Open Access Article
Open Access ArticleA reversible single-molecule ligand-gating ion transportation switch of ON–OFF–ON type through a photoresponsive pillar[6]arene channel complex†
Xinyu Hu *a and
Haishen Yangb
*a and
Haishen Yangb
aKey Laboratory of Micro-Nano Optoelectronic Devices (Wenzhou), College of Electrical and Electronic Engineering, Wenzhou University, Wenzhou 325035, People's Republic of China. E-mail: huxinyu@wzu.edu.cn
bShanghai Key Laboratory of Materials Protection and Advanced Materials in Electric Power, Shanghai University of Electric Power, Shanghai, 200090, People's Republic of China
First published on 15th February 2021
Abstract
A reversible pseudo-single-ligand-gated ion transportation switch of ON–OFF–ON type was achieved through host–guest complexation with pillar[6]arene (P[6]) as the ion channel, and a photoresponsive azobenzene as the dual-role (open and close) ligand.
Stimuli-responsive synthetic transmembrane channels have attracted much attention due to their broad applications, such as molecular recognition, drug delivery and functional nanodevices.1,2 Various stimuli including potential, mechanical force, light, and ligand have been utilized in this field.3,4 Amongst these, ligand gating is one of the typical approaches. So far, limited synthetic ligand-gated channels have been reported.4–7 Most of such channels are operated merely in an irreversible manner, i.e. the channels are either opened or blocked upon the addition of stimuli ligands, achieving the OFF–ON or ON–OFF behavior.8–11 However, synthetic channels with a reversible ligand-gating capability, which exist widely in nature, are much more fascinating because of their much more extensive applications. Only a few reversible switches of OFF–ON–OFF type have been reported.3 Those examples are featured with the successive introduction of two guests; the first one is to trigger the formation of channels (host), realizing the OFF–ON capability; and then introduction of the second one is to disrupt the channeling capability, realizing the transformation from ON to OFF states.12–14 However, the reversible ligand-gated switches of ON–OFF–ON type have not been reported yet, except for the only one reported by our group;15 the switch featured with calix[6]arene (CX6) as the channel, methylene blue (MB) as the channel blocker, and 4-sulfonated calix[6]arene with stronger affinity for MB as the channel-opener. However, all the reversible ligand-gating switches reported, thus far, require successive introduction of two extra components to realize the transformations either from ON to OFF then back to ON states, or from OFF to ON then back to OFF states. Obviously, limited repetition numbers of such bi-component-gating switches are foreseeable due to the accumulation of intruders, leading to the disruption of the labile membrane.16 Noninvasive photoresponsive ligand-gating switch should have prolonged durability. To the best of our knowledge, Gin's group and Woolley's group reported the only two examples of such switch, which was of OFF–ON–OFF type and synthesized through elaborate route.17,18 However, photoresponsive ligand-gating switch of ON–OFF–ON type has not been reported yet.
Pillar[n]arenes are a class of host cavitands and have been widely studied.19–22 Herein, we reported a reversible “single-molecule ligand-gating switch” of ON–OFF–ON type by using commercial available unmodified P[6] (pillar[6]arene) as the ion channel and readily obtainable azobenzene molecule as the photoresponsive gating ligand. Though the channeling capabilities of pillararene derivatives, and the host–guest behavior of P[6] with the cis-/trans-isomers of azobenzene have been reported previously.23,24 There are still some questions to be answered.21 First is the channeling activity mediated by internal or external cavities of pillararenes; second, whether the guest molecules will affect channel activity or rupture the membrane or not; finally, whether the host and guest molecules will still associate/dissociate efficiently in lipid bilayer membrane similar as they are in solution thereby realizing multiple recurring applications of “single-molecule ligand-gating switch”.
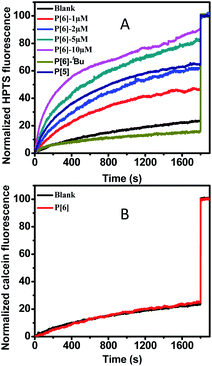
The transmembrane channeling capability of unmodified P[6] has not been studied yet, which was examined firstly by using HPTS (a pH sensitive fluorescence dye) entrapped LUVs (large unilamellar vesicles).25 Firstly, the pH gradient was establish by using a intravesicular aqueous buffer solution at pH 7.0 and an extravesicular aqueous buffer solution at pH = 7.6. Then, upon addition of P[6] solution in THF into the HPTS-encapsulated LUVs suspension, the fluorescence emission was recorded immediately until 1800 s. Finally, Triton X-100 (a surfactant) was added to disrupt LUVs and achieve the maximal fluorescence emission as the 100% reference point. As shown in Fig. 1A, a rapid increase of HPTS fluorescence emission was observed upon the introduction of P[6], which reaches 90% of the maximal level within 1800 s. This result indicates the increase of intravesicular pH through proton efflux or OH− influx in response to the transmembrane pH gradient (ΔpH = 0.6). Very small increase (≤10%) of fluorescence emission was observed in control experiment in the absence of P[6] (Fig. 1A), suggesting that P[6] is indeed responsible for the observed fluorescence increase. Furthermore, we examined the concentration dependence of channel activities of P[6]. As shown in Fig. 1A, the proton transport activity of P[6] is concentration dependent. When the concentration dropped to 5 μM, the activity can still reach to 83%. Even if the concentration is as low as 1 μM, the channelling efficiency still could remain 46%. Such activity of P[6] is comparable to many reported synthetic ion channels, including calixs and phenylene vinylene macrocycles in our previous reports.15,26 The above experimental results shows that the unmodified P[6] is able to serve as an efficient transmembrane ion channel. Such activity of P[6] is comparable to the reported ion channels composed of modified pillararenes, if not better.25
Then, we explored the channel activity influence of internal cavity using P[5] and P[6]–tBu (pillar[5]arenes and tert-butyl substituted pillar[6]arenes) by same assays. Higher activity was observed with P[6] containing larger cavity size (∼0.67 nm) while P[5] with smaller cavity size (∼0.47 nm) shows moderate HPTS fluorescence emission (around 65%) increase even extended incubation period.27 The impaired activity of P[5] may be due to the reduced cavity size leading to slowdown proton efflux. Furthermore, P[6]–tBu shows almost negligible fluorescence increase which is close to the blank experiment. The shutdown transportation of P[6]–tBu may be owing to that the tert-butyl groups located on the entrance of channel internal cavity may induce static hindrance and thus prohibiting proton efflux.
In order to confirm the ion transportation through the interior cavity of P[6], we examined the transportation disability of a larger fluorescence dye-calcein to rule out the transportation possibility from outside of the cavity. The hydrated radius of calcein is about 0.8 nm which is more than twice that of internal cavity of P[6].28 Therefore, calcein could not be transported from the cavity of P[6]. The calcein-encapsulated LUVs (∼40 mM) were prepared for calcein assay. Very minor increase of fluorescence intensity was observed after addition of P[6], even the concentration was up to 10 μM, which was only increased to 24%. The negligible increase was close to the controlling experiment showing no leakage of calcein from LUVs, indicating invalid transportation for calcein from internal cavity of P[6] (Fig. 1B). The transport disability supported the fact that the transmembrane transportation should be attributed to native internal voids of P[6] and ruled out the carpet-like disruption or barrel stave mechanism.29
In order to demonstrate the transportation activity of the internal cavity directly, the guest molecules were utilized to block the cavity and then disable channeling activity. The previous research showed that the trans form of an azobenzene-containing guest 3 could bind with P[6] forming 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
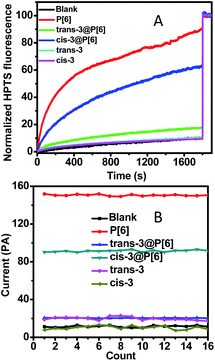
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex.21 The reported host–guest binding affinity may realize an ON–OFF switch in theory. We prepared the trans-3@P[6] complex as previously reported and measured its proton transport activity by HPTS assay.21 As shown in Fig. 2, trans-3@P[6] shows very low ionophoric activity (≤18%) compared with P[6] alone (90%), indicating a successful channel blockage by trans-3 and forming an ON–OFF type channel switch. These significantly reducing fluorescence results suggest that the internal cavity of P[6] plays a critical role in the transport of ions and also provide a coherent evidence supporting that the channeling process is mediated through the P[6] cavity rather than the barrel stave or carpet-like rupture.
1 host–guest complex.21 The reported host–guest binding affinity may realize an ON–OFF switch in theory. We prepared the trans-3@P[6] complex as previously reported and measured its proton transport activity by HPTS assay.21 As shown in Fig. 2, trans-3@P[6] shows very low ionophoric activity (≤18%) compared with P[6] alone (90%), indicating a successful channel blockage by trans-3 and forming an ON–OFF type channel switch. These significantly reducing fluorescence results suggest that the internal cavity of P[6] plays a critical role in the transport of ions and also provide a coherent evidence supporting that the channeling process is mediated through the P[6] cavity rather than the barrel stave or carpet-like rupture.
Next, we demonstrated that the guest molecules neither rupture bilayer membrane nor act as transport channels. As shown in Fig. 2, neither trans-3 nor cis-3 displays transportation efficiency, reaching only up to 10% of the maximal which is almost comparable to blank experiment. Conductance researches of the guest 3 on planar lipid bilayer showed repeatable and feeble current intensities upon addition of trans-3 or cis-3, which demonstrated the invalid transportation and no ruption behavior to membrane.
Finally, we investigated further ON–OFF–ON type switch based on photoisomerization of guest 3 from the trans to cis conformation. The previous report has demonstrated that the self-assembly trans-3@P[6] complex could be converted into cis-3@P[6] complex under irradiation of UV light. The binding affinity of cis-3@P[6] significantly decreased to (2.64 ± 0.29) × 102 M−1, which is as low as about 1/8 of trans-3@P[6] ((2.22 ± 0.34) × 103 M−1).21 The cis-3@P[6] complex was constructed and then added into HPTS entrapped-LUVs suspension. As expected, we observed 63% fluorescence intensity increasing which rebounded to 70% transportation performance of P[6] channel (90%). The results demonstrate that cis-3 partially reopened P[6] channel. The diameter of cis-3 is about 0.80 nm which is bigger than the internal cavity of P[6] channel leading to the formation of unthreaded host–guest complex, cis-3@P[6]. The cis-3 was only bound by a rim of P[6], while the rest of cis-3 was outside the cavity.21 In order to eliminate interference, the HPTS assay was constructed using cis-3 or trans-3, only slightly fluorescence increasing was observed which was close to the blank experiment which indicating neither cis-3 nor trans-3 alone affects transportation activity. These results show that P[6] can act as an excellent transmembrane channel, and trans-3 is able to shut down the P[6] channel through host–guest complexation. Then, cis-3 could reopen the blocked P[6] channel due to the decreasing affinity after trans–cis photoisomerization of guest 3, thereby achieving ON–OFF–ON channel switch.
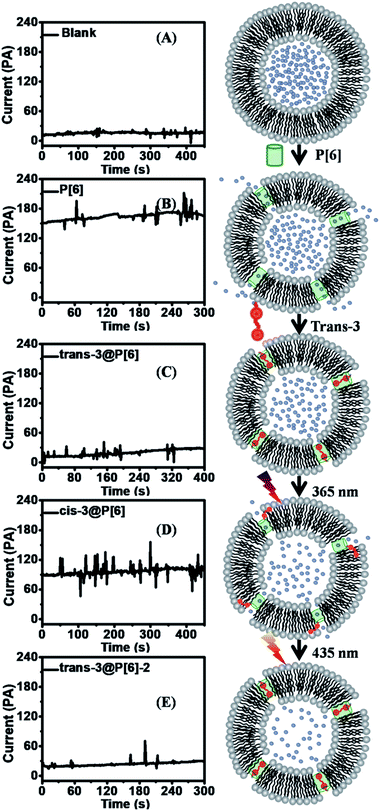
In order to achieve further supporting evidence for channelling switches, the conductance examinations were utilized to provide current recordings for ion transportation on planar lipid bilayer.15 At first, the addition of P[6] triggered a significant increase up to 150 pA in the current recordings under applied voltage of 2 V during 16 successive repeat experiments, indicating successful ion transportation through P[6] channel. Then, trans-3@P[6] complex hardly affected current profiles, suggesting P[6] channel was blocked by trans-3 thus obtaining ON–OFF type channel switch. Finally, cis-3@P[6] was introduced to investigate the channeling activity under identical experimental conditions retrieving 90 pA current flows, accounting for 60% of the highest current. Trans-3 or cis-3 alone hardly showed any current which was consistent with fluorescence results. The almost maintaining constant conductance profiles likely to confirm the formation of “single-molecule ligand-gating switch”. Further conductance investigation supplied straightforward evidence to verify the formation of reversible “single-molecule ligand-gating switch”. The current profiles were recorded as a function of time in Fig. 3. In fact, the current flows significantly increased to 150–170 pA after adding P[6] to drive the transmembrane channel formation in planar lipid bilayer and then decreased to 15–25 pA which almost close to planar lipid bilayer alone after further addition of trans-3 to form trans-3@P[6] complex. And then, the current flow enhanced to 90–100 pA after irradiation of UV light, mainly due to the trans-3@P[6] complex undergo trans–cis conversion upon irradiation with UV light at 365 nm for 3 min. Finally, the current flow reduced to 20–30 pA after irradiation of visible light at 435 nm for 3 min. This reversible single-molecule ligand-gating switch could be operated over three times (Fig. S5†). The conductance investigation clearly demonstrated that opening/blocking transition of P[6] channel could be controlled reversibly by a “single-molecule ligand-gating switch”.
In summary, we constructed an innovative reversible pseudo-single-ligand-gated ion transportation switch of ON–OFF–ON type using P[6] as an efficient channel and a photoresponsive azobenzene (guest 3) as the ligand. Firstly, the open internal cavity of P[6] channel could be blocked by trans-3 through host–guest complexation, thereby achieving ON–OFF type switch. Then, the switch could realize the channeling state from OFF to ON and then back to OFF state based on photoisomerization of guest 3, thus obtaining a reversible ON–OFF–ON type switch. Moreover, the “single-molecule ligand-gating switch” could realize multiple recurring applications. Together, “cavitand-channelling”–“photoisomer-blocking–reopening–reblocking” suggests the formation of reversible pseudo-single-ligand-gated ion transportation switch of ON–OFF–ON type. The reversible channelling switch will have a broad application in living organic and pharmaceutical field such as controlling drug release.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
X. H. thanks the Zhejiang Provincial Natural Science Foundation (LQ21B020001), Wenzhou Municipal Science and Technology Bureau (Y20190176, G2020004), Wenzhou University (JW10351029, 202010351041) and H. Y. thanks the financial supports from Science and Technology Commission of Shanghai Municipality (19DZ2271100), and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning.Notes and references
- S. Matile and T. Fyles, Acc. Chem. Res., 2013, 46, 2741 CrossRef CAS.
- A. D. Peters, S. Borsley, F. Sala, D. F. Cairns-Gibson, M. Leonidou, J. Clayden, G. F. S. Whitehead, I. J. Vitórica-Yrezábal, E. Takano, J. Burthem, S. L. Cockroft and S. J. Webb, Chem. Sci., 2020, 11, 7023 RSC.
- J.-Y. Chen and J.-L. Hou, Org. Chem. Front., 2018, 5, 1728 RSC.
- R. S. Hector and M. S. Gin, Supramol. Chem., 2005, 17, 129 CrossRef CAS.
- M. M. Tedesco, B. Ghebremariam, N. Sakai and S. Matile, Angew. Chem., Int. Ed., 1999, 38, 540 CrossRef CAS.
- T. Kiwada, K. Sonomura, Y. Sugiura, K. Asami and S. Futaki, J. Am. Chem. Soc., 2006, 128, 6010 CrossRef CAS.
- D. Lemoine, R. Jiang, A. Taly, T. Chataigneau, A. Specht and T. Grutter, Chem. Rev., 2012, 112, 6285 CrossRef CAS.
- G. A. Woolley and B. A. Wallace, Biochemistry, 1993, 32, 9819 CrossRef CAS.
- J.-H. Fuhrhop, U. Liman and V. Koesling, J. Am. Chem. Soc., 1988, 110, 6840 CrossRef CAS.
- H. Bayley and P. S. Cremer, Nature, 2001, 413, 226 CrossRef CAS.
- S. Litvinchuk, G. Bollot, J. Mareda, A. Som, D. Ronan, M. R. Shah, P. Perrottet, N. Sakai and S. Matile, J. Am. Chem. Soc., 2004, 126, 10067 CrossRef CAS.
- C. P. Wilson and S. J. Webb, Chem. Commun., 2008, 4007 RSC.
- C. P. Wilson, C. Boglio, L. Ma, S. L. Cockroft and S. J. Webb, Chem.–Eur. J., 2011, 17, 3465 CrossRef CAS.
- T. Muraoka, T. Endo, K. V. Tabata, H. Noji, S. Nagatoishi, K. Tsumoto, R. Li and K. Kinbara, J. Am. Chem. Soc., 2014, 136, 15584 CrossRef CAS.
- X. Hu, N. Liu, H. Yang, F. Wu, X. Chen, C. Li and X. Chen, Chem. Commun., 2019, 55, 3008 RSC.
- A. Cazacu, C. Tong, A. van der Lee, T. M. Fyles and M. Barboiu, J. Am. Chem. Soc., 2006, 128, 9541 CrossRef CAS.
- L. Lien, D. C. J. Jaikaran, Z. Zhang and G. A. Woolley, J. Am. Chem. Soc., 1996, 118, 12222 CrossRef CAS.
- P. V. Jog and M. S. Gin, Org. Lett., 2008, 10, 3693 CrossRef CAS.
- S. Sun, M. Geng, L. Huang, Y. Chen, M. Cen, D. Lu, A. Wang, Y. Wang, Y. Shi and Y. Yao, Chem. Commun., 2018, 54, 13006 RSC.
- Y. Cai, Z. Zhang, Y. Ding, L. Hu, J. Wang, T. Chen and Y. Yao, Chin. Chem. Lett., 2020 DOI:10.1016/j.cclet.2020.10.036.
- G. Yu, C. Han, Z. Zhang, J. Chen, X. Yan, B. Zheng, S. Liu and F. Huang, J. Am. Chem. Soc., 2012, 134, 8711 CrossRef CAS.
- T. Ogoshi, S. Kanai, S. Fujinami, T. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022 CrossRef CAS.
- X.-Y. Hu, K. Jia, Y. Cao, Y. Li, S. Qin, F. Zhou, C. Lin, D. Zhang and L. Wang, Chem.–Eur. J., 2015, 21, 1208 CrossRef CAS.
- Y. Sun, J. Ma, F. Zhang, F. Zhu, Y. Mei, L. Liu, D. Tian and H. Li, Nat. Commun., 2017, 8, 260 CrossRef.
- X.-B. Hu, Z. Chen, G. Tang, J.-L. Hou and Z.-T. Li, J. Am. Chem. Soc., 2012, 134, 8384 CrossRef CAS.
- X. Hu, C. Yu, K. D. Okochi, Y. Jin, Z. Liu and W. Zhang, Chem. Commun., 2016, 52, 5848 RSC.
- A. Harada, A. Hashidzume, H. Yamaguchi and Y. Takashima, Chem. Rev., 2009, 109, 5974 CrossRef CAS.
- D. A. Edwards, M. R. Prausnitz, R. Langer and J. C. Weaver, J. Controlled Release, 1995, 34, 211 CrossRef CAS.
- M. Danial, C. M. N. Tran, K. A. Jolliffe and S. Perrier, J. Am. Chem. Soc., 2014, 136, 8018 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fluorescence assay, conductance assay and photoisomerization assay. See DOI: 10.1039/d0ra10871e |
| This journal is © The Royal Society of Chemistry 2021 |