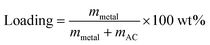Open Access Article
Open Access ArticleA facile synthesis of high entropy alloy nanoparticle–activated carbon nanocomposites for synergetic degradation of methylene blue
Yuyu Liua,
Zheng Chen *a,
Xiaoqin Yanga,
Jinyong Zhang
*a,
Xiaoqin Yanga,
Jinyong Zhang a,
Zhonggang Sunb,
Yuzeng Chenc and
Feng Liuc
a,
Zhonggang Sunb,
Yuzeng Chenc and
Feng Liuc
aSchool of Material Science and Engineering, China University of Mining and Technology, Xuzhou, Jiangsu 221008, China. E-mail: chenzheng1218@163.com; xiaoqinyang0530@163.com; Fax: +86 516 83591870; Tel: +86 516 83897715
bTech Institute for Advanced Materials, College of Materials Science and Technology, Nanjing Tech University, Nanjing, 210009, China
cState Key Laboratory of Solidification Processing, Northwestern Polytechnical University, Xi'an, Shaanxi 710072, China
First published on 14th July 2021
Abstract
Nanocomposites of CoCrFeMnNi high entropy alloy nanoparticle–activated carbon (HEA NPs–AC) were prepared by a facile and controllable impregnation–adsorption method. The HEA NPs–AC showed excellent catalytic performance in the degradation of methylene blue (MB) without any peroxide addition. Besides, their reaction rate is also competitive among single-element and other catalysts. The outstanding efficiency is attributed to the coupling effects of the solid-solution structure of HEA NPs, and the large specific surface area and substantial reaction channels of AC. Moreover, the HEA NPs embedded in distinctive porous architectures accelerate the electron transfer and the mass transport as nanoscale galvanic cells in active bond breaking of MB. The nanocomposites of HEA NPs–AC are distinguished by containing non-noble metals and having high catalytic performance due to the synergetic degradation, providing a better alternative for efficient metal catalysis.
1. Introduction
High entropy alloys (HEAs) are composed of equiatomic or near-equiatomic mixing of multiple elements rather than the conventional single major composition. Tremendous advances have been achieved since this new class of metallic material was proposed in the last decade.1–5 Recently, high entropy alloy nanoparticles (HEA NPs) have become one of the research frontiers for overcoming the difficulty of mixing elements with vastly different chemical and physical properties.6 It is creative to downsize bulk HEAs to HEA NPs which shows greatly prospective candidates for a wide range of applications in catalysis,7,8 energy storage,9 superparamagnetic and superconducting materials.10 Löffler et al.11 have reported that a surprisingly high intrinsic activity of the multinary CrMnFeCoNi NPs is at least comparable to that of Pt NPs under the same conditions. Furthermore, HEA NPs prepared by the carbothermal shock method can realize 100% conversion of ammonia and ultrahigh selectivity toward nitrogen oxide.6 These encouraging works reveal remarkable catalytic performance of HEA NPs, which inspires us to broaden its application for synthetic dye degradation.Currently, the methods of azo dye degradation are various including photocatalysis, redox reduction, and Fenton-like reaction. Visible light photocatalysis could offer an eco-friendly alternative for the selective transformation of organic molecules.12 Photocatalysis is generally supposed to be the catalysis of a photochemical reaction at a solid surface, especially a semiconductor such as TiO2, BaTiO3, Ag2O, etc.13–16 The factor of light is indispensable for titanium dioxide via photocatalytic decomposition of pollutants due to photogenerated holes and photogenerated electrons. Han et al. developed an alveoli-like bilayer Janus membrane as a highly efficient dye-sensitized photocatalyst with fluorescence peak at around 682 nm for water purification under visible light irradiation and photosensitizer decoration for tetracycline was up to 36%.17 The methods of redox reduction and Fenton-like reaction for azo dye degradation are different from photocatalysis due to the insensitivity of HEA powders, Fe-based metallic glasses, and magnetite nanoparticles to solar light. Fe-based metallic glasses have been firstly investigated for dyes decomposition and mineralization via Fenton-like process due to its far-from-equilibrium states, chemical homogeneity, and random atomic packing.18,19 However, highly efficient advanced oxidation processes are limited by many parameters, such as strong acid environments and peroxides that may induce secondary contamination. What's more, ball-milled HEA powders present good performance in the decoloration of azo dye (Direct Blue 6) as well,20,21 while the difficulty in recovering the powder seriously limits its industrial application. Nevertheless, porous carbon shows particularly distinguishing features with network geometry, co-existing of microstructure and mesostructure, high specific surface area, and convenience for nitrogen-doping.22–24 HEA NPs loaded on carbon nanostructures have attracted growing attention due to its distinctive structures and extensive application prospects in energy conversion and storage, and heterogeneous catalysis.25,26
In this work, a facile impregnation–adsorption method was utilized to fabricate noble metal-free CoCrFeMnNi HEA NPs and single-element NPs loaded on activated carbon (AC). The lattice structure and specific surface area of HEA NPs–AC were discussed in detail. In addition, the microstructure, morphology and electronic valence state were featured to reveal the stability and activity of NPs. To evaluate catalytic performance, the as-prepared nanocomposites were used for synthetic dye degradation via redox process to cleave active bonds. Finally, the mechanism of dye degradation and possible fracture pathways of active bonds were illustrated.
2. Experimental
The nanocomposites of HEA NPs–AC were fabricated by a simple impregnation–adsorption method just via the precursor solution absorption and calcination. The initial precursor included cobalt nitrate hexahydrate (Co(NO3)2·6H2O), chromic nitrate nonahydrate (Cr(NO3)2·9H2O), manganese nitrate tetrahydrate (Mn(NO3)2·4H2O), Aluminum nitrate nonahydrate (Al(NO3)3·9H2O), ferric nitrate nonahydrate (Fe(NO3)3·9H2O), nickel nitrate hexahydrate (Ni(NO3)2·6H2O), and ethanol (AR grade), which were employed to prepare precursor solution. Commercially available AC was firstly crushed to 20–40 mesh and then cleaned with ultrasonic shock in deionized water, followed by filtration and drying at 323 K for 36 h as carbon supports. The quantitative metal-nitrates of CoCrFeMnNi were respectively dissolved in 50 mL ethanol as precursor solutions that were designed for equal atomic ratio, while the total theoretical loading of AC was various (3 wt%, 5 wt%, and 10 wt%):where mmetal is the mass of loaded HEA, mAC is the mass of AC. The washed AC (3 g) was dipped into the precursor solutions of mixed metal salts for 4 h. Then excess ethanol was removed by rotary evaporator at 313 K for 40 min and the obtained AC was left to dry at room temperature. The ethanol-based precursor solution benefited uniform loading and particle dispersion as a result of favorable wettability to carbon. The precursor-loaded AC was placed into the quartz tube of the furnace and then the furnace was pumped into the vacuum. Protective gas (volume ratio of Ar
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2, 95%
H2, 95%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5%) was subsequently introduced into the furnace with heating to 1273 K for 3 h, and then cooling to room temperature.
5%) was subsequently introduced into the furnace with heating to 1273 K for 3 h, and then cooling to room temperature.
The Brunauer–Emmett–Teller (BET) surface area analysis of the catalysts was performed using the nitrogen adsorption method (ASAP2020Plus). Microstructural morphologies of the samples were characterized by a scanning electron microscope (SEM, Hitachi SU8220) and a high-resolution transmission electron microscope (HRTEM, Tecnai G2 F20). Compositional information and EDS maps of the microstructures were measured in a scanning transmission electron microscope equipped with high angle annular dark field detectors (HAADF-STEM, FEI Talos F200s TEM/STEM). Chemical composition and valence state of the samples were characterized using an X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi). Atomic structure and phase were revealed by X-ray diffractometer (XRD, Bruker D8 ADVANCE) with Cu Kα radiation operating at 40 kV and 40 mA. High-resolution mass spectra (HRMS, Q EXTRACTIVE) was employed to detect functional groups of dye solution after degradation. The total organic carbon (TOC) results of MB dye mineralization were obtained by a TOC analyzer (TOC-5000, Shimadzu). Fourier transform infrared spectroscopy (FT-IR, Bruker Vertex 80v) of CoCrMnFeNi NPs/AC before and after decomposition were recorded in the spectral range 4000 to 500 cm−1.
The as-prepared nanocomposites of HEA NPs–AC were added into methylene blue (MB, C16H18ClN3S·3H2O, AR grade) solution of 100 mL (catalyst dosage of 4 g L−1, dye concentration of 200 ppm, and constant temperature of 298 K, if not specifically mentioned). In the process of degradation, the reaction solution was stirred at a fixed speed and taken out of 2 mL at a selected time. The sucked solutions were centrifuged at a speed of 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and then determined by ultraviolet visible spectrophotometer (UV-vis) to obtain the absorbance spectrum.
000 rpm and then determined by ultraviolet visible spectrophotometer (UV-vis) to obtain the absorbance spectrum.
3. Results and discussion
3.1 Microstructure of the CoCrFeMnNi HEA NPs–AC
XRD patterns of the CoCrFeMnNi HEA NPs–AC with different loadings are shown in Fig. 1. The result of AC demonstrates that there are broad and amorphous diffraction peaks at 22°–26° and 42°–45° with (002) planes (2θ = 26.5°). The sharply crystallized peaks are the HEA NPs. The intensity of peaks heightens gradually with loading from 3 wt% to 10 wt% in Fig. 1. The crystalline peak (2θ = 43.7°) assigned to (111) plane without splitting is the multicomponent face-centered cubic (FCC) phase for 10 wt% CoCrFeMnNi HEA NPs–AC. The peaks at 50.9° and 74.9° correspond to (200) and (220), respectively.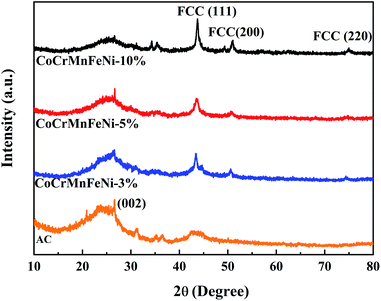 | ||
| Fig. 1 XRD patterns of CoCrFeMnNi HEA NPs–AC with different loadings (0 wt%, 3 wt%, 5 wt%, and 10 wt%). | ||
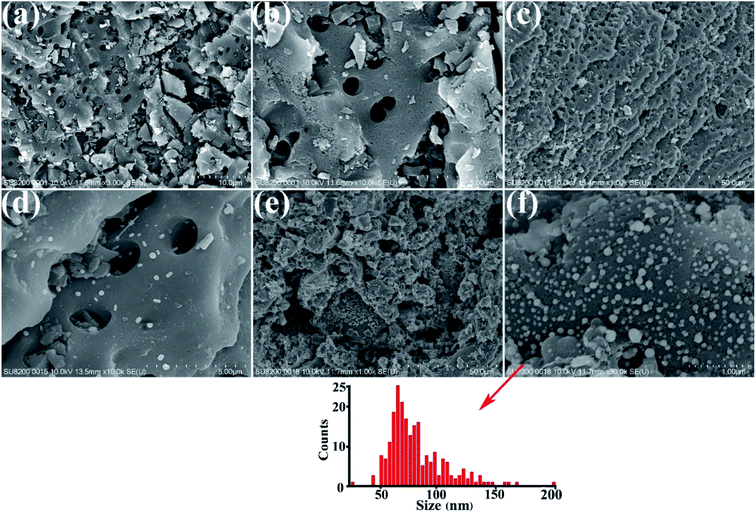
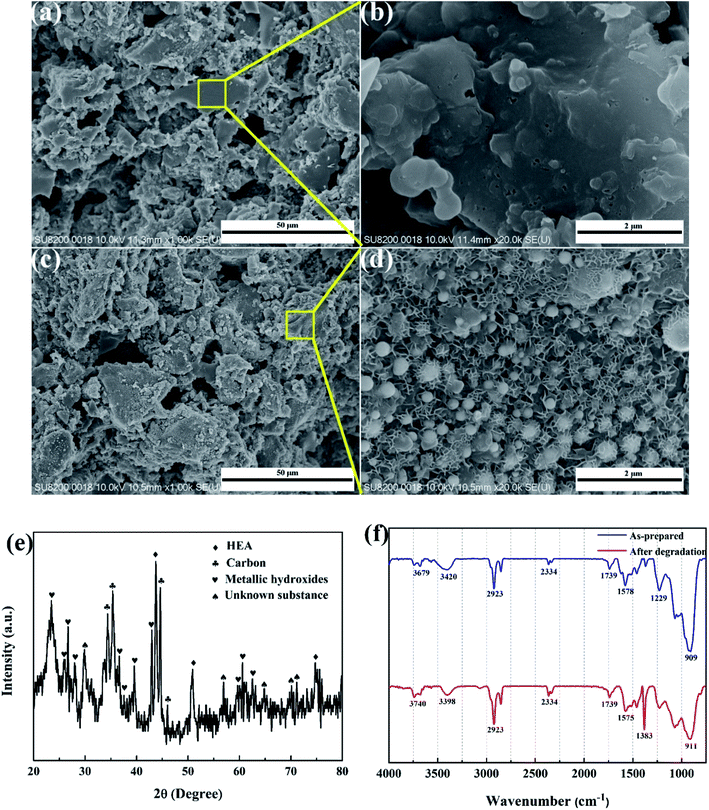
SEM images of the HEA NPs–AC and AC are exhibited in Fig. 2. Fig. 2a and b display a smooth surface of non-loaded AC with no impurity substance and few AC exfoliations after high temperature calcination. The circular-like macropores are abundantly distributed on the surface and form a developed porosity framework. The microstructure of 3 wt% sample begins to change with NPs appearing on the surface and the macropores channels in Fig. 2c and d. Nevertheless, Fig. 2e and f show that the morphology of pores seems to collapse and merge into unlike the former and large amounts of CoCrFeMnNi HEA NPs uniformly disperse with an average diameter of 89 nm in the inset of the statistical size distribution. Specific surface area of different loadings for CoCrFeMnNi NPs–AC are shown in Fig. 3. The SBET of 3–10 wt% samples is lower than that of AC (968.48 m2 g−1) and decreases with the increase of loading because the reduced SBET provides more space for the growth of the HEA NPs. Though the loading of the HEA NPs leads to the pore collapse of AC and reduction of SBET, the collapses facilitate the formation of massive reaction channels, which provides an effect route of reactants and dye molecule contacting the HEA NPs.27 It also improves catalytic efficiency through an effective pre-adsorption of organic molecules. Satisfied catalytic performance demands large specific surface area and massive active sites of the HEA NPs.
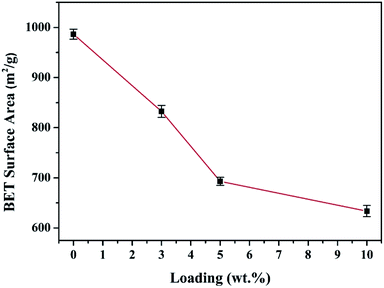 | ||
| Fig. 3 Comparison of the measured BET specific surface area (m2 g−1) with different loadings of the CoCrFeMnNi NPs–AC. | ||
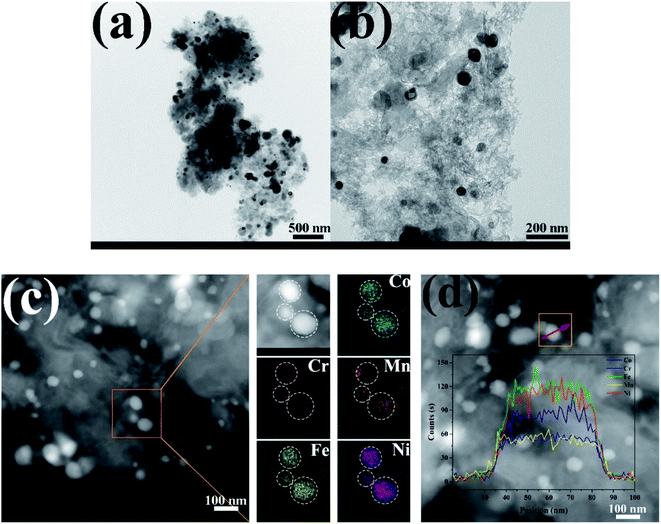
TEM images of the CoCrFeMnNi HEA NPs are illustrated in Fig. 4. The inter-planar spacing of the lattice planes is 0.207 nm in Fig. 4b, which is in agreement with the (111) plane of FCC metal crystal phase (Fig. 1). The inter-planar spacing is measured to be 0.335 nm corresponding to (002) plane of carbon. The yellow line in Fig. 4c mainly emphasizes long-range disordering of carbon which conforms to the broad peak in Fig. 1 and amorphous diffuse rings in Fig. 4d. Different region outlined by yellow line shows different orientation that means many defects. XRD patterns are characterized with intrinsic feature and the same situation as described in Fig. 4c is common. SAED image in Fig. 4d shows amorphous diffuse rings of carbon and FCC structure of CoCrFeMnNi HEA with (012) zone axis.
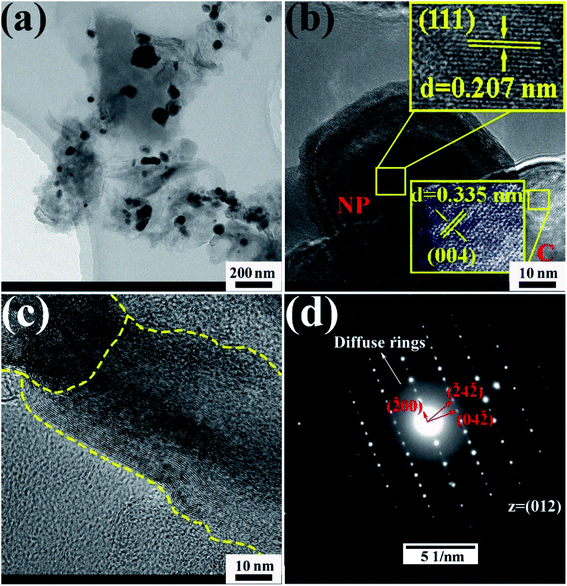 | ||
| Fig. 4 (a–c) HRTEM images of 10 wt% CoCrFeMnNi HEA NPs–AC with different magnification, (d) SAED pattern. | ||
3.2 Chemical composition of the CoCrFeMnNi HEA NPs
In order to obtain the information of electronic structure for transition metal HEA NPs, XPS spectra and characteristic peaks of the individual element are presented in Fig. 5. The peaks of C1s, O1s, Co2p, Cr2p, Mn2p, Fe2p and Ni2p are observed in full range pattern. The binding energies of the CoCrFeMnNi NPs at 778.2 eV, 574.3 eV, 638.7 eV, 706.7 eV and 852.68 eV are characterized for Co, Cr, Mn, Fe, and Ni, which reveals that quinary metal elements are in zero valence state in Fig. 5. While XPS results also show few fitting peaks of mixed metallic bonding and slightly oxidized bonding states due to inadequate reduction reaction and oxidation in the air, especially for the non-noble metals.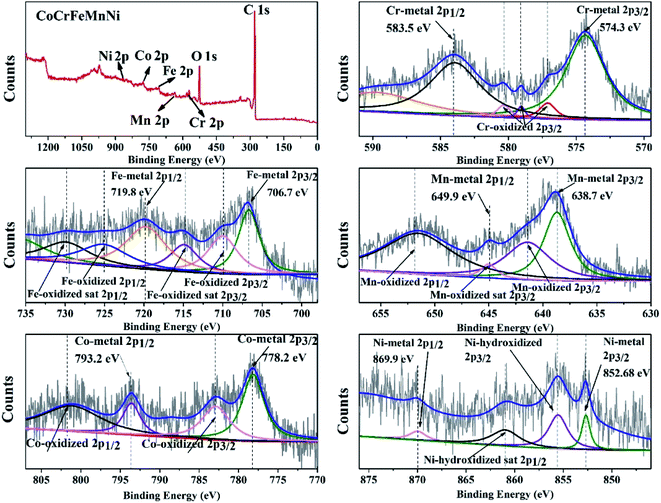 | ||
| Fig. 5 Characteristic XPS peaks of the10 wt% CoCrFeMnNi HEA NPs with individual element of Cr, Fe, Mn, Co and Ni. | ||
As displayed in Fig. 6, the morphology and elemental maps of the CoCrFeMnNi NPs were featured by HAADF-STEM. The as-synthesized HEA NPs in Fig. 6a and b are nearly spherical in shape dispersing on the carbon supports. STEM EDS elemental maps of individual HEA NP reveal that the distribution of elements is uniform while the counterpart of Cr and Mn is relatively less due to the low vapor pressure. The quantificational composition ratio of CoCrFeMnNi NPs for individual element are 17.08 at%, 9.77 at%, 29.15 at%, 15.75 at% and 28.25% respectively in Fig. 6c. EDS line-scanning result in Fig. 6d for a another HEA NP visually displays the elemental distribution that is roughly same as Fig. 6c.
3.3 Degradation performance of the as-prepared nanocomposites of HEA NPs–AC
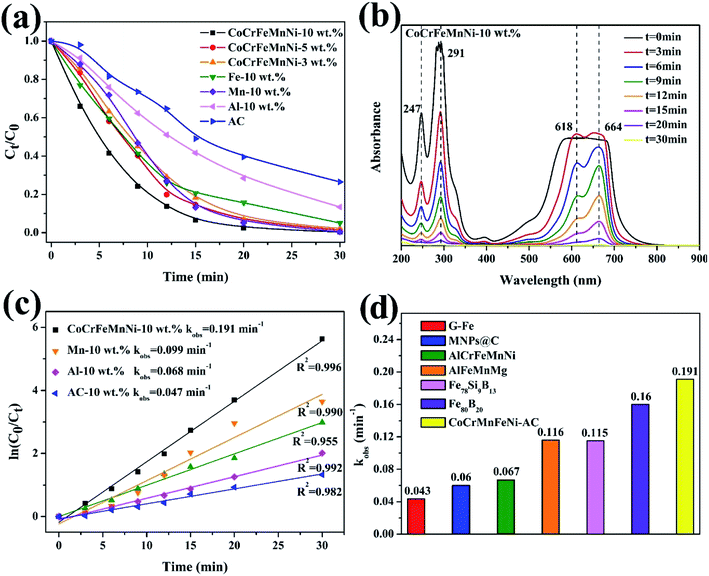
Normalized concentrations of the MB solution are obtained (depending on the chromogenic group peak at 291 nm) as shown in Fig. 7a. According to UV-vis absorbance results, it takes twelve minutes for 10 wt% CoCrFeMnNi NPs–AC to reduce dye concentration to less than 15% via redox reduction, while AC as catalyst shows an almost linear and sluggish adsorption curve. The decomposition efficiency of single-element NPs for Mn, Fe, and Al is slower than that of multicomponent catalysts owing to the lack of high entropy effects. Furthermore, the increase of loading (from 3 wt% to 10 wt%) can result in more generation of the NPs and consequent more exposure of active sites to accelerate degradation. The UV-vis spectrums in Fig. 7b demonstrate the effective degradation of the MB, the breakage and conversion of chemical bonds over loaded and non-loaded catalysts compared with AC. Four absorption peaks are observed at 247 nm, 291 nm, 618 nm and 664 nm approximately in MB solutions. The peaks at 247 nm and 291 nm stem from triazine groups. Besides, auxochrome and chromophore groups are the other two peaks at 618 nm and 664 nm.28The dye degradation efficiency of an environmental catalyst is reflected by the value of reaction. The comparable results about other catalysts for decomposition are summarized in Table 1. The reaction rates (kobs) can be fitted by the pseudo-first-order kinetic model, ln(C0/Ct) = kobst, where C0 is the initial concentration of dye at t = 0 and Ct is the dye concentration at time t. Fig. 7c shows that the order of decomposition efficiency for these catalysts is as follow: CoCrFeMnNi > Fe > Mn > Al > AC. The comparison of the reaction rates (kobs) among zero value metals, MNPs@C, ball-milled HEA, and metallic glass indicates that CoCrFeMnNi NPs–AC have surprisingly high intrinsic activity and competitive catalytic performance in Fig. 7d and Table 1. Deekshitha et al. prepared AgO/Ag2O@TiO2 with a reduction of Photoluminescence intensity on decorating TiO2@Ag2O-nanoparticle to degrade Reactive Blue 220 dye completely (100 ppm, 5 g L−1) under visible light irradiation in 60 min.14 Compared with photocatalysis, HEA NPs present superior catalytic performance as well.
| Catalysts | Organic dye | Concentration (ppm) | Dosage (g L−1) | kobs (min−1) | Ref. |
|---|---|---|---|---|---|
| G-Fe | Direct Blue 6 | 200 | 13.4 | 0.043 | 39 |
| MNPs@C | Industrial wastewater | 800 | 5 | 0.06 | 40 |
| AlCrFeMnNi | Direct Blue 6 | 200 | 0.5 | 0.067 | 21 |
| AlFeMnMg | Direct Blue 6 | 200 | 0.5 | 0.116 | 21 |
| Fe78Si9B13 | Acid orange II | 200 | 13.3 | 0.115 | 41 |
| Fe80B20 | Direct Blue 6 | 200 | 13.3 | 0.160 | 42 |
| CoCrFeMnNi–AC | Methylene blue | 200 | 4 | 0.191 | This work |
As shown in Fig. 8a and b, the non-loaded AC after degradation retains the analogously original morphological structure like Fig. 3a and b with no intermediates or decomposition products adhering to carbon supports. However, massive cotton-like sediments are around CoCrFeMnNi HEA NPs in Fig. 8c and d, which dramatically differ from those of carbon. The degradation products are similar to that of Fe-based amorphous powder and ball-milled HEA for organic dye degradation indicated by Wang et al. and Wu et al.21,29 XRD result in Fig. 8e remains FCC phase of HEA NPs and shows multiple metallic hydroxides which are generated by the combination of metallic ions and hydroxyl ions during degradation. The nanoscale surface features provided more active sites for the reaction to proceed and thus improved the degradation efficiency. Moreover, the surface of the HEA NPs uncovered by the decomposed products is still smooth without apparent corrosion pits, which demonstrates the stability and strong catalytic ability of the HEA NPs in the degradation process. The typical redox reaction is responsible for the zero valent metal degradation of dyes.30,31 However, HEA NPs are superior to conventional single or binary component in catalysis.
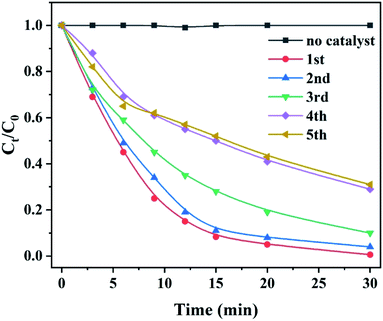
It is reported that the functional groups of MB around 2923 and 2852 cm−1 correspond to C–H stretching of –CH3 and –CH2. The absorption peaks around 1383 cm−1 are attributed to the presence of –COOH group.32 As shown in Fig. 8f, the intensity of the peaks at 2923 and 1383 cm−1 increases for the post-degradation sample due to a few undegraded and residual MB dye. The FT-IR spectra of other peaks remain consistent compared to as-prepared sample and post-degradation sample which means the stability of the catalyst. The sustainability and stability of the outstanding catalyst is a critically precious in environmental pollutants remediation. Fig. 9 demonstrates the reusability of 10 wt% CoCrFeMnNi HEA NPs–AC catalysts for MB degradation. The previous three cycles present satisfied decomposition performance and the efficiency of other cycles begins to decay but still sustained at nearly 75%.
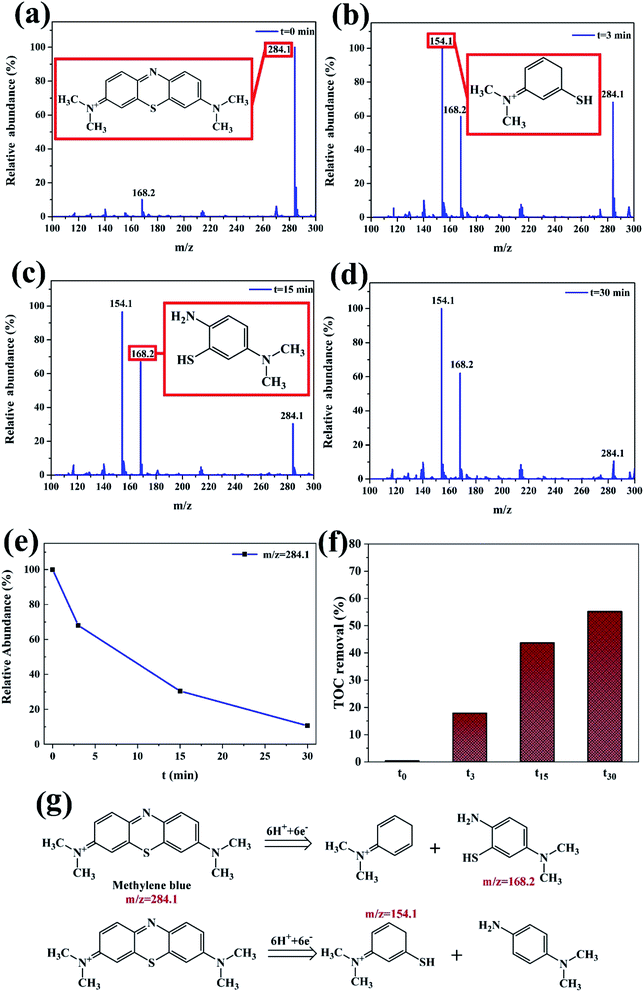
With the increase of reaction time, the macromolecule MB (m/z = 284.1) in Fig. 10a is firstly initiated by fracture of chromophore center, and then cracked into smaller molecules (m/z = 154.1, and 168.2) at different time in Fig. 10b–d, which are in agreement with the above UV-vis analysis in Fig. 7b. The relative abundance of m/z = 284.1 at different time gradually decreases with increasing degradation time in Fig. 10e. Two possible decomposition pathways of the MB are speculated, according to the m/z peaks of 154 and 168 as shown in Fig. 10g. The other two reaction intermediate products are not detected, probably due to that they are further decomposed into smaller molecules. It is also reported that the azo bond ‘–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–’ is reduced into ‘–NH2’ by reducing agents firstly, then hydrogen ions and electrons that combine with broken dye molecules are generated between metals.33 Moreover, the TOC removal of MB dye increases with degradation time and the TOC can be more than 50% at 30 min with nearly 100% decoloration in Fig. 10f.
N–’ is reduced into ‘–NH2’ by reducing agents firstly, then hydrogen ions and electrons that combine with broken dye molecules are generated between metals.33 Moreover, the TOC removal of MB dye increases with degradation time and the TOC can be more than 50% at 30 min with nearly 100% decoloration in Fig. 10f.
3.4 Synergetic degradation mechanism of the CoCrFeMnNi HEA NPs–AC
Fig. 11 shows the schematic diagram for catalytic reaction mechanism of the MB for CoCrFeMnNi HEA NPs–AC. The as-synthesized sample with convenient preparation, large specific surface area and uniform distribution are expected to have promising applications for environmental catalysts. Therefore, the noble metal-free HEA NPs and single-element NPs supported by carbon were employed as heterogeneous catalysts to evaluate catalytic performance of dye water degradation.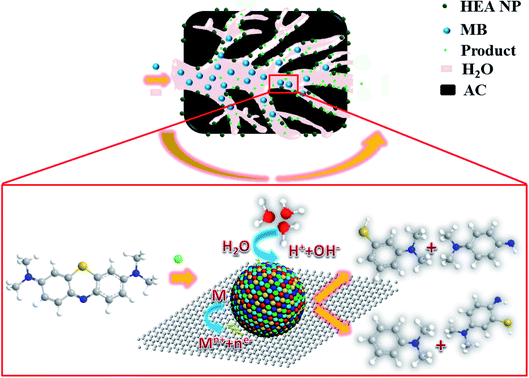 | ||
| Fig. 11 Schematic diagram for synthesis of the HEA NPs–AC and catalytic reaction mechanism of MB (M refers to HEA NP). | ||
4. Conclusion
The nanocomposites of the HEA NPs–AC were fabricated from precursor solution by a facile and scalable impregnation–adsorption method via thermal pyrolysis conditions for efficient dye degradation without peroxides. The main conclusions are as follows.1. The CoCrFeMnNi HEA NPs consist of the single FCC phase and uniformly disperse with average diameter of 89 nm. Specific surface area of the composites decreases with growing loading of HEA NPs. The combination of homogeneous phase formation with distinctive porous architectures provides solid foundation for preeminent catalytic.
2. Adding 10 wt% CoCrFeMnNi HEA NPs/AC, the time required to reduce MB concentration to less than 15% without the addition of peroxides can be as short as 12 minutes with the kobs of 0.191 min−1. Under the same condition (10 wt%), the order of decomposition efficiency for these catalysts is: CoCrFeMnNi > Fe > Mn > Al > AC. Meanwhile it is also competitive among other catalysts for dye degradation.
3. Large amounts of HEA NPs as nanoscale galvanic cells embedding in distinctive porous architectures have advantages in the decomposition of the MB dye by accelerating the electron transfer and the mass transport.
The nanocomposites of the HEA NPs–AC are distinguished by noble metal-free and high catalytic performance with porous nanostructure, achieving prospective performance in industrial catalytic applications due to the synergetic degradation. This work provides a novel approach to synthesize HEA NPs and expands its promising catalytic application as environmentally friendly materials.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for Fundamental Research Funds for the Central Universities (2019ZDPY12).References
- J. W. Yeh, S. K. Chen, S. J. Lin, J. Y. Gan, T. S. Chin, T. T. Shun, C. H. Tsau and S. Y. Chang, Adv. Eng. Mater., 2004, 6, 299–303 CrossRef CAS.
- B. Cantor, Entropy, 2014, 16, 4749–4768 CrossRef.
- Z. Lei, X. Liu, Y. Wu, H. Wang, S. Jiang, S. Wang, X. Hui, Y. Wu, B. Gault, P. Kontis, D. Raabe, L. Gu, Q. Zhang, H. Chen, H. Wang, J. Liu, K. An, Q. Zeng, T.-G. Nieh and Z. Lu, Nature, 2018, 563, 546 CrossRef CAS.
- Y. Lu, X. Gao, Y. Dong, T. Wang, H. L. Chen, H. Maob, Y. Zhao, H. Jiang, Z. Cao, T. Li and S. Guo, Nanoscale, 2018, 10, 1912–1919 RSC.
- Y. Lu, X. Gao, L. Jiang, Z. Chen, T. Wang, J. Jie, H. Kang, Y. Zhang, S. Guo, H. Ruan, Y. Zhao, Z. Cao and T. Li, Acta Mater., 2017, 124, 143–150 CrossRef CAS.
- Y. G. Yao, Z. N. Huang, P. F. Xie, S. D. Lacey, R. J. Jacob, H. Xie, F. J. Chen, A. M. Nie, T. C. Pu, M. Rehwoldt, D. W. Yu, M. R. Zachariah, C. Wang, R. Shahbazian-Yassar, J. Li and L. B. Hu, Science, 2018, 359, 1489–1494 CrossRef CAS PubMed.
- P. C. Chen, X. Liu, J. L. Hedrick, Z. Xie, S. Wang, Q.-Y. Lin, M. C. Hersam, V. P. Dravid and C. A. Mirkin, Science, 2016, 352, 1565–1569 CrossRef CAS.
- M. Takahashi, H. Koizumi, W.-J. Chun, M. Kori, T. Imaoka and K. Yamamoto, Sci. Adv., 2017, 3, e1700101 CrossRef PubMed.
- N. A. Frey, S. Peng, K. Cheng and S. H. Sun, Chem. Soc. Rev., 2009, 38, 2532–2542 RSC.
- Y. F. Ye, Q. Wang, J. Lu, C. T. Liu and Y. Yang, Mater. Today, 2015, 19, 349–362 CrossRef.
- T. Löffler, H. Meyer, A. Savan, P. Wilde, A. Garzón Manjón, Y.-T. Chen, E. Ventosa, C. Scheu, A. Ludwig and W. Schuhmann, Adv. Energy Mater., 2018, 8, 1802269 CrossRef.
- J. Zhou, X. Li, X. Ma, W. Sheng and X. Lang, Appl. Catal., B, 2021, 296, 120368 CrossRef CAS.
- A. Fujishima, X. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63, 515–582 CrossRef CAS.
- K. Deekshitha Vidya Shetty, Mater. Sci. Semicond. Process., 2021, 132, 105923 CrossRef.
- Y. Wang, N. Bi, H. Zhang, W. Tian, T. Zhang, P. Wu and W. Jiang, Colloids Surf., A, 2020, 585, 124105 CrossRef CAS.
- V. P. Singh, M. Sharma and R. Vaish, Mater. Chem. Phys., 2020, 252, 123311 CrossRef CAS.
- Z. Han, J. Fei, J. Li, Y. Deng, M. Lv, J. Zhao, C. Wang and X. Zhao, Chem. Eng. J., 2021, 407, 127214 CrossRef CAS.
- S. X. Liang, Z. Jia, W. C. Zhang, X. F. Li, W. M. Wang, H. C. Lin and L. C. Zhang, Appl. Catal., B, 2018, 221, 108–118 CrossRef CAS.
- S. H. Xie, P. Huang, J. J. Kruzic, X. R. Zeng and H. X. Qian, Sci. Rep., 2016, 6, 21947 CrossRef CAS.
- Z. Y. Lv, X. J. Liu, B. Jia, H. Wang, Y. Wu and Z. P. Lu, Sci. Rep., 2016, 6, 34213 CrossRef CAS.
- S. K. Wu, Y. Pan, J. Lu, N. Wang, W. J. Dai and T. Lu, J. Mater. Sci. Technol., 2019, 35, 1629–1635 CrossRef.
- X. Ke, X. Qin, X. Wang, Y. Wang, H. Tao, Q. Wu, L. Yang and Z. Hu, Adv. Mater., 2012, 24, 347–352 CrossRef PubMed.
- Z. Y. Lyu, D. Xu, L. J. Yang, R. C. Che, R. Feng, J. Zhao, Y. Li, Q. Wu, X. Z. Wang and Z. Hu, Nano Energy, 2015, 12, 657–665 CrossRef CAS.
- J. Zhao, H. Lai, Z. Lyu, Y. Jiang, K. Xie, X. Wang, Q. Wu, L. Yang, Z. Jin and Y. Ma, Adv. Mater., 2015, 27, 3541–3545 CrossRef CAS.
- Z. Y. Jin, J. Lv, H. L. Jia, W. H. Liu, H. L. Li, Z. H. Chen, X. Lin, G. Q. Xie, X. J. Liu, S. H. Sun and H. J. Qiu, Small, 2019, 15, 1904180 CrossRef CAS.
- K. Shen, X. Chen, J. Chen and Y. Li, ACS Catal., 2016, 6, 5887–5903 CrossRef CAS.
- R. Coll, J. Salvadó, X. Farriol and D. Montané, Fuel Process. Technol., 2001, 74, 19–31 CrossRef CAS.
- Q. Wang, M. Chen, P. Lin, Z. Cui, C. L. Chu and B. Shen, J. Mater. Chem. A, 2018, 6, 10686–10699 RSC.
- J.-Q. Wang, Y.-H. Liu, M.-W. Chen, G.-Q. Xie, D. V. Louzguine-Luzgin, A. Inoue and J. H. Perepezko, Adv. Funct. Mater., 2012, 22, 2567–2570 CrossRef CAS.
- N. Ruiz, S. Seal and D. Reinhart, J. Hazard. Mater., 2000, 80, 107–117 CrossRef CAS.
- A. M. Moore, C. H. De Leon and T. M. Young, Environ. Sci. Technol., 2003, 37, 3189–3198 CrossRef CAS.
- H. Wang, Q. Yao, C. Wang, B. Fan, Q. Sun, C. Jin, Y. Xiong and Y. Chen, Sci. Rep., 2016, 6, 35549 CrossRef PubMed.
- Z. Jia, J. Kang, W. C. Zhang, W. M. Wang, C. Yang, H. Sun, D. Habibi and L. C. Zhang, Appl. Catal., B, 2017, 204, 537–547 CrossRef CAS.
- Z. Wang, W. Qiu, Y. Yang and C. T. Liu, Intermetallics, 2015, 64, 63–69 CrossRef CAS.
- M. Mavrikakis, B. Hammer and J. Nørskov, Phys. Rev. Lett., 1998, 81, 2819–2822 CrossRef.
- Y. E. Seidel, A. Schneider, Z. Jusys, B. Wickman, B. Kasemo and R. J. Behm, Faraday Discuss., 2008, 140, 167–184 RSC.
- S. Q. Chen, G. N. Yang, S. T. Luo, S. J. Yin, J. L. Jia, Z. Li, S. G. Gao, Y. Shao and K. F. Yao, J. Mater. Chem. A, 2017, 5, 14230–14240 RSC.
- L. Yang, Y. L. Lv and D. P. Cao, J. Mater. Chem. A, 2018, 6, 3926–3932 RSC.
- Z. Xiong, B. Lai, P. Yang, Y. Zhou, J. Wang and S. Fang, J. Hazard. Mater., 2015, 297, 261–268 CrossRef CAS.
- B. Kakavandi and A. A. Babaei, RSC Adv., 2016, 6, 84999–85011 RSC.
- Y. Tang, Y. Shao, N. Chen, X. Liu, S. Q. Chen and K. F. Yao, RSC Adv., 2015, 5, 34032–34039 RSC.
- Y. Tang, Y. Shao, N. Chen and K.-F. Yao, RSC Adv., 2015, 5, 6215–6221 RSC.
| This journal is © The Royal Society of Chemistry 2021 |