of 18%. IR ν/cm−1: 736 (Ar–H), 1080 (Si–O), 1240 (C–O), 1506 (C–N); Raman ν/cm−1: 681 (σC–H), 1342 (C–N), 1524 (C
10) as eluents. Tet-SiPc was obtained as a dark blue-green solid in a yield of 18%. IR ν/cm−1: 736 (Ar–H), 1080 (Si–O), 1240 (C–O), 1506 (C–N); Raman ν/cm−1: 681 (σC–H), 1342 (C–N), 1524 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1612 (σC
N), 1612 (σC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H NMR (400 MHz, DMSO-d6) δ/ppm: 8.95 (2H, s, SH, D2O exchange, H1), 5.17 (4H, d, J = 8Hz, H2), 2.28 (4H, d, J = 8Hz, H3), 8.53–8.56 (8H, m, H4), 9.68–9.70 (8H, m, H5); ESI-MS calcd for m/z = 926.16, found: m/z = 925.63 [M]+.
C); 1H NMR (400 MHz, DMSO-d6) δ/ppm: 8.95 (2H, s, SH, D2O exchange, H1), 5.17 (4H, d, J = 8Hz, H2), 2.28 (4H, d, J = 8Hz, H3), 8.53–8.56 (8H, m, H4), 9.68–9.70 (8H, m, H5); ESI-MS calcd for m/z = 926.16, found: m/z = 925.63 [M]+.
2.3. Preparation of mesoporous silica-coated gold nanorods loaded with bis-(1-(4-hydroxyphenyl)-5-mercapto-tetrazolyl)silicon(IV) phthalocyanine (Tet-SiPc@AuNR@SiO2)
AuNRs were synthesized according to the seed-mediated growth method described by Babak et al.38 Firstly, CTAB solution (10 mL, 0.10 M) was mixed with HAuCl4 solution (50 μL, 50 mM). Subsequently, ice-cold NaBH4 solution (600 μL, 0.01 M) was added to the mixture, followed by stirring at 25 °C for 3 min, resulting in the formation of a brown seed solution. In order to grow the gold seeds, the seed solution was allowed to stand for 2 h. The nanorod growth solution was prepared by mixing HAuCl4 solution (300 μL, 50 mM) and CTAB solution (30 mL, 0.10 M) with gentle stirring, then AgNO3 solution (300 μL, 0.10 M), HCl solution (300 μL, 1.0 M) and ascorbic acid (240 μL, 0.10 M) were added in sequence to prepare the growth solution.To grow the gold nanorods, the seed solution (75 μL) was added to the growth solution under slow stirring, and the mixture was continuously stirred at 25 °C overnight to obtain a purple-red gold nanorod mixture, then the mixture was centrifuged at 8000 rpm for 10 min and washed with ultra-pure water three times. Finally, the product, AuNRs, was dispersed in 30 mL of ultra-pure water for further use.
For preparing the mesoporous silica-coated gold nanorods,39 NaOH solution (0.10 M) was added into the above prepared AuNR solution with stirring to adjust the pH of the mixed solution to 10. Next, tetraethyl orthosilicate (TEOS) (20%, 30 μL) in methanol and 10 μL of 2% 3-aminopropyltriethoxysilane (APTES) in methanol were injected into the AuNR solution three times at 30 min intervals. The mixed solution was stirred for 24 h at 25 °C to obtain AuNR@SiO2. Finally, the synthesized AuNR@SiO2 was collected via centrifugation at 8000 rpm for 5 min and washed three times with ultra-pure water to remove CTAB. The product was dispersed in 30 mL of ultra-pure water for further use. The concentrations of gold in AuNRs and AuNR@SiO2 were determined by using element analysis via Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES).
To prepare Tet-SiPc@AuNR@SiO2, Tet-SiPc DMSO solution (50 μL, 1 mM) was added to the AuNR@SiO2 solution (10 mL), the gold concentration of which was 200 μg mL−1 as determined via ICP-OES. The mixture was stirred at room temperature for 48 h. The obtained product solution was centrifuged at 8000 rpm for 5 min, followed by consecutive washing three times with ultra-pure water, and Tet-SiPc@AuNR@SiO2 was then dispersed in 10 mL of ultrapure water for further use.
2.4. Fluorescence quantum yields of Tet-SiPc
Fluorescence quantum yields (ΦF) of Tet-SiPc in DMSO were determined by using the comparative method of Eq. 1:40| ΦF = ΦF(std)·F·AStd·n2/(FStd·A·n2std) | (1) |
2.5. Singlet oxygen quantum yields of Tet-SiPc
Singlet oxygen quantum yields (ΦΔ) of Tet-SiPc were measured by the chemical trapping method based on the singlet oxygen quencher 1,3-diphenylisobenzofuran (DPBF).42 Tet-SiPc (3 mL, 3 × 10−6 M) and DPBF (6 × 10−5 M) were mixed using DMSO as the solvent, and the mixture was continuously irradiated with a laser (671 nm, 100 mW cm−2). The decrease of DPBF absorbance at 417 nm was detected from the UV-Vis spectra. Using n-ZnPc as the reference (ΦΔ = 0.67 for n-ZnPc in DMSO),43 the ΦΔ value of Tet-SiPc was calculated using Eq. 2: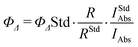 | (2) |
2.6. Singlet oxygen quantum yields of AuNRs, AuNR@SiO2 and Tet-SiPc@AuNR@SiO2
Singlet oxygen was monitored via chemical oxidation of 9,10-anthracenediyl-bis(methylene)dimalonic acid (ABDA) (3 × 10−4 M) in the presence of AuNRs, AuNR@SiO2 or Tet-SiPc@AuNR@SiO2, the gold concentration of which was found to be 100 μg mL−1via ICP-OES.20 The decrease in ABDA absorbance at 378 nm was monitored upon laser irradiation (671 nm, 100 mW cm−2). The irradiation was stopped every 3 min and UV-Vis absorption spectra were recorded.2.7. The loading rate of Tet-SiPc on AuNR@SiO2
The loading rate of Tet-SiPc on AuNR@SiO2 was measured according to the method described by Chen et al.10 The loading rate of Tet-SiPc on AuNR@SiO2 was calculated according to eqn (3):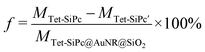 | (3) |
2.8. The photo-induced release of Tet-SiPc from Tet-SiPc@AuNR@SiO2
The photo-induced release of Tet-SiPc from Tet-SiPc@AuNR@SiO2 was studied via fluorescence spectroscopy.10 In brief, 3 mL of Tet-SiPc@AuNR@SiO2 solution with a 100 μg mL−1 gold concentration was added to seven centrifuge tubes, respectively. Each centrifuge tube was irradiated with an infrared laser (808 nm, 0.5 W cm−2) and a thermocouple thermometer was used to record the temperature of the Tet-SiPc@AuNR@SiO2 solution every one minute. AuNR@SiO2, AuNRs and ultra-pure water were used as controls. At the same time, the Tet-SiPc released from Tet-SiPc@AuNR@SiO2 was monitored via fluorescence spectroscopy. Tet-SiPc@AuNR@SiO2 solution was irradiated to release Tet-SiPc which was insoluble in solution at 808 nm (0.5 W cm−2). After the solutions were irradiated for 0, 5, 10, 15, 20 and 25 min. Tet-SiPc is insoluble in the solution, the precipitation of Tet-SiPc was centrifuged and re-dissolved in DMSO. The fluorescence of the Tet-SiPc solution obtained above was measured and the concentration of Tet-SiPc was calculated.2.9. Construction of antibiotic-resistant E. coli DH5α strains and expression of β-galactosidase in E. coli DH5α cells
E. coli DH5α cells are not resistant to Ampicillin, Kanamycin and Zeocin. The Ampicillin-, Kanamycin- or Zeocin-resistant E. coli DH5α strain could be created by transforming a plasmid containing a corresponding antibiotic-resistant gene. In this study, pUC18, pET28a and pPICZa plasmids were used to transform E. coli DH5α. pUC18, pET28a and pPICZa contain an Ampicillin-, a Kanamycin- and a Zeocin-resistant gene, respectively. After successful transformation, E. coli DH5α will gain the ability to resist Ampicillin, Kanamycin or Zeocin. The LB plates containing Ampicillin (100 μg mL−1), Kanamycin (50 μg mL−1) or Zeocin (25 μg mL−1) were used to confirm the ability of the transformed E. coli DH5α to resist the corresponding antibiotics.β-Galactosidase is an important enzyme to metabolize the lactose in E. coli, but the LacZ gene encoding β-galactosidase is mutated in E. coli DH5α, which causes the loss of the β-galactosidase activity. To regain the activity of β-galactosidase in E. coli DH5α, a recombinant plasmid expressing the functional β-galactosidase was constructed by using the pAO815 plasmid as the backbone. The pAO815 was linearized via digestion with the EcoR I restriction enzyme. The LacZ gene with full length was amplified using the plasmid pAd/CMV/V5-GW/lacZ as a template and a pair of specific primers (forward primer: 5′CCGGAATTCACCATGATAGATCCCGTCG 3′, reverse primer: 5′CCGGAATTCTATTTTTGACACCAGACCAACTG 3′) flanked by an EcoR I site at both 5′ and 3′ ends via PCR. The PCR product was purified by using the Promega Wizard SV Gel and PCR Clean-up System (Promega (Beijing) Biotech Co., Ltd. Beijing, China). The purified PCR product was digested using EcoR I and then ligated with the linearized pAO815 plasmid using T4 DNA ligase. The recombinant plasmid was confirmed by DNA sequencing and transformed into E. coli DH5α. The expression of β-galactosidase was verified on the LB plate containing 5-bromo-4-chloro-3-indolyl β-D-galactoside (X-Gal), a chromogenic substrate for β-galactosidase.
2.10. Antibacterial activity of Tet-SiPc@AuNR@SiO2 against E. coli DH5α and antibiotic-resistant E. coli DH5α strains
E. coli DH5α or antibiotic-resistant strains were cultured in the LB (Luria–Bertani) medium at 37 °C overnight until OD600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm reached about 0.5. The culture was then diluted 1
nm reached about 0.5. The culture was then diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in the LB medium. 40 μL of Tet-SiPc@AuNR@SiO2 (CAu = 100 μg mL−1) in sterile distilled water was added into 20 μL of bacterial dilution. The suspension was illuminated using 808 nm laser light at 0.5 W cm−2 for 5 min and then irradiated using a 671 nm laser for 10 min with a power density of 100 mW cm−2. After the combined irradiation at 808 nm and 671 nm, a series of 10-fold dilutions of the bacteria were performed with the LB medium, and 5 μL of each dilution was dropped on LB agar plates and incubated at 37 °C for 12–16 h until the colonies appeared. For the colony forming unit (CFU) assay or relative survival rate analysis, an aliquot (200 μL) of the above diluted bacterial suspension was plated on a 10 cm LB agar plate. The plates were incubated at 37 °C for 12–16 h until the colonies appeared, and then the total number of colonies on each plate was counted. The number of CFU mL−1 was calculated as the number of colonies counted on a plate/0.2 mL and the dilution factor. The cell survival rate was calculated via normalization with respect to the CFU value of the control group performed without the treatment of Tet-SiPc@AuNR@SiO2. Each experiment was repeated three times.
10 in the LB medium. 40 μL of Tet-SiPc@AuNR@SiO2 (CAu = 100 μg mL−1) in sterile distilled water was added into 20 μL of bacterial dilution. The suspension was illuminated using 808 nm laser light at 0.5 W cm−2 for 5 min and then irradiated using a 671 nm laser for 10 min with a power density of 100 mW cm−2. After the combined irradiation at 808 nm and 671 nm, a series of 10-fold dilutions of the bacteria were performed with the LB medium, and 5 μL of each dilution was dropped on LB agar plates and incubated at 37 °C for 12–16 h until the colonies appeared. For the colony forming unit (CFU) assay or relative survival rate analysis, an aliquot (200 μL) of the above diluted bacterial suspension was plated on a 10 cm LB agar plate. The plates were incubated at 37 °C for 12–16 h until the colonies appeared, and then the total number of colonies on each plate was counted. The number of CFU mL−1 was calculated as the number of colonies counted on a plate/0.2 mL and the dilution factor. The cell survival rate was calculated via normalization with respect to the CFU value of the control group performed without the treatment of Tet-SiPc@AuNR@SiO2. Each experiment was repeated three times.
2.11. Effect of Tet-SiPc@AuNR@SiO2 on the genomic DNA damage of E. coli DH5α
The genomic DNA of E. coli DH5α with or without the treatment of Tet-SiPc@AuNR@SiO2 under combined laser irradiation at 808 nm and 671 nm as mentioned above was extracted by using the genomic DNA extraction kit (Tiangen (Beijing) Biotech Co., Ltd Beijing, China) and quantified using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). 500 μg of genomic DNA was loaded into wells of a 1% agarose gel, and then electrophoresis was performed at 100 V for 30 min. The gel was stained with ethidium bromide and photographed.2.12. Effect of Tet-SiPc@AuNR@SiO2 on the enzyme activity of β-galactosidase
The engineered E. coli DH5α cells expressing the functional β-galactosidase were treated with or without Tet-SiPc@AuNR@SiO2 under combined laser irradiation at 808 nm and 671 nm as mentioned above. The bacterial cells were collected via centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min. After discarding the supernatant, the pellets were resuspended with Z-buffer (Na2HPO4 60 mM, NaH2PO4 40 mM, KCl 10 mM, MgSO4 1 mM, pH = 7.0) with β-mercaptoethanol (adding 0.27 mL β-mercaptoethanol to 100 mL of Z-buffer), and then 3 drops of chloroform and 2 drops of 0.1% sodium dodecyl sulfonate (SDS) were added, followed by vortexing at 3000 rpm for 10 s, and this procedure was repeated three times. After incubating the bacterial solution at 28 °C for 5 min, 200 μL of 2-nitrophenyl-β-D-galactopyranoside (ONPG) (4 mg mL−1 in Z-buffer) was added and incubated at 30 °C until a yellow colour developed. The elapsed time was recorded in minutes. After the yellow colour developed, 500 μL of 1% Na2CO3 solution was added. The reaction tubes were centrifuged for 10 min at 14
000 rpm for 5 min. After discarding the supernatant, the pellets were resuspended with Z-buffer (Na2HPO4 60 mM, NaH2PO4 40 mM, KCl 10 mM, MgSO4 1 mM, pH = 7.0) with β-mercaptoethanol (adding 0.27 mL β-mercaptoethanol to 100 mL of Z-buffer), and then 3 drops of chloroform and 2 drops of 0.1% sodium dodecyl sulfonate (SDS) were added, followed by vortexing at 3000 rpm for 10 s, and this procedure was repeated three times. After incubating the bacterial solution at 28 °C for 5 min, 200 μL of 2-nitrophenyl-β-D-galactopyranoside (ONPG) (4 mg mL−1 in Z-buffer) was added and incubated at 30 °C until a yellow colour developed. The elapsed time was recorded in minutes. After the yellow colour developed, 500 μL of 1% Na2CO3 solution was added. The reaction tubes were centrifuged for 10 min at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm to pellet cell debris, and the supernatants were carefully transferred into a 96-well plate. Finally, the developed yellow colour was measured at 420 nm on a spectrophotometer. The enzyme activity of β-galactosidase was calculated according to eqn (4) and the enzyme activity of control (no treatment with Tet-SiPc@AuNR@SiO2) was normalized to 100%:
000 rpm to pellet cell debris, and the supernatants were carefully transferred into a 96-well plate. Finally, the developed yellow colour was measured at 420 nm on a spectrophotometer. The enzyme activity of β-galactosidase was calculated according to eqn (4) and the enzyme activity of control (no treatment with Tet-SiPc@AuNR@SiO2) was normalized to 100%: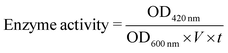 | (4) |
3. Results and discussion
3.1. Synthesis and characterization of Tet-SiPc
Tet-SiPc was obtained via a nucleophilic substitution reaction between 1-(4-hydroxyphenyl)-5-mercapto-tetrazole and dichloro-silicon phthalocyanine (SiPcCl2) using anhydrous K2CO3 as the catalyst in a yield of 18% (Scheme 1). The chemical structure of Tet-SiPc was characterized by various methods including 1H NMR, ESI-MS, FT-IR and Raman spectroscopy (Fig. S1–S4, ESI†). The UV/Vis spectra of Tet-SiPc in dimethyl sulfoxide (DMSO) showed typical spectra of Pc with a B band at 355 nm and a Q band at 683 nm (Fig. 1a). With the increase of the concentration of Tet-SiPc, the intensity of the Q band was enhanced, while the shape and position of the Q band did not change, indicating that the introduction of mercapto-tetrazole functional groups could reduce the aggregation of Pcs. Upon excitation at 615 nm, Tet-SiPc showed emission at 677 nm (Fig. 1b). The fluorescence lifetime of Tet-SiPc was found to be 5.23 ns, and the fluorescence quantum yield (ΦF) was calculated to be 0.0522 (Table S1 and Fig. S5, ESI†). The quantum yield of singlet oxygen (1O2) (ΦΔ) was found to be 0.355 (Table S1 and Fig. S6, ESI†).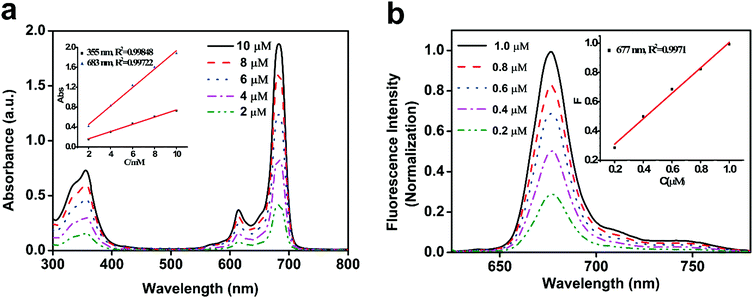 | ||
| Fig. 1 (a) UV-visible spectra of Tet-SiPc in DMSO; (b) fluorescence spectra of Tet-SiPc in DMSO (λex = 615 nm). | ||
3.2. Preparation and characterization of AuNRs, AuNR@SiO2 and Tet-SiPc@AuNR@SiO2
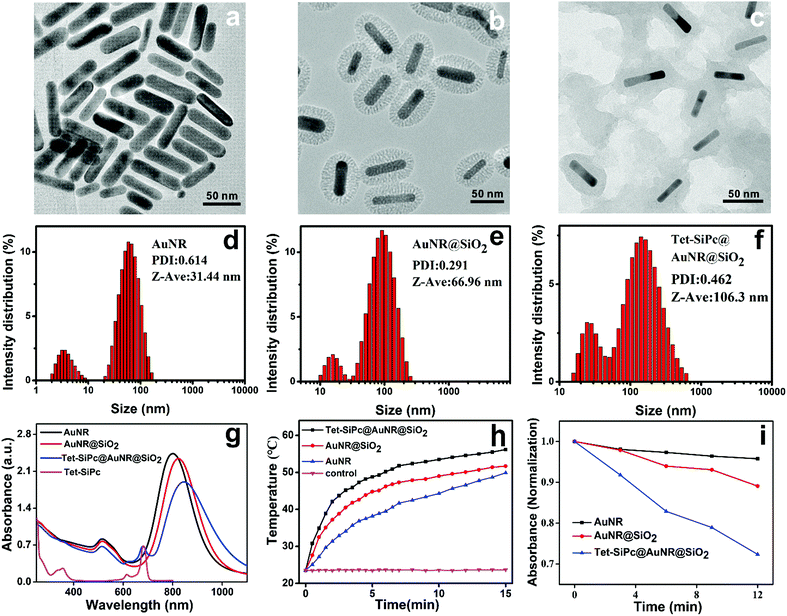
In order to enhance the antibacterial efficiency of Tet-SiPc and reduce its phototoxicity, AuNR-SiO2 was used as a nanocarrier to load Tet-SiPc to form a novel light control nano-switch (Tet-SiPc@AuNR-SiO2). The Tet-SiPc@AuNR-SiO2 was prepared through the adsorption of Tet-SiPc on AuNR-SiO2. Briefly, AuNRs were synthesized using the seed-mediated growth method.38 AuNR@SiO2, a mesoporous silica layer wrapped on the surface of AuNRs, was prepared by an improved method.39 Tet-SiPc (1 mM) was added to the aqueous solution of AuNR@SiO2 (200 μg mL−1) and stirred for 48 h. After being centrifuged and dialyzed, Tet-SiPc@AuNR@SiO2 was obtained (Scheme 1). The TEM images showed that AuNRs, AuNR@SiO2 and Tet-SiPc@AuNR@SiO2 exhibited uniform size and good dispersion in solution (Fig. 2a–c). The average length and width of AuNRs were about 59 nm and 16 nm, respectively, and the aspect ratio (AR) was calculated to be about 3.70. The thickness of the initial silica coating was measured to be about 22 nm for AuNR@SiO2. After the Tet-SiPc was successfully loaded into the mesoporous silica of AuNR@SiO2, the colour of the silica coating layer became darker due to the contribution of the high electron density of Tet-SiPc. The dynamic light scattering (DLS) size of AuNRs was found to be 31 nm. After the mesoporous silica was coated on AuNRs, the DLS size of AuNR@SiO2 increased from 31 nm to 67 nm. With further loading of Tet-SiPc on AuNR@SiO2, the DLS size of Tet-SiPc@AuNR@SiO2 was increased to 106 nm (Fig. 2d–f). The average particle sizes measured via DLS were larger than those measured via TEM, which could be related to the swelling of nanoparticles in solution.44 Energy dispersive X-ray spectroscopy (EDX) was used to demonstrate the successful loading of Tet-SiPc on AuNR@SiO2. The content of N element in AuNR@SiO2 was significantly higher than that in AuNRs, and the element of Si appeared at AuNR@SiO2, indicating the successful coating of SiO2 on AuNRs (Fig. S7 and S8, Table S2 and S3, ESI†). The presence of S element in Tet-SiPc@AuNR@SiO2 proved the successful loading of Tet-SiPc on Tet-SiPc@AuNR@SiO2 (Fig. S9, Table S4, ESI†). The successful preparation of Tet-SiPc@AuNR@SiO2 was also confirmed by the UV/Vis absorption spectra (Fig. 2g). The AuNRs exhibited two surface plasmon resonance absorption peaks. The lateral surface plasmon resonance absorption peak was located at 514 nm and the longitudinal surface plasmon resonance absorption peak was found to be at 801 nm. After the AuNRs were coated with mesoporous silica, the shape and position of the lateral characteristic absorption peak at 514 nm were unchanged, while the longitudinal surface plasmon resonance was red-shifted from 801 nm to 824 nm. In the absorption spectrum of Tet-SiPc@AuNR@SiO2, a characteristic absorption peak of Tet-SiPc was observed at 683 nm and the longitudinal surface plasmon resonance absorption peak of AuNRs continued to redshift to 845 nm, indicating that Tet-SiPc was successfully loaded in AuNR@SiO2. The loading ratio of Tet-SiPc in Tet-SiPc@AuNR@SiO2 was calculated to be 48% using UV-Vis absorption spectra. The photothermal effect of AuNRs, AuNR@SiO2 and Tet-SiPc@AuNR@SiO2 irradiated using an 808 nm laser with a power density of 0.5 W cm−2 for 15 min was shown in Fig. 2h. AuNRs, AuNR@SiO2 and Tet-SiPc@AuNR@SiO2 exhibited excellent photothermal properties. The temperature of AuNRs, AuNR@SiO2 and Tet- SiPc@AuNR@SiO2 increased to 49.9, 51.7 and 56.2 °C after 15 min illumination, respectively. Tet-SiPc@AuNR@SiO2 possessed the highest photothermal conversion efficiency, which is probably due to the theranostic photothermal effect of Tet-SiPc loaded on the mesoporous silica and AuNR@SiO2.45The 1O2 generation ability of AuNRs, AuNR@SiO2 and Tet-SiPc@AuNR@SiO2 was evaluated by using 9,10-anthracenediyl-bis(methylene)dimalonic acid (ABDA) as a probe.20 The decrease of the ABDA absorption at 378 nm as a function of irradiation time was observed upon irradiation at 671 nm (Fig. S10–S12, ESI†). The Tet-SiPc@AuNR@SiO2 exhibited the highest ability to produce 1O2, followed by AuNR@SiO2 and AuNRs (Fig. 2i). The best 1O2 generation ability of Tet-SiPc@AuNR@SiO2 may be related to the synergistic generation of 1O2 by both Tet-SiPc and AuNR@SiO2 in the nanosystem.46
3.3. Light-controlled Tet-SiPc release from the nano-switch Tet-SiPc@AuNR@SiO2
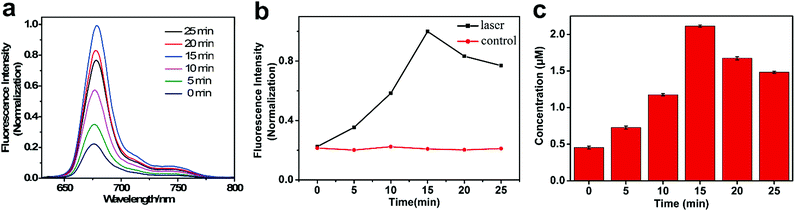
Most of the theranostic agents were “always-on” models for therapeutic intervention, leading to a low signal-to-noise ratio and microbial drug resistance. The nanomaterials for controlled release can effectively overcome these deficiencies.In order to confirm that Tet-SiPc@AuNR@SiO2 is an excellent light-controlled nano-switch, a series of experiments were carried out. The fluorescence of Tet-SiPc in Tet-SiPc@AuNR@SiO2 was quenched by AuNR@SiO2 without irradiation. Upon irradiation with NIR light, Tet-SiPc was released from Tet-SiPc@AuNR@SiO2 (Fig. 3a). The amount of Tet-SiPc released from Tet-SiPc@AuNR@SiO2 was quantified using fluorescence spectra (Fig. 3b and c). The photothermal effect of Tet-SiPc@AuNR@SiO2 could change the exothermic adsorption equilibrium and then promoted the release of Tet-SiPc. But the irradiation time was over 15 min, and a decrease in the fluorescence intensity of Tet-SiPc was observed, which may be caused by the photobleaching of Tet-SiPc by irradiation or the aggregation behaviour of Tet-SiPc released into water. Light can manipulate very precisely to release the Tet-SiPc in the Tet-SiPc@AuNR@SiO2, which provides a strategy to prevent the bacteria from developing drug resistance by long exposure to the Tet-SiPc@AuNR@SiO2 in antibacterial application.
3.4. Antibacterial activity of the nano-switch Tet-SiPc@AuNR@SiO2 against E. coli DH5α
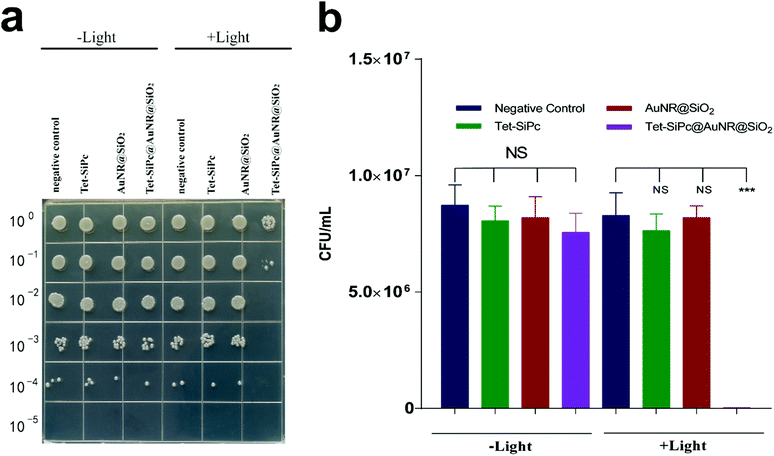
E. coli DH5α was selected as a model to evaluate the antibacterial activity of Tet-SiPc@AuNR@SiO2. Upon irradiation, both Tet-SiPc and AuNR@SiO2 did not show obvious antibacterial efficacies against DH5α, indicating that the photodynamic efficacy of Tet-SiPc or the photothermal efficacy of AuNR@SiO2 alone did not present enough antibacterial activity against DH5α. The Tet-SiPc@AuNR@SiO2 exhibited a significant antibacterial efficacy with a killing rate of 99.83% (Fig. 4), which can be explained by the fact that AuNR@SiO2 served as a photothermal agent to absorb the energy of the NIR laser and convert it into heat energy, which triggered the release of Tet-SiPc from Tet-SiPc@AuNR@SiO2 and promoted the released Tet-SiPc to produce 1O2 to kill the bacteria through the synergistic photodynamic and photothermal effects.473.5. Antibacterial activity of the nano-switch Tet-SiPc@AuNR@SiO2 against antibiotic-resistant E. coli DH5α strains
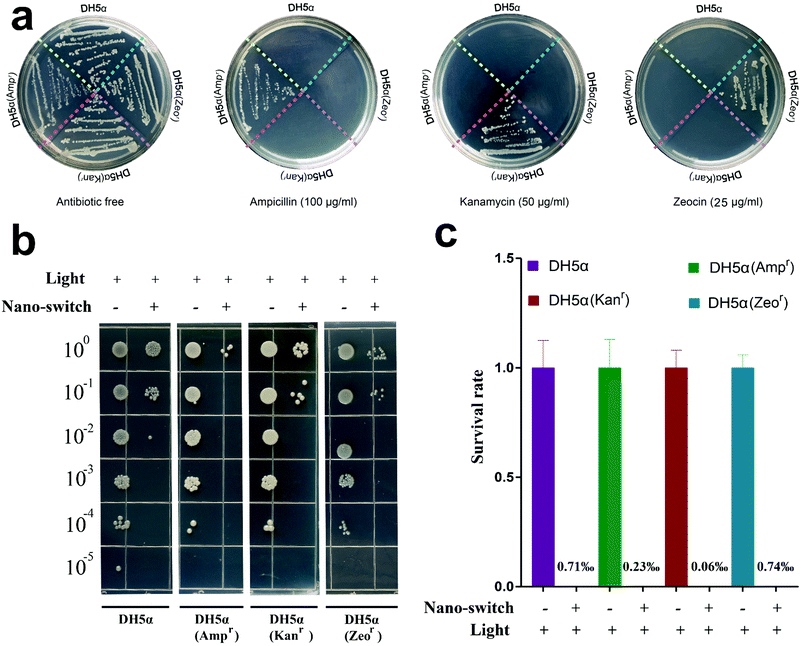
We wondered whether the Tet-SiPc@AuNR@SiO2 exhibited the same effect in killing the antibiotic-resistant bacteria as the non-resistant bacteria. Three antibiotic-resistant DH5α strains, DH5α (Ampr), DH5α (Kanr) and DH5α (Zeor), were obtained through the transformation of plasmids pUC18, pET28a and pPICZa into DH5α cells, respectively. Their ability to resist antibiotics was confirmed on the plates containing the corresponding antibiotics (Fig. 5a). The antibacterial activity of Tet-SiPc@AuNR@SiO2 against the antibiotic-resistant DH5α was evaluated. The results showed that the Tet-SiPc@AuNR@SiO2 also exhibited strong antibacterial activity against all DH5α (Ampr), DH5α (Kanr) and DH5α (Zeor) strains (Fig. 5b and c).3.6. Antibacterial mechanism of the nano-switch Tet-SiPc@AuNR@SiO2
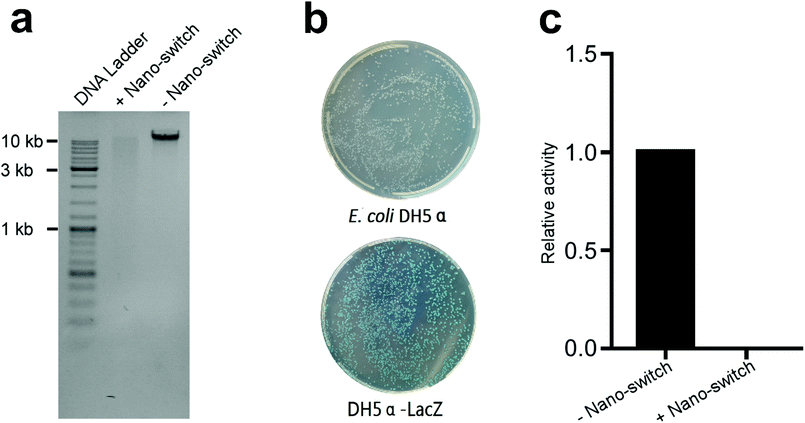
DNA and enzymes are the most important biological macromolecules in bacterial cells. DNA stores the genetic information of bacteria and controls the metabolism of bacteria.48 Enzymes catalyse nearly all the chemical reactions in cells and are also essential for cell survival. We suspected that Tet-SiPc@AuNR@SiO2 impaired DNA and enzymes in cells under laser light, which caused the death of cells. The genomic DNA of DH5α treated with or without Tet-SiPc@AuNR@SiO2 was isolated and analysed via agarose gel electrophoresis. The result was shown in Fig. 6a. The genomic DNA treated with Tet-SiPc@AuNR@SiO2 upon irradiation showed a smear band in the agarose gel, suggesting that DNA was fragmented.The β-galactosidase encoded by the LacZ gene is a favourable reporter for the quantitative analysis of enzymatic activity in microorganisms. In this study, β-galactosidase was used as a reporter to evaluate the enzymatic damage of DH5α treated with Tet-SiPc@AuNR@SiO2 under laser irradiation. β-galactosidase activity is deficient in DH5α because of the ΔM15 mutation of the LacZ gene. In order to restore the β-galactosidase activity, we constructed a plasmid expressing the LacZ gene and transformed it into DH5α, named DH5α-LacZ. The DH5α-LacZ strain showed blue colonies in the plate containing X-Gal, indicating that the β-galactosidase activity was regained in DH5α-LacZ (Fig. 6b). The β-galactosidase activity of the DH5α-LacZ strain treated with or without Tet-SiPc@AuNR@SiO2 was analysed. The result showed that the β-galactosidase activity of DH5α-LacZ was totally undetectable after the treatment of Tet-SiPc@AuNR@SiO2 under laser irradiation (Fig. 6c).
The possible antibacterial mechanism is that the Tet-SiPc@AuNR@SiO2 more efficiently generated heat and ROS upon irradiation. The enzymes and DNA in cells are denatured at high temperatures, and these denatured macromolecules are more vulnerable to impair by ROS. The synergistic photothermal and photodynamic effect gives the Tet-SiPc@AuNR@SiO2 a more effective ability to kill E. coli cells.
4. Conclusions
In this work, we successfully constructed a near-infrared light-controlled nano-switch Tet-SiPc@AuNR@SiO2. This nano-switch was assembled through adsorption of Tet-SiPc in the mesoporous silica layer of AuNR@SiO2. Tet-SiPc@AuNR@SiO2 realized precisely controlled release of Tet-SiPc from AuNR@SiO2 and the generation of ROS as well as excellent photothermal conversion efficiency through simple light irradiation and exhibited a synergistic photothermal and photodynamic effects in killing both E. coli and antibiotic-resistant E. coli strains. The degradation of genomic DNA and the loss of enzyme activity in E. coli cells after the treatment with Tet-SiPc@AuNR@SiO2 under irradiation could be the main causes of bacteria killing.Author contributions
Qiuhao Ye: methodology, data curation, formal analysis, writing – review & editing. Shuanghuang Xiao: investigation, writing – original draft. Ting Lin: investigation. Yufeng Jiang: investigation, methodology, formal analysis. Yiru Peng: conceptualization, methodology, project administration, supervision, writing – review & editing, funding acquisition. Yide Huang: methodology, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Basic Research Program of China (973 project) (2015CB35200), the National Natural Science Foundation of China (21274021), the Natural Science Foundation of Fujian (2019Y0007), the Joint Funds of Fujian Provincial Health and Education Research (2019-WJ-23), the Scientific Research Innovation Team Construction Programme of Fujian Normal University (IRTL 1702), the scientific research innovation program “Xiyuanjiang River Scholarship” of College of Life Sciences, Fujian Normal University and the Special Funds of the Central Government Guiding Local Science and Technology Development (2020L3008).Notes and references
- M. Bassetti, G. Poulakou, E. Ruppe, E. Bouza, S. J. Van Hal and A. Brink, Intensive Care Med., 2017, 43, 1464–1475 CrossRef CAS.
- J. M. Stokes, K. Yang, K. Swanson, W. Jin, A. Cubillos-Ruiz, N. M. Donghia, C. R. MacNair, S. French, L. A. Carfrae, Z. Bloom-Ackerman, V. M. Tran, A. Chiappino-Pepe, A. H. Badran, I. W. Andrews, E. J. Chory, G. M. Church, E. D. Brown, T. S. Jaakkola, R. Barzilay and J. J. Collins, Cell, 2020, 180, 688–702 CrossRef CAS.
- T. Wi, M. M. Lahra, F. Ndowa, M. Bala, J. R. Dillon, P. Ramon-Pardo, S. R. Eremin, G. Bolan and M. Unemo, PLoS Med., 2017, 14, e1002344 CrossRef.
- M. E. A. D. Kraker, A. J. Stewardson and S. Harbarth, PLoS Med., 2016, 13, e1002184 CrossRef.
- E. Tacconelli, E. Carrara, A. Savoldi, S. Harbarth, M. Mendelson, D. L. Monnet, C. Pulcini and G. Kahlmeter, Lancet Infect. Dis., 2018, 18, 318–327 CrossRef.
- T. Guo, Y. Lin, G. Jin, R. Weng, J. Song, X. Liu, G. Huang, L. Hou and H. Yang, Chem. Commun., 2019, 55, 850–853 RSC.
- N. Yang, C. Wang, X. Wang and L. Li, Nanotechnology, 2018, 29, 175601 CrossRef.
- W.-Y. Chen, H.-Y. Chang, J.-K. Lu, Y.-C. Huang, S. G. Harroun, Y.-T. Tseng, Y.-J. Li, C.-C. Huang and H.-T. Chang, Adv. Funct. Mater., 2015, 25, 7189–7199 CrossRef CAS.
- M. L. Xu, L. Y. Guan, S. K. Li, L. Chen and Z. Chen, Chem. Commun., 2019, 55, 5359–5362 RSC.
- X. Chen, S. Wu, D. Ma, J. Chen, Q. Guo, X. Han, K. Chen, H. Yang, Y. Huang and Y. Peng, Chem. Commun., 2018, 54, 13279–13282 RSC.
- X. Yang, J. Li, T. Liang, C. Ma, Y. Zhang, H. Chen, N. Hanagata, H. Su and M. Xu, Nanoscale, 2014, 6, 10126–10133 RSC.
- W. Zeng, H. Zhang, Y. Deng, A. Jiang, X. Bao, M. Guo, Z. Li, M. Wu, X. Ji, X. Zeng and L. Mei, Chem. Eng. J., 2020, 389, 124494 CrossRef CAS.
- Z. H. Yu, X. Li, F. Xu, X. L. Hu, J. Yan, N. Kwon, G. R. Chen, T. Tang, X. Dong, Y. Mai, D. Chen, J. Yoon, X. P. He and H. Tian, Angew. Chem., Int. Ed., 2020, 59, 3658–3664 CrossRef CAS.
- X. Chen, Q. Zhang, J. Li, M. Yang, N. Zhao and F. J. Xu, ACS Nano, 2018, 12, 5646–5656 CrossRef CAS.
- Y. Wang, Q. Cui, X. Zhao, T. Qin, W. Wang, H. Sun, H. Zhu, H. Guo and H. Sun, RSC Adv., 2018, 8, 41454–41463 RSC.
- C. Li, Y. Zhang, Z. Li, E. Mei, J. Lin, F. Li, C. Chen, X. Qing, L. Hou, L. Xiong, H. Hao, Y. Yang and P. Huang, Adv. Mater., 2018, 30, 1706150 CrossRef.
- Y. Liu, P. Bhattarai, Z. Dai and X. Chen, Chem. Soc. Rev., 2019, 48, 2053–2108 RSC.
- J. Liu, C. Detrembleur, M. C. De Pauw-Gillet, S. Mornet, C. Jerome and E. Duguet, Small, 2015, 11, 2323–2332 CrossRef CAS.
- X. Cui, W. Cheng and X. Han, J. Mater. Chem. B, 2018, 6, 8078–8084 RSC.
- K. Turcheniuk, V. Turcheniuk, C. H. Hage, T. Dumych, R. Bilyy, J. Bouckaert, L. Heliot, V. Zaitsev, R. Boukherroub and S. Szunerits, Chem. Commun., 2015, 51, 16365–16368 RSC.
- J. Sun, Y. Zhang, J. Su, T. Dai, J. Chen, L. Zhang, H. Wang, W. Liu, M. Huang and Z. Chen, Dyes Pigm., 2020, 179, 108392 CrossRef CAS.
- W. Liu, Y. Zhang, W. You, J. Su, S. Yu, T. Dai, Y. Huang, X. Chen, X. Song and Z. Chen, Nanoscale, 2020, 12, 13948–13957 RSC.
- E. Dube, D. O. Oluwole, N. Nwaji and T. Nyokong, Spectrochim. Acta, Part A, 2018, 203, 85–95 CrossRef CAS.
- P. Garcia Calavia, I. Chambrier, M. J. Cook, A. H. Haines, R. A. Field and D. A. Russell, J. Colloid Interface Sci., 2018, 512, 249–259 CrossRef CAS.
- L. Lamch, J. Kulbacka, M. Dubinska-Magiera, J. Saczko and K. A. Wilk, Photodiagn. Photodyn. Ther., 2019, 25, 480–491 CrossRef CAS.
- Z. Wang, T. Jia, Q. Sun, Y. Kuang, B. Liu, M. Xu, H. Zhu, F. He, S. Gai and P. Yang, Biomaterials, 2020, 228, 119569 CrossRef CAS.
- X. Li, D. Lee, J. D. Huang and J. Yoon, Angew. Chem., Int. Ed., 2018, 57, 9885–9890 CrossRef CAS.
- E. Lee, X. Li, J. Oh, N. Kwon, G. Kim, D. Kim and J. Yoon, Chem. Sci., 2020, 11, 5735–5739 RSC.
- H. H. Mohamed, I. Hammami, S. Akhtar and T. E. Youssef, Composites, Part B, 2019, 176, 107314 CrossRef CAS.
- A. Galstyan, A. Ricker, H. Nüsse, J. Klingauf and U. Dobrindt, ACS Appl. Bio Mater., 2019, 3, 400–411 CrossRef.
- Y. Jia, J. Li, J. Chen, P. Hu, L. Jiang, X. Chen, M. Huang, Z. Chen and P. Xu, ACS Appl. Mater. Interfaces, 2018, 10, 15369–15380 CrossRef CAS.
- K. Kuninobu, A. Tadaaki, M. Noriyuki, H. Makoto and S. Tamotsu, Chem. Lett., 2002, 966–967 Search PubMed.
- N. Nishiyama, Y. Nakagishi, Y. Morimoto, P. S. Lai, K. Miyazaki, K. Urano, S. Horie, M. Kumagai, S. Fukushima, Y. Cheng, W. D. Jang, M. Kikuchi and K. Kataoka, J. Controlled Release, 2009, 133, 245–251 CrossRef CAS.
- S. Bhana, R. O'Connor, J. Johnson, J. D. Ziebarth, L. Henderson and X. Huang, J. Colloid Interface Sci., 2016, 469, 8–16 CrossRef CAS.
- S. J. Wittenberger, Org. Prep. Proc. Int., 1994, 26, 499–531 CrossRef CAS.
- S. Ganapaty, P. Ramalingam and C. B. Rao, Indian J. Heterocycl. Chem., 2007, 16, 283–286 CAS.
- T. I. Bruce, N. Diel, K. F. Schoch, T. J. Marks, J. W. Lyding and C. R. Kannewurf, J. Am. Chem. Soc., 1983, 105, 1551–1567 CrossRef.
- N. Babak and A. E. Mostafa, Chem. Mater., 2003, 15, 1957–1962 CrossRef.
- I. Gorelikov and N. Matsuura, Nano Lett., 2008, 8, 369–373 CrossRef CAS.
- X. H. Peng, S. F. Chen, B. Y. Zheng, B. D. Zheng, Q. F. Zheng, X. S. Li, M. R. Ke and J. D. Huang, Tetrahedron, 2017, 73, 378–384 CrossRef CAS.
- X.-F. Zhang and H.-J. Xu, J. Chem. Soc., Faraday Trans., 1993, 89, 3347–3351 RSC.
- N. Nwahara, J. Britton and T. Nyokong, J. Coord. Chem., 2017, 70, 1601–1616 CrossRef CAS.
- N. A. Kuznetsova, N. S. Gretsova and E. A. Kalmykova, Russ. J. Gen. Chem., 2000, 70, 133–140 CAS.
- J. Geng, Z. Zhu, W. Qin, L. Ma, Y. Hu, G. G. Gurzadyan, B. Z. Tang and B. Liu, Nanoscale, 2014, 6, 939–945 RSC.
- J. Peng, L. Zhao, X. Zhu, Y. Sun, W. Feng, Y. Gao, L. Wang and F. Li, Biomaterials, 2013, 34, 7905–7912 CrossRef CAS.
- N. T. Chen, K. C. Tang, M. F. Chung, S. H. Cheng, C. M. Huang, C. H. Chu, P. T. Chou, J. S. Souris, C. T. Chen, C. Y. Mou and L. W. Lo, Theranostics, 2014, 4, 798–807 CrossRef.
- J. Wang, Y. Liu, Y. Ma, C. Sun, W. Tao, Y. Wang, X. Yang and J. Wang, Adv. Funct. Mater., 2016, 26, 7516–7525 CrossRef CAS.
- N. Jimenez-Garrido, L. Perello, R. Ortiz, G. Alzuet, M. Gonzalez-Alvarez, E. Canton, M. Liu-Gonzalez, S. Garcia-Granda and M. Perez-Priede, J. Inorg. Biochem., 2005, 99, 677–689 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterization data. See DOI: 10.1039/d0ma00782j |
| This journal is © The Royal Society of Chemistry 2021 |