Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Catalytic enantioselective intramolecular Tishchenko reaction of meso-dialdehyde: synthesis of (S)-cedarmycins†
Ismiyartoa,
Nobuki Kishib,
Yuki Adachia,
Rui Jianga,
Takahiro Doia,
Da-Yang Zhoua,
Kaori Asanoa,
Yasushi Obora b,
Takayoshi Suzukia,
Hiroaki Sasaia and
Takeyuki Suzuki
b,
Takayoshi Suzukia,
Hiroaki Sasaia and
Takeyuki Suzuki *a
*a
aThe Institute of Scientific and Industrial Research, Osaka University, Mihogaoka, Ibaraki, Osaka 567-0047, Japan. E-mail: suzuki-t@sanken.osaka-u.ac.jp
bDepartment of Chemistry and Materials Engineering, Faculty of Chemistry, Materials, and Bioengineering, Kansai University, Suita, Osaka 564-8680, Japan
First published on 22nd March 2021
Abstract
The first successful example of a catalytic enantioselective intramolecular Tishchenko reaction of a meso-dialdehyde in the presence of a chiral iridium complex is described. Chiral lactones were obtained in good yields with up to 91% ee. The obtained enantioenriched lactones were utilized for the first synthesis of (S)-cedarmycins A and B.
The catalytic dimerization of aldehydes giving the corresponding esters was first discovered by Claisen in 1887,1 and is now well known as the “Tishchenko reaction” (Scheme 1).
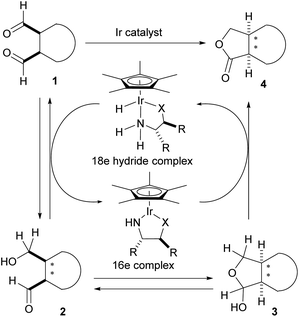
Claisen's method utilizing sodium alkoxides, however, could only be applied to nonenolizable aldehydes like benzaldehyde, because enolizable aldehydes undergo aldol reactions when treated with strong bases such as sodium alkoxides.1 In 1906, a Russian chemist, Tishchenko, reported that aluminum alkoxides were superior to sodium alkoxides in the reaction, because they were more Lewis acidic and less basic.2 The transformation of acetaldehyde into ethyl acetate is the representative application of the Tishchenko reaction in the chemical industry.3 So far, a number of homogeneous catalysts exhibiting high catalytic performance for the Tishchenko reaction have been developed to compensate for the drawback of the classical aluminum alkoxide catalysts.4 In 2005, we reported a mild Tishchenko dimerization using a Ir catalyst.5 The reaction proceeds at room temperature and is effective with a wide range of aldehydes. We also reported the enantioselective oxidative lactonization6 of meso-diols for the preparation of chiral lactones.7 Although asymmetric aldol-Tishchenko reactions8 for chiral 1,3-diols are known, there is no report of an asymmetric intramolecular Tishchenko reaction of meso-dialdehydes for chiral lactones. We envisaged that the asymmetric borrowing hydrogen methodology9 could be applied to achieve an enantioselective intramolecular Tishchenko reaction of meso-dialdehydes (Scheme 2).
The initial enantioselective reduction of meso-dialdehyde 1 could occur by a chiral 18 electron Ir hydride complex to afford chiral hydroxy aldehyde 2. The hydroxy aldehyde-lactol equilibrium would generate lactol 3. Finally, irreversible oxidation of 3 would produce the desired lactone 4.
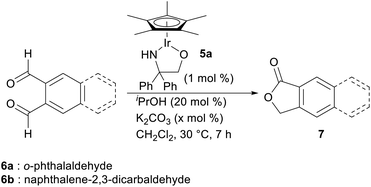
With this hypothesis in mind, we began the investigation of the intramolecular Tishchenko reaction using o-phthalaldehyde as a model substrate (Table 1). When a mixture of o-phthalaldehyde (6a) in CH2Cl2 containing the Ir complex 5a,10 iPrOH, and K2CO3 (6a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a
5a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) iPrOH
iPrOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K2CO3 = 100
K2CO3 = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 mol ratio) was stirred at 30 °C for 7 h, lactone (7a) was obtained in 97% yield (entry 1). The reaction did not proceed at all in the absence of Ir catalyst (entry 2). Interestingly, the reaction without the additional hydrogen donor, iPrOH, also gave the lactone, but the yield was low (entry 3). To enhance the reactivity, the addition of a base was necessary.5a Without base or with a lower amount of base, the reaction proceeded in less than 28% yield (entries 4, 5). The reaction of 2,3-naphthalene dicarboxaldehyde 6b also proceeded similarly under optimized conditions (entry 6).
20 mol ratio) was stirred at 30 °C for 7 h, lactone (7a) was obtained in 97% yield (entry 1). The reaction did not proceed at all in the absence of Ir catalyst (entry 2). Interestingly, the reaction without the additional hydrogen donor, iPrOH, also gave the lactone, but the yield was low (entry 3). To enhance the reactivity, the addition of a base was necessary.5a Without base or with a lower amount of base, the reaction proceeded in less than 28% yield (entries 4, 5). The reaction of 2,3-naphthalene dicarboxaldehyde 6b also proceeded similarly under optimized conditions (entry 6).
| Entry | Aldehyde | Ir (5a) | iPrOH | K2CO3 (x mol%) | Yield (%) |
|---|---|---|---|---|---|
| a All the reactions were carried out on a 0.15 mmol scale of 6. | |||||
| 1 | 6a | + | + | 20 | 97 |
| 2 | 6a | — | + | 20 | 0 |
| 3 | 6a | + | — | 20 | 24 |
| 4 | 6a | + | + | — | 6 |
| 5 | 6a | + | + | 10 | 28 |
| 6 | 6b | + | + | 20 | 97 |
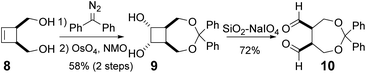
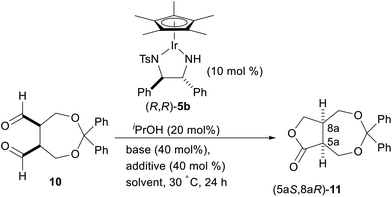
Encouraged by these results, we next examined the enantioselective intramolecular Tishchenko reaction of a meso-dialdehyde. With the utility of the product in mind, we selected the meso-aldehyde 10 as a model substrate (Scheme 3). The requisite aldehyde was prepared in 3 steps from cis-3-cyclobutene-1,2-dimethanol 8.11 Protection of the diol with diphenyldiazomethane followed by dihydroxylation gave the anti-diol 9 in 58% yield as the major isomer. The relative configuration of 9 was unambiguously determined by the crystalline sponge (CS) method.12 Oxidative cleavage13 of 9 by silica gel-supported NaIO4 gave the desired meso-dialdehyde 10.
With the meso-dialdehyde 10 in hand, we investigated the enantioselective intramolecular Tishchenko reaction using a chiral Ir complex. After screening the catalysts and reaction conditions, we were pleased to find that the enantioselective intramolecular Tishchenko reaction was realized for the first time. Thus, treatment of 10 with Cp*Ir [(R,R)-Tsdpen] (5b;14 TsDPEN = N-(p-toluenesulfonyl)-1,2-diphenylethylenediamine) (10 mol%) and iPrOH (20 mol%) in the presence of K2CO3 (40 mol%) in CH2Cl2 at 30 °C for 24 h provided the desired 11 in 83% yield with 78% ee (Table 2, entry 1). The reaction in acetonitrile proceeded quantitatively, but the enantioselectivity was diminished (entry 2). The reaction in the presence of K3PO4 slightly increased the chemical yield and ee (entry 3). Finally, the addition of a phosphoric acid,15 (PhO)2PO2H, increased the enantioselectivity (91% ee, entry 4). Similar positive effects of the phosphoric acid on the enantioselectivity were also observed with 10 mol% of chiral phosphoric acids such as TRIP (TRIP = 3,3′-bis(2,4,6-triisopropylphenyl)-1,1′-binaphthyl-2,2′-diylhydrogen phosphate). However (R)-TRIP and (S)-TRIP gave the same enantioselectivity.
The absolute configuration of 11 was unambiguously determined by single crystal X-ray crystallographic analysis using the optically pure lactone obtained by recrystallization (Fig. 1).
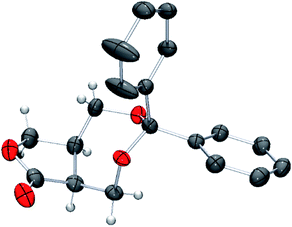 | ||
| Fig. 1 POV-ray depiction of (5aS,8aR)-11. Thermal ellipsoid is drawn at the 50% probability level. H atoms on benzene rings are omitted for clarify. | ||
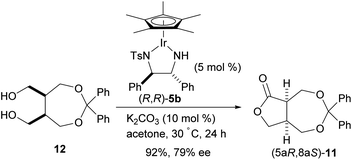
To compare the enantioselective intramolecular Tishchenko reaction with the enantioselective oxidative lactonization,6 the corresponding diol 12 was treated with the same catalyst in the presence of acetone as an oxidant, to afford the desired lactone 11 in 92% yield and 79% ee with (5aS,8aR) configuration (Scheme 4). The addition of (PhO)2PO2H did not improve the enantioselectivity in this reaction system (87%, 78% ee).
These results show that both enantiomers of the lactone can be prepared using the same catalyst by selecting the appropriate reaction system (Scheme 5). The enantioselective intramolecular Tishchenko reaction and the enantioselective oxidative lactonization are complementary, indicating both reactions pass through the same transition state.
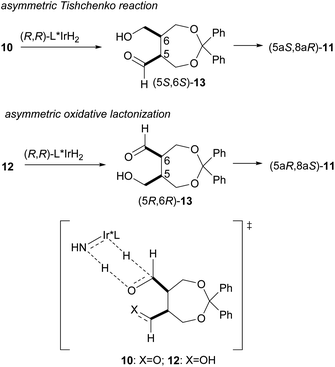 | ||
| Scheme 5 Comparison of enantioselective intramolecular Tishchenko reaction with enantioselective oxidative lactonization. | ||
In this reaction, we used 16 electron Ir complex 5b and iPrOH to generate the 18 electron Ir hydride complex. To obtain information on the catalyst, cold spray ionization mass spectrometry (CSI-MS)16 was conducted. The sample prepared from 5b and iPrOH gave a prominent peak at m/z 695 corresponding to the 18 electron Ir hydride complex, Cp*IrH[TsNCHPhCHPhNH2] + H+. The Ir complex formed from 5b and 1 equiv. of (PhO)2PO2H in iPrOH exhibited a peak at m/z 965 due to Cp*IrH[TsNCHPhCHPhNH2][OPO(OPh)2] + Na+. These results indicate that Cp*Ir, TsDPEN, and phosphoric acid contribute to the formation of an efficient asymmetric environment.15
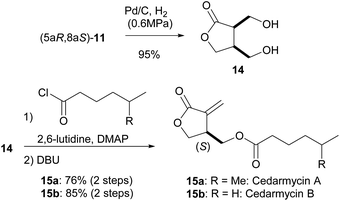
Having succeeded in the first intramolecular enantioselective Tishchenko reaction of meso-dialdehyde, we then applied our method to the synthesis of several natural products. Cedarmycins were isolated in 2001 by the Frumai and Igarashi groups from a plant called Cryptomeria japonica.17 Cedarmycins exhibit antibiotic activity with cedarmycin A showing potent activity against candida glabrata IFO 0622, comparable to amphotericin B. To date, two groups have reported different methods for the racemic synthesis of the cedarmycins.18 However, no reports on the catalytic asymmetric synthesis or the absolute configurations of these compounds have been published. As shown in Scheme 6, the deprotection of 11 under hydrogenolysis conditions afforded the desired cis-diol 14. Sequential double acylation/elimination of 14 proceeded in the presence of DBU to give cedarmycins A (15a) and B (15b) in 76% and 85% yields, respectively. By comparison of their optical rotations, the absolute configurations of cedarmycins A and B were determined to be (S). It should be noted that cleavage of the acetal moiety of 11 by acid hydrolysis caused partial racemization of 15. The racemization likely occurred by the recyclization of cis-14 by the nucleophilic attack of the γ-hydroxy group under acidic conditions.
In conclusion, we have achieved the first enantioselective intramolecular Tishchenko reaction of meso-dialdehydes and applied this methodology to the synthesis of natural products. Chiral lactones are useful chiral building block for the organic synthesis. The direct conversion of 1,4-dialdehydes to γ-lactones is attractive from the view point of environmentally benign redox neutral process.19 Additional studies on the substrate scope and reaction mechanism are currently in progress in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI (Grant No. 26410047, 17K05784, 20K05494) and Uehara Memorial Foundation. This work was also supported in part by “Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials” in “Network Joint Research Center for Materials and Devices” (No. 20194015) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT). We are also grateful to Tsuyoshi Matsuzaki, Hitoshi Haneoka, and Tsunayoshi Takehara of the Comprehensive Analysis Center of ISIR for their assistance.Notes and references
- L. Claisen, Ber. Dtsch. Chem. Ges., 1887, 20, 646–650 CrossRef.
- W. Tischtschenko, J. Russ. Phys. Chem., 1906, 38, 355–418 Search PubMed.
- B. N. Pattanaik and H. C. Mandalia, Int. J. Curr. Res. Rev., 2011, 3, 23–40 CAS.
- (a) O. P. Tormakangas and A. M. P. Koskinen, Recent Res. Dev. Org. Chem., 2001, 5, 225–255 CAS; (b) T. Seki, T. Nakajo and M. Onaka, Chem. Lett., 2006, 35, 824–829 CrossRef CAS; (c) A. M. P. Koskinen and A. O. Kataja, in Organic Reactions, ed. S. E. Denmark, John Wiley & Sons, Inc., Hoboken, 2015, vol. 86, pp. 105–410 Search PubMed; (d) R. Dhanya, T. Shilpa, S. Saranya and G. Anilkumar, ChemistrySelect, 2020, 5, 754–763 CrossRef CAS.
- (a) T. Suzuki, T. Yamada, T. Matsuo, K. Watanabe and T. Katoh, Synlett, 2005, 2005, 1450–1452 CrossRef; (b) T. Suzuki, T. Yamada, K. Watanabe and T. Katoh, Bioorg. Med. Chem. Lett., 2005, 15, 2583–2585 CrossRef CAS PubMed.
- T. Suzuki, K. Morita, Y. Matsuo and K. Hiroi, Tetrahedron Lett., 2003, 44, 2003–2006 CrossRef CAS.
- (a) Z. Shen, H. A. Khan and V. M. Dong, J. Am. Chem. Soc., 2008, 130, 2916–2917 CrossRef CAS PubMed; (b) D. Hojo, K. Noguchi and K. Tanaka, Angew. Chem., Int. Ed., 2009, 48, 8129–8132 CrossRef CAS PubMed; (c) D. H. Phan, B. Kim and V. M. Dong, J. Am. Chem. Soc., 2009, 131, 15608–15609 CrossRef CAS PubMed; (d) Z. Shen, P. K. Dornan, H. A. Khan, T. K. Woo and V. M. Dong, J. Am. Chem. Soc., 2009, 131, 1077–1091 CrossRef CAS PubMed; (e) M. C. Willis, Angew. Chem., Int. Ed., 2010, 49, 6026–6027 CrossRef CAS PubMed; (f) H. A. Khan, K. G. M. Kou and V. M. Dong, Chem. Sci., 2011, 2, 407–410 RSC; (g) S. K. Murphy and V. M. Dong, J. Am. Chem. Soc., 2013, 135, 5553–5556 CrossRef CAS PubMed; (h) L. Souillart and N. Cramer, Angew. Chem., Int. Ed., 2014, 53, 9640–9644 CrossRef CAS PubMed; (i) X. Wu, Z. Chen, Y. B. Bai and V. M. Dong, J. Am. Chem. Soc., 2016, 138, 12013–12016 CrossRef CAS PubMed; (j) T. Shirai, T. Iwasaki, K. Kanemoto and Y. Yamamoto, Chem.–Asian J., 2020, 15, 1858–1862 CrossRef CAS PubMed.
- (a) C. M. Mascarenhas, S. P. Miller, P. S. White and J. P. Morken, Angew. Chem., Int. Ed., 2001, 40, 601–603 CrossRef CAS; (b) C. Schneider and M. Hansch, Synlett, 2003, 2003, 837–840 CrossRef; (c) V. Gnanadesikan, Y. Horiuchi, T. Ohshima and M. Shibasaki, J. Am. Chem. Soc., 2004, 126, 7782–7783 CrossRef CAS PubMed; (d) J. Mlynarski and M. Mitura, Tetrahedron Lett., 2004, 45, 7549–7552 CrossRef CAS; (e) Y. Horiuchi, V. Gnanadesikan, T. Ohshima, H. Masu, K. Katagiri, Y. Sei, K. Yamaguchi and M. Shibasaki, Chem. –Eur. J., 2005, 11, 5195–5204 CrossRef CAS PubMed; (f) J. Mlynarski, J. Jankowska and B. Rakiel, Chem. Commun., 2005, 4854–4856 RSC; (g) J. Mlynarski, J. Jankowska and B. Rakiel, Tetrahedron: Asymmetry, 2005, 16, 1521–1526 CrossRef CAS; (h) K. Rohr, R. Herre and R. Mahrwald, Org. Lett., 2005, 7, 4499–4501 CrossRef CAS PubMed; (i) M. Stodulski, J. Jazwinski and J. Mlynarski, Eur. J. Org. Chem., 2008, 2008, 5553–5562 CrossRef; (j) T. Asano, S. Kotani and M. Nakajima, Org. Lett., 2019, 21, 4192–4196 CrossRef CAS PubMed.
- (a) M. H. S. A. Hamid, P. A. Slatford and J. M. J. Williams, Adv. Synth. Catal., 2007, 349, 1555–1575 CrossRef CAS; (b) T. Suzuki, Chem. Rev., 2011, 111, 1825–1845 CrossRef CAS PubMed; (c) J. M. Ketcham, I. Shin, T. P. Montgomery and M. J. Krische, Angew. Chem., Int. Ed ., 2014, 53, 9142–9150 CrossRef CAS PubMed; (d) T. Kwok, O. Hoff, R. J. Armstrong and T. J. Donohoe, Chem. –Eur. J., 2020, 26, 12912–12926 CrossRef CAS PubMed.
- T. Suzuki, K. Morita, M. Tsuchida and K. Hiroi, Org. Lett., 2002, 4, 2361–2363 CrossRef CAS PubMed.
- N. Gauvry, C. Comoy, C. Lescop and F. Huet, Synthesis, 1999, 1999, 574–576 CrossRef.
- Y. Inokuma, S. Yoshioka, J. Ariyoshi, T. Arai, Y. Hitora, K. Takada, S. Matsunaga, K. Rissanen and M. Fujita, Nature, 2013, 495, 461–466 CrossRef CAS PubMed.
- Y. L. Zhong and T. K. Shing, J. Org. Chem., 1997, 62, 2622–2624 CrossRef CAS PubMed.
- K. Mashima, T. Abe and K. Tani, Chem. Lett., 1998, 1201–1202 CrossRef CAS.
- (a) C. Li, B. Villa-Marcos and J. Xiao, J. Am. Chem. Soc., 2009, 131, 6967–6969 CrossRef CAS PubMed; (b) W. J. Tang and J. L. Xiao, Synthesis, 2014, 46, 1297–1302 CrossRef; (c) S. Tribedi, C. M. Hadad and R. B. Sunoj, Chem. Sci., 2018, 9, 6126–6133 RSC.
- S. Sakamoto, M. Fujita, K. Kim and K. Yamaguchi, Tetrahedron, 2000, 56, 955–964 CrossRef CAS.
- T. Sasaki, Y. Igarashi, N. Saito and T. Furumai, J. Antibiot., 2001, 54, 567–572 CrossRef CAS PubMed.
- (a) H. S. Yang, X. X. Qiao, Q. Cui and X. H. Xu, Chin. Chem. Lett., 2009, 20, 1023–1024 CrossRef CAS; (b) M. G. Lloyd, M. D'Acunto, R. J. K. Taylor and W. P. Unsworth, Tetrahedron, 2015, 71, 7107–7123 CrossRef CAS.
- S. H. Bergens, D. P. Fairlie and B. Bosnich, Organometallics, 1990, 9, 566–571 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, compond characterization, NMR and CSI spectra. CCDC 2019330 (9) and 2022569 (11). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra00915j |
| This journal is © The Royal Society of Chemistry 2021 |