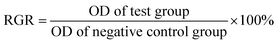Open Access Article
Open Access ArticleIn vitro biocompatibility of a sandblasted, acid-etched HA composite coating on ultrafine-grained titanium
Yanxia Chi,
Sipeng An ,
Yunpeng Xu,
Mingda Liu and
Jie Zhang
,
Yunpeng Xu,
Mingda Liu and
Jie Zhang *
*
Jiamusi University, Jiamusi, heilongjiang province, China. E-mail: zjie612@163.com
First published on 3rd February 2021
Abstract
A sandblasted, acid-etching hydroxyapatite (SLA-HA) composite coating on ultrafine-grained titanium was synthesized by the sandblasting, acid etching and electrophoresis deposition. Mouse osteoblasts (MC3T3-E1) were cultured in vitro and inoculated on the SLA-HA composite coating of the ultrafine-grained titanium. Using ultrafine-grained titanium with SLA coating as the control group, the adhesion and proliferation of the osteoblasts were analyzed using the CCK-8 assay. The number and morphology of the cells were observed using a laser confocal microscope. Cells toxicity of the cytotoxicity to osteoblasts was studied by culturing them in an immersion solution of the SLA-HA composite coating. The hemolysis properties of the obtained material were assessed using fresh rabbit blood. Ultrafine-grained titanium with the SLA-HA composite coating was found to have no significant toxicity to osteoblasts, as well as good blood compatibility, playing a positive role in the adhesion of osteoblasts and promoting their proliferation and differentiation.
Introduction
Implant dentures have become an important device for treating clinical dentition defects and replacing missing teeth.1 Titanium has become the first choice among implant materials because of its good biocompatibility, corrosion resistance and osseointegration properties.2 However, the mechanical strength of titanium is relatively low, which can cause the implant to crack or even fracture, resulting in failure in clinical usage.2,3 Thus, as an implant material, the mechanical properties of titanium still need to be improved. The equal-channel angular pressing (ECAP) technique, developed by Segal in the 1980s, is a processing method that causes large plastic deformation.4 Its basic principle is to achieve a large amount of shear deformation through an isometric corner channel, resulting in refined material grain and improved material performance. Existing research has shown that the mechanical properties of titanium materials have been improved significantly with grain refinement. As the grain size decreases from 30 μm to 300–400 nm, its hardness increases from 1496 MPa to 2458 MPa, while the dynamic and static compressive yield strengths increase from 900 MPa to 1240 MPa and 300 MPa to 772 MPa, respectively.1,2 These findings revealed the bright prospects for ultrafine-grained titanium materials in oral implantology applications.2,3When ultrafine-grained titanium materials are used in clinical implants, they need to undergo surface treatment. As a result, the implant can obtain good surface morphology and biological activity, which can promote osseointegration and reduce the risk of implantation failure.5–9 Surface treatment technologies that combine sandblasting and acid etching (SLA) yield good results. Sandblasting involves coarsening the surface of the material to obtain a certain scale of pit. During acid erosion treatment, the surface and surface residual materials of the contaminated layer are cleaned with an acid solution, which forms a micron or even submicron hole structure. Thus, the surface morphology of the material is changed and its biological activity is improved, which is beneficial to the formation of bone bonding.10,11 At the same time, if titanium is exposed to air it can easily form an oxide film. Therefore, acid etching is performed after the sandblasting treatment. This means that the contaminated layer and the remaining spray on the surface can be cleaned up, while the effect of oxidation on the experiment can be minimized. Hydroxyapatite (HA) is a major inorganic component of bones and teeth in humans and animals; its main components include calcium and phosphorus. After HA implantation in an organism, HA can form a chemical bond with biological tissue. Thus, calcium and phosphorus will be freed from the surface of the material, and promote the growth of new tissue. HA can also stimulate bone hyperplasia and bone growth.12–14 That is, new bone and osteoblasts can grow at the junction between the HA implant and the original bone, which is helpful for the combination of implants and bone tissue.15,16
In this study, a HA coating treated with sandblasting and acid erosion was obtained on the surface of ultrafine-grained titanium by electrophoresis deposition, and the biological activity of the composite coating was studied.
Results and discussion
Absorbance values of MC3T3-E1 osteoblasts in an ultrafine-grained titanium with SLA-HA group, an ultrafine-grained titanium with SLA group, a DMEM culture medium group and a PBS group after inoculation and cultivation for 4 h, 6 h and 8 h are shown in Table 1. In Table 1, the absorbance values for the ultrafine-grained titanium with SLA-HA group, the ultrafine-grained titanium with SLA group and DMEM culture medium group show an increasing trend over time, while those of the PBS group do not significantly change with time. The absorbances of the ultrafine-grained titanium with SLA-HA group and DMEM medium group are significantly higher than those of the ultrafine-grained titanium with SLA group (P < 0.05), with statistical significance. There are no significant differences between the ultrafine-grained titanium with SLA-HA group and the DMEM medium group (P > 0.05). The results show that the ultrafine-grained titanium with SLA-HA group was more favorable to cell adhesion than the ultrafine-grained titanium with SLA group. This might be because the porosity of the HA coating surface in the ultrafine-grained titanium with SLA-HA group was more conducive to cell adhesion and growth, and could provide a good place for the exchange of nutrients and metabolites for cells, as shown in Fig. 1.| Group | 4 h | 6 h | 8 h |
|---|---|---|---|
| a * indicates contrast with the control group, P < 0.05. | |||
| Extracts of the ultrafine-grained titanium SLA-HA | 0.510 ± 0.042 | 0.678 ± 0.144* | 0.845 ± 0.297* |
| Extracts of the ultrafine-grained titanium SLA | 0.516 ± 0.033 | 0.520 ± 0.021* | 0.633 ± 0.101* |
| DMEM culture medium | 0.670 ± 0.399* | 0.724 ± 0.045* | 0.763 ± 0.456* |
| PBS | 0.057 ± 0.001 | 0.057 ± 0.001 | 0.056 ± 0.001 |
Absorbance values of the osteoblasts in the ultrafine-grained titanium with SLA-HA group, the ultrafine-grained titanium with SLA group, the DMEM culture medium group and the PBS group, after inoculation and cultivation for 1 d, 3 d, 5 d, are shown in Table 2. It can be seen from Table 2 that the absorbance values of the ultrafine-grained titanium with SLA-HA group, the ultrafine-grained titanium with SLA group and DMEM culture medium group show an increasing trend with increasing cultivation time. The absorbance value of the PBS group does not significantly change with time, as shown in Table 2. At the same time, it can be seen that the absorbance values of the ultrafine-grained titanium with SLA-HA group and the DMEM medium group are significantly higher than those of the ultrafine-grained titanium with SLA group, with statistical significance (P < 0.05). The absorbance values of the DMEM medium group are significantly higher than those of the ultrafine-grained titanium with SLA-HA group, with statistical significance (P < 0.05). The results show that the ultrafine-grained titanium with SLA-HA group had better biological activity than the ultrafine-grained titanium with SLA group, and was more conducive to cell proliferation. However, the cell proliferation ability of the ultrafine-grained titanium with SLA-HA group was weaker than that of the DMEM medium group, which might be because the high concentration of Ca ions in the extracellular environment inhibited cell proliferation to a certain extent.
| Group | 1 d | 3 d | 5 d |
|---|---|---|---|
| a * indicates contrast with the control group, P < 0.05. | |||
| Extracts of the ultrafine-grained titanium SLA-HA | 1.075 ± 0.092* | 1.275 ± 0.372* | 1.338 ± 0.459* |
| Extracts of the ultrafine-grained titanium SLA | 0.836 ± 0.166* | 0.866 ± 0.095* | 0.846 ± 0.055* |
| DMEM culture medium | 1.510 ± 0.098* | 1.737 ± 0.225* | 2.055 ± 0.095* |
| PBS | 0.060 ± 0.006 | 0.071 ± 0.012 | 0.071 ± 0.013 |
Fig. 2 shows the morphologies of cells in the ultrafine-grained titanium with SLA-HA group and the ultrafine-grained titanium with SLA group under a laser confocal microscope. It can be seen from Fig. 2 that the cell numbers and morphologies of the two groups are significantly different; the state of the former group is better than the latter group. The osteoblasts in the ultrafine-grained titanium with SLA-HA group are fully stretched and closely linked, and the shape of their spreading is preferable. The cell number in the ultrafine-grained titanium with SLA group is lower in comparison with the former group, and the links between cells are loose and their spreading morphology is slightly worse. This indicates that the ultrafine-grained titanium with SLA-HA group is more favorable to the proliferation and differentiation of MC3T3-E1 osteoblasts than the ultrafine-grained titanium with SLA group. This could be due to the intracytoplasmic calcium concentration changing after a few hydroxyapatite particles were swallowed by the cells. Thus, the osteogenic differentiation was initiated to promote cell maturation and achieve the ideal state of osteogenic differentiation.
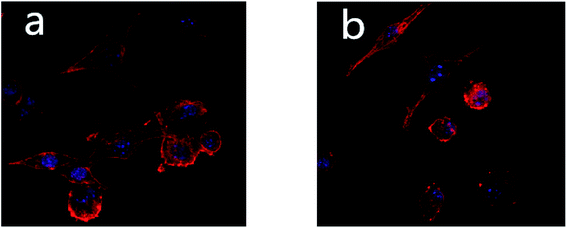 | ||
| Fig. 2 Morphologies of two groups of cells under the laser confocal microscope (600 times): (a) ultrafine-grained titanium with SLA-HA group; (b) ultrafine-grained titanium with SLA group. | ||
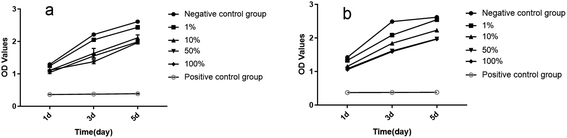
The CCK8 detection method was used in this study. The CCK8 reagent contains WST-8, which was reduced to form highly water-soluble yellow Formazan dye by dehydrogenase in cells under the action of 1-methoxy PMS. The amount of the produced Formazan is proportional to the number of living cells, so this property could be directly used to analyze cell proliferation and toxicity. The depth of color is directly proportional to the cell proliferation and inversely proportional to the cytotoxicity. Fig. 3a shows the growth curve of cells in the ultrafine-grained titanium with SLA-HA group. At the 1 d, 3 d and 5 d timepoints, the numbers of cells in the ultrafine-grained titanium with SLA-HA group with concentrations of 1%, 10%, 50% and 100% and the negative control group show an increasing tendency, while the numbers of cells in the positive control group show no significant change, as shown in Fig. 3. Statistical analysis shows that the absorbance values (OD) of the ultrafine-grained titanium with SLA-HA extract at different concentrations are higher than those of the positive control group, with statistical significance (P < 0.05), while the absorbance value (OD) of the negative control group is not statistically significant (P > 0.05), as shown in Table 3.
| Group | OD values | RGR (%) | Toxicity analysis |
|---|---|---|---|
| 1% | 2.437 | 93.34 | 1 |
| 10% | 2.114 | 80.97 | 1 |
| 50% | 1.988 | 76.14 | 1 |
| 100% | 1.964 | 75.22 | 1 |
| Negative control group | 2.611 | 100 | 0 |
| Positive control group | 0.388 | 14.86 | 4 |
Fig. 3b shows the growth curve of cells in the ultrafine-grained titanium with SLA group. At the 1 d, 3 d and 5 d timepoints, the numbers of cells in the ultrafine-grained titanium with SLA group with concentrations of 1%, 10%, 50% and 100% and the negative control group show a proliferation trend, while the numbers of cells in the positive control group show no significant change, as shown in Fig. 3. Statistical analysis shows that the absorbance values of the ultrafine-grained titanium with SLA group at different concentrations are higher than those of the positive control group, with statistical significance (P < 0.05), while the OD value of the negative control group is not statistically significant (P > 0.05), as shown in Table 4.
| Group | OD values | RGR (%) | Toxicity analysis |
|---|---|---|---|
| 1% | 2.544 | 97.14 | 1 |
| 10% | 2.232 | 85.22 | 1 |
| 50% | 1.974 | 75.37 | 1 |
| 100% | 1.966 | 75.07 | 1 |
| Negative control group | 2.619 | 100 | 0 |
| Positive control group | 0.381 | 14.55 | 4 |
There is no significant difference in absorbance between the ultrafine-grained titanium with SLA-HA group and the ultrafine-grained titanium with SLA group (P < 0.05), as shown in Tables 3 and 4. The results show that the number of cells in these two groups increased with time, and there is no significant difference in comparison with the negative control group. As a result, there was no obvious toxicity observed for the ultrafine-grained titanium with SLA-HA group and the ultrafine-grained titanium with SLA group.
Toxicological grade was evaluated according to the 6-grade toxicity standard in the United States pharmacopoeia. For an RGR value of ≥75%, the cytotoxicity grade is 0 or 1, qualified; for 50% < RGR < 74%, the cytotoxicity grade is 2, requiring a comprehensive evaluation in combination with the cell morphology; for an RGR value of ≤49%, the cytotoxicity level is 3 to 5, unqualified.
According to the toxicity evaluation, the toxicity grade of ultrafine-grained titanium with SLA-HA and ultrafine-grained titanium with SLA are grade 1, indicating that they meet the standards of GB/T16886 “biological evaluation of medical devices” in vitro cytotoxicity test, which require a low cytotoxicity grade and no obvious effect on cells.
The hemolysis test is one of the most basic methods to evaluate the acute hemolytic activity and blood compatibility of materials in vitro. It can reflect the damage to red blood cells caused by the material itself and is an important index to evaluate biocompatibility. When an implant material is in contact with blood in the body, a certain component in the material can cause the denaturation of an active component in the blood. As a result, the red blood cells and hemoglobin in the plasma become dissociated, resulting in toxic damage in the body and changes in the function of vascular endothelial cells, thus leading to blood coagulation and thrombosis in the body. The hemolysis rate can reflect the relationship between a coating material and red blood cells; if the hemolysis rate of the coating material is low, it can be concluded that the coating material causes little damage to red blood cells and has good biocompatibility.17 The “Biological evaluation of medical equipment” ISO 10993 was adopted as the evaluation criteria of the hemolysis test. With a hemolysis rate of 5% as a reference, a hemolysis rate of >5% was positive, indicating that the material causes detectable hemolysis, while a hemolysis rate of <5% was negative, indicating that the material does not cause hemolysis.
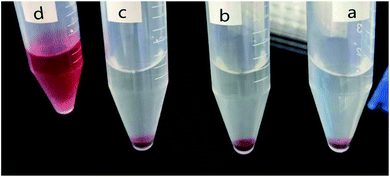
Fig. 4 shows the changes of internal liquid observed by the naked eye after centrifugation in each group of the hemolysis test. Delamination phenomena were observed in the test tubes containing the ultrafine-grained titanium with SLA-HA group, the ultrafine-grained titanium with SLA group and the saline group (negative control group). It can be seen by the naked eye that the centrifugal tube containing the immersion liquid and rabbit blood exhibited upper and lower layers; the upper layer was a clear liquid, while the lower layer was a dark red precipitate. The distilled water group (positive control group) exhibited a red transparent liquid in the centrifugal tube. The results show that there was no significant hemolytic reaction in the ultrafine-grained titanium with SLA-HA group (group A), the ultrafine-grained titanium with SLA group (group B) and the normal saline group, while a hemolytic reaction was obvious in the positive control group.
Table 5 shows the absorbance values (OD values) of the different groups in the hemolysis test. The hemolysis rate was calculated using OD values (formula (1)). The hemolysis rates of the ultrafine-grained titanium with SLA-HA group and ultrafine-grained titanium with SLA group were 0.5% and 0.8%, respectively, conforming to the standard of the biological evaluation. Thus, the ultrafine-grained titanium with SLA-HA group and ultrafine-grained titanium with SLA group did not cause the occurrence of hemolysis.
| Test number | Ultrafine-grained titanium with SLA-HA group | Ultrafine-grained titanium with SLA group | 0.9% saline group | Distilled water group |
|---|---|---|---|---|
| 1 | 0.058 | 0.059 | 0.057 | 0.663 |
| 2 | 0.078 | 0.062 | 0.055 | 0.687 |
| 3 | 0.061 | 0.072 | 0.070 | 0.679 |
| 4 | 0.059 | 0.071 | 0.060 | 0.692 |
| Average value | 0.064 | 0.066 | 0.061 | 0.680 |
Experimental
Preparation of the SLA-HA composite coating
Ultrafine-grained titanium as a matrix was cut into round sheets with a diameter of 10 mm and a thickness of 2 mm. The prepared specimens were subjected to the sandblasting treatment at a pressure of 0.5 MPa with Al2O3 particles of 110 μm. Samples treated with sandblasting were acidified with a mixed acid solution (37 wt% hydrochloric acid, 98 wt% sulfuric acid, deionized water, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 5 min at 80 °C. Hydroxyapatite (HA) coating was prepared on the surface of ultrafine-grained titanium treated with the sandblasting and acid corrosion (SLA) by electrophoresis deposition (voltage 25 V, time 60 s).
1) for 5 min at 80 °C. Hydroxyapatite (HA) coating was prepared on the surface of ultrafine-grained titanium treated with the sandblasting and acid corrosion (SLA) by electrophoresis deposition (voltage 25 V, time 60 s).
Cell adhesion assay
Experiment subjects were divided into four groups: (a) ultrafine-grained titanium with SLA-HA group; (b) ultrafine-grained titanium with SLA group; (c) DMEM culture medium group; (d) PBS group. Among these groups, the ultrafine-grained titanium with SLA-HA group and ultrafine-grained titanium with SLA group were the experimental groups. The DMEM culture medium group was the negative control group, and the PBS group was the positive control group.The samples were sterilized at high temperature and put into 48-well cell culture plates. The osteoblast cell density was adjusted to 5.0 × 104 ml−1 after three generations, at 0.2 ml per well, and six duplicated wells were set up under each condition in each group. The cells were cultured at a constant temperature of 37 °C in an incubator with a CO2 concentration of 5% for 4 h to allow the cells adhere to the walls. Then the culture medium was slowly injected along the edge of the wells. These 48-well cell culture plates were cultured for 4 h, 6 h or 8 h; the supernatant was then discarded, and the cells were washed three times with PBS. The samples were placed into new 48-well plates, adding 0.2 ml per well CCK-8 culture medium, and were then cultured for 4 h in the incubator. 100 μl of liquid was taken out and transferred into a 96-well plate, and its absorption value was detected under 490 nm by an Enzyme Standard Instrument.
Cell proliferation assay
Experimental groupings are the same as in the previous section. Samples were sterilized at high temperature and put into 48-well cell culture plates. The cell density was 5.0 × 104 ml−1 after three generations, at 0.2 ml per well, and six duplicated wells were set up under each condition in each group, for 1 d, 3 d or 5 d of culturing. The following operations were the same as the cell adhesion testing.Detection of cell morphology
After the ultrafine-grained titanium with SLA-HA group and the ultrafine-grained titanium with SLA group were sterilized at high temperature and put into 48-well cell culture plates, the cell density was adjusted to 5.0 × 104 ml−1 after three generations and cells were put into the above cell culture plates at 0.2 ml per well. Meanwhile, three duplicated wells were set up under each condition in each group and were cultured for 24 h. Every sample was fixed with 4% formaldehyde solution at 0.5 ml per well for 10 min and washed 3 times with PBS. Then membrane filtration was carried out with 5 ml of 0.1% Triton X-100 solution on the sample surface for 20 min at room temperature; the wasted liquid was discarded and the samples were washed 3 times with PBS. 0.5 ml of Hoechst33342 staining solution was added into the cell culture plates, and the samples were dyed at room temperature for 5 min and washed three times with PBS. Rhodamine ghost pen cyclic peptide was added at 0.2 ml per well at the darkroom temperature, and the samples were washed three times with PBS after 40 min. Finally, anti-fluorescent quenching agent was added and the cell culture plates were sealed. Cell morphology was observed using a laser confocal microscope.Toxicity test
The relative proliferation rate of cells is calculated as follows:
Hemolysis experiment
Experiment subjects were divided into four groups: (a) the ultrafine-grained titanium with SLA-HA group; (b) the ultrafine-grained titanium with SLA group; (c) 0.9% saline group; (d) distilled water group. Among them, the ultrafine-grained titanium with SLA-HA group and ultrafine-grained titanium with SLA group are the test groups, the 0.9% saline group was a negative control group and the distilled water group was a positive control group.The ultrafine-grained titanium with SLA-HA group and the ultrafine-grained titanium with SLA group were immersed into physiological saline at the ratio of specimen surface area to physiological saline volume of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (cm2 ml−1). The diluted fresh blood from New Zealand rabbits' heart was used as the anticoagulation blood.
1 (cm2 ml−1). The diluted fresh blood from New Zealand rabbits' heart was used as the anticoagulation blood.
The diluted rabbit blood (0.2 ml) was added into 10 ml centrifuge tubes containing the above immersion liquid. The obtained mixture was settled for 60 min under 37 °C and separated in the centrifugal machine for 5 min at a speed of 2500 rpm. The supernatant absorbance was measured at a wavelength of 490 nm using an Enzyme Standard Instrument. The hemolysis rate was calculated according to the following formula (1).
 | (1) |
Statistical analysis
Statistical analysis of the overall data was carried out using GraphPad statistical software. The resulting datum was expressed as the mean ± standard deviation by variance analysis, and the test level was α = 0.05. LSD was employed in the variance analysis among the groups.Conclusions
The SLA-HA coating on the ultrafine-grained titanium had no significant toxicity to osteoblasts, and had good blood compatibility. It played a positive role in the adhesion of osteoblasts and was also conducive to cell proliferation and differentiation. The obtained ultrafine-grained titanium with SLA-HA had good biological activity. After the surface of the ultrafine-grained titanium was treated with a sandblasted, acid-etched HA coating, it showed better biological properties, making it a promising alternative to titanium implants and demonstrating its potential as a new type of oral cavity implant material.Author contributions
Yanxia Chi and Jie Zhang helped with the design of the experimental methods. Yunpeng Xu, Mingda Liu and Sipeng An had completed the experiments and collated the data.Ethical statement
In this study, all animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Jiamusi University in China and approved by the biological and medical Ethics Committee of School of Stomatology in Jiamusi University.Examination and approval no. JMSU-KQ-2021-004.
RSC Advances - RA-ART-12-2020-010146.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81601616), Excellent innovation team based on the basic scientific research vocational cost for the provincial undergraduate universities in Heilongjiang (No. 2018-KYYWF-0914), Health and planning commission research project in Heilongjiang province (No. 2017-419).Notes and references
- F. Findik, Surface Treatment of Ti-Alloys, Current Trends in Biomedical Engineering& Biosciences, 2018, 15(3), 555911 Search PubMed.
- D. J. Fernandes, C. N. Elias and R. Z. Valiev, Properties and performance of ultrafine grained titanium for biomedical applications, Mater. Res., 2015, 18(6), 1163–1175 CrossRef CAS.
- C. N. Elias, D. J. Fernandes and R. S. de Biasi, Comparative study of compressive and fatigue strength of dental implants made of nanocrystalline Ti Hard and microcrystalline Ti G4, Fatigue Fract. Eng. Mater. Struct., 2017, 40(5), 696–705 CrossRef CAS.
- V. M. Segal, V. I. Reznikov and A. E. Drobyshevskii, et al., Plastic Metal Working by Simple Shear., Russ. Metall., 1981, 1, 99–105 Search PubMed.
- A. Wennerberg and T. Albrektsson, On implant surfaces: a review of current knowledge and opinions, Int. J. Oral Maxillofac. Implants, 2010, 25(1), 63–74 Search PubMed.
- D. V. Nazarov, E. G. Zemtsova and A. Y. Solokhin, et al., Modification of the surface topography and composition of ultrafine and coarse grained titanium by chemical etching, Nanomaterials, 2017, 7(1), 15 CrossRef.
- H. Deppe, S. Warmuth and A. Heinrich, et al., Laser-assisted three-dimensional surface modifications of titanium implants: preliminary data, Lasers Med. Sci., 2005, 19(4), 229–233 CrossRef.
- C. Massaro, P. Rotolo and F. De Riccardis, et al., Comparative investigation of the surface properties of commercial titanium dental implants. Part I: chemical composition, J. Mater. Sci.: Mater. Med., 2002, 13(6), 535–548 CrossRef CAS.
- A. Siddiqui, J. Jacob, A. Barket Fidai and D. C. Rodrigues, Biological characterization of surface-treated dental implant materials in contact with mammalian host and bacterial cells: titanium versus zirconia, RSC Adv., 2019, 9(55), 32097–32109 RSC.
- C. M. Han, H. E. Kim and Y. S. Kim, et al., Enhanced biocompatibility of Co-Cr implant material by Ti coating and micro-arc oxidation, J. Biomed. Mater. Res., Part B, 2009, 90(1), 165–170 CrossRef.
- Di Carlo, A. Di Crescenzo and S. Pilato, et al., Osteoblastic Differentiation on Graphene Oxide-Functionalized Titanium Surfaces: An In Vitro Study, NANOMATERIALS, 2020, 10(4), 654 CrossRef CAS.
- Y. Estrin, E. P. Ivanova and A. Michalska, et al., Accelerated stem cell attachment to ultrafine grained titanium, Acta Biomater., 2011, 7(2), 900–906 CrossRef CAS.
- L. Zhao, S. Mei and P. K. Chu, et al., The influence of hierarchical hybrid micro/nano-textured titanium surface with titania nanotubes on osteoblast functions, Biomaterials, 2010, 31(19), 5072–5082 CrossRef CAS.
- H. C. Lai, L. F. Zhuang and X. Liu, et al., The influence of surface energy on early adherent events of osteoblast on titanium substrates, J. Biomed. Mater. Res., Part A, 2010, 93(1), 289–296 Search PubMed.
- T. Kokubo and H. Takadama, How useful is SBF in predicting in vivo bone bioactivity, Biomaterials, 2006, 27(15), 2907–2915 CrossRef CAS.
- H. J. Rønold, S. P. Lyngstadaas and J. E. Ellingsen, Analysing the optimal value for titanium implant roughness in bone attachment using a tensile test, Biomaterials, 2003, 24(25), 4559–4564 CrossRef.
- E. N. Alves, R. F. Presgrave and O. A. Presgrave, et al., A reassessment of the in vitro RBC haemolysis assay with defibrinated sheep blood for the determination of the ocular irritation potential of cosmetic products: comparison with the in vivo Draize rabbit test, Altern. Lab. Anim., 2008, 36(3), 275–284 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |