Open Access Article
Open Access ArticleSolid-phase reactive chromatography (SPRC): a sustainable method for the synthesis of benzimidazol-diphenyl-2-imino-thiazolidine-4-ols (hemiaminals) which are active against lung cancer†
V. M. Bangade *ab,
P. R. Malia and
H. M. Meshram
*ab,
P. R. Malia and
H. M. Meshram *a
*a
aMedicinal Chemistry and Pharmacology Division, CSIR-Indian Institute of Chemical Technology, Hyderabad, 500007, India
bDepartment of Chemistry, The Institute of Science, Dr Homi Bhabha State University, 15, Madame Cama Road, Mumbai-32, India. E-mail: vikasmb2009@gmail.com
First published on 8th January 2021
Abstract
The concept of a green approach has been applied in the reaction, separation and solidification of a single unit. The products, 2-benzimidazol-diphenyl-2-imino-thiazolidine-4-ols, are purely hemiaminals. They have a tendency to decompose at a high rate. The solid-phase reactive chromatographic technique has been applied to avoid extensive liquid–liquid extraction and stabilised the product by precipitation in solvents. Benzimidazole phenyl thiourea and phenacyl bromide have been used as starting materials in this study. Both the starting materials are adsorbed on dry silica and packed in a glass column with a systematic arrangement of layers. A directly solid product was precipitated in cold hexane. Anticancer activities have been recorded against four cell lines, human colon, prostate, lung and breast cancer, with reference to doxorubicin as a standard. These compounds show a promising effect on human lung cancer, products B9 (IC50 = 3.890 μM) and B10 (IC50 = 2.798 μM) and B13 (IC50 = 3.140 μM) which very close to doxorubicine (IC50 = 1.750 μM). It was observed that fluoro phenyl functionalities are effective compared to trifluoro methyl phenyl functionalities for anticancer activities. These small molecules lose their activities, except fluorination, on bulky substitution. This convenient metal-free approach is highly efficient for unstable hemiaminals such as the potent anticancer benzimidazol-diphenyl-2-imino-thiazolidine-4-ols.
Introduction
The solid-supported on-column reactions are extremely popular because of their interesting applications like separation and purification by skipping liquid–liquid extraction in a single unit. Industrially viable chemical reactions have been developed in a manner that used recyclable solvents and reagents. Because of bulky batches, industrial techniques avoid the extraction process. Synthetic chemists are attentive towards cumulative single unit reactions for directly converting reactants into a pure product. There are important reactions performed in a column using solid phase reagents and catalysts:1 asymmetric synthesis of beta-lactam,2 Wittig and Horner–Emmons reaction,3 esterification,4 metathesis,5 Suzuki coupling reaction,6 and organocatalyzed Diels–Alder reaction.7 The continuous flow method is one of the sequential column methods in which reagents and catalysts on a solid phase support are loaded onto sequential columns.8Compounds with a tetrahedral carbon attached to both amino nitrogen and hydroxyl groups are assigned as hemiaminals (carbinolamines).9 These are highly unstable intermediates in the reaction of amine with carbonyl compounds. In the synthesis of thiazoles, hemiaminals appeared only in the mechanism point of view but were difficult to isolate as stable compounds.10,11 Synthesis of a stable hemiaminal is a hefty task for a synthetic chemist.
A literature survey reveals that 2-imino-thiazolidine-4-ols can be prepared by the reaction of thiourea and 2-bromo-acetophenone.12 Recently, Dalmal et al. synthesized 2-imino-4-(trifluoromethyl)thiazolidin-4-alcohols (hemiaminals) using 3-bromo-1,1,1-trifluoropropan-2-one, followed by subsequent modifications for the various derivatives of isoxazoles, propargylamine and triazoles, which are biologically important compounds.13 Not only Gunal et al. isolated hemiaminals10 but also Tuncel and Dogan synthesized chiral hemiaminals by the reduction of 2-iminothiazolidine-4-ones using LiAlH4.9 Hemiaminals can be stabilized using synthetic receptors,14 dendrimers15 and triazole rings.16
In this study, we stabilized benzimidazol-diphenyl-2-imino-thiazolidine-4-ols (hemiaminals) as the main products using benzimidazol-phenyl thiourea derivative 1a and substituted 2-bromo-acetophenone. Benzimidazole is a very important heterocyclic component in medicinal chemistry and synthetic chemistry. It is a unique bicyclic member of heterocyclic compound for its nucleophilic and electrophilic reactions. The reaction proceedings are diverse compared to other heterocyclic compounds. In the reported list of the WHO (in year 2019), triclabendazole is a medically approved essential medicine used for fascioliasis and paragonimiasis in treating liver flukes.17 Tetra-substituted benzimidazole derivatives have anti-urease activity.18 Benzimidazole derivatives are used for multipurpose activities, such as antiviral, antitumor, antipyretic, and anti-ulcer activities. Recently, Ozdemir et al. studied a series of benzimidazole-piperazine hybrids for cytotoxic activity against human lung cancer and breast cancer cell lines.19 Thus, the synthesis of benzimidazole-based medicinally important compounds is a robust target for a chemist.
It was observed that the reported methods for the synthesis of 2-imino-thiazolidine-4-ol are acceptable but there are many parameters that should be under modification for the best results of hemiaminals. The time required for the reaction, separation, purification and stability of product are major factors responsible for the quality work of 2-imino-thiazolidine-4-ol. The reported methods are not applicable to unstable hemiaminals such as benzimidazol-diphenyl-2-imino-thiazolidine-4-ol, which is a strong anticancer agents. Thus, it is appreciated to develop a more viable and easy method for synthesizing 2-imino-thiazolidine-4-ol. By compiling these major factors and the combined advantage of benzimidazole and 2-imino-thiazolidine, we envisaged to synthesize benzimidazole-diphenyl-2-imino-thiazolidine-4-ol by column chromatography. To our knowledge, there is no report for the synthesis of hemiaminals such as benzimidazole-diphenyl-2-imino-thiazolidine-4-ol by reaction, separation and solidification in a single unit.
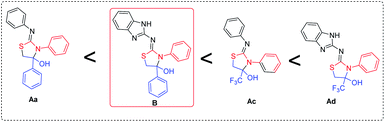
There are different structural hemiaminals synthesized but benzimidazole-diphenyl-2-imino-thiazolidine-4-ol hemiaminal (B1) is exclusively different than other hemiaminals shown in Fig. 1.
Fig. 1 shows the hemiaminals of trifluoromethyl 2-bromoacetophenone, Ac and Ad, which are highly stable. Hemiaminal Ad may be highly stable due to benzimidazole ring. Similarly, hemiaminal B1 is more stable than Aa because it has benzimidazole ring and Aa will appear as a mechanism point of view. Though B1 is stable in a solid state, it has an unstable nature in the solution form. Both Ac and Ad are highly stable in solid and solution. However, because of the benzimidazole ring, it may be possible that Ad is stable than Ac.
We disclose herein an industrially viable method for the synthesis of benzimidazole-diphenyl-2-imino-thiazolidine-4-ol (hemiaminal) by regular column chromatography, which combines reaction, separation and stabilization of product by solidification in a cumulative single unit (Scheme 1). Note that amino thiazolidine-4-ols show promising anticancer activities.
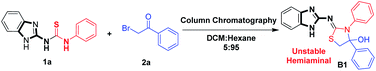 | ||
| Scheme 1 Synthesis of benzimidazol-diphenyl-2-imino-thiazolidine-4-ol (hemiaminal) by column chromatography. | ||
Results and discussion
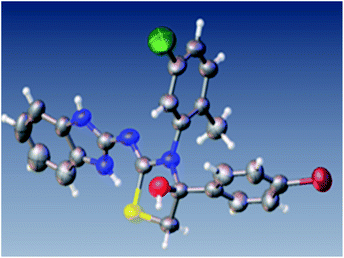
We Initiated our efforts by attempting the reaction of 1-(1H-benzo[d]imidazol-2-yl)-3-phenylthiourea 1a and 2-bromoacetophenone 2a in THF at room temperature, and within a minute we verified the progress of the reaction. The reaction was monitored by TLC; to our surprise, it was completed with a high rate of reaction. The reaction was extracted by ethyl acetate and separated by column chromatography. At the time of separation by column chromatography, we identified that the original product started to decompose. Literature survey and our practical approaches reveal that 2-imino-thiazolidine-4-ol usually undergoes decomposition.As the rate of reaction was very high, due to the problem of decomposition, we planned a silica-adsorbed column-based reaction. Benzimidazol-phenyl thiourea 1a was dissolved in minimum THF and adsorbed on silica (Merck 60–120 mesh silica gel). 2-Bromoacetophenone 2a was then dissolved in minimum THF and adsorbed on a silica gel. Both adsorbed silica of 1a and 2a were packed in a glass column, which was eluted by 5% (DCM: hexane). The eluent was then collected in ice-cold hexane. After some time, we reported that a milky precipitate started to appear in ice cold hexane. The column was eluted till the precipitate appeared. The solid precipitate was then filtered out with a sintered funnel, and the filtrate was evaporated under the vacuum partially such that only DCM was evaporated and the remaining product precipitated in hexane. The product was then filtered and dried using a vacuum pump. The product was characterized by NMR, ESI-mass, HRMS and finally confirmed as benzimidazole-2-imino-thiazolidine-4-ol (Fig. 2) by single crystal X-ray diffraction study.21
The column was prepared in such a manner that the total length of the glass column was 100 cm, and there were five different silica layers we have packed in the column. After a cotton plug at the bottom close to the stopcock, the first layer was packed by dry silica up to 5 cm for good separation. Then, the second layer was packed by adsorbed silica of thiourea 1a (should be long layer, up to 30–40 cm long). The third layer was packed by dry silica gel up to 1–2 cm long. The fourth layer was packed with silica adsorbed by 2a up to 05 cm long and was covered by 1–2 cm long dry silica followed by a cotton plug to stabilize the silica layer (Fig. 3).
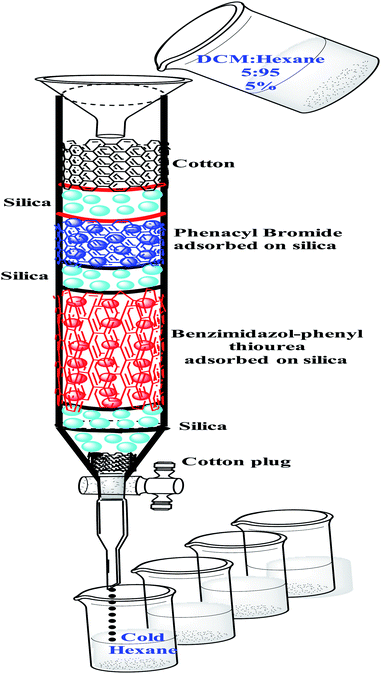 | ||
| Fig. 3 Schematic diagram of column chromatography for the synthesis of benzimidazol-diphenyl-2-imino-thiazolidine-4-ol. | ||
The reaction was screened for a combination of solvents in column chromatography. First, we attempted with 5% ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, but we could not get a good yield because the product could not properly precipitate and was difficult to recover the stable product from solvent during vacuum evaporation at a low temperature. 5% DCM
hexane, but we could not get a good yield because the product could not properly precipitate and was difficult to recover the stable product from solvent during vacuum evaporation at a low temperature. 5% DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane was best for good product yield because the product easily precipitated in cold hexane and DCM, and then easily evaporated at a low temperature followed by precipitation. The 5% was justified from TLC of the reaction such that the reaction in the column was completed in the stipulated time. We have screened the percentage of solvent from 1% to 10% DCM: hexane, and finally confirmed that 5% was suitable for optimum conversion of the product.
hexane was best for good product yield because the product easily precipitated in cold hexane and DCM, and then easily evaporated at a low temperature followed by precipitation. The 5% was justified from TLC of the reaction such that the reaction in the column was completed in the stipulated time. We have screened the percentage of solvent from 1% to 10% DCM: hexane, and finally confirmed that 5% was suitable for optimum conversion of the product.
As the retention factor (Rf-value) of thiourea 1 was low, it was moving slowly and Rf-value of 2a was higher than thiourea 1, which was moving fast. Therefore, the height of the second layer of silica adsorbed by thiourea 1 should be long enough such that 2a will react with it and should not elute without reaction.
We explored the scope of this reaction with respect to the substituents. We examined by choosing a synthesis of benzimidazol-2-imino-thiazolidine-4-ol B1 as a model reaction for optimization (Table 1, B1), the reaction of benzimidazol-phenyl-thiourea 1 and 2-bromoacetophenone 2a in column at room temperature using 5% DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
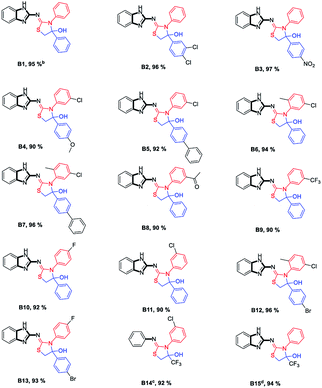
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane as a solvent for column chromatography. The eluent from the column was collected in ice cold hexane. The stabilized solid product was afforded with high yield (95%, Table 1) of benzimidazole-2-imino-thiazolidine-4-ol B1. In the electronic study of the substituent, the electron withdrawing group on 2-bromoacetophenone led to a high yield compared to electron donating groups (Table 1). The efficiency of this protocol was successfully demonstrated by the reaction of thiourea 1 and 3-bromo-1,1,1-trifluoropropan-2-one (Table 1, B14 and B15), although with a high yield (96 and 98%). The reaction was sluggish for donating and fusing ring 2-((1H-benzo[d]imidazol-2-yl)imino)-4-(naphthalen-2-yl)-3-phenylthiazolidin-4-ol. This method provides easy access for synthesizing hitherto unknown benzimidazole-biphenyl-thiazolidin-4-ol and benzimidazole-phenyl-trifluoro methyl-thiazolidin-4-ol compounds (B1–B13 and B15).
hexane as a solvent for column chromatography. The eluent from the column was collected in ice cold hexane. The stabilized solid product was afforded with high yield (95%, Table 1) of benzimidazole-2-imino-thiazolidine-4-ol B1. In the electronic study of the substituent, the electron withdrawing group on 2-bromoacetophenone led to a high yield compared to electron donating groups (Table 1). The efficiency of this protocol was successfully demonstrated by the reaction of thiourea 1 and 3-bromo-1,1,1-trifluoropropan-2-one (Table 1, B14 and B15), although with a high yield (96 and 98%). The reaction was sluggish for donating and fusing ring 2-((1H-benzo[d]imidazol-2-yl)imino)-4-(naphthalen-2-yl)-3-phenylthiazolidin-4-ol. This method provides easy access for synthesizing hitherto unknown benzimidazole-biphenyl-thiazolidin-4-ol and benzimidazole-phenyl-trifluoro methyl-thiazolidin-4-ol compounds (B1–B13 and B15).
a Reaction condition: benzimidazol-phenyl thiourea 1a (1 mmol) and phenacyl bromide 2a (1 mmol), under column chromatography using (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95) (DCM 95) (DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) solvent at room temperature.b Yields are reported after solidification.c B14 prepared from diphenyl thiourea and trifluoro methane 2-bromoacetophenone.d B15 prepared from 1a and trifluoro methane 2-bromoacetophenone. hexane) solvent at room temperature.b Yields are reported after solidification.c B14 prepared from diphenyl thiourea and trifluoro methane 2-bromoacetophenone.d B15 prepared from 1a and trifluoro methane 2-bromoacetophenone. |
|---|
 |
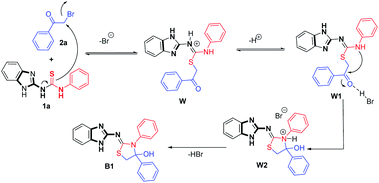
The genesis of mechanism depends on the pKa values of amines,20 i.e., the basicity of respective nitrogen of amines (Scheme 2). Obviously, the nitrogen of benzimidazole is more basic than aniline; thus, benzimidazole nitrogen was converted to imine by attacking the 2-bromoacetophenone through sulfur. In the next step, aniline nitrogen of thiourea underwent cyclization by attacking carbonyl carbon and formed a benzimidazol-diphenyl-2-imino-thiazolidine-4-ol (B1). Thus, the stability of product was maintained by the four bond distant benzimidazole ring as compared to hemiaminal Aa.
In terms of importance of these compounds, curiously we observed that the anticancer activities of benzimidazol-diphenyl-2-imino-thiazolidine-4-ols are really promising. Though these moieties are smaller in size, their anticancer activities are attractive than other hemiaminals prepared by earlier methods. All products were screened against the human colon, prostate, lung and breast cancer cell lines by using doxorubicin as a standard. Average values of three individual experiments were recorded by 50% inhibitory concentration after 48 h of actual drug treatment. It is again worthy to note that fluorinating phenyl rings are exclusively effective against lung cancer than trifluoro methylating phenyl rings on benzimidazol-diphenyl-2-imino-thiazolidine-4-ol. Thus, by the collected data, products B9 (IC50 = 3.890 μM) and B10 (IC50 = 2.798 μM) and B13 (IC50 = 3.140 μM) have been extensively studied. It is again important to point out that due to the increase in the size of this thiazolidin-4-ol by bulky substitution on phenyl rings, there is a continuous decrease in the anticancer activity of the respective compound. Thus, only fluorination or a small size benzimidazol-diphenyl-2-imino-thiazolidine-4-ol is efficient for its anticancer activity.
Conclusions
In conclusion, the chemistry of the synthesis of hemiaminal products is really challenging. We have proposed the synthesis of hemiaminals, benzimidazol-diphenyl-2-imino-thiazolidine-4-ol by column chromatography. The method is metal free, catalyst free and highly efficient. The stability of hemiaminal has been increased by the insertion of four bond distant benzimidazole ring. We have escaped the process of extraction and protected the stability of product. Simultaneously, we performed reaction, separation and solidification in a single unit. The method is purely efficient, ecofriendly and sustainable for a green approach. The benzimidazol-diphenyl-2-imino-thiazolidine-4-ol products were screened against four human colon, breast, lung and prostate cancer cell lines with respect to doxorubicin as a standard. We are happy to mention that fluorinating phenyl rings in benzimidazol-diphenyl-2-imino-thiazolidine-4-ol enhance anticancer activity, which is hitherto unknown for lung cancer. Increasing the activity of these types of compounds is going on in our laboratory.Experimental section
Reactions were performed in oven-dried borosil glassware. Commercial reagents were purchased from Sigma Aldrich, Fluka or Alfa Aesar and used as-received. Anhydrous solvents were purchased from Rankem. All solvents were distilled before use. Thin layer chromatography (TLC) was performed using precoated glass silica gel plates and visualized by UV fluorescence of 254 nm short wavelength ultraviolet light. TLC was exposed to iodine vapours and a solution of p-anisaldehyde (3 g of p-anisaldehyde, 1 mL of AcOH and 3 mL of concentrated H2SO4 in 125 mL of HPLC grade MeOH) followed by direct heating on hot plate. Melting points were uncorrected. 1H NMR and 13C NMR spectra were recorded on Bruker Avance 300 MHz, 500 MHz and 700 MHz NMR spectrometer. For 1H NMR, chemical shifts (δ) were expressed in parts per million (ppm) with respect to TMS as internal standards (0.00 ppm) and CDCl3 (δ = 77.0)/DMSO-d6 (39.43) used as internal standards for 13C NMR. Coupling constants (J) were expressed in Hertz (Hz). Mass spectra were recorded by using 70 eV spectrometer.General procedure for the preparation of product benzimidazol-2-imino-thiazolidine-4-ol B1
Benzimidazol-phenyl-thiourea 1a (1 mmol, 268 mg) was dissolved in minimum THF and 2-bromoacetophenone 2a (1 mmol, 199 mg) was dissolved in minimum DCM at room temperature, and both the components adsorbed separately on 60–120 mess silica gel using vacuum pump.The glass column of 100 cm length was prepared by five different silica layers. After cotton plug at bottom near to stopcock, the first layer was packed by dry silica up to 05 cm for the good separation. Following the first layer, the second layer was packed by adsorbed silica of thiourea 1a (should be long layer, up to 30–40 cm long). The third layer was packed by dry silica gel up to 1–2 cm long. The fourth layer was packed with silica adsorbed by 2a up to 05 cm long and this fourth layer covered by 1–2 cm long dry silica followed by cotton plug to stabilize silica layer. The reaction was performed in column using 5% (DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) as a solvent for column chromatography. The eluent from column was collected in ice cold hexane. After some time, white precipitate started to appear in cold hexane, and the elution of column was continued without any break using 5% (DCM
hexane) as a solvent for column chromatography. The eluent from column was collected in ice cold hexane. After some time, white precipitate started to appear in cold hexane, and the elution of column was continued without any break using 5% (DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) as a solvent. The beakers with precipitated product were maintained at ice cold temperature. When the white precipitate stopped to appear, we have closed the stopcock to finish the reaction. The solid precipitate was filtered out using vacuum pump. The filtrate was evaporated for slow evaporation of DCM, and the solid product was precipitated again in hexane which was filtered out using vacuum pump. The dried solid benzimidazole-2-imino-thiazolidine-4-ol B1 product was afforded with high yield (95%). The product B1 was characterized by 1H-NMR, 13C-NMR and ESI mass-HRMS.
hexane) as a solvent. The beakers with precipitated product were maintained at ice cold temperature. When the white precipitate stopped to appear, we have closed the stopcock to finish the reaction. The solid precipitate was filtered out using vacuum pump. The filtrate was evaporated for slow evaporation of DCM, and the solid product was precipitated again in hexane which was filtered out using vacuum pump. The dried solid benzimidazole-2-imino-thiazolidine-4-ol B1 product was afforded with high yield (95%). The product B1 was characterized by 1H-NMR, 13C-NMR and ESI mass-HRMS.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
H. M. M. is thankful to Director, IICT, for continued support and encouragement. V. M. B. is thankful to the Director Institute of Science and P. R. M. is thankful to the CSIR, New Delhi, for a fellowship.Notes and references
- S. V. Ley, I. R. Baxendale, R. N. Bream, P. S. Jacson, A. G. Leach, D. A. Longbottom, M. Nesi, J. S. Scott, R. I. Storer and S. J. taylor, J. Chem. Soc., Perkin Trans. 1, 2000, 3815–4195 RSC.
- A. M. Hafez, A. E. Taggi, T. Dudding and T. Lectka, J. Am. Chem. Soc., 2001, 123, 10853–10859 CrossRef CAS.
- S. C. Dakdouki, D. Villemin and N. Bar, Eur. J. Org. Chem., 2010, 333–337 CrossRef CAS.
- G. Strohlein, Y. Assuncao, N. Dube, A. Bardow, M. Mazzotti and M. Morbidelli, Chem. Eng. Sci., 2006, 61, 5296–5306 CrossRef.
- O. Trapp, S. K. Weber, S. Bauch and W. Hafstadt, Angew. Chem., Int. Ed., 2007, 14, 7307–7310 CrossRef.
- M. Ejlersen, C. Lou, Y. S. Sanghvi, Y. Tor and J. Wengel, Chem. Commun., 2018, 54, 8003–8006 RSC.
- V. Chiroli, M. Benaglia, F. Cozzi, A. Puglisi, R. Annunziata and G. Celentano, Org. Lett., 2013, 15, 3590–3593 CrossRef CAS.
- A. M. Hafez, A. E Taggi and T. Lectka, Chem.–Eur. J., 2002, 8, 4114–4119 CrossRef CAS.
- S. T. Tuncel and I. Dogan, Chirality, 2020, 32, 866–875 CrossRef CAS.
- S. E. Gunal, G. S. Gurses, S. S. Erdem and I. Dogan, Tetrahedron, 2016, 72, 2122–2131 CrossRef CAS.
- K. Tanaka, K. Nomura, H. Oda, S. Yoshida and K. Mitsuhashi, J. Heterocycl. Chem., 1991, 28, 907–911 CrossRef CAS.
- (a) V. S. Karavan and V. A. Nikiforov, Russ. J. Org. Chem., 1999, 35, 741–747 CAS; (b) M. N. Murawiewa and V. M. Schtschukina, J. Gen. Chem. USSR (Engl. Transl.), 1960, 30, 2327–2331 Search PubMed.
- T. Dalmal, K. Appalanaidu, U. B. Kosurkar, N. J. Babu and R. M. Kumbahre, Eur. J. Org. Chem., 2014, 2468–2479 CrossRef CAS.
- (a) T. Iwasawa, R. J. Hooley and J. Rebek, Science, 2007, 317, 493–496 CrossRef CAS; (b) R. J. Hooley, P. Restorp, T. Iwasawa and J. Rebek, J. Am. Chem. Soc., 2007, 129, 15639–15643 CrossRef CAS.
- P. Subik, B. Welc, B. Wisz and S. Wolowiec, Tetrahedron Lett., 2009, 50, 6512–6514 CrossRef CAS.
- M. Barys, Z. Ciunik, K. Drabent and A. Kwiecien, New J. Chem., 2010, 34, 2605–2611 RSC.
- (a) WHO Model Formulary, ed. M. C. Stuart, M. Kouimtzi and S. R. Hill, 2008, 94–96, ISBN 9789241547659 Search PubMed; (b) World Health Organization, Model list of essential medicines, 2019, Licence: CCBY-NC-SA 3.0 IGO Search PubMed.
- N. Karaali, S. Aydin, N. Baltas and E. Mentese, J. Heterocycl. Chem., 2020, 1–10 Search PubMed.
- A. Ozdemir, S. Turanh, b. Cahskn, M. Arka and E. Banoglu, Pharm. Chem. J., 2020, 53, 1036–1046 CrossRef CAS.
- S. Murru, C. B. Singh, V. Kavala and B. K. Patel, Tetrahedron, 2008, 64, 1931–1942 CrossRef CAS.
- CCDC: 1041069 contains crystal data with structure refinement data as ESI† for this paper. These data can be obtained at https://summary.ccdc.cam.ac.uk/structure-summary-form or by writing to the Cambridge Crystallographic Data Centre, Cambridge CB2 1EZ, UK, E-mail: E-mail: deposit@ccdc.cam.ac.uk.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization data, of compound, anticancer activities and single crystal X-ray analysis data. CCDC 1041069. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra09082d |
| This journal is © The Royal Society of Chemistry 2021 |