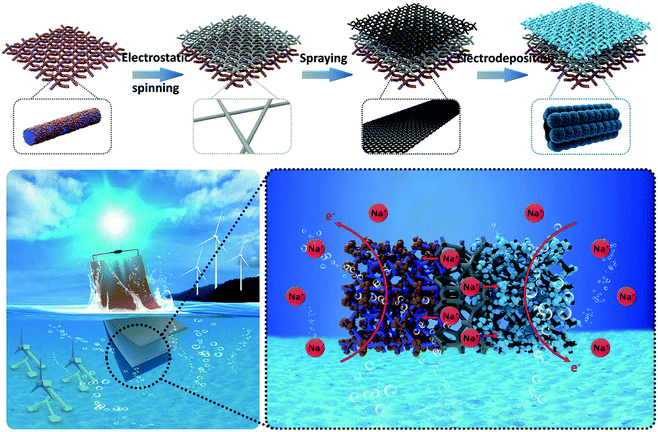
Towards large-scale electrochemical energy storage in the marine environment with a highly-extensible “paper-like” seawater supercapacitor device†
Situo
Cheng‡
,
Zhe
Dai‡
,
Jiecai
Fu
 *,
Peng
Cui
,
Kun
Wei
,
Yaxiong
Zhang
,
Yin
Wu
,
Yupeng
Liu
,
Zhenheng
Sun
,
Zhipeng
Shao
,
Xiaosha
Cui
,
Qing
Su
and
Erqing
Xie
*,
Peng
Cui
,
Kun
Wei
,
Yaxiong
Zhang
,
Yin
Wu
,
Yupeng
Liu
,
Zhenheng
Sun
,
Zhipeng
Shao
,
Xiaosha
Cui
,
Qing
Su
and
Erqing
Xie
 *
*
Key Laboratory for Magnetism and Magnetic Materials of the Ministry of Education, School of Physical Science and Technology, Lanzhou University, Lanzhou 730000, P. R. China. E-mail: fujc@lzu.edu.cn; xieeq@lzu.edu.cn
First published on 27th November 2020
Abstract
Harvesting energy from natural resources is of significant interest because of their abundance and sustainability. In particular, large-scale marine energy storage shows promising prospects because of the massive and diverse energy forms such as waves, tide and currents; however it is greatly hindered due to its complicated circumstances and intermittent nature. Storing and transporting locally generated energy has become a vital step for future sustainable energy supplies. Here, we proposed a highly-extensible “paper-like” all-in-one seawater supercapacitor constructed from a nanofiber-based film in operando towards electrochemical energy storage in the marine environment, which features lightweight and excellent mechanical properties with a typical thickness of about 100 μm. The single supercapacitor cell shows a remarkable performance with an energy density of 6.6 mW h cm−3 at a power density of 99.0 mW cm−3, and exhibits a capacitance retention of 100% under different bending operations. Moreover, the large-scale extensibility of the all-in-one seawater supercapacitor cell was fully demonstrated with an optimized circuit design. The integrated device connected with multiple cells in series and parallel can successfully drive a motor with a voltage of 12 V and a power of 2.5 W for operation. It shows prospective applications for future large-scale distributed energy storage systems in the marine environment.
Introduction
The gradual exhaustion of fossil energy and climate change has accelerated the exploration of renewable and clean power sources, such as solar energy, wind, ocean energy etc.1–3 Among them, the ocean, accounting for about 71% of the total earth's surface, represents a massive energy resource. It is estimated that the marine renewable energy resources available globally exceed our present and projected future energy needs, which shows the significant potential for future sustainable energy supplies.4,5 However, marine renewable energy forms including wave, tide and currents are greatly dependent on environmental variations, such as time of day, climate, season, etc., leading to the derived energy having unsteady, intermittent, and stochastic characteristics. Therefore, storing the harvested energy offers another option to enhance the energy system's feasibility and flexibility except for improving the climate forecasting and control. Nevertheless, because of the unique circumstances of marine energy, including the corrosive nature of seawater and unstable working conditions, energy storage technology for marine renewables remains rather embryonic. It is of great value and significance to develop universal energy-storing recipes/setups that use abundant seawater as a container for tidal energy and other new energy sources.6–11As an energy storage device and circuit element, supercapacitors have attracted tremendous interest for the potential application field of large-scale energy storage due to their merits, such as fast charging/discharging rates (within intervals of seconds), a high coulombic efficiency (>90%), and long life spans (>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles).12–17 In particular, the commonly used Na+ ion-based aqueous electrolyte in the supercapacitor perfectly matches the main component of seawater. However, traditional supercapacitors predominantly present a sandwiched architecture with the later stacking of the current collector, cathode, separator, anode and current collector in sequence, in which the discrete stacking form cannot deal with the volatile marine circumstances due to their weak mechanical integrity and stiff structures.18,19 Such a configuration will result in an unsatisfied energy storing capability and stability and even the device's failure. It is thus vital to develop high-performance supercapacitor devices with a rational configuration to better address these issues. New-style all-in-one supercapacitors enable monolithic integration of all of the components into one substrate, which can drastically minimize the proportion of inactive material and avoid the possibility of multilayer delamination. The substrates for the all-in-one supercapacitor can act as the separators simultaneously, where two sides of the substrate are combined with the conductive materials to form a conductive/nonconductive/conductive sandwiched structure. This unique 3D framework structure can not only facilitate an efficient ion/electron transportation, but can also break out of the traditional model, in which the structure of each part of the conventional supercapacitor is designed separately and then repackaged, realizing more flexible and diversified applications.20–26
000 cycles).12–17 In particular, the commonly used Na+ ion-based aqueous electrolyte in the supercapacitor perfectly matches the main component of seawater. However, traditional supercapacitors predominantly present a sandwiched architecture with the later stacking of the current collector, cathode, separator, anode and current collector in sequence, in which the discrete stacking form cannot deal with the volatile marine circumstances due to their weak mechanical integrity and stiff structures.18,19 Such a configuration will result in an unsatisfied energy storing capability and stability and even the device's failure. It is thus vital to develop high-performance supercapacitor devices with a rational configuration to better address these issues. New-style all-in-one supercapacitors enable monolithic integration of all of the components into one substrate, which can drastically minimize the proportion of inactive material and avoid the possibility of multilayer delamination. The substrates for the all-in-one supercapacitor can act as the separators simultaneously, where two sides of the substrate are combined with the conductive materials to form a conductive/nonconductive/conductive sandwiched structure. This unique 3D framework structure can not only facilitate an efficient ion/electron transportation, but can also break out of the traditional model, in which the structure of each part of the conventional supercapacitor is designed separately and then repackaged, realizing more flexible and diversified applications.20–26
In this work, we describe the construction of an all-in-one supercapacitor towards electrochemical energy storage in the marine environment, which is composed of an iron oxide incorporated carbon nanofiber matrix as the anode, in situ electrospun polyacrylonitrile (PAN) as the separator and MnO2 anchored woven carbon nanotubes as the cathode. Such an all-in-one architecture features lightweight and excellent mechanical properties with a typical thickness of about 100 μm (Fig. 1), showing the highly promising “paper-like” cell for robust energy storage in the marine environment. The typical charge–discharge process involves the rocking behaviors of seawater derived Na+ ions between the manganese dioxide cathode and the iron oxide anode by crossing the PAN separator. The assembled flexible all-in-one supercapacitor cell shows a remarkable performance with an energy density of 6.6 mW h cm−3 at a power density of 99.0 mW cm−3 and exhibits a capacitance retention of 100% under different bending angles. Furthermore, the large-scaling capability of the all-in-one supercapacitor was fully demonstrated with the optimized circuit design. The integrated device connected with multiple cells in series and parallel can successfully drive a motor with a voltage of 12 V and a power of 2.5 W for operation. Our results suggest that the all-in-one architecture design in this work offers a promising strategy for future high-performance energy storage devices in the marine environment.
Results and discussion
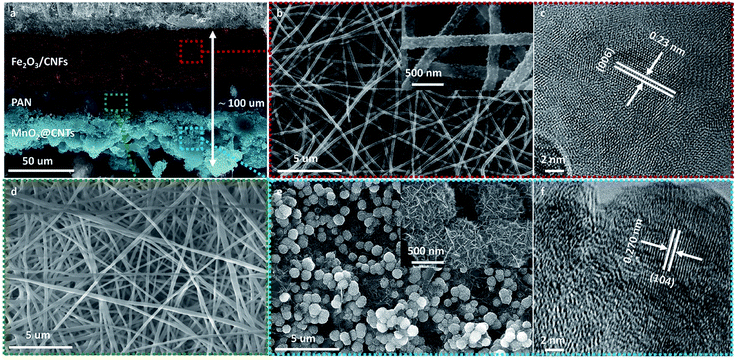
The schematic diagram presented in Fig. 1 illustrates the preparation procedure of the paper-like all-in-one seawater supercapacitor setup. It starts from an electrospun Fe2O3@CNFs nonwoven film, which possesses the advantages of being lightweight and possessing excellent mechanical properties and favorable hydrophilicity. Following a porous PAN nanofibers membrane, carbon nanotubes and MnO2 were deposited in situ successively on one side of the Fe2O3@CNFs film, ensuring the stable bonding and mechanical consistency of the obtained structure. In the marine environment, the porous and hydrophilic PAN nanofiber film contributes to unimpeded Na+ ion migration, which significantly facilitates the charge carrier transportation of both sides of the CNF and CNT networks. The Fe2O3 nanoparticles (anchored to the CNFs) and MnO2 nanosheets (adhered to the CNTs) with high theoretical specific capacitance, nontoxicity and high chemical stability endow the capacitance performance via Na+ ions involved in pseudocapacitive reactions in seawater without a benign environment in the sea. Therefore, the assembled paper-like all-in-one seawater supercapacitor cell exhibits the promising characteristics of high capacitance, long life span, environmentally friendliness, and excellent mechanical flexibility for the complicated circumstances in the marine environment. More impressively, benefiting from the smart and flexible structural design, multiple capacitor cells can be integrated together through a series or parallel connection, a higher output voltage or output current can be obtained, which offers a promising strategy for future high-performance energy storage devices in the marine environment.The cross-sectional SEM image of a typical paper-like all-in-one supercapacitor cell (Fig. 2a) shows the distinctive layer configuration with a tight adhesion between each layer (cathode, separator and anode), in which the thickness of the anode, separator and cathode corresponds to 40 μm, 20 μm and 40 μm, respectively, with the whole ASC thickness of about 100 μm. The anode Fe2O3@CNFs in Fig. 2b presents the uniform distribution and cross-linking characteristics, which provides an efficient ion transport channel and charge transferring conductive network. The active substance of the Fe2O3 nanoparticles can be identified by the lattice fringe (Fig. 2c), XPS spectrum27 (Fig. S2b†) and XRD (Fig. S2e†). Note that the Fe2O3 nanoparticles are evenly dispersed on the surface of the carbon fiber (inset of Fig. 2b), ensuring the effective pseudocapacitive reactions during the electrochemical charge–discharge process. Fig. 2d shows the texture nature of the intermediated PAN nanofibers film, which acts as the separator of the cell. It can be found that numerous PAN nanofibers are connected to each other and densely stacked, preventing the CNTs from penetrating to the other side of the PAN nanofiber film during the spraying but keeping the versatile channel for the electrolyte ions diffusion during electrochemical operations. The zeta potential of the carbon nanotube spray solution is 45.7 mV, which reflects the good dispersion of the carbon nanotubes. Fig. S5† displays the morphology of the CNTs on the PAN nanofibers, in which the CNTs are interwoven to form continuous conductive networks facilitating the electron transfer. Fig. 2d exhibits the morphology of the bottom-located cathode MnO2@CNTs, revealing the uniformly dispersed MnO2 nanospheres on carbon nanotubes. The detailed micrograph shows the nanosheets-like appearance of the MnO2 nanoparticles, which benefits the electrochemical performance due to the relatively high SSA and rich electrochemical active sites of the electrode.28 The crystal structure characterization of the HR-TEM image of the MnO2 cathode shows a typical lattice spacing of 0.27 nm, corresponding to the plane (104) of birnessite MnO2, as shown in Fig. 2d. The XRD also verifies the birnessite-MnO2 nature of the cathode (Fig. S6f†). The Brunauer–Emmett–Teller (BET) specific surface areas of Fe2O3@CNFs and MnO2@CNTs are estimated to be 317 m2 g−1 and 220 m2 g−1 (Fig. S8†). The cathode and anode with outstanding specific surface areas ensure the efficient ion diffusion and maximize the effective interfacial area. Considering those analyses above, it can be concluded that a robust all-in-one supercapacitor cell with a typical sandwich configuration of Fe2O3@CNFs (anode), PAN (separator) and MnO2@CNTs (cathode) has been successfully obtained in the experiments.
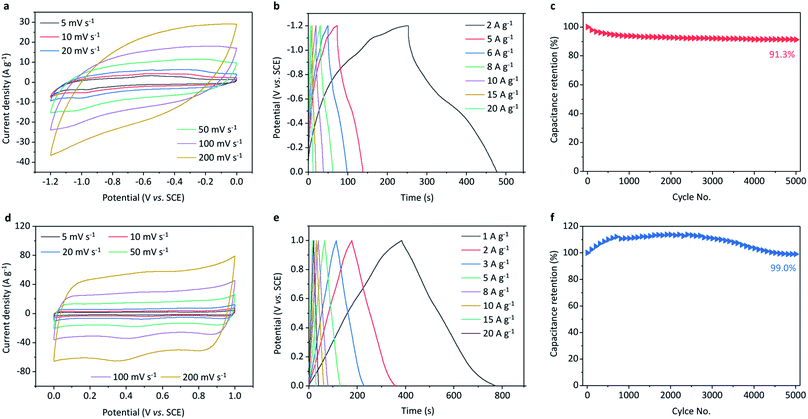
The electrochemical properties of the MnO2@CNTs cathode and Fe2O3@CNFs anode of the supercapacitor cell were studied comprehensively, as shown in Fig. 3. The CV curves of the Fe2O3@CNFs electrode at different scan rates show a distorted rectangular shape because of the essential reversible surface redox reactions of the Fe2O3 active material (Fig. 3a).29 The specific capacitance of the anode is calculated to be 328 F g−1 (based on the weight of Fe2O3@CNFs) at the scan rate of 5 mV s−1, and retains a value of 111 F g−1 when the scan rate reaches 100 mV s−1 (Fig. S7d†). The GCD curves (Fig. 3b) collected from 2 to 20 A g−1 display the shape of a typical curved triangle owing to the addition of the redox reaction of Fe(III/II). The equivalent series resistance (ESR) deconvoluted from the Nyquist plot (Fig. S7c†) is only 4.9 Ω, indicating the excellent ionic response of the electrode. Moreover, the anode exhibits a capacitance retention of 91.3% even after long cycling of 5000 times at a current density of 2 A g−1 (Fig. 3d and S7e†). Such a superior electrochemical performance of the anode should be attributed to the well-defined structure of the Fe2O3@CNFs with uniformly dispersed Fe2O3 nanoparticles on the conductive interwoven CNFs.
Fig. 3d shows the CV curves of the MnO2@CNTs electrode cycled at scan rates ranging from 5 to 200 mV s−1, revealing a typical rectangle-like shape because of the essential reversible successive surface redox reactions Mn(IV/III) of MnO2. Its capacitance reaches 372 F g−1 (based on the weight of MnO2@CNTs) at the scan rate of 5 mV s−1, and a capacitance retention percentage of 58% is kept even when the scan rate increases by 40-fold to 200 mV s−1 (Fig. S11a†). The GCD curves (Fig. 3e) of 1–20 A g−1 show the shape of a typical isosceles triangle, owing to the excellent ion and electron transport properties of the MnO2@CNTs electrode. This phenomenon can also be verified by the Nyquist plots and Bode plots (Fig. S11b and c†), in which a low equivalent series resistance of 3.4 Ω and a much smaller time constant of 0.39 s can be deduced. It is noteworthy that the capacitance retention can be achieved with 99.0% even after a successful test of 5000 cycles at the current density of 2 A g−1, demonstrating the superior stability of the electrode (Fig. 3f). The high performance of the cathode should be attributed to the combined high conductivity of the CNTs and the high electrochemical activity of MnO2, which ensures efficient electron transportation through the conductive networks. It is thus evident to expect the superior electrochemical performance of the configured all-in-one supercapacitor cell with the MnO2@CNTs cathode and Fe2O3@CNFs anode.
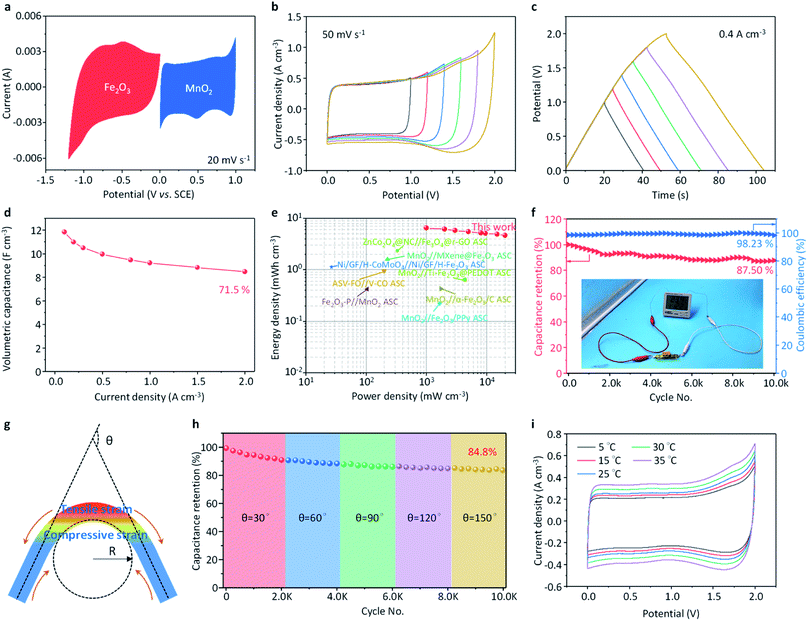
The electrochemical performance of the configured all-in-one supercapacitor cell was optimized and evaluated comprehensively, as shown in Fig. 4. To get optimized electrochemical performance of the ASC cell, the mass of the active substances in the cathode and anode should be matched according to the principle of charge conservation. Fig. 4a shows the representative CV curves of the Fe2O3@CNFs and MnO2@CNTs in simulated seawater at a scan rate of 20 mV s−1, in which the area of the CV curves of the two systems are almost the same, indicating the well-matched charges of the anode and cathode. Fig. 4b and c display the CV and GCD curves of the all-in-one supercapacitor cell operated at different voltage windows, respectively, implying a suitable operating voltage window of 0–2.0 V of the cell. Note that the CV curves with a voltage window of 0–2.0 V perform a quasi-rectangular profile and current densities increased with increasing scan rates, which suggests a pure capacitive behavior of the supercapacitor (Fig. S13a†). The GCD curves (Fig. S13b†) operated at 0.1 to 2 mA cm−3 show the shape of a typical isosceles triangle, further demonstrating the excellent electrochemical capacitive behaviors of the configured all-in-one supercapacitor cell. The volumetric capacitance (Fig. 4d) reaches 11.8 F cm−3 at the current density of 0.1 A cm−3, and the capacitance retention is about 71.5% when the scan rate increases to 2 A cm−3, revealing the outstanding rate performance of the all-in-one supercapacitor cell.
Ragone plots in Fig. 4e manifest the energy density and average power density of the all-in-one supercapacitor cell, which is derived from the galvanostatic charge/discharge curves at different current densities in Fig. 4e. Significantly, the all-in-one seawater supercapacitor cell delivers an energy density as high as 6.6 mW h cm−3 at a power density of 99.0 mW cm−3 and still retains 4.7 mW h cm−3 at an ultra-high power density of 2000.4 mW cm−3. Those values are substantially higher than those of previously reported ASC devices, such as ZnCo2O4@NC//Fe3O4@r-GO ASC, MnO2//MXene@Fe2O3 ASC, Ni/GF/H-CoMoO4//Ni/GF/H-Fe2O3 ASC, MnO2//Ti-Fe2O3@PEDOT ASC, ASV-FO//V-CO ASC, Fe2O3-P//MnO2 ASC, MnO2//α-Fe2O3/C ASC and MnO2//Fe2O3/PPy ASC.30–37 The mass specific electrochemical performance of the all-in-one seawater supercapacitor cell outperforms that of the reported results in the literature (Table S1†). Furthermore, the cyclic performance and coulombic efficiency of the all-in-one supercapacitor is conducted over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at a current density of 4 A g−1, as shown in Fig. 4f. The cell retains 87.50% of its initial capacitance after cycling for 10
000 cycles at a current density of 4 A g−1, as shown in Fig. 4f. The cell retains 87.50% of its initial capacitance after cycling for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles under the voltage window of 0–2.0 V, confirming its good stability at a high voltage window of 0–2.0 V. An almost 100% coulombic efficiency can be achieved for the cells. A demo experiment of powering an electronic circuit was performed in the inset of Fig. 4f. Besides, the EIS deconvoluted ESR is as low as 4.9 Ω (Fig. S14a†), which suggests the high charge-transfer behavior and electrochemical response at the interface between the electrolyte and the electrode at the high-frequency range. The nearly vertical line in the low frequency also indicates an ideal capacitive behavior of the supercapacitor, in which a very small time constant of 3.22 s (Fig. S14b†) indicates an excellent capacitance response of the electrode. To demonstrate the feasibility of its operation in seawater, we further measure the electrochemical performance of the all-in-one supercapacitor in the highly seawater-simulating electrolyte, which was also compared with that in the NaCl electrolyte (Fig. S15†). There is no obvious difference in the electrochemical performance of the all-in-one supercapacitor. At high potentials, due to the difference in electrolyte anions, the capacitance of the all-in-one “paper-like” supercapacitors in Na2SO4 electrolyte is smaller than that in NaCl electrolyte, but the cycle stability is slightly improved (Fig. S16†). At the same time, since the seawater shows weak alkalinity, we further compared the resultant electrochemical properties of the all-in-one “paper-like” supercapacitors working in the NaCl electrolyte with pH = 7 and pH = 8. It can be found that the capacity and cycle stability of the all-in-one supercapacitor are not affected by slight changes in pH (Fig. S17†).
000 cycles under the voltage window of 0–2.0 V, confirming its good stability at a high voltage window of 0–2.0 V. An almost 100% coulombic efficiency can be achieved for the cells. A demo experiment of powering an electronic circuit was performed in the inset of Fig. 4f. Besides, the EIS deconvoluted ESR is as low as 4.9 Ω (Fig. S14a†), which suggests the high charge-transfer behavior and electrochemical response at the interface between the electrolyte and the electrode at the high-frequency range. The nearly vertical line in the low frequency also indicates an ideal capacitive behavior of the supercapacitor, in which a very small time constant of 3.22 s (Fig. S14b†) indicates an excellent capacitance response of the electrode. To demonstrate the feasibility of its operation in seawater, we further measure the electrochemical performance of the all-in-one supercapacitor in the highly seawater-simulating electrolyte, which was also compared with that in the NaCl electrolyte (Fig. S15†). There is no obvious difference in the electrochemical performance of the all-in-one supercapacitor. At high potentials, due to the difference in electrolyte anions, the capacitance of the all-in-one “paper-like” supercapacitors in Na2SO4 electrolyte is smaller than that in NaCl electrolyte, but the cycle stability is slightly improved (Fig. S16†). At the same time, since the seawater shows weak alkalinity, we further compared the resultant electrochemical properties of the all-in-one “paper-like” supercapacitors working in the NaCl electrolyte with pH = 7 and pH = 8. It can be found that the capacity and cycle stability of the all-in-one supercapacitor are not affected by slight changes in pH (Fig. S17†).
Due to the complex circumstance of the marine environment, the electrochemical performance dependence of the environmental factors such as temperature and mechanical stability was highlighted to demonstrate the flexible adaptability of the all-in-one seawater supercapacitor cells. We use θ, R, and L (the length of the device) to precisely evaluate the bending durability of the all-in-one supercapacitor cell.38,39 A schematic diagram of these parameters is illustrated in Fig. 4g (L = 5 cm, R = 1 cm). In the case of the different curvatures (i.e., different θ values), the cyclic performance of the all-in-one supercapacitor is conducted over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at a current density of 4 A g−1, as shown in Fig. 4h. The cell retains 87.50% of its initial capacitance after 10
000 cycles at a current density of 4 A g−1, as shown in Fig. 4h. The cell retains 87.50% of its initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-cycle charging–discharging operations. Fig. 4g displays the CV curves of the all-in-one supercapacitor cell operated at different temperatures. It can be found that the capacitance of the cell at 5 °C and 35 °C is calculated to be ∼83% and 129% normalized with that of 25 °C, respectively, indicating a wide working temperature range with a high capacitance efficiency either in a cold climate or in hot conditions (Fig. 4i). Therefore, it is fully evident that the all-in-one supercapacitor cell here can easily cope with the environmental variations in the marine environment.
000-cycle charging–discharging operations. Fig. 4g displays the CV curves of the all-in-one supercapacitor cell operated at different temperatures. It can be found that the capacitance of the cell at 5 °C and 35 °C is calculated to be ∼83% and 129% normalized with that of 25 °C, respectively, indicating a wide working temperature range with a high capacitance efficiency either in a cold climate or in hot conditions (Fig. 4i). Therefore, it is fully evident that the all-in-one supercapacitor cell here can easily cope with the environmental variations in the marine environment.
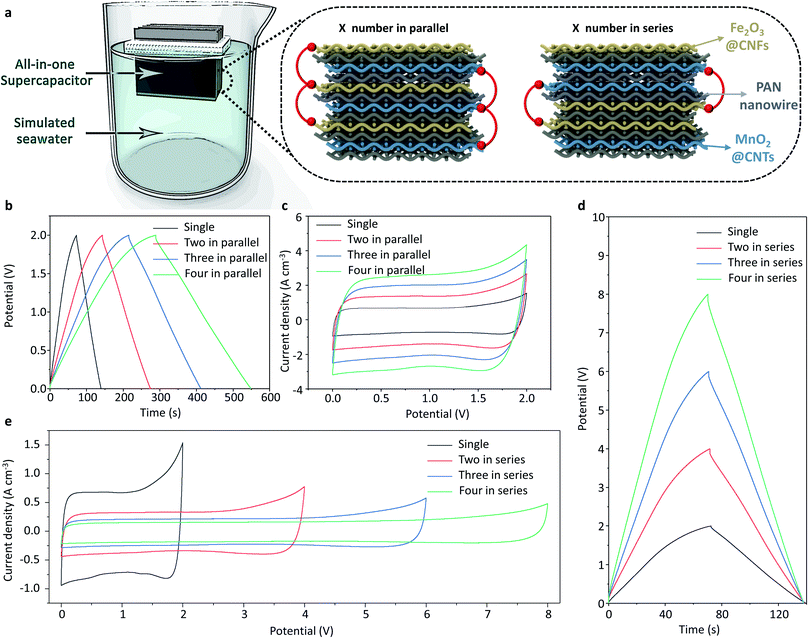
To demonstrate the concept of the paper-like all-in-one seawater supercapacitor device working in the marine environment for large-scale energy storage, the all-in-one seawater supercapacitor cells were integrated with the optimized circuit. Benefiting from the smart and flexible structural design, multiple capacitor cells can be integrated together to form a unified whole, achieving the purpose of series and parallel connections (Fig. 5a). The GCD and CV curves of the parallel-connected paper-like all-in-one seawater supercapacitors with units (1–4) are shown in Fig. 5b and c. The cycle time of completing a charge and discharge will increase with the increase of the parallel unit at the same current density. The output current increases with the increase of parallel units under the same voltage window, which provides strong evidence for the application of large current charging and discharging. The CV and GCD curves of the series-connected paper-like all-in-one seawater supercapacitor device with units (1–4) reveal that an output voltage of 8.0, 6.0 and 4.0 V can be achieved with the four, three and two serially connected cells, indicating the electrochemical performance multiplication effect of the integrated device compared with that of a single supercapacitor unit. Furthermore, for a potential application demonstration, three all-in-one supercapacitor cells were connected in series to expand the voltage window to 6.0 V, which can be used to charge a cell phone (HONOR V10 phone) as illustrated (Movie 1†). The high current and high voltage energy storage mode perfectly accords with the demand conditions of offshore energy storage, offering a promising strategy for future high-performance energy storage devices in the marine environment.
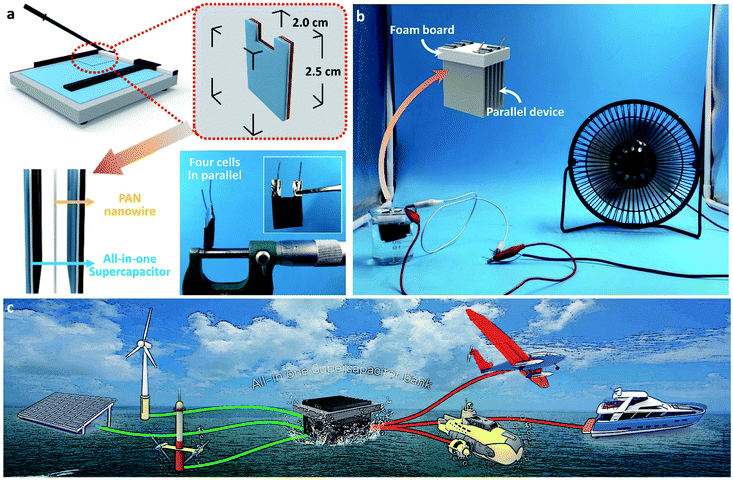
In order to verify that the all-in-one supercapacitor device meets the practical application requirements of a large current and a large voltage, we use a slicer to cut the all-in-one supercapacitor paper into 2.5 cm long and 2 cm wide sheets. The individual cells are separated from each other by PAN nanofiber film and connected in parallel to form a device group (Fig. 6a). The thickness of one device formed by connecting four separate cells in parallel is only 0.51 mm. We connected 6 parallel devices in series and suspended them in simulated seawater using a foam version. After the series device is fully charged, it successfully drives a small fan with a rated voltage of 12 V and a rated power of 2.5 W (Fig. 6b and Movie S1†). Unlike conventional rechargeable batteries, such as lithium-ion, lead–acid, nickel–metal hydride, and redox flow batteries, the proposed all-in-one seawater supercapacitor device here is charged and discharged by using natural seawater as the electrolyte; hence, they are suitable as main or auxiliary power sources in diverse marine sectors and typical energy storage applications. More impressively, the unique all-in-one 3D structure solves the problem of the increased cost caused by secondary packaging. The good flexibility makes it easier to face the unique geographical environment of the ocean. In this regard, the prospective applications have been made, as shown in Fig. 6c. Integrated all-in-one seawater supercapacitor devices could be distributed across the ocean serving as gas stations on the ocean, providing power for seaplanes, vessels and underwater detectors. This convenient and quick way of replenishing energy solves the energy barrier caused by the use of time and distance of marine equipment.
Conclusions
In summary, we proposed a paper-like all-in-one supercapacitor constructed from a nanofiber-based film in operando towards electrochemical energy storage in the marine environment, which features lightweight and excellent mechanical properties with a typical thickness of about 100 μm. The assembled flexible all-in-one supercapacitor device shows a remarkable performance of an energy density of 6.6 mW h cm−3 at a power density of 99.0 mW cm−3. The supercapacitor cell has a capacitance preservation rate of almost 100% under different bending conditions and a working temperature ranging from freezing point to summer heat, proving a robust environmental adaptability for potential applications in the marine environment. More impressively, benefiting from the smart and flexible structural design, multiple capacitor cells can be integrated to achieve a higher output voltage and output current through optimized circuit configuration series and parallel connections, which demonstrates a prospective solution for large-scale energy storage systems in the marine environment. It is believed that the proposed paper-like all-in-one seawater supercapacitor cell here offers a promising strategy for future high-performance energy storage devices in the marine environment.Experimental methods and details
Synthesis of the Fe2O3@CNFs based free-standing anode
Firstly, 0.34 g of polyacrylonitrile (PAN, Mw = 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Sigma-Aldrich) and a certain amount of iron(III) acetylacetonate (Fe(acac)3, Weiyi Chemical Technology Co. Ltd.) was successively dissolved in 4 mL N,N-dimethyl formamide (DMF, Rionlon-Bohua Pharmaceutical & Chemical Co., Ltd), followed by vigorous magnetic stirring overnight. Then, the obtained sol–gel was sent to the dedicated single needle electrospinning equipment to fabricate the nanofiber-shaped Fe(acac)3/PAN precursors (the typical electrospun parameters with the applied voltage of 1 kV cm−1), and the collected Fe(acac)3/PAN nonwoven nanofiber films were dried at 80 °C for 24 h. After that, the dried precursors were placed into a tube furnace for pre-oxidizing at 260 °C for 4 h in air, and then heated up to 800 °C with a rate of 4 °C min−1 under Ar atmosphere, maintained for 2 h to carbonize the PAN nanofibers. Finally, the carbonized Fe(acac)3/PAN (should be FeC@CNFs at this stage) was subjected to oxygen plasma etching for 10 minutes to transform the FeC to Fe2O3, then heated up to 300 °C with a rate of 2 °C min−1 for 2 h to obtain the eventual products of the Fe2O3@CNFs based free-standing anode.
000, Sigma-Aldrich) and a certain amount of iron(III) acetylacetonate (Fe(acac)3, Weiyi Chemical Technology Co. Ltd.) was successively dissolved in 4 mL N,N-dimethyl formamide (DMF, Rionlon-Bohua Pharmaceutical & Chemical Co., Ltd), followed by vigorous magnetic stirring overnight. Then, the obtained sol–gel was sent to the dedicated single needle electrospinning equipment to fabricate the nanofiber-shaped Fe(acac)3/PAN precursors (the typical electrospun parameters with the applied voltage of 1 kV cm−1), and the collected Fe(acac)3/PAN nonwoven nanofiber films were dried at 80 °C for 24 h. After that, the dried precursors were placed into a tube furnace for pre-oxidizing at 260 °C for 4 h in air, and then heated up to 800 °C with a rate of 4 °C min−1 under Ar atmosphere, maintained for 2 h to carbonize the PAN nanofibers. Finally, the carbonized Fe(acac)3/PAN (should be FeC@CNFs at this stage) was subjected to oxygen plasma etching for 10 minutes to transform the FeC to Fe2O3, then heated up to 300 °C with a rate of 2 °C min−1 for 2 h to obtain the eventual products of the Fe2O3@CNFs based free-standing anode.
Fabrication of the “paper-like” all-in-one supercapacitor device
Firstly, a PAN nanofibers film was deposited as the separator on the one side of the Fe2O3@CNFs film by in situ electrospinning, in which the PAN nanofibers film was fabricated by electrospinning the PAN contained solution (0.34 g dissolved in 4 mL DMF). Secondly, a carbon nanotubes (CNTs) film was sprayed at the surface of PAN with the CNTs solution (0.1 mg mL−1). After drying at 60 °C, the MnO2 film was electrodeposited on the surface of the CNTs with an aqueous electrolyte containing 20 mM Mn(NO3)2 and 100 mM NaNO3 (Aladdin Chem Inc., AR). Eventually, a paper-like all-in-one supercapacitor device was formed with the Fe2O3@CNFs as the anode, PAN as the separator and MnO2@CNTs as the cathode.Materials and structure characterization
The morphological microstructures of the Fe2O3@CNFs, MnO2@CNTs and all-in-one supercapacitor device were characterized by field-emission scanning electron microscopy (FESEM, Tescan Lyra 3) and transmission electron microscopy (TEM, Thermo Fisher Talos 200S), equipped with a super-X energy dispersive X-ray spectrometer. X-ray photoelectron spectroscopy (XPS, PHI5702) was employed to determine the oxide state of Mn, Fe, C and O. The crystallographic information and phase purity of the products were investigated by XRD (Rigaku D-max 2400) and Raman spectroscopy (HORIBA HR800).Assembling and regulating of the supercapacitor cell and integrated device
To optimize the electrochemical properties of the all-in-one supercapacitor, the mass of the active substances in the cathode and anode should be matched according to the principle of charge conservation (eqn (1)). A normal seawater-simulating electrolyte of 0.5 M NaCl (Shantou Chem Inc., AR) and a highly seawater-simulating electrolyte composed of 0.5 M NaCl, 0.036 M MgSO4, 0.0088 M CaSO4 and 0.0087 M KBr (Shantou Chem Inc., AR) are used as the electrolytes to evaluate the energy storage ability of the all-in-one supercapacitor device in the marine environment.| Q = mcathodeCcathodeVcathode = manodeCanodeVanode | (1) |
Among them, mcathode and manode represent the active material quality of the cathode (i.e., MnO2@CNTs) and the anode (i.e., Fe2O3@CNTs), Ccathode and Canode are the specific capacitance of the cathode and anode, Vcathode and Vanode are the voltage windows of the redox reaction at the cathode and anode, respectively.
Electrochemical measurements
Cyclic voltammetry (CV), galvanostatic charging–discharging (GCD), and electrochemical impedance spectroscopy (EIS) experiments were performed on a calibrated electrochemical station (CorrTest CS310) in the 0.5 M NaCl electrolyte to evaluate the electrochemical performance of the Fe2O3@CNFs and MnO2@CNTs based electrodes. The voltage window during CV measurements was set at between 0 and −1.2 V at scan rates of 5, 10, 20, 50, 100, and 200 mV s−1, respectively. The EIS spectra were measured in frequencies from 0.01 Hz to 100 kHz at an open-circuit voltage with an AC perturbation amplitude of 5 mV.Author contributions
S. C. and Z. D. contributed equally to this paper. S. C. and J. F. conceived the ideas, S. C. and Z. D. designed the experiments, fabricated the sample, measured the electrochemical performance and wrote the paper. P. C., K. W. Y. W. and Z. S. performed part of the device fabrication and integration; Y. Z. and X. C. helped with the deposition of MnO2 for the integrated device; and Y. L., Z. S. and Q. S. contributed to the discussion of the paper. J. F. and E. X. supervised the project, analyzed the data and revised the paper.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51772138, 51572118, 51601082 and 11974150), the Fundamental Research Funds for the Central Universities (No. lzujbky-2020-59), Special Funding for Open and Shared Large-Scale Instruments and Equipments of Lanzhou University. We are grateful for the fruitful discussion with Dr Minghao Yu from TU Dresden.References
- M. Z. Jacobson, M. A. Delucchi, Z. A. F. Bauer, S. C. Goodman, W. E. Chapman, M. A. Cameron, C. Bozonnat, L. Chobadi, H. A. Clonts, P. Enevoldsen, J. R. Erwin, S. N. Fobi, O. K. Goldstrom, E. M. Hennessy, J. Liu, J. Lo, C. B. Meyer, S. B. Morris, K. R. Moy, P. L. O'Neill, I. Petkov, S. Redfern, R. Schucker, M. A. Sontag, J. Wang, E. Weiner and A. S. Yachanin, Joule, 2017, 1, 108–121 CrossRef.
- S. Chu, Y. Cui and N. Liu, Nat. Mater., 2016, 16, 16–22 CrossRef PubMed.
- R. C. Armstrong, C. Wolfram, K. P. de Jong, R. Gross, N. S. Lewis, B. Boardman, A. J. Ragauskas, K. Ehrhardt-Martinez, G. Crabtree and M. V. Ramana, Nat. Energy, 2016, 1, 15020 CrossRef.
- M. Baumann, L. Barelli and S. Passerini, Adv. Energy Mater., 2020, 10, 2001002 CrossRef CAS.
- Z. Wang, R. Carriveau, D. S. K. Ting, W. Xiong and Z. Wang, Int. J. Energy Res., 2019, 43, 6108–6150 CrossRef.
- D. Desai, E. S. Beh, S. Sahu, V. Vedharathinam, Q. van Overmeere, C. F. de Lannoy, A. P. Jose, A. R. Völkel and J. B. Rivest, ACS Energy Lett., 2018, 3, 375–379 CrossRef CAS.
- S. H. Hsu, J. Miao, L. Zhang, J. Gao, H. Wang, H. Tao, S. F. Hung, A. Vasileff, S. Z. Qiao and B. Liu, Adv. Mater., 2018, 30, e1707261 CrossRef PubMed.
- S. M. Hwang, J. S. Park, Y. Kim, W. Go, J. Han, Y. Kim and Y. Kim, Adv. Mater., 2019, 31, e1804936 CrossRef PubMed.
- A. Alabastri, P. D. Dongare, O. Neumann, J. Metz, I. Adebiyi, P. Nordlander and N. J. Halas, Energy Environ. Sci., 2020, 13, 968–976 RSC.
- G. Zhou, Z. Guo, Y. Shan, S. Wu, J. Zhang, K. Yan, L. Liu, P. K. Chu and X. Wu, Nano Energy, 2019, 55, 42–48 CrossRef CAS.
- K. Mase, M. Yoneda, Y. Yamada and S. Fukuzumi, Nat. Commun., 2016, 7, 11470 CrossRef CAS PubMed.
- J. Zhao, Y. Jiang, H. Fan, M. Liu, O. Zhuo, X. Wang, Q. Wu, L. Yang, Y. Ma and Z. Hu, Adv. Mater., 2017, 29, 1604569 CrossRef PubMed.
- P. Geng, S. Zheng, H. Tang, R. Zhu, L. Zhang, S. Cao, H. Xue and H. Pang, Adv. Energy Mater., 2018, 8, 1703259 CrossRef.
- C. Liu, X. Yan, F. Hu, G. Gao, G. Wu and X. Yang, Adv. Mater., 2018, 30, 1705713 CrossRef PubMed.
- G. Wang, S.-K. Kim, M. C. Wang, T. Zhai, S. Munukutla, G. S. Girolami, P. J. Sempsrott, S. Nam, P. V. Braun and J. W. Lyding, ACS Nano, 2020, 14, 632–639 CrossRef CAS PubMed.
- Y. Zhou, K. Maleski, B. Anasori, J. O. Thostenson, Y. Pang, Y. Feng, K. Zeng, C. B. Parker, S. Zauscher, Y. Gogotsi, J. T. Glass and C. Cao, ACS Nano, 2020, 14, 3576–3586 CrossRef CAS PubMed.
- S. Cheng, Y. Zhang, Y. Liu, Z. Sun, P. Cui, J. Zhang, X. Hua, Q. Su, J. Fu and E. Xie, Chem. Eng. J., 2021, 406, 126875 CrossRef CAS.
- K. Krishnamoorthy, P. Pazhamalai and S.-J. Kim, Energy Environ. Sci., 2018, 11, 1595–1602 RSC.
- M. R. Lukatskaya, S. Kota, Z. Lin, M.-Q. Zhao, N. Shpigel, M. D. Levi, J. Halim, P.-L. Taberna, M. W. Barsoum, P. Simon and Y. Gogotsi, Nat. Energy, 2017, 2, 17105 CrossRef CAS.
- H. Guo, M. H. Yeh, Y. C. Lai, Y. Zi, C. Wu, Z. Wen, C. Hu and Z. L. Wang, ACS Nano, 2016, 10, 10580–10588 CrossRef CAS PubMed.
- H. Park, J. W. Kim, S. Y. Hong, G. Lee, H. Lee, C. Song, K. Keum, Y. R. Jeong, S. W. Jin, D. S. Kim and J. S. Ha, ACS Nano, 2019, 13, 10469–10480 CrossRef CAS PubMed.
- M. Yao, R. Wang, Z. Zhao, Y. Liu, Z. Niu and J. Chen, ACS Nano, 2018, 12, 12503–12511 CrossRef CAS PubMed.
- K. Wang, X. Zhang, C. Li, X. Sun, Q. Meng, Y. Ma and Z. Wei, Adv. Mater., 2015, 27, 7451–7457 CrossRef CAS PubMed.
- L. Liu, B. Shen, D. Jiang, R. Guo, L. Kong and X. Yan, Adv. Energy Mater., 2016, 6, 1600763 CrossRef.
- Y. Wang, S. Su, L. Cai, B. Qiu, N. Wang, J. Xiong, C. Yang, X. Tao and Y. Chai, Adv. Energy Mater., 2019, 9, 1900037 CrossRef.
- Z. Zhou, Q. Li, L. Yuan, L. Tang, X. Wang, B. He, P. Man, C. Li, L. Xie, W. Lu, L. Wei, Q. Zhang and Y. Yao, Energy Storage Materials, 2020, 25, 893–902 CrossRef.
- T. Yamashita and P. Hayes, Appl. Surf. Sci., 2008, 254, 2441–2449 CrossRef CAS.
- N. Liu, Y. Su, Z. Wang, Z. Wang, J. Xia, Y. Chen, Z. Zhao, Q. Li and F. Geng, ACS Nano, 2017, 11, 7879–7888 CrossRef CAS PubMed.
- H. Wang, Z. Xu, H. Yi, H. Wei, Z. Guo and X. Wang, Nano Energy, 2014, 7, 86–96 CrossRef CAS.
- K. Chi, Z. Zhang, Q. Lv, C. Xie, J. Xiao, F. Xiao and S. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 6044–6053 CrossRef CAS PubMed.
- X. Lu, Y. Zeng, M. Yu, T. Zhai, C. Liang, S. Xie, M. S. Balogun and Y. Tong, Adv. Mater., 2014, 26, 3148–3155 CrossRef CAS PubMed.
- Y. Zeng, Y. Han, Y. Zhao, Y. Zeng, M. Yu, Y. Liu, H. Tang, Y. Tong and X. Lu, Adv. Energy Mater., 2015, 5, 1402176 CrossRef.
- L. Wang, H. Yang, X. Liu, R. Zeng, M. Li, Y. Huang and X. Hu, Angew. Chem., Int. Ed., 2017, 56, 1105–1110 CrossRef CAS PubMed.
- D. Chen, S. Zhou, H. Quan, R. Zou, W. Gao, X. Luo and L. Guo, Chem. Eng. J., 2018, 341, 102–111 CrossRef CAS.
- F. Li, Y.-L. Liu, G.-G. Wang, H.-Y. Zhang, B. Zhang, G.-Z. Li, Z.-P. Wu, L.-Y. Dang and J.-C. Han, J. Mater. Chem. A, 2019, 7, 22631–22641 RSC.
- H. Liang, C. Xia, A.-H. Emwas, D. H. Anjum, X. Miao and H. N. Alshareef, Nano Energy, 2018, 49, 155–162 CrossRef CAS.
- S. Sun, T. Zhai, C. Liang, S. V. Savilov and H. Xia, Nano Energy, 2018, 45, 390–397 CrossRef CAS.
- J. Zhou, J. Cheng, B. Wang, H. Peng and J. Lu, Energy Environ. Sci., 2020, 13, 1933–1970 RSC.
- H. Li, Z. Tang, Z. Liu and C. Zhi, Joule, 2019, 3, 613–619 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ta09643a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |