Open Access Article
Open Access ArticleSeasonal dynamics of the phenolic constituents of the cones and leaves of oriental Thuja (Platycladus orientalis L.) reveal their anti-inflammatory biomarkers†
Reham S. Darwisha,
Hala M. Hammodaa,
Doaa A. Ghareebbc,
Ali S. A. Abdelhamidc,
Fathallah M. Harraza and
Eman Shawky *a
*a
aDepartment of Pharmacognosy, Faculty of Pharmacy, Alexandria University, Alkhartoom Square, Alexandria 21521, Egypt. E-mail: shawkyeman@yahoo.com; eman.m.shawky@alexu.edu.eg; Fax: +20-34871668-4873273
bBiological Screening and Preclinical Trial Laboratory, Department of Biochemistry, Faculty of Science, Alexandria University, Alexandria, Egypt
cPharmaceutical and Fermentation Industries Development Centre, City of Scientific Research and Technological Applications (SRTA-City), Borg Al-Arab, Alexandria, Egypt
First published on 14th July 2021
Abstract
In this study, the seasonal dynamics of the flavonoids in the cones and leaves of oriental Thuja (Platycladus orientalis L. Franco) as well as the in vitro anti-inflammatory activity of their extracts were investigated. The important chemical markers of the studied extracts were determined using untargeted HPTLC profiling, which was further utilized to assess the seasonality effect on the composition of these metabolites over three seasonal cycles. A quantitative HPTLC method was developed and validated for the identified chemical markers of oriental Thuja: hyperoside, quercetrin, isoscutellarein-7-O-β-xyloside, cupressuflavone, hinokiflavone, sotetsuflavone and isoscutellarein-8-methyl ether. The highest amounts of flavonoids were observed during the summer and winter seasons, where the leaves possessed higher contents of flavonoids compared to cones. Flavone glycosides are a major class of flavones encountered in leaves, while the cones mainly accumulated biflavones. The results showed that the effect of seasonal variation on the accumulation of flavonoids within the cones was less pronounced than in the leaves. The summer leaves showed a remarkable reduction in the levels of INF-γ, where the value decreased to 80.7 ± 1.25 pg mL−1, a significantly lower level than that obtained with piroxicam (180 ± 1.47 pg mL−1); this suggests a noteworthy anti-inflammatory potential. OPLS (orthogonal projection to latent structures) models showed that flavonoidal glycosides, quercetrin, hyperoside and isoscutellarein-7-O-β-xyloside were the most contributing biomarkers to the reduction in pro-inflammatory mediators in LPS-stimulated WBCs. The results obtained in the study can thus be exploited to establish the best organs as well as the optimal periods of the year for collecting and obtaining certain biomarkers at high concentrations to guarantee the efficacy of the obtained extracts.
1. Introduction
Oriental Thuja (Platycladus orientalis L Franco, synonyms: Thuja orientalis and Biota orientalis L. Endl) is a coniferous evergreen tree1–3 and an ornamental plant with many folk uses and diverse biological activities. Traditionally, it is used for treatment of health problems related to inflammation, such as rheumatism, gout, rheumatoid arthritis, cystitis, cough and bronchial catarrh.4–6 Many studies have proved that Platycladus possesses several biological activities, including antioxidant,7,8 anti-inflammatory,9,10 antimicrobial,7,8 anticancer7,11 and neuroprotective activities.12,13 The previous phytochemical investigations of oriental Thuja revealed that flavones (aglycones and glycosides), biflavones, terpenes (diterpenes and sesquiterpenes), phenolics, neolignans, and lignans are the main chemical classes found in this plant.3,14–16 Many of these compounds were found to be active and to possess diverse biological activities; a new gernaylated flavone glycoside (apigenin 8-gernayl-4′-O-α-glucopyranoside) and two new pernylated flavonoid glycosides (apigenin 8-pernyl-4′-glucopyranosyl-7-O-α-glucopyranoside and apigenin 5-pernyl-7-glucopyranosyl-4′-O-β-D-glucopyranoside) were tested against lung adenocarcinoma (A549), human hepatocellular liver carcinoma (HepG2), and human breast carcinoma (MCF-7) cell lines as well as the mouse fibroblast cell line NIH/3T3 as normal cells. All of them showed cytotoxic effects on cancer cells without toxic effects on normal cells.17,18 Furthermore, four new compounds, namely, an abietane, two isopimarane diterpenes, and a dihydrobenzofuran neolignan, in addition to a new sesquiterpene glycoside were tested for their inhibitory effects on nitric oxide and TNF-α production; all tested compounds showed inhibitory effects.19 Moreover, isocupressic acid reduced cognitive deficits in an AD (Alzheimer's disease) model via modulation of the Aβ peptide aggregation pathway.12Flavonoids and biflavonoids constitute a large and diverse group of polyphenols, which are commonly found in Cupressaceae family members. They have valuable actions in plants, such as pigmentation of flowers and seeds in order to attract pollinators and seed-dispersing animals; also, they have roles in protection from drought stress, UV radiation, and microbe infection as well as in reduction of oxidative damage risks.20 In recent years, the pharmacological effects of plant flavonoids have been supported by a plethora of reports.20 It has been found that flavonoids are capable of induction of human protective enzyme systems. In addition, flavonoids play a role in protection against degenerative diseases, such as cardiovascular diseases, cancers, and age-related diseases.21,22 There are many previous reports indicating that flavonoids possess anti-inflammatory effects;23 also, they can modulate the synthesis and release of different mediators of inflammation.24–26
Plants develop mechanisms by which they can cope with unfavorable biotic and abiotic stress conditions. Synthesis of secondary metabolites (i.e., flavonoids) is one of those mechanisms.27–29 Many factors were found to influence the levels of plant flavonoids, including light, ultraviolet (UV) radiation, low/high temperature, the circadian cycle, seasonality, and the plant part used.29
Many attempts have been reported to investigate the effects of environmental, seasonal and abiotic factors on phenolic and flavonoid contents in plants, such as the investigation of the abiotic factors in the phenolic content of grapes,30 seasonal variation in production of antioxidant phenolic compounds in Morus nigra leaves,31 investigation of the seasonal effect on flavonol content in willow species32 and the effects of abiotic stress factors on the antioxidant properties and polyphenolic profile of green barley (Hordeum vulgare L.).33 Consequently, studying the seasonal dynamics of the phenolic constituents is necessary because each species exerts its chief biological effect in distinct seasons, which can aid precise selection of the harvesting time of the plant.
Within the same context, the objective of the current work was the investigation of the seasonal dynamics of the phenolic contents (i.e., flavones and biflavones) as well as the anti-inflammatory activity of the leaves and cones of oriental Thuja, correlating them with climatic data; we examined the production of the pro-inflammatory cytokines TNF-α, IL-1β and INF-γ, as well as the cytotoxicity induced by the extract in LPS-stimulated WBCs. The determination of the biomarkers of the extracts of the different seasons as well as their efficacy-directed discrimination was achieved with the aid of chemometrics, aiming at the selection of the best time of harvesting of each organ of the plant to achieve the highest efficacy.
2. Experimental
2.1. Instruments
2.2. Plant collection
Aerial parts of P. orientalis L. were collected from Antoniadis Garden, Alexandria, Egypt, in February, April, August and November of 2017, 2018 and 2019. Three samples were collected in every season. Plant identification was confirmed by Dr Therese Labib, specialist of plant identification at El Orman Garden, Cairo, Egypt. The voucher specimen (No. TO-2018) was maintained in the herbarium of the Pharmacognosy Department, Faculty of Pharmacy, Alexandria University. Aerial parts were separated into cones and leaves for each season sample except for winter, where the samples contained leaves but no developed cones. Three samples were collected over three consecutive years for a total of nine samples for each organ during each season, except for the winter season.2.3. Preparation of samples and isolation of chemical markers
25 g of each plant part (leaves and cones) were collected over four seasons in 2017, 2018 and 2019 and air-dried, ground and separately macerated in 1 L 95% ethanol; the extracts were then filtered and concentrated under reduced pressure. After that, each ethanol extract was re-dissolved in methanol to obtain a suitable concentration for each extract sample. Samples were filtered using disk filters (0.45 μm) before their application. For chemical marker isolation, 150 g of air dried-ground leaves of P. orientalis collected in the summer season (August 2018) was macerated in 95% ethanol for two weeks, filtered and concentrated under reduced pressure. After that, the ethanol extract was re-dissolved in a mixture of water and methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain a hydroalcoholic extract, and then liquid–liquid extraction was performed using n-hexane, methylene chloride, ethyl acetate and n-butanol, subsequently. Finally, concentration of each fraction to dryness under reduced pressure was carried out. The crude extracts of the methylene chloride and ethyl acetate (10 mL) fractions were fractionated by means of flash column chromatography; then, PTLC was performed on the subfractions containing the targeted compounds with 1 mm thickness and normal phase preparative plates. The mobile phase used was ethyl acetate/methanol/water/acetic acid 45
1) to obtain a hydroalcoholic extract, and then liquid–liquid extraction was performed using n-hexane, methylene chloride, ethyl acetate and n-butanol, subsequently. Finally, concentration of each fraction to dryness under reduced pressure was carried out. The crude extracts of the methylene chloride and ethyl acetate (10 mL) fractions were fractionated by means of flash column chromatography; then, PTLC was performed on the subfractions containing the targeted compounds with 1 mm thickness and normal phase preparative plates. The mobile phase used was ethyl acetate/methanol/water/acetic acid 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35 (v/v) for compounds 1–3 and toluene/ethyl acetate (2
0.35 (v/v) for compounds 1–3 and toluene/ethyl acetate (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for isolation of compounds 4–7. Spots were scraped off and eluted by a mixture of methanol and chloroform (2
1) for isolation of compounds 4–7. Spots were scraped off and eluted by a mixture of methanol and chloroform (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and after that, the compounds were purified by a Sephadex LH-20 column using pure methanol (analytical grade) as the eluent. 2D-HPTLC was used to confirm the purity of the compounds, and the percent recovery of the compounds was in the range of 98–99%. Finally, the isolated compounds were subjected to NMR and MS techniques for their identification. The structures of the isolated chemical markers were confirmed by comparing the NMR data for each compound with those reported in the literature; hyperoside and quercetrin,22 isoscutellarein-7-O-β-D-xyloside,23 cupressuflavone and hinokiflavone,24 sotetsuflavone34,35 and isoscutellarein-8-methyl ether.27,28
1), and after that, the compounds were purified by a Sephadex LH-20 column using pure methanol (analytical grade) as the eluent. 2D-HPTLC was used to confirm the purity of the compounds, and the percent recovery of the compounds was in the range of 98–99%. Finally, the isolated compounds were subjected to NMR and MS techniques for their identification. The structures of the isolated chemical markers were confirmed by comparing the NMR data for each compound with those reported in the literature; hyperoside and quercetrin,22 isoscutellarein-7-O-β-D-xyloside,23 cupressuflavone and hinokiflavone,24 sotetsuflavone34,35 and isoscutellarein-8-methyl ether.27,28
2.4. High-performance thin-layer chromatography
The band-wise application of the samples and standard solutions (standards: hyperoside, quercetrin, isoscutellarein-7-O-β-xyloside, cupressuflavone, hinokiflavone, sotetsuflavone and isoscutellarein-8-methyl ether), each with a concentration of 1 mg mL−1, was performed by means of a Camag (Wilmington, NC) Linomat V automated spray-on band applicator equipped with a 100 μL syringe. Standard solutions were applied to pre-coated HPTLC plates (10 cm × 20 cm). Application was performed from the left to the right of the plate at a distance of 1.5 cm from the bottom and the margins of the plate. The bandwidth was set to 6 mm and the inter-band spaces to 4 mm to obtain 18 tracks for six different concentrations each, and this method was applied in triplicate. For the sample plates, the bandwidth was set to 8 mm and the inter-band spaces to 5 mm to obtain 16 tracks; 14 tracks were for the samples, and the last two tracks were for the application of standards with a concentration of 4 μg per spot. Standard plates were used to construct the calibration curves and to calculate some of the validation parameters of the studied method. On the other hand, the sample plates were plates of different extracts which were used to carry out targeted and untargeted chemical profiling of the different extracts. The mobile phase system used was ethyl acetate/methanol/water/acetic acid 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35 (v/v) (system I) for the standards hyperoside, quercetrin, and isoscutellarein-7-O-β-xyloside (standard plate I) and sample plate I, while a mobile phase of toluene/ethyl acetate (2
0.35 (v/v) (system I) for the standards hyperoside, quercetrin, and isoscutellarein-7-O-β-xyloside (standard plate I) and sample plate I, while a mobile phase of toluene/ethyl acetate (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (system II) was used for the standards cupressuflavone, hinokiflavone, sotetsuflavone and isoscutellarein-8-methyl ether (standard plate II) and sample plate II. After the development of the plates, post-chromatographic derivatization of the plates was performed using anisaldehyde/H2SO4 reagent. The visualizing reagent was prepared by mixing 5 mL of concentrated sulfuric acid with 85 mL of cold methanol and cooling; after that, 10 mL of glacial acetic acid was added, followed by cooling, and finally, 0.5 mL of anisaldehyde was added to the previous mixture, which was then stored in a refrigerator.
1) (system II) was used for the standards cupressuflavone, hinokiflavone, sotetsuflavone and isoscutellarein-8-methyl ether (standard plate II) and sample plate II. After the development of the plates, post-chromatographic derivatization of the plates was performed using anisaldehyde/H2SO4 reagent. The visualizing reagent was prepared by mixing 5 mL of concentrated sulfuric acid with 85 mL of cold methanol and cooling; after that, 10 mL of glacial acetic acid was added, followed by cooling, and finally, 0.5 mL of anisaldehyde was added to the previous mixture, which was then stored in a refrigerator.
2.5. The image processing methodology and multivariate analysis
2.6. Development and validation of a quantitative HPTLC method for simultaneous detection of flavones and biflavones
The HPTLC method followed by the image analysis method was developed and validated for the quantitation of flavones and biflavones in the extracts of P. orientalis cones and leaves.The calibration function for each standard, hyperoside, quercetrin, isoscutellarein-7-O-β-xyloside, cupressuflavone, hinokiflavone, sotetsuflavone and isoscutellarein-8-methyl ether was built as follows: successive spots from each stock solution (1 mg mL−1) were applied in the following order: 2, 3, 4, 5, 6, and 7 μL. Each of these calibration levels was applied in triplicate. Development was performed as mentioned in Section 2.5. The plates were dipped in a solution of anisaldehyde/H2SO4 followed by heating in an oven at 150 °C. In order to construct the calibration curve, the peak area was plotted against the standard concentration in μg per spot. After that, the quantitative HPTLC method was validated according to ICH guidelines (ICH, 2005); the validation parameters were linearity, sensitivity, precision accuracy and specificity (see the ESI†).
2.7. Cytotoxicity and anti-inflammatory activity tests
Assessment of the cytotoxicities of the different extracts compared to piroxicam was carried out using the MTT assay followed by detection of the effective anti-inflammatory concentrations (EAICs) of each extract in lipopolysaccharides (LPS)-stimulated human WBC culture. After that, the investigation of the anti-inflammatory effect of each plant extract effective dose (EAICs) in LPS-stimulated WBCs was performed. Determination of pro-oxidant or pro-inflammatory mediator levels, including lipid peroxidation products (TBARS), ELISA detection of tumor necrosis factor alpha (TNF-α), ELISA detection of interleukin-1 beta (IL-1β) and ELISA detection of interferon gamma (INF-γ), was carried out. Details of the procedures can be found in the ESI section.† Statistical analysis of the results was performed using one-way variance of analysis (ANOVA). Results are expressed as the means ± standard deviations of three individual replicates.2.8. Multivariate analysis
The data matrices were imported into SIMCA-P ver 14.0 software (Umetrics, Sweden) for multivariate data analysis, including principal component analysis (PCA), hierarchical cluster analysis (HCA) and orthogonal projection to latent structures (OPLS).In order to determine the chemical markers of the leaf and cone extracts of P. orientalis, pixel data matrices of sample plates I and II obtained from the Image J program were subjected to principle component analysis (PCA).
The HPTLC quantitative data matrix was subjected to PCA and HCA analyses in order to investigate the clustering patterns of the different samples based on their quantitation for targeted analysis.
The data matrix of the anti-inflammatory activities of the different samples together with the data matrix obtained from the quantitative HPTLC method and the total phenolic data were subjected to multivariate orthogonal projection to latent structures (OPLS) analyses to determine the biomarker flavones and/or biflavones that are correlated to the anti-inflammatory activity and to investigate the clustering pattern based on the anti-inflammatory activity and total phenolic contents. A VIP plot was used to identify the components which significantly contributed to the model; variables with VIP values greater than 1 were considered important variables. Meanwhile, coefficient plots were used to identify variables which can be considered positive or negative contributors to the anti-inflammatory activity. The p value for statistical significance for all analyses in this study was set to less than 0.05. SIMCA-P Version 14.0 (Umetrics, Umeå, Sweden) was utilized for implementation of all statistical analyses, and a p value <0.05 was selected to indicate statistical significance.
3. Results and discussion
3.1. HPTLC-PCA untargeted chemical profiling of P. orientalis samples
Recently, HPTLC has been considered to be an evolution of planar chromatography, specifically in the field of natural products analysis. HPTLC has the advantage of representing complex plant matrices in a simple image. HPTLC plates can be evaluated qualitatively using different visualizing agents and quantitatively by densitometry or image analysis. A fingerprint can be defined as the individual chromatographic track that possibly represents a mixture of metabolites. HPTLC can be used to explore the variability between different extracts qualitatively and quantitatively, and it is considered a valuable tool for targeted and untargeted metabolomics.36The HPTLC fingerprint was exploited for chemical profiling of P. orientalis leaf and cone extracts collected in different seasons over a period of three years to investigate the impact of the season and plant organ on the chemical profile of the relevant extracts.
Flavonoids of P. orientalis can be categorized into three groups, flavone aglycones, biflavones and flavonoidal glycosides, which show variable polarities.35 Several trials were attempted to choose a stationary phase and mobile phases that provided the best separation of polar and non-polar compounds to obtain a holistic picture of the flavonoidal content of P. orientalis extracts. For polar compounds, the stationary phase selected was normal phase silica, and the mobile phase was ethyl acetate/methanol/water/acetic acid 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35 (v/v) (system I) for standard plate I and sample plate I; meanwhile, for non-polar compounds, the stationary phase selected was normal phase silica and the mobile phase was toluene/ethyl acetate (2
0.35 (v/v) (system I) for standard plate I and sample plate I; meanwhile, for non-polar compounds, the stationary phase selected was normal phase silica and the mobile phase was toluene/ethyl acetate (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (system II) for standard plate II and sample plate II.
1) (system II) for standard plate II and sample plate II.
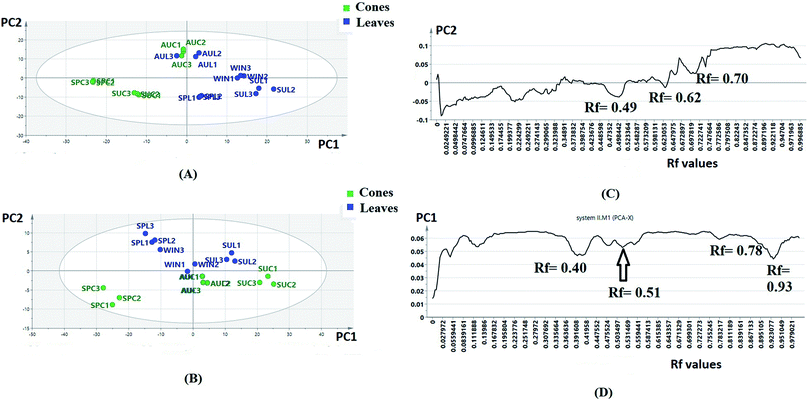
The obtained HPTLC plates (Fig. S1†) were utilized for untargeted chemical profiling of the collected extracts, where the data matrices subjected to unsupervised analysis were obtained by digitization of sample plate I (7 samples × 250 variables) and sample plate II (7 samples × 290 variables), respectively. Each data set was subjected to PCA separately; after that, score and loading line plots were obtained (Fig. 1A–D).
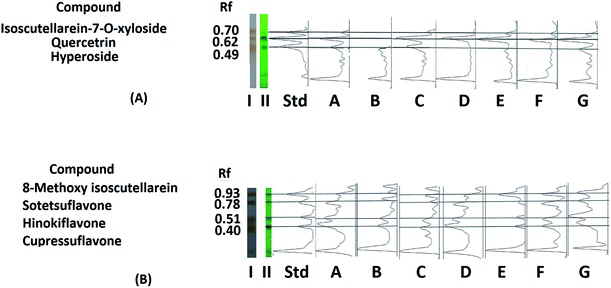
The score plots of sample plates I and II (Fig. 1A and B) were composed of seven principle components (PCs). The first two PCs explained 80.23% of the variation among the samples for sample plate I, while for sample plate II, they explained 87.43% of the variation. Regarding sample plate I, the leaf samples of all seasons were clustered along the positive side of PC1, while all the cone samples were clustered along the negative side of PC1. On the other hand, the score plot of sample plate II showed the clustering of all leaf samples except that of autumn along the positive side of PC2 as well as the clustering of all cone samples and autumn leaf samples along the negative side of the same principle component. It could be observed that the separation of samples in plates I and II was based on the organ selected, suggesting that different organs respond to the seasonality effect in different ways; this indicated that the data from both plates could be further used for differentiation between P. orientalis samples according to the organ and season. The loading line plot of the sample plate I data (Fig. 1C) showed that the zones with Rf values of 0.49, 0.62 and 0.70 had the highest impact on the PC1 and PC2 directions, while the loading plot of the sample II data (Fig. 1D) revealed the high impact of the zones with Rf values of 0.40, 0.51, 0.78 and 0.93 on both PC components. These compounds were targeted for further isolation and were subsequently used for quantitation and discrimination of the different extracts. Full characterization of the compounds was achieved through extensive studying of their NMR spectral data34 (ESI†).
3.2. Seasonal dynamics of the flavonoid and biflavonoid content of P. orientalis cone and leaf extracts using targeted chemical profiling
Among the factors affecting the flavonoid profiles in plants, seasonality is considered to be important.37,38 The climate is characterized by four distinct seasons with extremely different climate conditions; the dry seasons are spring and summer, while the wet seasons are autumn and winter. There are differences in the minimum and maximum temperatures, day lengths and average rainfall (Table 1).| Season/month | Average minimum temperature (°C) | Average maximum temperature (°C) | Average day length (hours) | Average rainfall (mm) |
|---|---|---|---|---|
| April (spring) | 15 | 27.8 | 12 | — |
| August (summer) | 22.2 | 33.3 | 14 | — |
| November (autumn) | 14.4 | 23.9 | 12 | 2.5 |
| February (winter) | 10 | 20 | 10 | 5.8 |
A quantitative HPTLC method was developed and validated according to ICH guidelines for the identified chemical markers of oriental Thuja, namely, hyperoside, quercetrin, isoscutellarein-7-O-β-xyloside, cupressuflavone, hinokiflavone, sotetsuflavone and isoscutellarein-8-methyl ether, were used as model analytes (Fig. 2). The developed method showed good linearity for each standard, with R2 ≥ 0.990 (Table S1†), high sensitivity, and low LOD and LOQ values (Table S1†). The intra- and inter-day precision and accuracy values were acceptable (Tables S2 and S3†). Seven chemical markers (Fig. 2) were targeted for quantitation in different P. orientalis extracts.
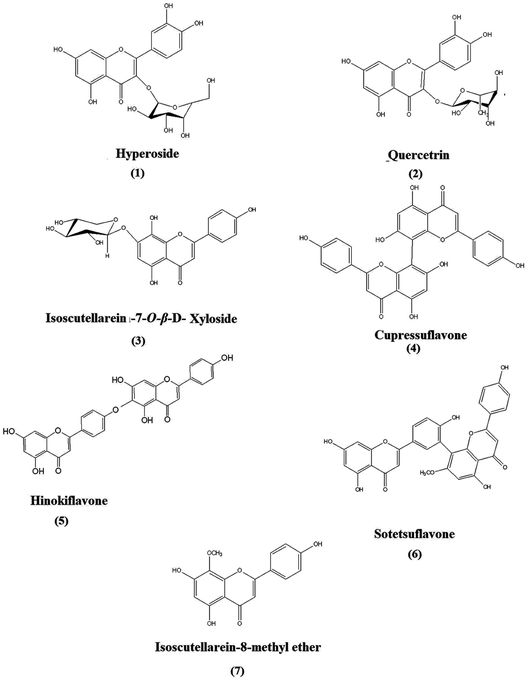 | ||
| Fig. 2 Structures of the targeted chemical markers (compounds 1 to 7): flavones and biflavones isolated from P. orientalis L. | ||
The positions of the targeted compounds were determined by superimposition of the Image J chromatograms of the different extracts and standards acquired before (under 254 nm UV light) and after derivatization of the standard and sample plates (Fig. 3A and B). The quantitation results revealed that the variation in the content of each compound was not statistically influenced by the year of collection; thus, they were further represented as the average contents of the nine samples collected for each organ during each season over the three-year period. The obtained quantitation results for the targeted flavonoids were in accordance with those previously reported in the literature for the plant.39
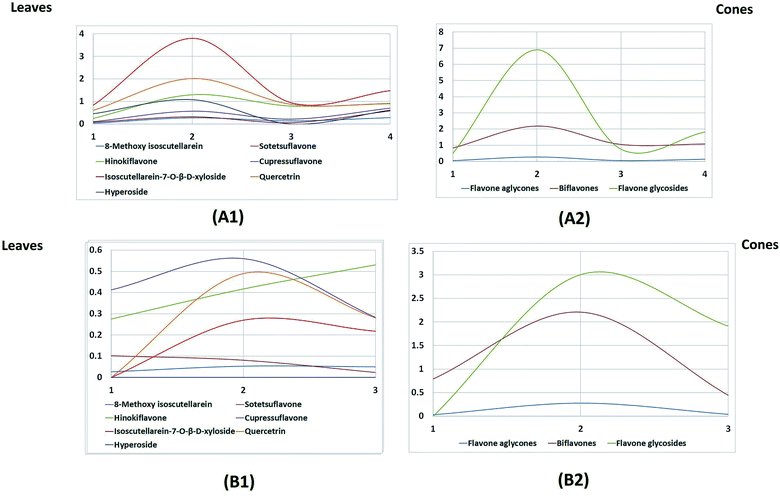
The leaves possessed higher contents of flavonoids compared to the cones (Fig. 4A). In particular, the highest amount was found in the summer leaf extract, followed by the winter, autumn and spring leaf extracts, respectively.
Regarding the flavone aglycone isoscutellarein-8-methyl ether, it was found in reasonable amounts in the leaves, but its content was almost negligible in the cones. Flavone glycosides were the major class of flavones encountered in the leaves, while the cones mainly accumulated biflavones. Regarding the leaf extracts, the flavone glycosides were represented by isoscutellarein-7-O-β-D-xyloside, quercetrin and hyperoside; these accumulated in high amounts during the summer season and then started to decline during autumn, followed by another significant increase during the winter and finally a slight deterioration during the spring season. The same pattern was also observed for the biflavones, represented by sotetsuflavone, hinokiflavone and cupressuflavone, except that during the spring season, there was a greater decrease in the biflavone content.
On the other hand, the seasonal dynamics of flavones were less pronounced in the cones, as only slight fluctuations in the content of biflavones were observed; however, a great decline in the content of flavone glycosides was observed during the spring season compared to the autumn and summer cones. The fact that the new, immature spring cones possessed no flavone glycosides can be attributed to the absence of enzymes responsible for glycosylation in the newly developed cones.
Studying Table 2 and Fig. 4, which was built based on the HPTLC quantitation results, revealed that isoscutellarein-7-O-β-D-xyloside followed by quercetrin were the major flavones in the leaves in all seasons, and they both showed the maximum accumulation during the summer season (3.804 and 2.021 mg g−1 dry weight of the plant, respectively). Meanwhile, the biflavone cupressuflavone followed by hinokiflavone were the major flavones detected in the cones, and their contents showed slight fluctuations during the different seasons.
| Compound | Spring leaves | Spring cones | Summer leaves | Summer cones | Autumn leaves | Autumn cones | Winter leaves |
|---|---|---|---|---|---|---|---|
| Absolute amount of flavones and biflavones (mg g−1 dry weight of the plant) | |||||||
| 8-Methoxy isoscutellarein | 0.037 ± 0.007 | 0.027 ± 0.002 | 0.272 ± 0.015 | 0.053 ± 0.002 | 0.14 ± 0.015 | 0.05 ± 0.002 | 0.28 ± 0.022 |
| Sotetsuflavone | 0.086 ± 0.002 | 0.103 ± 0.020 | 0.327 ± 0.022 | 0.082 ± 0.001 | 0.07 ± 0.002 | 0.024 ± 0.002 | 0.588 ± 0.017 |
| Hinokiflavone | 0.259 ± 0.014 | 0.276 ± 0.011 | 1.300 ± 0.040 | 0.417 ± 0.055 | 0.8 ± 0.013 | 0.53 ± 0.015 | 0.918 ± 0.025 |
| Cupressuflavone | 0.101 ± 0.011 | 0.413 ± 0.020 | 0.568 ± 0.044 | 0.56 ± 0.045 | 0.215 ± 0.015 | 0.283 ± 0.011 | 0.704 ± 0.030 |
| Isoscutellarein-7-O-β-D-xyloside | 0.840 ± 0.11 | 0 | 3.804 ± 0.1 | 0.27 ± 0.027 | 0.94 ± 0.01 | 0.218 ± 0.014 | 1.482 ± 0.15 |
| Quercetrin | 0.615 ± 0.018 | 0 | 2.021 ± 0.050 | 0.49 ± 0.015 | 0.879 ± 0.02 | 0.281 ± 0.015 | 0.905 ± 0.09 |
| Hyperoside | 0.458 ± 0.015 | 0 | 1.082 ± 0.064 | 0 | 0 | 0 | 0.619 ± 0.075 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Absolute amount of each class (mg g−1 dry weight of the plant) | |||||||
| Flavone aglycone | 0.037 | 0.027 | 0.272 | 0.053 | 0.14 | 0.05 | 0.28 |
| Biflavones | 0.446 | 0.792 | 2.195 | 1.059 | 1.085 | 0.837 | 2.21 |
| Flavone glycosides | 1.913 | 0 | 6.907 | 0.76 | 1.819 | 0.499 | 3.006 |
| Total | 2.396 | 0.819 | 9.374 | 1.872 | 3.044 | 1.386 | 5.496 |
Flavonoids are considered as a protective shield for plants against many abiotic stresses,22 and their concentrations are affected by many environmental and developmental factors, including tissue selection, drought, light intensity, quality and photoperiod. The accumulation of flavones in summer in high concentration may be a result of the stress conditions of long day length (14 h) (Table 1), leading to longer time of direct exposure to light and intense UV light as well as low humidity of soil moisture.40 The high content of flavones also found in winter leaf extracts could be attributed to the colder temperatures (Table 1), which can lead to increase in the flavonoid production; however, the accumulation of flavonoids in cooler temperatures is light dependent,38 which is the reason why the winter leaf extracts showed a total flavonoid content that was high but still lower than that of the summer leaf extracts. Flavonoids act as a protective shield for plants against environmental conditions, and their accumulation is enhanced by stress factors, such as high light intensity, longer day length and cold temperature. The extreme environmental conditions during summer and winter potentiate the production of flavonoids, and this justifies the high flavonoidal contents detected in summer and winter leaf extracts.40
It was obvious that the cone extracts possessed lower flavonoid contents than the leaf extracts; the reason for this could be the fact that cones are embedded between leaves, so they are exposed to less light than the leaves are. It was previously reported that shading of the foliage in the case of grapes decreased the flavonoid content significantly.41
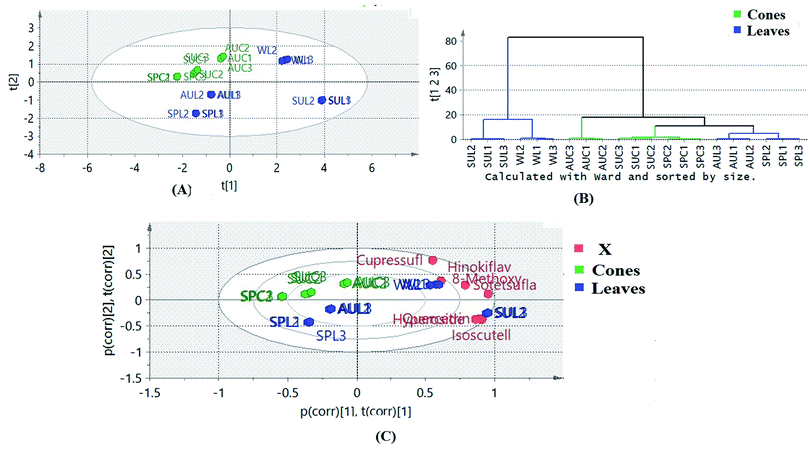
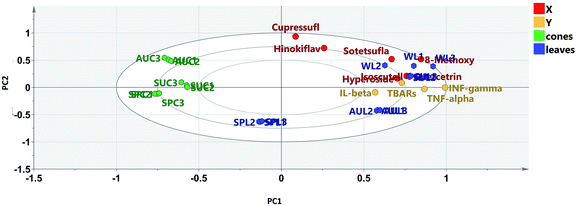
Multivariate statistical analysis was implemented in order to gain more holistic knowledge about the seasonal variability in the flavone and biflavone contents of the samples and their clustering patterns. Principle component analysis (PCA) of the data helped to explore the variability in the amounts of the targeted flavones and biflavones of the extracts of cones and leaves during the different seasons of collection. The quantitative results of the different extracts of cones and leaves during different seasons were subjected to PCA. PC1 and PC2 explained about 82% of the variation in all the samples. The PCA biplot (Fig. 5A) revealed that the autumn leaf and cone extracts, spring leaf and cone extracts and summer cone samples were clustered along the negative side of PC1, where the summer and winter leaf extracts were clustered together along the positive side of PC1. The winter leaf extracts clustered along the positive side of PC2, while the summer leaf extracts clustered along the negative side of PC2. Meanwhile, all cone samples were clustered together along the positive side of PC2. On the other hand, the samples of spring and autumn leaves were clustered together along the negative side of PC2. It was obvious that the cone samples were more consistent in their clustering pattern than the leaf samples, indicating that the cones were less affected by seasonal variation. Hierarchical clustering analysis of the data (Fig. 5B) showed that the summer and winter leaf extracts formed a separate cluster because both samples contained higher amounts of the targeted flavones and biflavones than the other tested samples.
A biplot was used to co-chart the scores and loadings together for their display and interpretation, as it shows the similarities and the dissimilarities between observations and allows the interpretation of the observations in terms of the variables. Observations situated near variables are high in those variables and are low in variables situated opposite. As shown in Fig. 5A, biflavones were correlated to winter leaf extracts, in which they accumulated the most, while glycosides were the important loadings of summer leaf extracts. The results obtained confirmed the effects of different seasons on the contents of the targeted chemical markers according to the organ selected.
3.3. Cytotoxicity and anti-inflammatory activities of the different extracts
It is known that inflammatory stimuli, such as immune stimulatory lipopolysaccharides, activate macrophages which produce different pro-inflammatory mediators. Interleukin-1 beta (IL-1β) is a pro-inflammatory cytokine produced by activated macrophages which enhances the secretion of tumor necrosis factor alpha (TNF-α)42 involved in various inflammatory diseases. Meanwhile, excessive release of gamma interferon (INF-γ) has been linked to the pathogenesis of chronic inflammatory and autoimmune diseases.43In this work, the cytotoxicity induced by the different extracts was evaluated on normal white blood cells with several dilutions of the extracts in the presence or absence of LPS. The EC100 values of all extracts were evaluated and were all found to be higher than that of the positive control piroxicam (Table 2). The effective anti-inflammatory concentration (EAIC) of each extract was then determined, which is the concentration that can bring the excessive proliferation of LPS-induced cells to normal proliferation of non-stimulated cells (stimulation index = 1). As shown in Table 2, summer leaves possessed the lowest EAIC of 40 ± 1.56 μg mL−1, while the spring cones exhibited the highest value of 210 ± 4.21 μg mL−1. The effect of each extract on lipid peroxidation and pro-inflammatory mediators in LPS-stimulated WBCs at its EAIC was determined. LPS administration to WBCs elevated the level of malonaldehyde (MDA) from 0.92 to 3.09 mmol mL−1. Summer and winter leaves showed the most significant reductions in the level of malonaldehyde in LPS-stimulated WBCs compared to the untreated LPS-stimulated cells (Table 2) in the thiobarbituric acid reactive substances (TBARS) assay, followed by autumn leaves, spring leaves and summer cones. It could also be observed that the summer leaves significantly reduced the pro-inflammatory mediators TNF-α (200.82 ± 4.52 pg mL−1), which was comparable to the effect of piroxicam (165.21 ± 1.15 pg mL−1). Meanwhile, the winter leaves were the most potent inhibitors of IL-1β, causing its reduction to a concentration of 250 ± 3.65 pg mL−1 compared to a value of 265.21 ± 2.12 pg mL−1 in normal cells. Summer leaf extracts significantly reduced the elevated levels of INF-γ to a value of 80.7 ± 1.25 pg mL−1; this value was significantly lower than that obtained with the positive control piroxicam (180 ± 1.47 pg mL−1), suggesting noteworthy anti-inflammatory potential for this particular extract.
3.4. OPLS modeling of flavonoid and biflavonoid profiles for determination of biomarkers for anti-inflammatory activities
To identify which flavonoids and biflavonoids acted as biomarkers that positively contributed to the anti-inflammatory activities of the different tested samples and to investigate the clustering pattern of the samples according to their biological activities, orthogonal projection to latent structures (OPLS) was implemented using the flavonoid and biflavonoid quantitation results dataset as X variables against the data obtained from the anti-inflammatory activities as Y variables. The performance of the established OPLS model (Fig. 6A) was indicated by the values of the coefficient of determination (R2 = 0.966), which represents the goodness of the model, and the cross-validation redundancy (Q2 = 0.959), which indicates how well the model predicts new data. The first and second latent variables explained 89% of the variation among the tested samples. The OPLS model is a discriminatory model; it showed between-class discrimination along PC1 and within-class discrimination along PC2. Between-class discrimination could be observed between the leaf samples, which were clustered along the positive side of PC1, and spring leaves, which were clustered with the cone samples along the negative side of PC1 (Fig. 6A). Meanwhile, within-class discrimination could be observed between the winter and summer leaf samples, which were clustered along the positive side of PC2, and the autumn leaf samples, which were clustered along the negative side of PC2. It was clear that the plant organ had a more significant effect on the activity-directed discrimination of the samples than the season of collection, which had a less pronounced effect (Table 3).| Plant | Cytotoxicity EC100 (μg mL−1) ± SD | Effective anti-inflammatory concentrations (μg mL−1) ± SD | Lipid peroxidation level (nmol mL−1) ± SD | TNF-α level (pg mL−1) ± SD | IL-1β (pg mL−1) ± SD | INF-γ (pg mL−1) ± SD |
|---|---|---|---|---|---|---|
| Spring leaves | 144 ± 4.12 | 98 ± 3.36 | 1.91 ± 0.021 | 260 ± 4.01 | 255 ± 2.56 | 370 ± 4.50 |
| Spring cones | 183 ± 1.56 | 210 ± 4.21 | 2.2 ± 0.015 | 330 ± 3.02 | 654 ± 5.24 | 830 ± 2.52 |
| Summer leaves | 42 ± 1.05 | 40 ± 1.56 | 1.84 ± 0.025 | 200.82 ± 4.52 | 303.8 ± 4.25 | 80.7 ± 1.25 |
| Summer cones | 156 ± 2.51 | 147 ± 3.01 | 1.91 ± 0.021 | 230.49 ± 3.69 | 264.571 ± 5.05 | 570 ± 5.25 |
| Autumn leaves | 123 ± 2.16 | 68 ± 2.56 | 1.87 ± 0.026 | 210 ± 5.25 | 280 ± 4.36 | 100 ± 1.95 |
| Autumn cones | 78 ± 3.12 | 76 ± 2.65 | 1.96 ± 0.014 | 320 ± 2.96 | 367.6 ± 2.01 | 760 ± 4.32 |
| Winter leaves | 71 ± 1.82 | 56 ± 1.85 | 1.84 ± 0.012 | 205 ± 4.24 | 250 ± 3.65 | 100 ± 1.54 |
| Piroxicam | 35.20 ± 1.25 | 23.98 ± 1.54 | 0.95 ± 0.011 | 165.21 ± 1.15 | 175 ± 2.52 | 180 ± 1.47 |
| LPS-stimulated WBCs | — | — | 3.09 ± 0.20 | 450.43 ± 2.3 | 551.66 ± 4.31 | 770 ± 3.1 |
| Normal cells (control) | — | — | 0.92 ± 0.10 | 260.21 ± 2.1 | 265.21 ± 2.12 | 420 ± 2.4 |
Examining the biplot (Fig. 6) revealed that the summer and winter leaf extracts showed proximity to all the tested reduced pro-inflammatory mediators, where the flavonoid glycosides rather than the biflavonoids, particularly quercetrin, isoscutellarein-7-O-β-D-xyloside and hyperoside, were closely related to the tested biological activity.
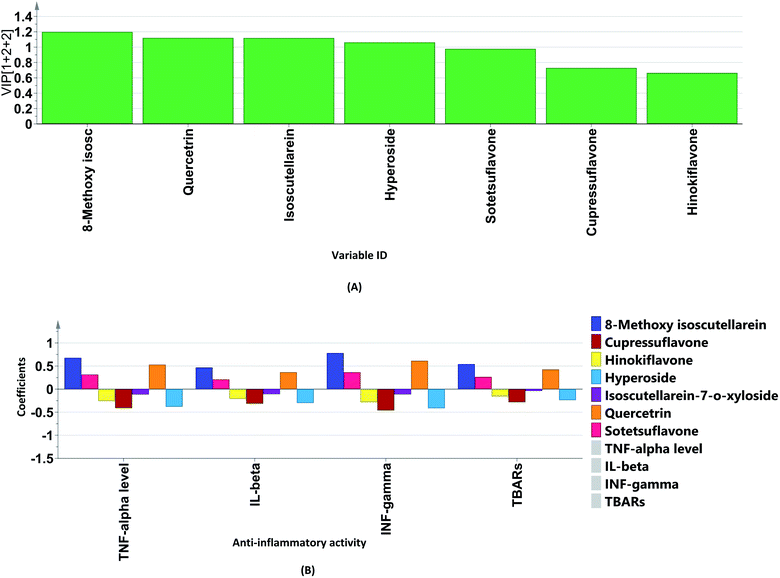
The variable importance in projection (VIP) plot together with the coefficient plots (Fig. 7A and B) were exploited to identify the biomarkers in the different extracts of P. orientalis. The VIP plot condenses the importance of each X variable to the model over all components and weighs it according to the Y variation accounted for by each component. Variables can be considered important when they have VIP values above 1. On the other hand, in coefficient plots, the size of the coefficient represents the change in the Y-variable when the X-variable varies from 0 to 1. It was found that the flavonoid glycosides, quercetrin, hyperoside and isoscutellarein-7-O-β-D-xyloside were the positive contributors to anti-inflammatory activity. This justifies the low anti-inflammatory activity shown by the cone extracts due to their null or minimal content of flavonoid glycosides. On the other hand, cupressuflavone was the major negative contributor to the activity, as it was equally present in extracts possessing high and low bioactivity.
4. Conclusion
The study in hand indicated that the leaves and cones of oriental Thuja (P. orientalis L.) contain considerable amounts of flavonoids, the contents of which demonstrate seasonal dynamics. The seasonal variation of the flavonoids of oriental Thuja was investigated to establish the optimal periods of the year for collecting and obtaining certain biomarkers at high concentrations to guarantee the efficacy of the obtained extracts.Untargeted HPTLC profiling of different P. orientalis extracts was effectively used to determine the chemical markers, which were further utilized to assess the seasonality effects on the composition of these metabolites over three seasonal cycles using a validated quantitative HPTLC method coupled to image analysis and chemometrics. The results showed that the targeted chemical markers can be classified into three classes: flavone aglycones, biflavones and flavonoidal glycosides. The highest amounts were found for flavonoids; they are considered to be protective shields for plants against many environmental stresses, and their content is affected by many abiotic and ontogenic factors. Extreme environmental and climate conditions during summer and winter were the reason for the accumulation of flavonoids in the leaves in both seasons. Leaves and cones responded differently to the seasonal variations, where the cone extracts exhibited lower flavonoid contents than the leaf extracts, and the effect of seasonal variation on the accumulation of flavonoids within the cones was less pronounced than in the case of leaves. Summer leaves showed remarkable reductions in all pro-inflammatory mediators, especially in the levels of INF-γ. The OPLS model showed that the flavonoidal glycosides, quercetrin, hyperoside and isoscutellarein-7-O-β-xyloside were more correlated to the anti-inflammatory activity. It is worthy of mention that this is the first time that the seasonal effects on the flavone and biflavone contents in different P. orientalis extracts and their anti-inflammatory activities were investigated. This study revealed that seasonality has pronounced effects on the contents and compositions of secondary metabolites in addition to its distinct effects on the biological activities of plants. It is essential to determine the best time of harvesting of plants to obtain the maximum yield of secondary metabolites and to achieve the highest biological activity required.
Conflicts of interest
There are no conflicts to declare.References
- L. Zhu and A. Lou, PLoS One, 2013, 8, 1–7 Search PubMed.
- G.-H. Xu, I.-J. Ryoo, Y.-H. Kim, S.-J. Choo and I.-D. Yoo, Arch. Pharmacal Res., 2009, 32, 175–282 Search PubMed.
- I. Amri, M. Hanana, B. Jamoussi, L. Hamrouni, A. Ismail, H. Mohsen, J. Bassem and H. Lamia, Arch. Phytopathol. Plant Prot., 2015, 48, 18–27 CrossRef.
- N. Jain, Int. J. Pure Appl. Biosci., 2017, 5, 73–83 CrossRef.
- S. M. Hashemi and S. A. Safavi, Chil. J. Agric. Res., 2012, 72, 188–194 CrossRef.
- N. Zhang, D. K. Park and H. J. Park, BMC Complementary Altern. Med., 2013, 13, 1–11 CrossRef.
- J.-J. Zhu, J.-J. Yang, G.-J. Wu and J.-G. Jiang, Ind. Crops Prod., 2020, 146, 1–9 CrossRef.
- N. D. Jasuja, S. K. Sharma, R. Saxena, J. Choudhary, R. Sharma and S. C. Joshi, J. Med. Plants Res., 2013, 7, 1886–1893 Search PubMed.
- D.-l. Gan, Y. Yao, H.-w. Su, Y.-yi. Huang, J.-f. Shi, X.-b. Liu and M. x. Xiang, Curr. Med. Sci., 2020, 41, 180–186 CrossRef.
- S.-Y. Fan, H.-W. Zeng, Y.-H. Pei, L. Lia, J. Ye, Y.-X. Pan, J.-G. Zhang, X. Yuan and W.-D. Zhang, J. Ethnopharmacol., 2012, 141, 647–652 CrossRef CAS PubMed.
- E. R. Elsharkawy, H. Aljohar, A. E. R. M. Donia and D. M. Abdel Raheim, Br. J. Pharm. Res., 2017, 15, 1–9 CrossRef.
- L. Yan, X. He, Y. Jin, J. Wang, F. Liang, R. Pei, P. Li, Y. Wang and W. Su, Front. Aging Neurosci., 2020, 12, 1–11 CrossRef.
- M.-Q. Shan, J. Shang and A.-W. Ding, Am. J. Chin. Med., 2014, 42(3), 523–542 CrossRef.
- L. R. Ad and S. Owonikoko, IOSR J. Appl. Chem., 2014, 7, 6–10 Search PubMed.
- M. A. Lila, J. Biomed. Biotechnol., 2004, 2004, 306–313 CrossRef.
- Y.-Z. Wang, C.-P. Tang, C.-Q. Ke, H.-C. Weiss, E.-R. Gesing and Y. Ye, Phytochemistry, 2008, 69, 518–526 CrossRef CAS PubMed.
- J. X. Shi, Y.-H. Li, X. Wang, Y.-Q. Qin, C.-Y. Zhang, X. Zhang, C.-R. Li, M.-Q. An, L.-R. Li, S.-H. Lu and J. Huang, J. Asian Nat. Prod. Res., 2018, 20, 1075–1080 CrossRef CAS PubMed.
- Y. Selim, E. El-Sharkawy and M. M. Abd El-Azim, Nat. Prod. Res., 2020, 34, 1–9 CrossRef PubMed.
- S.-Y. Fan, Y.-H. Pei, H.-W. Zeng, S.-D. Zhang, Y.-L. Li, L. Li, J. Ye, Y.-X. Pan, H.-L. Li and W.-D. Zhang, Planta Med., 2011, 77, 1623–1630 CrossRef CAS PubMed.
- H. Wan, J. Zhang, T. Song, J. Tian and Y. Yao, Front. Plant Sci., 2015, 6, 1–13 Search PubMed.
- H. P. V. Rupasinghe, Molecules, 2020, 25, 1–7 Search PubMed.
- S. Kumar and A. K. Pandey, Sci. World J., 2013, 2013, 1–16 Search PubMed.
- R. E. Mutha, A. U. Tatiya and S. J. Surana, Future J. Pharm. Sci., 2021, 7, 1–13 CrossRef.
- S. J. Maleki, J. F. Crespo and B. Cabanillas, Food Chem., 2019, 299, 1–11 CrossRef.
- A. García-Lafuente, E. Guillamón, A. Villares, M. A. Rostagno and J. A. Martínez, Inflammation Res., 2007, 58, 537–552 CrossRef.
- R. A. Milella, D. Antonacci, P. Crupi, F. Incampo, C. Carrieri, N. Semeraro and M. Colucci, J. Food Sci., 2012, 77, H154–H159 CrossRef CAS PubMed.
- http://www.petroleum.gov.eg/en/AboutEgypt/Pages/LocationandClimate.aspx.
- http://database.prota.org/PROTAhtml/Platycladusorientalis_En.htm.
- A. F. Gomes, M. P. Almeida, M. F. Leite, S. Schwaiger, H. Stuppner, M. Halabalaki, J. G. Amaral and J. M. David, Food Chem., 2019, 273, 186–193 CrossRef CAS PubMed.
- E. H. Blancquaert, A. Oberholster, J. M. Ricardo-da-Silva and A. J. Deloire, S. Afr. J. Enol. Vitic., 2019, 40, 1–14 Search PubMed.
- A. P. Dalmagro, A. Camargo, d. S. Filho, H. Higino, M. M. Valcanaia, P. Cesar de Jesus and A. L. B. Zeni, Ind. Crops Prod., 2018, 123, 323–330 CrossRef CAS.
- S. Wiesneth, G. Aas, J. Heilmann and G. Jürgenliemk, Phytochemistry, 2018, 145, 26–39 CrossRef CAS PubMed.
- P. L. Kowalczewski, D. Radzikowska, E. Ivanišová, A. Szwengiel, M. Kačániová and S. Zuzanna, Int. J. Mol. Sci., 2020, 21, 1–14 Search PubMed.
- R. S. Darwish, E. Shawky, H. M. Hammoda and F. M. Harraz, Nat. Prod. Res., 2019, 1–5 CrossRef.
- Q. Liu, Y. Geng, X. Wang, J. Li and J. Yu, Anal. Methods, 2019, 11, 4260–4266 RSC.
- C. Tonioloa, M. Nicolettia, F. Maggib and A. Vendittiac, Nat. Prod. Res., 2014, 28, 119–126 CrossRef PubMed.
- L. Jaakola and A. Hohtola, Plant, Cell Environ., 2010, 33, 1239–1247 CAS.
- A. Mouradov and G. Spangenberg, Front. Plant Sci., 2014, 5, 1–16 Search PubMed.
- M. Shan, S. F. Y. Li, S. Yu, Y. Qian, S. Guo, L. Zhang and A. Ding, J. Chromatogr. Sci., 2018, 56, 41–48 CAS.
- J. Mierziak, K. Kostyn and A. Kulma, Molecules, 2014, 19, 16240–16265 CrossRef PubMed.
- M. O. Downey, N. K. Dokoozlian and M. P. Krstic, Am. J. Enol. Vitic., 2006, 57, 257–268 CAS.
- L. M. Beaulieu, E. Lin, E. Mick, M. Koupenova, E. O. Weinberg, C. D. Kramer, C. A. Genco, K. Tanriverdi, M. G. Larson, E. J. Benjamin and J. E. Freedman, Arterioscler., Thromb., Vasc. Biol., 2014, 34, 552–564 CrossRef CAS PubMed.
- O. Meyer, Jt., Bone, Spine, 2009, 76, 464–473 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra01681d |
| This journal is © The Royal Society of Chemistry 2021 |