Open Access Article
Open Access ArticlePyridylphosphonium salts as alternatives to cyanopyridines in radical–radical coupling reactions†
Jacob W.
Greenwood
 ,
Benjamin T.
Boyle
,
Benjamin T.
Boyle
 and
Andrew
McNally
and
Andrew
McNally
 *
*
Department of Chemistry, Colorado State University, Fort Collins, Colorado 80523, USA. E-mail: andy.mcnally@colostate.edu
First published on 2nd July 2021
Abstract
Radical couplings of cyanopyridine radical anions represent a valuable technology for functionalizing pyridines, which are prevalent throughout pharmaceuticals, agrochemicals, and materials. Installing the cyano group, which facilitates the necessary radical anion formation and stabilization, is challenging and limits the use of this chemistry to simple cyanopyridines. We discovered that pyridylphosphonium salts, installed directly and regioselectively from C–H precursors, are useful alternatives to cyanopyridines in radical–radical coupling reactions, expanding the scope of this reaction manifold to complex pyridines. Methods for both alkylation and amination of pyridines mediated by photoredox catalysis are described. Additionally, we demonstrate late-stage functionalization of pharmaceuticals, highlighting an advantage of pyridylphosphonium salts over cyanopyridines.
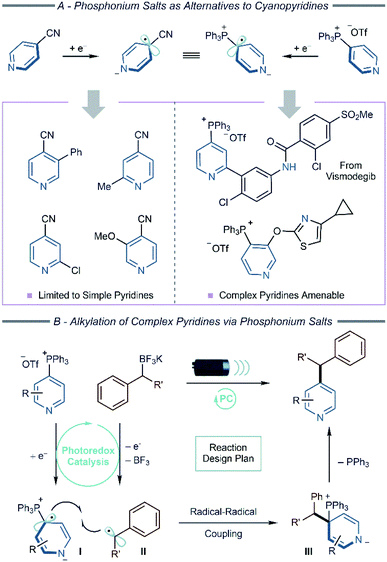
Modern photoredox catalysis and electrochemistry have enabled new synthetic methods that proceed via open-shell intermediates.1 Under this regime, pyridine functionalization strategies have been developed where 4-cyanopyridines undergo single-electron reduction to form dearomatized radical species that couple with other stabilized radicals (Scheme 1A).2 The cyano group is critical for efficient reactivity via pyridyl radical anions; alternatives such as 4-halopyridines more readily undergo elimination to pyridyl radicals after single-electron reduction resulting in a distinct set of coupling processes.3 We aimed to show that pyridylphosphonium salts could replicate the reactivity of cyanopyridines and allow a broader set of inputs into dearomatized pyridyl radical coupling reactions.4
Cyanopyridines have facilitated pyridine alkylation, allylation, and alkenylation reactions providing access to valuable building blocks for medicinal and agrochemical programs.5 The cyano group is essential for these methods, but a problem arises when applying this chemistry to complex pyridines, such as those found in pharmaceutical and agrochemical candidates. These structures are often devoid of pre-installed functional groups, and it is often challenging to install a cyano group from C–H precursors regioselectively.6 We envisioned pyridylphosphonium salts, regioselectively constructed from the C–H bonds of a diverse set of pyridines, could serve as alternatives to cyanopyridines.7 Herein, we report couplings between alkyl BF3K salts and preliminary studies of carboxylic acids and amines with pyridylphosphonium salts, including late-stage functionalization of complex pyridine-containing pharmaceuticals using this strategy.
Recently, we reported a radical coupling reaction between a boryl-stabilized cyanopyridyl radical and a boryl-stabilized pyridylphosphonium radical.7a The intermediate radicals arose via an unusual inner-sphere process that would be difficult to extend to other coupling reactions. A significant advance would be to show that pyridylphosphonium salts could function more generally as radical anion precursors and mimic the reactivity of cyano-pyridines. In particular, showing their viability in photoredox and electrochemical processes would translate to numerous synthetic transformations. To demonstrate this principle, we envisioned a redox-neutral alkylation reaction (Scheme 1B) via a radical coupling between radical zwitterion I, formed through single-electron reduction of a pyridylphosphonium salt (Eredp/2 = −1.51 V vs. SCE) and benzyl radical II, resulting from single-electron oxidation of a BF3K salt (Ered = +1.10 V vs. SCE for a primary benzylic salt).8 Loss of triphenylphosphine from dearomatized intermediate (III) would furnish the alkylated pyridine product. Notably, the redox events could invert, where the photocatalyst oxidizes the BF3K salt first and reduces the pyridylphosphonium salt second, broadening the scope of amenable photocatalysts.
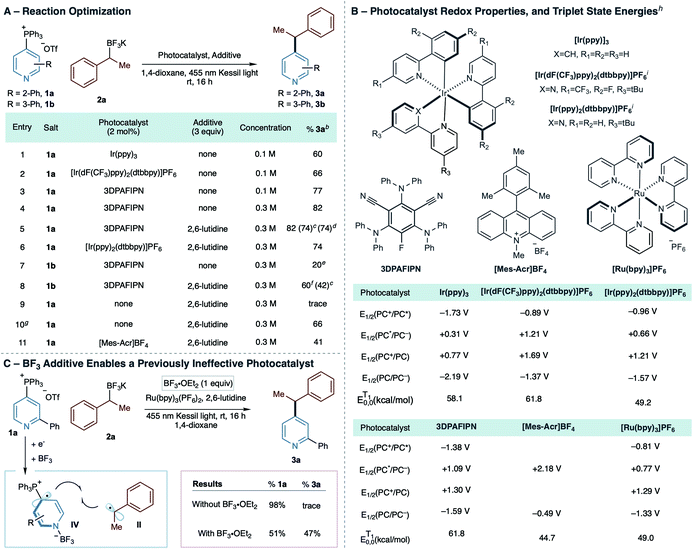
We began our investigation by examining a series of photocatalysts for the coupling reaction of phosphonium salt 1a, formed with complete regioselectivity for the 4-position from 2-phenylpyridine, and benzylic BF3K salt 2a under irradiation from a 455 nm Kessil light (Table 1A and available redox potentials and triplet state energies of photocatalysts shown in Table 1B). We discovered that both Ir(ppy)3 and [Ir(dF(CF3)ppy)2(dtbbpy)]PF6 catalyze the transformation despite markedly different redox properties (entries 1 and 2), suggesting that the order of redox events in Scheme 1B are potentially interchangeable.1b The Adachi-type photocatalyst 3DPAFIPN improved the yield to 77% with a further increase to 82% after increasing the reaction concentration (entries 3 and 4). Adding 2,6-lutidine, previously shown as an effective additive for photoredox cross-coupling reactions of BF3K salts by the Molander group,9 had no impact on the yield of 2-phenylpyridine salt 1a (entry 5) and the [Ir(ppy)2(dtbbpy)]PF6 catalyst was marginally less efficient under the same conditions (entry 6). We observed that 2,6-lutidine did substantially improve the yield when isomeric 3-Ph salt 1b was employed (entries 7 and 8); without 2,6-lutidine, the crude 1H NMR indicates significant amounts of decomposition occurred, including 3-phenylpyridine, and the 4- vs. 2-position product ratio was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This outcome suggests that protiodephosphination and non-selective Minisci-type pathways can occur under these conditions. With 2,6-lutidine, the crude reaction pathway is cleaner, and the 4- vs. 2-position ratio improved to 8
1. This outcome suggests that protiodephosphination and non-selective Minisci-type pathways can occur under these conditions. With 2,6-lutidine, the crude reaction pathway is cleaner, and the 4- vs. 2-position ratio improved to 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. At this point, we have not established the role of 2,6-lutidine, although it is conceivable that it reacts with BF3 produced as the reaction progresses. In 2-substituted systems, steric hindrance around the pyridine N-atom of the salt would deter BF3-coordination, whereas, in 3-substituted systems, such as salt 1b, coordination is more likely and may have a deleterious effect on the reaction (vide infra). Given the structural variation of pyridines that we anticipated applying to this process and how those structures could impact boron speciation during the reaction, we elected to use 2,6-lutidine as an additive in all subsequent reactions.10
1. At this point, we have not established the role of 2,6-lutidine, although it is conceivable that it reacts with BF3 produced as the reaction progresses. In 2-substituted systems, steric hindrance around the pyridine N-atom of the salt would deter BF3-coordination, whereas, in 3-substituted systems, such as salt 1b, coordination is more likely and may have a deleterious effect on the reaction (vide infra). Given the structural variation of pyridines that we anticipated applying to this process and how those structures could impact boron speciation during the reaction, we elected to use 2,6-lutidine as an additive in all subsequent reactions.10
a Conditions: 1a (1.0 equiv.), 2a (2.0 equiv.), photocatalyst (2 mol%), additive (3.0 equiv.), rt.
b Yields determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as internal standard.
c Isolated yield on 0.50 mmol scale.
d Isolated yield on 2.00 mmol scale.
e 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 4- vs. 2-regioisomeric ratio determined from the crude 1H NMR.
f 8 1 4- vs. 2-regioisomeric ratio determined from the crude 1H NMR.
f 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 4- vs. 2-regioisomeric ratio determined from the crude 1H NMR.
g Used 365 nm LEDs instead of 455 nm Kessil light for 89 h.
h All redox potentials reported vs. SCE and all values compiled from previous literature reports.1
i Counterion omitted in structure for simplicity. 1 4- vs. 2-regioisomeric ratio determined from the crude 1H NMR.
g Used 365 nm LEDs instead of 455 nm Kessil light for 89 h.
h All redox potentials reported vs. SCE and all values compiled from previous literature reports.1
i Counterion omitted in structure for simplicity.
|
|---|
|
We conducted a series of further experiments to explore the effect of light and photocatalyst type on the reaction (Table 1, entries 9–11). Irradiating the reaction at 455 nm without photocatalyst resulted in traces of 3a, but we did observe 66% yield of the product when we used a 365 nm light (entries 8 and 9). No evidence of an electron donor–acceptor (EDA) complex was observable by UV-Vis spectroscopy, and we propose that the reaction starts by an overlap of the tails of the LEDs emission with the absorption of 1a at 345 nm (see ESI†).11 Furthermore, a photocatalyst with a redox potential window misaligned with the redox events in Scheme 1B, [Mes-Acr]BF4, is also competent (entry 11). An energy transfer mechanism was considered based on entry 9, but the low triplet state energies for [Mes-Acr]BF4 make this pathway unlikely (Table 1B). However, this result suggests that other factors could influence the reaction mechanism. The results in Table 1C show that pyridine N-activation can play a significant role in some instances. Using Ru(bpy)3 and 455 nm irradiation resulted in trace products but adding 1 equivalent of BF3·OEt2 formed 3a in 47% yield. Here, we hypothesize that 1a reacts with BF3 to enable single-electron reduction, and radical zwitterions of type IV are intermediates in the reaction. At this stage of our investigations, we conclude that radical zwitterions I and IV are likely present to varying extents depending on the photocatalyst and reaction conditions employed. Using optimal catalyst 3DPAFIPN and adding 2,6-lutidine to the reaction may favor I over IV and potentially indicate that I is more reactive in the C–C bond-forming step. Stern–Volmer quenching of 3DPAFIPN by pyridylphosphonium salt 1a occurs (KSV = 14.9 M−1 and Kq = 3.55 × 109 L mol−1 s−1), indicating that type I intermediates are accessible under the reaction conditions without BF3.12–14
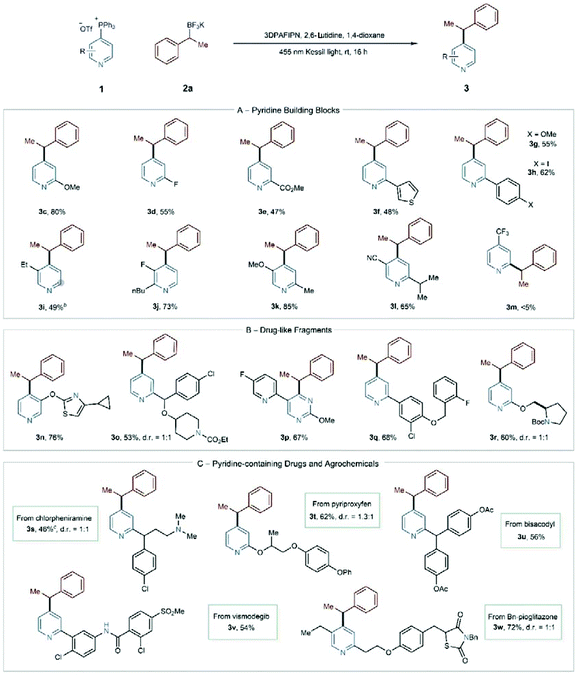
Employing the optimized conditions, we investigated the scope of pyridylphosphonium salts in this coupling process (Table 2). Starting with building block-type pyridines, 2-substituted pyridines with electron-withdrawing and electron-donating groups couple effectively (3c–3e). Aryl and heteroaryl groups are also compatible at the 2-position (3f & 3g), and we did not observe any undesired reactivity of the carbon–iodine bond in 3h. An 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 regioisomeric mixture of compounds formed when a 3-ethyl substituent was present (3i), but single isomers formed from 2,3- and 2,5-disubstituted pyridines (3j–3l). At present, 2-substituted pyridylphosphonium salts are not successful coupling partners; as a representative example, when we attempted to synthesize 3m, we observed significant decomposition of the starting material and only minor amounts (<5%) of the desired compound among other unidentified products.
1 regioisomeric mixture of compounds formed when a 3-ethyl substituent was present (3i), but single isomers formed from 2,3- and 2,5-disubstituted pyridines (3j–3l). At present, 2-substituted pyridylphosphonium salts are not successful coupling partners; as a representative example, when we attempted to synthesize 3m, we observed significant decomposition of the starting material and only minor amounts (<5%) of the desired compound among other unidentified products.
a Isolated yields of single regioisomers. Conditions: 1 (1.0 equiv.), 2a (2.0 equiv.), 3DPAFIPN (2 mol%), 2,6-lutidine (3.0 equiv.), 1,4-dioxane (0.3 M), rt.
b 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 crude regioisomeric ratio. Isolated as a single regioisomer. Grey circle denotes the site of alkylation for the minor regioisomer.
c With 1 equiv. TfOH. 1 crude regioisomeric ratio. Isolated as a single regioisomer. Grey circle denotes the site of alkylation for the minor regioisomer.
c With 1 equiv. TfOH.
|
|---|
|
Next, we converted a series of drug-like fragments and pharmaceuticals into phosphonium salts in this alkylation reaction. These examples represent the most significant advantage of this chemistry as installing a cyano group would be challenging from the C–H bond and limits the ability to make analog compounds. In addition, these structures contain multiple reactive sites and functional groups that could interfere with the coupling process. Nevertheless, we synthesized benzylated fragments 3n–3r without difficulty. Notably, other heterocycles are compatible, such as thiazoles and protected piperidines and pyrrolidines. The pyridine-pyrimidine biaryl 3p is particularly interesting as the phosphonium salt formed site-selectively on the pyrimidine ring, and the photoredox coupling proceeded in good yield on this heterocycle. Lastly, we demonstrated coupling with four FDA-approved pharmaceuticals and an agrochemical that illustrates functional group tolerance for protonated tertiary amines, amides, aryl halides, benzyl ethers, and sulfones (3s–3w). These examples validate this tactic for late-stage functionalization of complex pyridines.
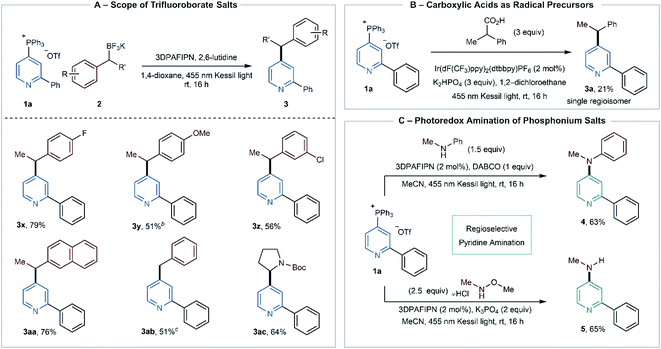
Scheme 2A shows the scope of the BF3K salts in the photoredox alkylation reaction. Secondary benzylic salts with electron-withdrawing and electron-donating groups are suitable coupling partners (3x–3z). In the case of 3y, we added a 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of benzylic and homobenzylic BF3K salts but only observed the benzylated product, presumably because the primary isomer is more difficult to oxidize. Secondary naphthyl and primary benzylic BF3K salts are proficient, resulting in 3aa and 3ab. The reaction also tolerates α-amino BF3K salts as evidenced by heterobenzylic amine derivative 3ac. At this stage, non-stabilized radicals were not successful in this process.
1 mixture of benzylic and homobenzylic BF3K salts but only observed the benzylated product, presumably because the primary isomer is more difficult to oxidize. Secondary naphthyl and primary benzylic BF3K salts are proficient, resulting in 3aa and 3ab. The reaction also tolerates α-amino BF3K salts as evidenced by heterobenzylic amine derivative 3ac. At this stage, non-stabilized radicals were not successful in this process.
Finally, we investigated whether pyridylphosphonium salts are competent with other radical precursors. In Scheme 2B, we obtained a preliminary result (unoptimized) of coupling with a carboxylic acid. These abundant compounds would improve the scope of radical coupling partners, and further studies are currently underway in our laboratory. In addition, Wu recently reported a method for photoredox catalyzed amination using cyanopyridines as coupling partners, and we attempted to replicate this transformation using pyridylphosphonium salts (Scheme 2C).15 Applying salt 1a to the reaction protocol with N-methyl aniline resulted in diaryl amine 4.16 Similarly, using N,O-dimethylhydroxylamine as a coupling partner, followed by in situ cleavage of the N–O bond, formed aniline 5 in reasonable yield. Consistent with the results in Table 2, we expect that this reaction will be compatible with more complex pyridine phosphonium salts and further suggests that phosphonium ions can serve as surrogates for cyanopyridines in other radical anion coupling reactions.
Conclusions
In conclusion, we report that pyridylphosphonium salts behave as alternatives to cyanopyridines to extend the utility of radical–radical coupling reactions to more complex substrates. We showed that two distinct reactions, pyridine alkylation, and amination, can proceed via phosphonium-stabilized radical intermediates. Our lab is currently investigating the capacity of pyridylphosphonium salts to participate in other open-shell reactions, as well as the mechanisms described in this study.Data availability
All experimental procedures and data related to this study can be found in the ESI.Author contributions
AMC and JWG conceptualized the work. JWG and BTB performed the experiments in this project. AMC and JWG prepared the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The National Science Foundation supported this work under Grant No. (1753087). We also thank the Sloan Foundation, Amgen, and Eli Lilly for generous gifts that partially supported this work. We gratefully acknowledge the Shores and Miyake groups at CSU for their expertise and generous use of their equipment.Notes and references
- (a) J. Twilton, C. Le, P. Zhang, M. H. Shaw, R. W. Evans and D. W. C. MacMillan, Nat. Rev. Chem., 2017, 1, 0052 CrossRef CAS; (b) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed; (c) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102–113 RSC; (d) N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075–10166 CrossRef CAS PubMed; (e) F. Strieth-Kalthoff, M. J. James, M. Teders, L. Pitzer and F. Glorius, Chem. Soc. Rev., 2018, 47, 7190–7202 RSC; (f) E. J. Horn, B. R. Rosen and P. S. Baran, ACS Cent. Sci., 2016, 2, 302–308 CrossRef CAS PubMed.
- For selected examples, see: (a) A. McNally, C. K. Prier and D. W. C. MacMillan, Science, 2011, 334, 1114–1116 CrossRef CAS PubMed; (b) T. Hoshikawa and M. Inoue, Chem. Sci., 2013, 4, 3118 RSC; (c) Z. Zuo and D. W. C. MacMillan, J. Am. Chem. Soc., 2014, 136, 5257–5260 CrossRef CAS PubMed; (d) J. D. Cuthbertson and D. W. C. MacMillan, Nature, 2015, 519, 74–77 CrossRef CAS PubMed; (e) F. Lima, M. A. Kabeshov, D. N. Tran, C. Battilocchio, J. Sedelmeier, G. Sedelmeier, B. Schenkel and S. V. Ley, Angew. Chem., Int. Ed., 2016, 55, 14085–14089 CrossRef CAS PubMed; (f) S. Zhu, J. Qin, F. Wang, H. Li and L. Chu, Nat. Commun., 2019, 10, 749 CrossRef PubMed; (g) M. C. Nicastri, D. Lehnherr, Y. Lam, D. A. DiRocco and T. Rovis, J. Am. Chem. Soc., 2020, 142, 987–998 CrossRef CAS PubMed; (h) Y. Ma, X. Yao, L. Zhang, P. Ni, R. Cheng and J. Ye, Angew. Chem., Int. Ed., 2019, 58, 16548–16552 CrossRef CAS PubMed; (i) D. Lehnherr, Y. Lam, M. C. Nicastri, J. Liu, J. A. Newman, E. L. Regalado, D. A. DiRocco and T. Rovis, J. Am. Chem. Soc., 2020, 142, 468–478 CrossRef CAS PubMed; (j) A. Yu. Vorob'ev, Chem. Heterocycl. Compd., 2019, 55, 90–92 CrossRef.
- In azoles, halides can facilitate homolytic aromatic substitution pathways, see: (a) C. K. Prier and D. W. C. MacMillan, Chem. Sci., 2014, 5, 4173–4178 RSC; (b) A. Singh, A. Arora and J. D. Weaver, Org. Lett., 2013, 15, 5390–5393 CrossRef CAS PubMed.
- (a) R. A. Aycock, H. Wang and N. T. Jui, Chem. Sci., 2017, 8, 3121–3125 RSC; (b) C. P. Seath, D. B. Vogt, Z. Xu, A. J. Boyington and N. T. Jui, J. Am. Chem. Soc., 2018, 140, 15525–15534 CrossRef CAS PubMed; (c) A. J. Boyington, C. P. Seath, A. M. Zearfoss, Z. Xu and N. T. Jui, J. Am. Chem. Soc., 2019, 141, 4147–4153 CrossRef CAS PubMed; (d) C. P. Seath and N. T. Jui, Synlett, 2019, 30, 1607–1614 CrossRef CAS PubMed; (e) A. Singh, J. J. Kubik and J. D. Weaver, Chem. Sci., 2015, 6, 7206–7212 RSC; (f) S. Senaweera and J. D. Weaver, J. Am. Chem. Soc., 2016, 138, 2520–2523 CrossRef CAS PubMed.
- (a) M. Baumann and I. R. Baxendale, Beilstein J. Org. Chem., 2013, 9, 2265–2319 CrossRef PubMed; (b) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed; (c) T. Eicher, S. Hauptmann and A. Speicher, The Chemistry of Heterocycles: Structure, Reactions, Syntheses, and Applications, Wiley-VCH, Weinheim, 3rd edn, 2012 Search PubMed.
- (a) X. Yu, J. Tang, X. Jin, Y. Yamamoto and M. Bao, Asian J. Org. Chem., 2018, 7, 550–553 CrossRef CAS; (b) D. Zhao, P. Xu and T. Ritter, Chem, 2019, 5, 97–107 CrossRef CAS; (c) B. L. Elbert, A. J. M. Farley, T. W. Gorman, T. C. Johnson, C. Genicot, B. Lallemand, P. Pasau, J. Flasz, J. L. Castro, M. MacCoss, R. S. Paton, C. J. Schofield, M. D. Smith, M. C. Willis and D. J. Dixon, Chem.–Eur. J., 2017, 23, 14733–14737 CrossRef CAS PubMed.
- (a) J. L. Koniarczyk, J. W. Greenwood, J. V. Alegre-Requena, R. S. Paton and A. McNally, Angew. Chem., Int. Ed., 2019, 58, 14882–14886 CrossRef CAS PubMed; (b) J. L. Koniarczyk, D. Hesk, A. Overgard, I. W. Davies and A. McNally, J. Am. Chem. Soc., 2018, 140, 1990–1993 CrossRef CAS PubMed; (c) X. Zhang and A. McNally, ACS Catal., 2019, 9, 4862–4866 CrossRef CAS PubMed; (d) M. C. Hilton, R. D. Dolewski and A. McNally, J. Am. Chem. Soc., 2016, 138, 13806–13809 CrossRef CAS PubMed; (e) C. B. Kelly and R. Padilla-Salinas, Chem. Sci., 2020, 11, 10047–10060 RSC.
- J. K. Matsui, D. N. Primer and G. A. Molander, Chem. Sci., 2017, 8, 3512–3522 RSC.
- (a) J. C. Tellis, D. N. Primer and G. A. Molander, Science, 2014, 345, 433–436 CrossRef CAS PubMed; (b) J. C. Tellis, J. Amani and G. A. Molander, Org. Lett., 2016, 18, 2994–2997 CrossRef CAS PubMed; (c) J. K. Matsui, Á. Gutiérrez-Bonet, M. Rotella, R. Alam, O. Gutierrez and G. A. Molander, Angew. Chem., Int. Ed., 2018, 57, 15847–15851 CrossRef CAS PubMed.
- Using benzyltrifluoroborate, the 1H NMR yield of product 3aa decreased from 50% to 29% when 2,6-lutidine was omitted.
- G. H. Lovett, S. Chen, X.-S. Xue, K. N. Houk and D. W. C. MacMillan, J. Am. Chem. Soc., 2019, 141, 20031–20036 CrossRef CAS PubMed.
- We thank an anonymous reviewer for suggesting that intermediates I are more reactive than IV to explain the role of 2,6-lutidine.
- L. Cardinale, M. O. Konev and A. Jacobivon Wangelin, Chem.–Eur. J., 2020, 26, 8239–8243 CrossRef CAS PubMed.
- Despite the similarities in photophysical properties between photocatalysts Ru(bpy)3(PF6)2 and Ir(ppy)2(dtbbpy)PF6, the iridium photocatalyst works well in the absence of BF3 additive while Ru(bpy)3(PF6)2 requires it. The reason for this is not yet understood and studies to explain this observation are ongoing.
- C. Zhou, T. Lei, X.-Z. Wei, C. Ye, Z. Liu, B. Chen, C.-H. Tung and L.-Z. Wu, J. Am. Chem. Soc., 2020, 142, 16805–16813 CrossRef CAS PubMed.
- Excluding the 3DPAFIPN resulted in traces (<1%) of 4. Control reactions also show that DABCO does not displace the phosphonium ion in 1a to form an ammonium salt.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc02324a |
| This journal is © The Royal Society of Chemistry 2021 |