Open Access Article
Open Access ArticleTwo-dimension on two-dimension growth: hierarchical Ni0.2Mo0.8N/Fe-doped Ni3N nanosheet array for overall water splitting†
Chen Liu,
Han Zhu *,
Shuanglong Lu
*,
Shuanglong Lu ,
Fangping Xu,
Fang Duan
,
Fangping Xu,
Fang Duan and
Mingliang Du
and
Mingliang Du *
*
Key Laboratory of Synthetic and Biological Colloids, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, Wuxi 214122, P. R. China. E-mail: zhysw@jiangnan.edu.cn; du@jiangnan.edu.cn
First published on 1st June 2021
Abstract
Developing advanced electrocatalysts with low cost for electrocatalytic water splitting are highly desirable. Herein, we report the design of two-dimension on two-dimension growth of hierarchical Ni0.2Mo0.8N nanosheets on Fe-doped Ni3N nanosheets supported on Ni foam (Ni0.2Mo0.8N/Fe–Ni3N/NF) via hydrothermal reaction and nitridation treatment. In the hierarchical structures, small Ni0.2Mo0.8N nanosheets were uniformly anchored on Fe–Ni3N nanosheets. Due to enhanced electron transfer between Ni0.2Mo0.8N and Fe–Ni3N, Ni0.2Mo0.8N/Fe–Ni3N/NF exhibits superior electrocatalytic activity for the oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER). After stability tests for 50 h, Ni0.2Mo0.8N/Fe–Ni3N/NF exhibits negligible degradation of the current density for the OER (91% remain) and HER (95% remain), suggesting excellent stability. Owing to the outstanding performance, Ni0.2Mo0.8N/Fe–Ni3N/NF display a cell voltage of 1.54 V (10 mA cm−2) for electrocatalytic overall water splitting.
Introduction
Hydrogen (H2) is a promising and attractive energy source owing to its high combustion efficiency, non-polluting production and sustainability.1–3 Currently, the industrial methods to produce H2 usually consume a lot of fossil energy, and are unfavorable for solving the problems of energy and the environment.4–6 Thus, production of H2 through efficient and facile methods are highly desirable. Electrocatalytic water splitting is an exciting strategy to sustainably produce hydrogen energy.7–10 Electrochemical water splitting consists of two half-reaction steps, the oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER), requiring higher overpotentials to obtain significant catalytic efficiency due to the existence of inherent energy barriers.11–14 Therefore, electrocatalysts are crucial to reduce the energy barrier of rate-determining steps. Pt-based noble metals are the best catalysts towards the OER and HER.15–18 However, their lower abundance and higher cost greatly suppress their wide commercial development. Therefore, exploring highly efficient and earth-abundant electrocatalysts toward water splitting are vital for large-scale practical application.Nowadays, transition metal-based materials19–21 including the chalcogenides,22–24 phosphides,25–27 nitrides,28–30 carbides31–33 and selenides34–36 have been extensively applied for electrocatalysis. Among of these electrocatalysts, transition metal nitrides (TMNs) are the new emerging alternative electrocatalyst for water splitting due to the high electrical conductivity and corrosion-resistance.37–39 However, amounts of electrocatalysts with highly performance are only suitable for OER or HER. Therefore, developing outstanding bifunctional electrocatalysts with excellent activity for OER and HER is crucial. Integrating multiple metal into one integrated material provide a powerful way to turn the electronic structure, further improving the OER and HER activity.40–43
Herein, we reported the design of two-dimension on two-dimension growth of hierarchical Ni0.2Mo0.8N nanosheets on Fe-doped Ni3N nanosheets supported on Ni foam (Ni0.2Mo0.8N/Fe–Ni3N/NF). In the hierarchical structures, small Ni0.2Mo0.8N nanosheets were homogenously distributed on Fe–Ni3N nanosheets. The Ni0.2Mo0.8N/Fe–Ni3N/NF exhibits lowest OER overpotential of 266 mV (20 mA cm−2), compared with the Fe–Ni3N/NF (292 mV), Ni0.2Mo0.8N/NF (320 mV) and commercial RuO2 (328 mV). The Ni0.2Mo0.8N/Fe–Ni3N/NF shows negligible degradation of the current density (91%) after OER stability test for 50 h. In addition, the Ni0.2Mo0.8N/Fe–Ni3N/NF also displays the lower overpotential (40 mV, 20 mA cm−2) and excellent durability (50 h, 95% current density remain). The Ni0.2Mo0.8N/Fe–Ni3N/NF shows a quite low cell voltage of 1.54 V (10 mA cm−2) for overall water splitting.
Experimental
Chemicals
Iron nitrate nonahydrate (Fe(NO3)3·9H2O), sodium molybdate (Na2(MoO4)2·H2O), urea, ammonium fluoride (NH4F), nickel nitrate hexahydrate (Ni(NO3)2·6H2O), ethanol, and ruthenium dioxide (RuO2) were obtained from Sinopharm Chemical Reagent Co. Ltd. Potassium hydroxide (KOH) and Commercial 20% Pt/C were purchased from Shanghai Macklin Biochemical Co. Ltd. The 5 wt% Nafion solution was obtained from Sigma Aldrich. Ni foam (NF) was brought from Kunshan Rongsheng company, China. Unless otherwise stated, all reagents were used without purification.Synthesis of NiMoO4 and Ni0.2Mo0.8N grown on NF (NiMoO4/NF and Ni0.2Mo0.8N/NF)
The NiMoO4 supported on NF were synthesized by the hydrothermal reaction. 2 mmol Ni(NO3)2·6H2O and 2 mmol Na2(MoO4)2·H2O were dissolved in 17.5 mL distilled water, respectively. Then, the mixed solution were transfer to the Teflon-lined stainless steel autoclave and heated at 150 °C for 6 h. Then, after cooling down to 25 °C, the as-prepared NiMoO4/NF materials were cleaned by the distilled water and keep ultrasonicate for 30 min. Furthermore, the Ni0.2Mo0.8N/NF were prepared by the following nitridation treatment.The dry NiMoO4/NF was placed at furnace and treated in a flow of 20 standard cubic centimeters (sccm) NH3 and 130 sccm Ar. The NiMoO4/NF kept at 500 °C for 1 h (5 °C min−1). Ni0.2Mo0.8N/NF was obtained after the treatment.
Synthesis of Fe-doped Ni(OH)2 precursor grown on NF and Fe-doped Ni3N grown on NF
Fe-doped Ni(OH)2 precursor grown on Ni foam (Fe–Ni(OH)2/NF) was obtained to prepare the Ni0.2Mo0.8N/Fe–Ni3N/NF. Before starting the reaction, the NF (2 × 3 cm2) was pre-treated with HCl solution (1 M) to get rid of the oxidation layer on the NF. After 20 min, the NF was washed by distilled water. 0.2 mmol Fe(NO3)3·9H2O, 3.8 mmol Ni(NO3)2·6H2O, 10 mmol urea and 20 mL distilled water were mixed to form the homogeneous solution by a magnetic stirrer. The 4 mmol NH4F and as-treated NF was immersed in the precursor solution and keep stirring for 30 min. Then the Teflon-lined stainless steel autoclave was used as the container of preparing the Fe–Ni(OH)2/NF and heated at 120 °C for 14 h. After cooling down to 25 °C, the Fe–Ni(OH)2/NF was cleaned by ultrasonicating with distilled water for 30 min and dried at 60 °C. Then the dry Fe–Ni(OH)2/NF was placed at furnace and treated in a flow of 20 standard cubic centimeters (sccm) NH3 and 130 sccm Ar. The Fe–Ni(OH)2/NF kept at 500 °C for 1 h (5 °C min−1). Fe–Ni3N/NF was obtained after the treatment.Fabrication of Ni0.2Mo0.8N/Fe-doped Ni3N nanosheets arrays supported on NF and Ni0.2Mo0.8N nanosheets arrays supported on NF
The Ni0.2Mo0.8N/Fe-doped Ni3N arrays supported on NF (Ni0.2Mo0.8N/Fe–Ni3N/NF) were synthesized through the combination of hydrothermal reaction and nitridation treatment. 2 mmol Ni(NO3)2·6H2O and 2 mmol Na2(MoO4)2·H2O were dissolved in 17.5 mL distilled water, respectively. The Fe–Ni(OH)2/NF were immersed in mixed solution containing Na2(MoO4)2·H2O and Ni(NO3)2·6H2O. After 30 min, the Fe–Ni(OH)2/NF and mixed solution were transfer to the Teflon-lined stainless steel autoclave and heated at 150 °C for 6 h. Then, after cooling down to 25 °C, the as-prepared Fe–Ni(OH)2/NiMoO4/NF materials were cleaned by the distilled water and keep ultrasonicate for 30 min. The Ni0.2Mo0.8N/Fe–Ni3N/NF was obtained through the NH3 treatment of Fe–Ni(OH)2/NiMoO4/NF.Characterization methods
The morphologies of the as-synthesized materials were examined by field emission scanning electron microscope (FE-SEM, S-4800, Hitachi, Japan) and transmission electron microscope (TEM, JEM-2010plus, JEOL, Japan). The phase structures were recorded by X-ray diffractometer (D8 Discover, Bruker AXS, Germany) with a Cu Kα radiation (λ = 1.5406 Å). Chemical states and composition of the as-synthesized materials were investigated by X-ray photoelectron spectroscopy (XPS, Axis supra, Kratos Analytical Ltd, UK). The results were obtained at 15 kV and 10 mA with monochromatic Al Kα radiation. All the XPS spectra of materials were corrected through C 1s spectrum at 284.8 eV.Electrochemical measurement
The electrocatalytic performances of as-synthesized materials were performed by a CHI660E electrochemical workstation (Chen Hua, Shanghai, China). All the electrochemical tests were operated in 1 M KOH solution. The three-electrode system was used to perform the OER and HER tests. The as-prepared materials (geometric area: 0.5 cm2) and a carbon rod were used as working electrode and counter electrode, respectively. Furthermore, the reference electrode was a saturated calomel electrode (SCE). The potential was corrected by the following equation: E(RHE) = E(SCE) + 0.059 pH + 0.244.The linear sweep voltammogram (LSV) for OER was measured in a potential range of 1.0 to 1.6 V versus RHE (vs. RHE). For HER measurements, the LSV was measured in a potential range of and 0 to −0.5 V vs. RHE. The scan rate is 1 mV s−1. OER and HER curves of were treated by iR correction. According to the equation: Ecorrected = Euncorrected − iRs, the curve of iR correction would be finished. Rs (solution resistance) was able to observed by the electrochemical impedance spectroscopy (EIS) tests. The EIS for OER and HER was tested at 1.47 V and −0.03 V vs. RHE from 10 kHz to 0.01 Hz with a 10 mV AC amplitude. The stability test for OER was operated at 1.515 V vs. RHE for 50 h. The stability test for HER was finished at −74 mV vs. RHE for 50 h. The double-layer capacitance (Cdl) was obtained by the CVs cycling tests from 0.1 to 0.2 V vs. RHE with scan rates ranged from 10–60 mV s−1. Cdl can be used to calculate the electrochemically active surface area (ECSA). Overall water splitting was measured in a two-electrode electrolyzer. The LSV was measured in a potential range of 1.0 to 1.9 V voltage. The stability test of overall water-splitting was operated at 1.59 V voltage for 20 h.
Results and discussion
As shown in Fig. 1a–c, after the hydrothermal reaction, the Fe–Ni(OH)2 nanosheets were densely and vertically grown on substrate Ni foam (NF), forming a unique three-dimensional (3D) architectures. The thickness of the Fe–Ni(OH)2 nanosheets ranged from 80–200 nm (Fig. S1†). After the NH3 treatment at 500 °C, the Fe–Ni(OH)2 were converted into Fe-doped Ni3N (Fe–Ni3N). The Fe–Ni(OH)2 nanosheets were transformed into three-dimensional porous network structures consist of interconnected nanospheres (Fig. 1d–f). The average diameters of Fe–Ni3N grains ranged from 160–320 nm (Fig. S1†). As shown in Fig. S2a–c,† the NiMoO4 precursor nanowires were synthesized on NF through the same hydrothermal reaction. After the NH3 treatment, the as-prepared Ni0.2Mo0.8N/NF also indicates the nanowire morphologies and structures (Fig. 1i).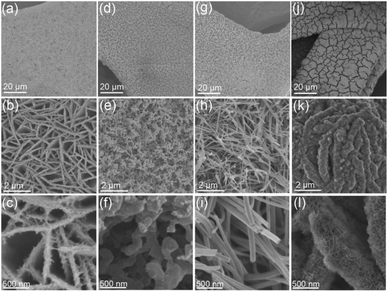 | ||
| Fig. 1 FE-SEM images of (a–c) Fe–Ni(OH)2/NF, (d–f) Fe–Ni3N/NF, (g–i) Ni0.2Mo0.8N/NF and (j–l) Ni0.2Mo0.8N/Fe–Ni3N/NF at different magnifications. | ||
The diameters of the Ni0.2Mo0.8N nanowires ranged from 80 to 200 nm (Fig. S1†). When NiMoO4 meets Fe–Ni(OH)2 (Fig. S2d–f†), the as-synthesized NiMoO4/Fe–Ni(OH)2/NF exhibited a hierarchical structure consist of Fe–Ni(OH)2 as substrates and NiMoO4 vertically grown on Fe–Ni(OH)2 nanosheets. Through the NH3 treatment at 500 °C, the NiMoO4/Fe–Ni(OH)2/NF were converted into Ni0.2Mo0.8N/Fe–Ni3N/NF (Fig. 1j–l) with two unique phases. The Fe–Ni3N nanosheets were coated by small Ni0.2Mo0.8N nanosheets. It is interesting that with the Ni0.2Mo0.8N, the Fe–Ni3N substrates still remain the morphology of NiMoO4/Fe–Ni(OH)2. The thickness of Ni0.2Mo0.8N/Fe–Ni3N/NF ranged from 210–350 nm (Fig. S3a and b†), while the thickness range of small nanosheet is 7–15 nm (Fig. S3c and d†). Different from the individual Fe–Ni3N (Fig. 1d–f), Fe–Ni3N substrates were not also transformed into the similar structure. The small Ni0.2Mo0.8N nanosheets could hinder the collapse of Fe–Ni3N from nanosheets to interconnected nanospheres. The unique structure of Ni0.2Mo0.8N/Fe–Ni3N/NF may be provide more active sites for electrocatalysis.
The crystal structures of materials were investigated by the XRD characterization. Fig. 2a and S4† show the sharp peaks near the 44.5°, 51.8° and 76.4°, corresponding to the metal Ni in the NF. The peaks of Fe–Ni(OH)2/NF were observed at 33.5°, 34.4° and 38.8° (Fig. S4†), indicating the formation of Ni(OH)2 phase (JCPDS 38-0715). The peaks at 38.9°, 42.1°, 58.5°, 70.6° and 78.4° of Fe–Ni3N/NF (Fig. 2a) were assigned to Ni3N phases (JCPDS 10-0280), indicating that the Ni(OH)2/NF were transformed to Fe–Ni3N phases after NH3 treatment. The visible diffraction peaks of Ni0.2Mo0.8N/NF at 32.2°, 36.5° and 49.4° belong to the crystal planes of Ni0.2Mo0.8N (JCPDS 29-0931). For the Ni0.2Mo0.8N/Fe–Ni3N/NF, the peaks for Ni3N phases were very weak due to the low crystallinity, while the peaks for Ni0.2Mo0.8N phases are strong. The HRTEM image of Fe–Ni3N/NF reveals a lattice fringe (0.20 nm) (Fig. S5a and b†), corresponding to the (100) plane of Ni3N. The TEM image of Ni0.2Mo0.8N/Fe–Ni3N/NF (Fig. S5c†) indicates that small Ni0.2Mo0.8N nanosheets were uniformly dispersed on Fe–Ni3N/NF nanosheets. Fig. 2b shows the HRTEM image of Ni0.2Mo0.8N/Fe–Ni3N/NF. The (100) and (101) planes of Ni0.2Mo0.8N was observed. Furthermore, the (002) plane (the interplanar distance of 0.21 nm) of Ni3N was observed in Fig. 2b. There were no peaks of metal Fe or Fe oxides and nitrides in the measured XRD patterns and HRTEM images, indicating that the Fe elements were doped into the Ni(OH)2 and the Ni3N crystal matrix.44–46 The STEM-EDS mapping images of Ni0.2Mo0.8N/Fe–Ni3N/NF were represented in Fig. 2c. As shown in new Fig. 2c, the Ni, Mo, Fe and N were uniformly distributed throughout the randomly examined Ni0.2Mo0.8N/Fe–Ni3N nanosheets. The Fe, Ni and N elements can be visible observed as substrates while the Ni, Mo and N elements also distributed as spots. The results confirmed the formation of hierarchical structures of Ni0.2Mo0.8N/Fe–Ni3N/NF using Fe–Ni3N as support.
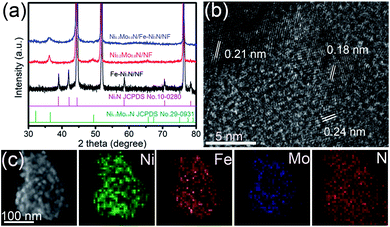 | ||
| Fig. 2 (a) XRD patterns of Ni0.2Mo0.8N/Fe–Ni3N/NF, Ni0.2Mo0.8N/NF and Fe–Ni3N/NF. (b) HRTEM image of Ni0.2Mo0.8N/Fe–Ni3N/NF. (c) STEM-EDS mapping images of Ni0.2Mo0.8N/Fe–Ni3N/NF. | ||
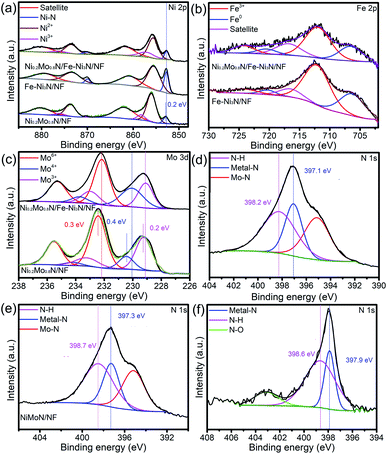
XPS spectra were shown in Fig. S6† and 3 to research the chemical compositions and surface chemical states of materials. Fig. S6† is the XPS survey of Ni0.2Mo0.8N/Fe–Ni3N/NF, indicating that the Ni, Fe, Mo and N were observed. In Fig. 3a, the two peaks of the Ni0.2Mo0.8N/Fe–Ni3N/NF at 852.6 and 870.2 eV belong to the Ni species in Ni–N bonds.47–49 Furthermore, the peaks located at 855.6, 873.5 eV and 857.1, 876.3 eV correspond to the oxidized Ni2+ and Ni3+ species, while the remaining two peaks (880.1 and 861.8 eV) belong to satellite peaks.50,51 Compared with the BE of Ni0.2Mo0.8N/NF, the binding energy (BE) values of Ni–N bond of Ni0.2Mo0.8N/Fe–Ni3N/NF shifts negatively by about 0.2 eV (Fig. 3a). The difference in BEs of the Ni–N bond between the Ni0.2Mo0.8N/Fe–Ni3N/NF and Fe–Ni3N/NF might be attributed to the electron interaction between the Fe–Ni3N nanosheets and Ni0.2Mo0.8N nanosheets or the interaction with underlying NF substrates.
The BEs shift in Mo 3d suggests the strong electron transfer from the Ni0.2Mo0.8N to Fe–N3N, leading to the strong interaction between the two phases. Fig. 3d shows the N 1s XPS spectra Ni0.2Mo0.8N/Fe–Ni3N/NF, the peak at about 397.1 eV corresponds to the N species of transition metal–N bonds, while the peak at about 395.2 eV was attributed to Mo–N bonds.53 The peak at 398.2 eV belongs to the N–H species on the surface of materials.54 Fig. 3e and f represented the N 1s XPS spectra of Ni0.2Mo0.8N/NF and Fe–Ni3N/NF. By analyzing the peak in Fig. 3d and f, the negative shift in BEs of the Ni0.2Mo0.8N/Fe–Ni3N/NF was observed. The changes in BEs of N 1s of Ni0.2Mo0.8N/Fe–Ni3N/NF suggests that the Fe–Ni3N integrated with Ni0.2Mo0.8N can lead to the enhanced charge transfers and electron interactions.
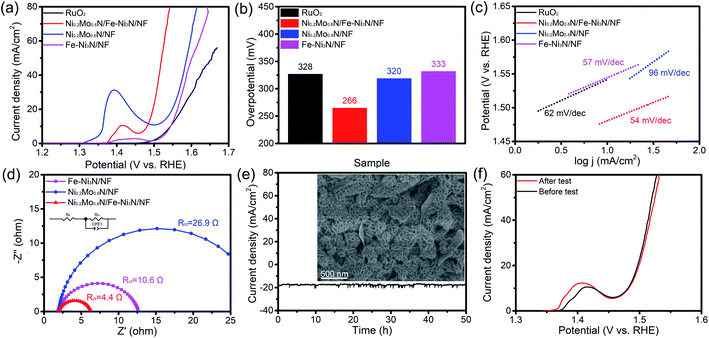
The OER performance of samples was examined. As shown in Fig. 4a and b, the Ni0.2Mo0.8N/Fe–Ni3N/NF displays the overpotential of 266 mV to acquire 20 mA cm−2. The overpotentials of Ni0.2Mo0.8N/NF (320 mV), Fe–Ni3N/NF (292 mV) and commercial RuO2 (328 mV) are higher than those of the Ni0.2Mo0.8N/Fe–Ni3N/NF. Fig. 4c shows the Tafel slopes of electrocatalysts. The Ni0.2Mo0.8N/Fe–Ni3N/NF had the lowest Tafel slopes (54 mV dec−1), indicating the best OER performance than those of the Fe–Ni3N/NF, Ni0.2Mo0.8N/NF and commercial RuO2, respectively. In addition, the performances of Ni0.2Mo0.8N/Fe–Ni3N/NF was superior to those of recently reported OER catalysts (Table S1†).
EIS measurements were performed to analyze the OER electrode kinetics. In the Nyquist plots (Fig. 4d), the Ni0.2Mo0.8N/Fe–Ni3N/NF reveals a small charge transfer resistance (Rct) of 4.4 Ω. The Rct of Fe–Ni3N/NF and Ni0.2Mo0.8N/NF is 10.6 Ω and 26.9 Ω, respectively. The relatively lower Tafel slope and Rct suggests the fast kinetics process of Ni0.2Mo0.8N/Fe–Ni3N/NF. The combination of Fe–Ni3N and Ni0.2Mo0.8N nanosheets lead to the superior OER activity.
The stability test was carried out by chronopotentiometry measurement to investigate the changes of the Ni0.2Mo0.8N/Fe–Ni3N/NF catalyst after prolonged water electrolysis. Fig. 4e shows the current–time curves of OER chronopotentiometry test. The current density of Ni0.2Mo0.8N/Fe–Ni3N/NF still retain 91% after continuous electrolysis for 50 h, and FE-SEM image of Ni0.2Mo0.8N/Fe–Ni3N/NF demonstrates that the hierarchical and two-dimensional features of the Ni0.2Mo0.8N/Fe–Ni3N/NF remain unchanged after the stability test. As displayed in Fig. 4f, the LSV curves before and after cycle tests also exhibit negligible decrease in current density, suggesting the excellent stability in alkaline condition.
The XRD patterns, XPS survey and XPS spectra of the Ni0.2Mo0.8N/Fe–Ni3N/NF after OER and HER stability test was shown in Fig. S6–S10† and 5. As shown in Fig. S7 and S9,† the diffraction peaks for Ni0.2Mo0.8N (JCPDS 29-0931) both become weaker after the HER and OER stability test, respectively. The XPS survey and XPS spectra of Ni0.2Mo0.8N/Fe–Ni3N/NF after OER and HER all indicate the existences of Ni, Mo, Fe and N elements. The chemical states display small changes, suggesting the superior stability of main structures. The N 1s XPS changes after the HER and OER suggests the surface oxidation of Ni0.2Mo0.8N and the formation of metal-oxyhydroxide or metal-hydroxide. In Fig. 5a, the Ni 2p XPS spectra after stability tests show the BEs of Ni3+ (874.5 and 856.8 eV) and Ni2+ (872.9 and 855.4 eV).50 In the Fe 2p XPS spectra (Fig. 5b), the peaks of Fe3+ (725.5 and 713.0 eV), Fe2+ (722.3 and 709.7 eV) and satellite can be observed.55 Fig. 5c and d represent the Mo 3d and N 1s spectra after OER test. The peaks of Mo6+ 235.0 and Mo4+ (233.8 and 230.1 eV), Mo3+ (233.0 and 229.0 eV) and N–H bond (399.4 eV), metal–N bond (397.9 eV), Mo–N bond (394.3 eV) can still be observed.38,49 The chemical states of elements exhibit changes after the OER stability tests, suggesting that the surface of electrocatalyst might be transformed into the metal-oxyhydroxide or metal-hydroxide.48
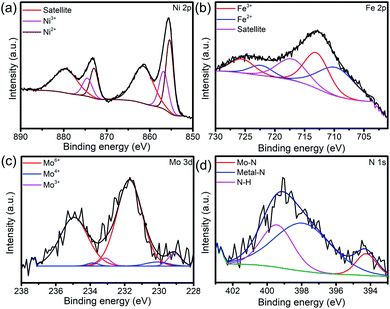 | ||
| Fig. 5 (a) Ni 2p, (b) Fe 2p, (c) Mo 3d and (d) N 1s XPS spectra of Ni0.2Mo0.8N/Fe–Ni3N/NF after OER stability test for 50 h. | ||
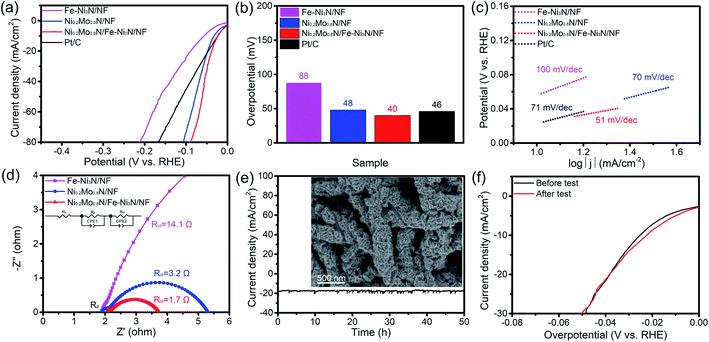
The electrochemical performance of as-synthesized materials toward HER has also been performed. As shown in Fig. 6a and b, the Ni0.2Mo0.8N/Fe–Ni3N/NF displays the best HER activity (40 mV, 20 mA cm−2), which is lower than other electrocatalysts. In addition, the Ni0.2Mo0.8N/Fe–Ni3N/NF also obtains the lowest Tafel slope of 51 mV dec−1 (Fig. 6c). The Nyquist plots under HER condition were shown in Fig. 6d. The Ni0.2Mo0.8N/Fe–Ni3N/NF displays a smaller charge-transfer resistance (Rct), indicating that the Ni0.2Mo0.8N/Fe–Ni3N/NF exhibits enhanced charge-transfer ability. The outstanding charge-transfer ability of electrocatalysts for HER lead to favorable electrocatalytic kinetics. Some recently reported HER electrocatalyst was summarized in Table S2,† the overpotential of Ni0.2Mo0.8N/Fe–Ni3N/NF can be comparable. It is noted that the outstanding HER performance of Ni0.2Mo0.8N/Fe–Ni3N/NF could be ascribed to the strong interactions between Fe–Ni3N and Ni0.2Mo0.8N/NF phases.
The durability of Ni0.2Mo0.8N/Fe–Ni3N/NF toward HER was tested at a potential of 74 mV vs. RHE. After HER chronopotentiometry test for 50 h, FE-SEM image (Fig. 6e) shows that the hierarchical and two-dimensional features of the Ni0.2Mo0.8N/Fe–Ni3N/NF remain the same after the HER stability test. The current density of Ni0.2Mo0.8N/Fe–Ni3N/NF retain 95% (Fig. 6e). The LSV curves (Fig. 6f) of Ni0.2Mo0.8N/Fe–Ni3N/NF after HER stability tests both show slight difference, suggesting the excellent stability for HER in alkaline condition.
The peak for Ni0.2Mo0.8N phases can be observed from the Fig. S9.† In XPS survey, the signal of N was observed (Fig. S10†). By analyzing the Fig. 7a, the peaks of Ni3+ (875.5 and 856.4 eV), Ni2+ (873.1 and 855.4 eV) and Ni–N bonds (870.0 and 852.6 eV) can be observed.45 For the Fe 2p XPS spectra (Fig. 7b), the peaks of Fe3+ (723.9 and 711.8 eV) and Fe0 (720.6 and 706.6 eV) still exist.52 Furthermore, Mo 3d (Fig. 7c) and N 1s (Fig. 7d) XPS spectra still show the existence of Mo6+, Mo4+, Mo3+ and N–H, metal–N, Mo–N.49 XRD pattern and XPS spectra indicated that the chemical compositions of sample were almost unchanged, revealing the superior stability of Ni0.2Mo0.8N/Fe–Ni3N/NF in HER process.
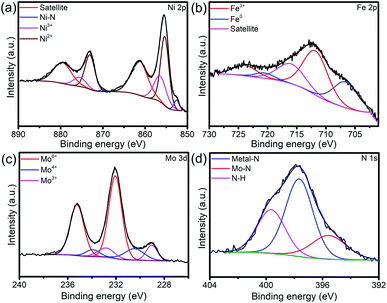 | ||
| Fig. 7 (a) Ni 2p, (b) Fe 2p, (c) Mo 3d and (d) N 1s XPS spectra of Ni0.2Mo0.8N/Fe–Ni3N/NF after HER stability test for 50 h. | ||
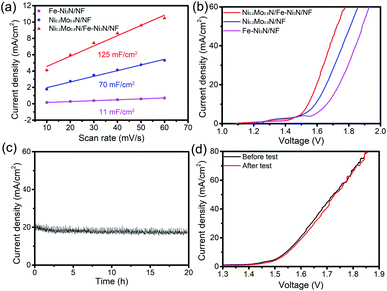
The ECSA of various electrocatalysts were roughly obtained by the Cdl. The Cdl were measured through CVs (Fig. S11†). In Fig. 8a, the Cdl of the Ni0.2Mo0.8N/Fe–Ni3N/NF, Fe–Ni3N/NF and Ni0.2Mo0.8N/NF were 129, 11 and 70 mF cm−2, respectively. The ECSA of the Ni0.2Mo0.8N/Fe–Ni3N/NF, Fe–Ni3N/NF and Ni0.2Mo0.8N/NF was 1612.5 cm2, 137.5 cm2 and 875.0 cm2, respectively. The bigger Cdl and ECSA of Ni0.2Mo0.8N/Fe–Ni3N/NF demonstrates that the integration of the small Ni0.2Mo0.8N nanosheets on Fe–Ni3N nanosheets can expose abundant catalytically active site.
Considering the excellent performance of OER and HER and practical applications of the material, Ni0.2Mo0.8N/Fe–Ni3N/NF was used as the bifunctional electrocatalysts for overall waters splitting. Fig. 8b shows the overall water splitting polarization curves of electrocatalysts. The Ni0.2Mo0.8N/Fe–Ni3N/NF only requires a potential of 1.54 V to achieve a current density of 10 mA cm−2, which is much smaller than that of Fe–Ni3N/NF (1.67 V) and Ni0.2Mo0.8N/NF (1.59 V). Furthermore, the Ni0.2Mo0.8N/Fe–Ni3N/NF bifunctional electrocatalyst shows strong stability, which displayed small degradation after stability test for 20 h (Fig. 8c). The Ni0.2Mo0.8N/Fe–Ni3N/NF electrode after stability test only need 1.55 V (10 mA cm−2) (Fig. 8d), which further demonstrate its excellent durability.
Conclusions
In summary, we have synthesized a hierarchical, well-organized nanostructure with small Ni0.2Mo0.8N nanosheets grown on the Fe–Ni3N nanosheets. Compared with the individual Fe–Ni3N/NF and Ni0.2Mo0.8N/NF, the integrated Ni0.2Mo0.8N/Fe–Ni3N/NF exhibits favorable electrical conductivity, resulting in exceptional performance of OER and HER in alkaline conditions. The experimental results indicated that Ni0.2Mo0.8N/Fe–Ni3N/NF exhibit superior stability during the OER and HER process. The remarkable electrocatalytic performance and durability of the Ni0.2Mo0.8N/Fe–Ni3N/NF can be attributed to the optimization of electronic properties under the interaction of Fe–Ni3N nanosheets and small Ni0.2Mo0.8N nanosheets. Benefit from the excellent chemical performance, stability, and higher ECSA of Ni0.2Mo0.8N/Fe–Ni3N/NF, the as-synthesis material can be used as the efficient electrocatalyst for overall water-splitting in alkaline conditions.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This study was supported by the National Natural Science Foundation of China (NSFC) (grant no. 51803077, 52073124), Natural Science Foundation of Jiangsu Province (grant no. BK20180627), Postdoctoral Science Foundation of China (2018M630517 and, 2019T120389), the MOE & SAFEA, 111 Project (B13025), the National First-Class Discipline Program of Light Industry Technology and Engineering (LITE2018-19), and the Fundamental Research Funds for the Central Universities (JUSRP221017).Notes and references
- P. Kuang, M. Sayed, J. Fan, B. Cheng and J. Yu, Adv. Energy Mater., 2020, 10, 1903802 CrossRef CAS.
- J. Cai, J. Shen, X. Zhang, Y. H. Ng, J. Huang, W. Guo, C. Lin and Y. Lai, Small Methods, 2019, 3, 1800184 CrossRef.
- G. Zhao, K. Rui, S. X. Dou and W. Sun, Adv. Funct. Mater., 2018, 28, 1803291 CrossRef.
- P. Li and H. C. Zeng, ACS Appl. Mater. Interfaces, 2019, 11, 46825–46838 CrossRef CAS PubMed.
- Q. Lu, Y. Yu, Q. Ma, B. Chen and H. Zhang, Adv. Mater., 2016, 28, 1917–1933 CrossRef CAS PubMed.
- M. Gong, D. Y. Wang, C. C. Chen, B. J. Hwang and H. Dai, Nano Res., 2016, 9, 28–46 CrossRef CAS.
- B. You and Y. Sun, Acc. Chem. Res., 2018, 51, 1571–1580 CrossRef CAS PubMed.
- S. Anantharaj, S. R. Ede, K. Karthick, S. S. Sankar, K. Sangeetha, P. E. Karthikc and S. Kundu, Energy Environ. Sci., 2018, 11, 744–771 RSC.
- J. Duan, S. Chen and C. Zhao, Nat. Commun., 2017, 8, 1–7 CrossRef PubMed.
- J. Hou, Y. Wu, B. Zhang, S. Cao, Z. Li and L. Sun, Adv. Funct. Mater., 2019, 29, 1808367 CrossRef.
- H. Zhu, J. Zhang, R. Yanzhang, M. Du, Q. Wang, G. Gao, J. Wu, G. Wu, M. Zhang, B. Liu, J. Yao and X. Zhang, Adv. Mater., 2015, 27, 4752–4759 CrossRef CAS PubMed.
- C. Zhu, A. L. Wang, W. Xiao, D. Chao, X. Zhang, N. H. Tiep, S. Chen, J. Kang, X. Wang, J. Ding, J. Wang, H. Zhang and H. J. Fan, Adv. Mater., 2018, 30, 1705516 CrossRef PubMed.
- X. Du, J. Huang, J. Zhang, Y. Yan, C. Wu, Y. Hu, C. Yan, T. Lei, W. Chen, C. Fan and J. Xiong, Angew. Chem., Int. Ed., 2019, 58, 4484–4502 CrossRef CAS PubMed.
- Y. Lin, Y. Pan, S. Liu, K. Sun, Y. Cheng, M. Liu, Z. Wang, X. Li and J. Zhang, Appl. Catal., B, 2019, 259, 118039 CrossRef CAS.
- J. B. Tan and G. R. Li, J. Mater. Chem. A, 2020, 8, 14326–14355 RSC.
- X. Liang, B. Zheng, L. Chen, J. Zhang, Z. Zhuang and B. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 23222–23229 CrossRef CAS PubMed.
- Q. Liang, L. Zhong, C. Du, Y. Luo, J. Zhao, Y. Zheng, J. Xu, J. Ma, C. Liu, S. Li and Q. Yan, ACS Nano, 2019, 13, 7975–7984 CrossRef CAS PubMed.
- G. Ou, P. Fan, H. Zhang, K. Huang, C. Yang, W. Yu, H. Wei, M. Zhong, H. Wu and Y. Li, Nano Energy, 2017, 35, 207–214 CrossRef CAS.
- V. R. Jothi, R. Bose, H. Rajan, C. Jung and S. C. Yi, Adv. Energy Mater., 2018, 8, 1802615 CrossRef.
- Y. Guo, T. Park, J. W. Yi, J. Henzie, J. Kim, Z. Wang, B. Jiang, Y. Bando, Y. Sugahara, J. Tang and Y. Yamauchi, Adv. Mater., 2019, 31, 1807134 CrossRef PubMed.
- J. Yin, J. Jin, H. Lin, Z. Yin, J. Li, M. Lu, L. Guo, P. Xi, Y. Tang and C. H. Yan, Adv. Sci., 2020, 7, 1903070 CrossRef CAS PubMed.
- J. Huang, Y. Jiang, T. An and M. Cao, J. Mater. Chem. A, 2020, 8, 25465–25498 RSC.
- S. Anantharaj, S. Kundu and S. Noda, J. Mater. Chem. A, 2020, 8, 4174–4192 RSC.
- W. Chen, X. Hou, X. Shi and H. Pan, ACS Appl. Mater. Interfaces, 2018, 10, 35289–35295 CrossRef CAS PubMed.
- X. Y. Yu, Y. Feng, B. Guan, X. W. Lou and U. Paik, Energy Environ. Sci., 2016, 9, 1246–1250 RSC.
- G. Zhang, G. Wang, Y. Liu, H. Liu, J. Qu and J. Li, J. Am. Chem. Soc., 2016, 138, 14686–14693 CrossRef CAS PubMed.
- H. Zhang, A. W. Maijenburg, X. Li, S. L. Schweizer and R. B. Wehrspohn, Adv. Funct. Mater., 2020, 30, 2003261 CrossRef CAS.
- Y. Wang, D. Liu, Z. Liu, C. Xie, J. Huo and S. Wang, Chem. Commun., 2016, 52, 12614–12617 RSC.
- Z. Liu, H. Tan, D. Liu, X. Liu, J. Xin, J. Xie, M. Zhao, L. Song, L. Dai and H. Liu, Adv. Sci., 2019, 6, 1801829 CrossRef PubMed.
- N. Han, P. Liu, J. Jiang, L. Ai, Z. Shao and S. Liu, J. Mater. Chem. A, 2018, 6, 19912–19933 RSC.
- C. C. Yang, S. F. Zai, Y. T. Zhou, L. Du and Q. Jiang, Adv. Funct. Mater., 2019, 29, 1901949 CrossRef.
- J. Jiang, Q. Liu, C. Zeng and L. Ai, J. Mater. Chem. A, 2017, 5, 16929–16935 RSC.
- J. Chen, B. Ren, H. Cui and C. Wang, Small, 2020, 16, 1907556 CrossRef CAS PubMed.
- X. Peng, Y. Yan, X. Jin, C. Huang, W. Jin, B. Gao and P. K. Chu, Nano Energy, 2020, 78, 105234 CrossRef CAS.
- Z. Zou, X. Wang, J. Huang, Z. Wu and F. Gao, J. Mater. Chem. A, 2019, 7, 2233–2241 RSC.
- F. Ming, H. Liang, H. Shi, X. Xu, G. Mei and Z. Wang, J. Mater. Chem. A, 2016, 4, 15148–15155 RSC.
- C. Ray, S. C. Lee, B. Jin, A. Kundu, J. H. Park and S. C. Jun, J. Mater. Chem. A, 2018, 6, 4466–4476 RSC.
- J. Jia, M. Zhai, J. Lv, B. Zhao, H. Du and J. Zhu, ACS Appl. Mater. Interfaces, 2018, 10, 30400–30408 CrossRef CAS PubMed.
- Y. Gu, S. Chen, J. Ren, Y. A. Jia, C. Chen, S. Komarneni, D. Yang and X. Yao, ACS Nano, 2018, 12, 245–253 CrossRef CAS PubMed.
- L. Zhou, S. Jiang, Y. Liu, M. Shao, M. Wei and X. Duan, ACS Appl. Energy Mater., 2018, 1, 623–631 CrossRef CAS.
- S. Sirisomboonchai, S. Li, A. Yoshida, X. Li, C. Samart, A. Abudula and G. Guan, ACS Sustainable Chem. Eng., 2019, 7, 2327–2334 CrossRef CAS.
- K. He, T. T. Tsega, X. Liu, J. Zai, X. H. Li, X. Liu, W. H. Li, N. Ali and X. Qian, Angew. Chem., Int. Ed., 2019, 58, 11903–11909 CrossRef CAS PubMed.
- Y. Zhou, Z. Wang, Z. Pan, L. Liu, J. Xi, X. Luo and Y. Shen, Adv. Mater., 2019, 31, 1806769 CrossRef PubMed.
- C. Tang, R. Zhang, W. Lu, L. He, X. Jiang, A. M. Asiri and X. Sun, Adv. Mater., 2017, 29, 1602441 CrossRef PubMed.
- Q. Zhang, D. Yan, Z. Nie, X. Qiu, S. Wang, J. Yuan, D. Su, G. Wang and Z. Wu, ACS Appl. Energy Mater., 2018, 1, 571–579 CrossRef CAS.
- Z. Wu, Z. Zou, J. Huang and F. Gao, J. Catal., 2018, 358, 243–252 CrossRef CAS.
- J. Lai, B. Huang, Y. Chao, X. Chen and S. Guo, Adv. Mater., 2019, 31, 1805541 CrossRef PubMed.
- L. Yu, Q. Zhu, S. Song, B. McElhenny, D. Wang, C. Wu, Z. Qin, J. Bao, Y. Yu, S. Chen and Z. Ren, Nat. Commun., 2019, 10, 1–10 CrossRef PubMed.
- A. Wu, Y. Xie, H. Ma, C. Tian, Y. Gu, H. Yan, X. Zhang, G. Yang and H. Fu, Nano Energy, 2018, 44, 353–363 CrossRef CAS.
- Z. Wang, P. Guo, S. Cao, H. Chen, S. Zhou, H. Liu, H. Wang, J. Zhang, S. Liu, S. Wei, D. Sun and X. Lu, Appl. Catal., B, 2021, 284, 119725 CrossRef CAS.
- Y. Wang, C. Xie, D. Liu, X. Huang, J. Huo and S. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 18652–18657 CrossRef CAS PubMed.
- Z. Liu, H. Tan, J. Xin, J. Duan, X. Su, P. Hao, J. Xie, J. Zhan, J. Zhang, J. J. Wang and H. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 3699–3706 CrossRef CAS PubMed.
- C. Tang, H. Zhang, K. Xu, Q. Zhang, J. Liu, C. He, L. Fan and T. Asefa, J. Mater. Chem. A, 2019, 7, 18030–18038 RSC.
- Z. Yin, Y. Sun, Y. Jiang, F. Yan, C. Zhu and Y. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 27751–27759 CrossRef CAS PubMed.
- Z. Cai, D. Zhou, M. Wang, S. M. Bak, Y. Wu, Z. Wu, Y. Tian, X. Xiong, Y. Li, W. Liu, S. Siahrostami, Y. Kuang, X. Q. Yang, H. Duan, Z. Feng, H. Wang and X. Sun, Angew. Chem., Int. Ed., 2018, 57, 9392–9396 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra01299a |
| This journal is © The Royal Society of Chemistry 2021 |