Activity of layered swedenborgite structured Y0.8Er0.2BaCo3.2Ga0.8O7+δ for oxygen electrode reactions in at intermediate temperature reversible ceramic cells†
Ji-Seop
Shin
a,
Hyunyoung
Park
 a,
Kwangho
Park
a,
Muhammad
Saqib
a,
Minkyeong
Jo
a,
Jung Hyun
Kim
b,
Hyung-Tae
Lim
c,
Minseuk
Kim
a,
Jongsoon
Kim
a,
Kwangho
Park
a,
Muhammad
Saqib
a,
Minkyeong
Jo
a,
Jung Hyun
Kim
b,
Hyung-Tae
Lim
c,
Minseuk
Kim
a,
Jongsoon
Kim
 a and
Jun-Young
Park
a and
Jun-Young
Park
 *a
*a
aHMC, Department of Nanotechnology and Advanced Materials Engineering, Sejong University, Seoul 05006, Korea. E-mail: jyoung@sejong.ac.kr; Tel: +82 2 3408 3848
bDepartment of Advanced Materials Science and Engineering, Hanbat National University, Daejeon 34158, Korea
cSchool of Materials Science and Engineering, Changwon National University, Changwon 51140, Korea
First published on 9th December 2020
Abstract
To improve the thermal stability and intrinsically sluggish kinetics of oxygen electrode reactions in solid oxide fuel cells (SOFCs) and reversible protonic ceramic cells (RPCCs) at intermediate temperatures, a novel layered swedenborgite structure Y0.8Er0.2BaCo3.2Ga0.8O7+δ (YEBCG) catalyst is introduced as an alternative to perovskite materials that contain cobalt. The thermal expansion coefficient of YEBCG is 8.41 × 10−6 K−1, which is relatively well matched to the state-of-the-art proton-conducting and oxygen-ion-conducting electrolytes. The chemical bulk diffusion and surface exchange coefficients of YEBCG are 7.12 × 10−4 and 8.01 × 10−3 cm2 s−1, respectively, at 650 °C, which leads to much faster action than with state-of-art perovskite structured materials at intermediate temperatures. The maximum power densities of YEBCG cells are notably high, reaching 0.77 and 0.83 W cm−2 at 650 °C in SOFC and protonic ceramic fuel cell modes, respectively. Under electrolysis, the YEBCG cells achieve outstanding current densities of −0.61 and −4.42 A cm−2 at 500 and 700 °C, respectively, under an applied voltage of 1.4 V. Furthermore, the RPCC with YEBCG present no degradation over an entire 1000 h in fuel cell and electrolysis cell modes. These results demonstrate the excellent properties, including good durability, of the YEBCG air electrode when used in high performance SOFCs and RPCCs.
Introduction
Solid oxide fuel cells (SOFCs) are well regarded as sustainable and efficient electrochemical energy conversion devices because of their high energy conversion efficiency, excellent fuel flexibility, and low environmental impact. However, conventional SOFCs operate at high temperatures (>700 °C), this leads to high costs due to constraints on material selection and limited long-term thermal stability.1–3 To overcome these limitations at high operating temperatures, many researchers have investigated reducing the operating temperatures of SOFCs to intermediate temperatures (IT, 500–700 °C) through the use of high ionic conductivity ceria-based electrolytes (e.g. Sm0.2Ce1.8O2−δ) that work well at reduced temperatures.4,5 Recently, protonic ceramic fuel cells (PCFCs), which show low activation energies (0.3–0.6 eV) for proton transport at reduced temperatures (<700 °C), have received a tremendous amount of interest as an alternative to high temperature SOFCs. PCFCs have various advantages, they alleviate problems in areas such as material compatibility for cell fabrication and long-term thermal stress durability as well as having a higher CH4 conversion ratio and better carbon coking resistance with hydrocarbon fuels compared to SOFCs.6–8More recently, the PCFC technology has been extended to include reversible protonic ceramic cells (RPCCs) that address some energy storage and conversion challenges.9 RPCCs, which can operate in protonic ceramic fuel cell (PCFC) mode and electrolysis cell (PCEC) mode as a single electrochemical device, supply versatile pathways for energy storage and conversion of the inherently intermittent electricity produced from renewable energy sources.10–12 However, the electrochemical performance of IT-SOFCs and RPCCs stills needs to be improved in terms of electrical performance and reliability at low-to-intermediate temperatures in order to make their use commercially feasible. In particular, research into materials with high intrinsic oxygen reduction (ORR) and evolution reaction (OER) activity for use in IT-SOFCs and RPCCs is still lacking, especially at low-to-intermediate temperatures. This is despite the fact that the poor polarization resistance (Rp) of ceramic cells primarily originates from the sluggish kinetics of the air electrode materials being used.13–15
Of late, several novel approaches have been proposed to enhance the simultaneous migration of protons, oxygen-ions, and electrons through morphological tuning (e.g., infiltration and exsolution) of the proton-conducting mixed ionic–electronic (MIE) conducting composites at reduced temperatures.16,17 However, research into highly electrocatalytic active air electrode materials for ORR/OER is even more crucial at this time because there is still possibility for further performance enhancement through morphological tuning once the optimum materials are identified. Until now, Co-containing single and double perovskite materials, such as Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF), PrBa0.5Sr0.5Co1.5Fe0.5O5+δ (PBSCF), and NdBa0.5Sr0.5Co1.5Fe0.5O5+δ (NBSCF) have been used as the air electrode materials for RPCCs because of their excellent ORR/OER activity with high mixed ionic and electronic conductivity at intermediate temperatures.18–20 In addition, recent studies have demonstrated substantial water uptake and proton transport in double perovskite cobaltite systems.21,22 This property allows the simultaneous migration of protons, oxygen-ions, and electrons, resulting in high-performance RPCCs at intermediate temperatures. This is because water generation and dissociation reactions take place with ORR and OER, respectively. However, the thermal behavior of most cobaltite materials differs considerably from that of state-of-the-art proton-conducting and oxygen-ion conducting electrolytes [e.g. BaCe0.7Zr0.1Y0.1Yb0.1O3−δ (BCZYYb), BaZr0.85Y0.15O3−δ, Gd0.1Ce0.8O2−δ, and Smd0.1Ce0.8O2−δ (SDC)]. For example, the thermal expansion coefficient (TEC) value of BCZYYb electrolyte is 9.5 × 10−6 K−1, while BSCF, NBSCF, and PBSCF show extraordinary high TEC values of 23.2 × 10−6, 24.7 × 10−6, and 23.7 × 10−6 K−1, respectively.23–25 The high TECs of Co-based perovskites are related to the Co3+ cation state in those materials, this can transit from low spin (t62ge0g), to intermediate (t52ge1g), to high spin (t42ge2g) progressively, increasing its ionic radius at high temperature.26 This indicates that Co-containing perovskites could possibly be more detrimental in terms of long-term thermal stability because of the mismatch in TEC can impose severe mechanical stress between cell components.27
Co-containing swedenborgite structured YBaCo4O7+δ (YBC) materials are promising oxygen electrodes for IT-SOFCs and RPCCs on account of their very low TECs (8–11 × 10−6 K−1), which are very close to that of the BCZYYb electrolyte.28,29 The crystal structure of YBC consists of layers formed by two different types of CoO4 tetrahedral, (Co1)O4 and (Co2)O4, which are characterized by different bond lengths with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio.30 Hence, the YBC materials maintain high-spin states in a wide temperature range (no spin transition in Co2+ and Co3+ at high temperature). Furthermore, an acceptable level of MIC conductivity, a significantly large oxygen-storage capacity (2600 μmol O per g), and structural similarity to other cobaltites, provide good prospects for their use as air electrode materials in PCFCs.31–33 However, the YBC materials undergo severe phase decomposition at 600–800 °C due to the preference of Co cations for octahedral coordination, which makes their application in IT-SOFCs and RPCCs difficult.34 More recently, Manthiram et al.35 reported that YBC is completely stabilized by partial substitution of Ga in Co sites at 600–800 °C. Danilov et al.36 also demonstrated that other dopants, such as Fe and Zn, in the Co sites improved the catalytic activity toward ORRs in SOFCs. However, a comprehensive study of the doping effects on electrochemical performances and phase stability in YBC-based swedenborgite oxides for SOFCs and RPCCs at intermediate temperature has yet to be carried out.
3 ratio.30 Hence, the YBC materials maintain high-spin states in a wide temperature range (no spin transition in Co2+ and Co3+ at high temperature). Furthermore, an acceptable level of MIC conductivity, a significantly large oxygen-storage capacity (2600 μmol O per g), and structural similarity to other cobaltites, provide good prospects for their use as air electrode materials in PCFCs.31–33 However, the YBC materials undergo severe phase decomposition at 600–800 °C due to the preference of Co cations for octahedral coordination, which makes their application in IT-SOFCs and RPCCs difficult.34 More recently, Manthiram et al.35 reported that YBC is completely stabilized by partial substitution of Ga in Co sites at 600–800 °C. Danilov et al.36 also demonstrated that other dopants, such as Fe and Zn, in the Co sites improved the catalytic activity toward ORRs in SOFCs. However, a comprehensive study of the doping effects on electrochemical performances and phase stability in YBC-based swedenborgite oxides for SOFCs and RPCCs at intermediate temperature has yet to be carried out.
Herein, a systematic examination to gain fundamental understanding is carried out with a goal to improve the ORR/OER catalytic activity and phase stability of YBC-based swedenborgite oxides used in SOFCs and RPCCs as the air electrode material. We selected various doping elements, including lanthanides (Nd, Ce, La, Gd, Er, and Sm) and (post-) transition metals (Co, Cu, Mn, Ni, Fe, Ga, Cr, Al, and Ti) for Y- and Co-sites, respectively, for the YBC-based materials. The doping effect on the electrical properties of the YBC is investigated as functions of the dopants and their composition in a symmetrical cell. The electrochemical impedance spectroscopy (EIS) results identified that Y0.8Er0.2BaCo3.2Ga0.8O7+δ (YEBCG) exhibits much lower area specific resistances (ASRs) in comparison to YBC. The maximum power densities of the YEBCG cell are outstandingly high, reaching 1.30 and 1.35 W cm−2 at 700 °C, respectively, in SOFC and PCFC mode. The YEBCG RPCC achieved remarkably high current densities of −0.61 A cm−2 at 500 °C at an applied voltage of 1.4 V and the faradaic efficiency of 97.2% at an applied current of 0.5 A cm−2 demonstrating that the YEBCG cell produces the theoretically expected amount of H2. In addition, the YEBCG cell presented no degradation during the entire 400 h operation in PCFC mode and 500 h in electrolysis cell mode at 600 °C.
Experimental
Synthesis and characterization of swedenborgite structured YBC materials
YBC-based oxides were synthesized using a solid-state reaction method with proper stoichiometric amounts of precursors; BaCO3, Y2O3, CeO2, Nd2O3, La2O3, Co3O4, CuO, MnO2, NiO, Fe2O3 (99–99.9% purity, Alfa Aesar), Ga2O3, CrO3, Al2O3, TiO2 (99.9% purity, Sigma Aldrich), Gd2O3, Er2O3, Yb2O3, and Sm2O3 (99.9% purity, LTS Chem). The metal oxides were ball-milled using zirconia balls at the appropriate stoichiometric ratio in ethanol for 24 h and then dried at 60 °C in oven overnight. The as-prepared Y1−xLnxBaCo4O7+δ (Ln = La, Nd, Ce, Gd, Sm, Er, and Yb) and YBaCo4−xTrxO7+δ (Tr = Fe, Ga, Ni, Mn, Cu, Cr, Al, and Ti) precursors were calcined at 1100 °C for 6 h to obtain single swedenborgite structured materials. The Er and Ga-doped YBC (Y0.8Er0.2BaCo3.2Ga0.8O7+δ, YEBCG) was calcined at 1200 °C for 12 h in air. After calcination, the powders were pulverized using a mortar and pestle to give a uniform particle size.To investigate the crystalline structure of materials, the X-ray diffraction (XRD, X'Pert, PAN analytical) technique was used with a step size of 0.026° in a 2θ range of 20°–80° under Cu-Kα radiation. The diffraction peaks of the miller index were utilized to obtain the lattice parameters of the swedenborgite structured materials from hexagonal interplanar distances. XRD Rietveld structural refinement was also carried out using FullProf software. In addition, XRD was performed to characterize potential phase reactions between cell components during high-temperature sintering/calcination. The morphology and microstructure of the cell components were characterized by the use of field emission scanning electron microscopy (FESEM, SU-8010, Hitachi) and high resolution transmission electron microscopy (HRTEM, JEM 2100F, JEOL, 200 kV) with energy-dispersive X-ray spectroscopy (EDX, HORIBA). The compositions and chemical bonding states of the YBC were determined using a K-Alpha Plus X-ray photoelectron spectrometer (XPS, Thermo Scientific) with an Al Kα X-ray source of hv = 1486.6 eV. The thermal expansion coefficient (TEC) values of YBC and YEBCG were acquired using a dilatometer (L75H, LINSEIS) from room temperature to 900 °C with a heating/cooling rate of 5 °C min−1 in air. For the TEC measurements, the YBC and YEBCG powders were uniaxially pressed to fabricate a 4.1 × 4.1 × 22 mm rectangular bar that was then sintered at 1250 °C (for YBC) or 1300 °C (for YEBCG) for 24 h in air.
Fabrications of symmetric and anode-supported cells
In order to measure the ASR for all YBC samples including the doped YBC, symmetric cells based on Sm0.2Ce0.8O2−δ (SDC) electrolytes were prepared. The SDC powders (produced by Kceracell, Korea) were uniaxially pressed under 18 MPa for 1 min in a circular mold then sintered at 1650 °C for 10 h in air to acquire very dense electrolyte pellets. Electrode inks were prepared by mixing YBC-based materials with ESL 441 vehicle (ESL ElectroScience), these were then screen-printed on both sides of the electrolyte pellet and sintered at 950 °C for 2 h.The NiO-SDC and NiO-BCZYYb (65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 wt%) anode-supported cells were fabricated using the tape-casting method. The NiO (Kceracell) and SDC (and BCZYYb, Kceracell) powders were ball-milled with poly methyl methacrylate (Alfa Aesar), fish oil (Aldrich), polyvinyl butyral (Butvar), di-n-butyl phthalate (Daejung Chemicals), and polyethylene glycol (Acros) in toluene (Aldrich) and ethyl alcohol solutions for 48 h. The NiO-SDC (and BCZYYb) slurries were cast into a polyester mylar film using a doctor blade system (Hansung Systems) before being pre-sintered at 900 °C for 2 h. The anode functional layer (AFL) and electrolyte slurries were mixed with polyvinyl butyral, di-n-butyl phthalate, and Solsperse (SG24000, Lubrizol) for 48 h, then they were coated on the anode substrate using the drop-coating method before sintering at 1550 and 1450 °C for 4 h for SDC and BCZYYb electrolytes, respectively. For the preparation of cathode ink, the as-prepared YBC-based materials and SDC (and BCZYYb) were composited in a ratio of 6
35 wt%) anode-supported cells were fabricated using the tape-casting method. The NiO (Kceracell) and SDC (and BCZYYb, Kceracell) powders were ball-milled with poly methyl methacrylate (Alfa Aesar), fish oil (Aldrich), polyvinyl butyral (Butvar), di-n-butyl phthalate (Daejung Chemicals), and polyethylene glycol (Acros) in toluene (Aldrich) and ethyl alcohol solutions for 48 h. The NiO-SDC (and BCZYYb) slurries were cast into a polyester mylar film using a doctor blade system (Hansung Systems) before being pre-sintered at 900 °C for 2 h. The anode functional layer (AFL) and electrolyte slurries were mixed with polyvinyl butyral, di-n-butyl phthalate, and Solsperse (SG24000, Lubrizol) for 48 h, then they were coated on the anode substrate using the drop-coating method before sintering at 1550 and 1450 °C for 4 h for SDC and BCZYYb electrolytes, respectively. For the preparation of cathode ink, the as-prepared YBC-based materials and SDC (and BCZYYb) were composited in a ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 wt% with a texanol-based vehicle (type-411, ESL). The composite cathode YBC-SDC and YBC-BCZYYb inks were screen-printed onto the sintered SDC and BCZYYb electrolytes surface, respectively, and then finally sintered at 950 °C for 2 h. Au wires were attached with an Ag mesh using platinum paste (Heraeus, USA) as the current collector.
4 wt% with a texanol-based vehicle (type-411, ESL). The composite cathode YBC-SDC and YBC-BCZYYb inks were screen-printed onto the sintered SDC and BCZYYb electrolytes surface, respectively, and then finally sintered at 950 °C for 2 h. Au wires were attached with an Ag mesh using platinum paste (Heraeus, USA) as the current collector.
Electrochemical performance measurements of YBC materials
The electrochemical activity for oxygen electrode reactions in YBC-based materials was evaluated using the polarization resistance (Rp) of the symmetric cells with the SDC measured by electrochemical impedance spectroscopy (EIS) (VSP, Biologic, Claix) from 500 to 800 °C in the frequency range of 1 MHz to 0.1 Hz (10 mV scanning amplitude) under ambient air and open-circuit voltage (OCV) conditions. To further understand the influence of doping YBC on the kinetics of the ORR, the ASR of the symmetric cell with the SDC electrolyte was tested under several oxygen partial pressures (pO2, from 0.21 to 0.04 atm) at 600–700 °C.The chemical diffusion (Dchem) and surface exchange coefficients (kchem) values for oxygen in the YBC-based materials were determined by electrical conductivity relaxation (ECR) experiments on thin rectangular slabs in a van der Pauw electrode configuration. The samples were prepared by cold-pressing the YBC-based powders then sintering at 1250 °C (for YBC) or 1300 °C (for YEBCG) for 24 h to fabricate a dense bar. DC electrical conductivity was measured using a potentiostat/galvanostat (VSP, Bio-Logic) according to oxygen partial pressure (−1.98 ≤ log(pO2 per atm) ≤ −0.68) that was controlled using a mixture of O2 and N2 gas at 600–750 °C. The normalized electrical conductivity (Г) as a function of time is given by eqn (1);
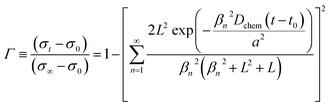 | (1) |
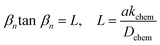 | (2) |
The anode-supported single cell performance measurements were performed using a fuel cell test station (Scitech Korea) equipped with humidifiers, gas mass flow controllers, and an alumina reactor. Ceramic adhesive (Ceramabond™ 668, Aremco) was used to seal the anode side of the single cells in an alumina tube. Current–voltage (I–V) polarization curve measurements were carried out using a potentiostat/galvanostat (SP-240, BioLogic) under 3 vol% humidified hydrogen and ambient air on the fuel and air electrode side, respectively, at a flow rate of 200 sccm for the SOFC and PCFC. While in PCEC mode, 20% steam mixed with air was fed into the air electrode side using a micro peristaltic pump (Ismatec). The steam partial pressure was controlled by a temperature-controlled water bubbler. The 10% hydrogen in the wet N2 stream was fed into the hydrogen electrode side at a flow rate of 200 sccm. The faradaic efficiency of the cells under PCEC operation was measured by analyzing the gas composition of the hydrogen electrode exhaust using gas chromatography.
Density functional theory calculations
All the density functional theory (DFT) calculations were performed using the Vienna Ab initio Simulation Package (VASP).40 We used projector-augmented wave (PAW) pseudopotentials41 with a plane-wave basis set as implemented in VASP. Perdew–Burke–Ernzerhof (PBE) parametrization of the generalized gradient approximation (GGA)42 was used for the exchange-correlation functional. For the DFT calculations, a 3 × 2 × 2 k-point grid was used to calculate a 1 × 2 × 1 supercell structure of YBC and YBC-based materials. The GGA+U method43 was adopted to address the localization of the d-orbital in Co and each dopant ions, as determined in a previous report.44–47 An appropriate number of k-points and a kinetic energy cutoff of 500 eV were used in all the calculations. All the structures were optimized until the force in the unit cell converged to within 0.03 eV Å−1.Results and discussion
Structural analysis of YBC-based powders
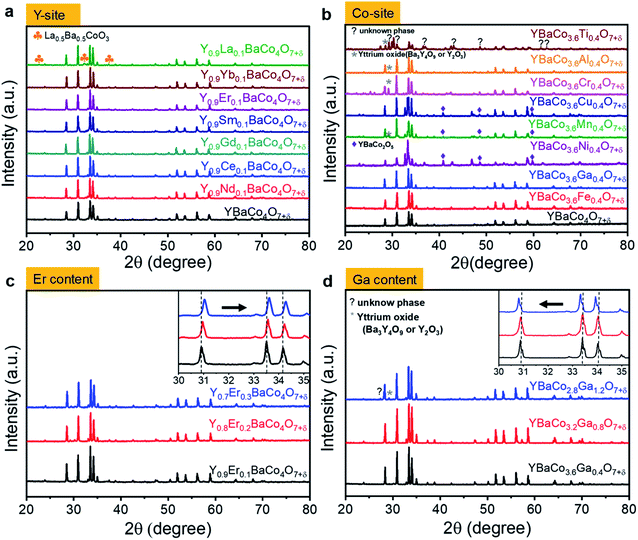
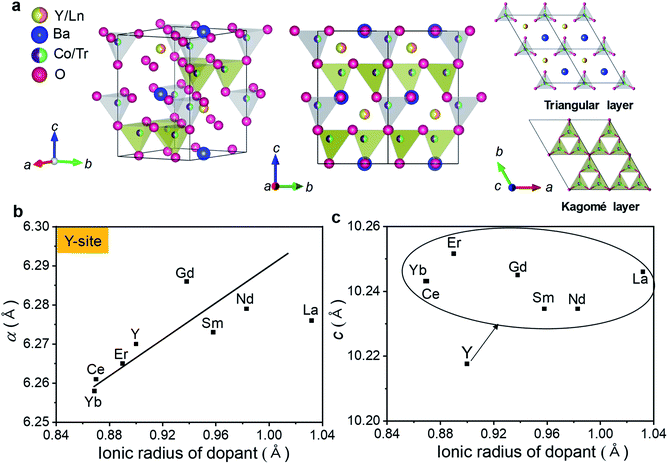
To evaluate the phase and structure of the as-prepared YBaCo4O7+δ (YBC)-based powders, XRD analysis was performed and the results are shown in Fig. 1. The Y-site in the YBC was substituted with various rare-earth metals (Ln = La, Nd, Ce, Gd, Sm, Er, and Yb) to fabricate Y0.9Ln0.1BaCo4O7+δ (YLBC) samples. XRD patterns of the synthesized YLBC powders, after using the solid-state reaction method, exhibited a swedenborgite (NaBe4SbO7) structure without any detectable impurities (except La) (Fig. 1a).48 Small traces of impurity phases, such as La0.5Ba0.5CoO3−δ (PDF#: 32-0480), were detected in the La-doped YBC sample. The transition and post-transition metals (Tr = Fe, Ga, Ni, Mn, Cu, Cr, Al, and Ti), which include the elements in groups 3–12 of the periodic table, were also doped into the Co-site of the YBC to synthesize YBaCo3.6Tr0.4O7+δ (YBCT) samples. Fig. 1b shows that only YBaCo3.6Fe0.4O7+δ (YBCF) and YBaCo3.6Ga0.4O7+δ (YBCG) powders have a pure phase without detectable impurities. Other dopants in the YBCT resulted in the formation of small amounts of impurities, such as Ba3Y4O9 (PDF#: 38-1377), Y2O3 (PDF#: 43-1036), YBaCo2O5 (PDF#: 47-0735), and unknown phases.As shown in Fig. 2a, the crystal structures of the YBC, YLBC, and YBCT were indexed as hexagonal structures with a space group of P63mc, in the agreement with the data seen in literature.49,50 The YBC-based oxides, belonging to the swedenborgite compound family, exhibited a special layered crystal structure that consists of an alternating stacking of Co2+/3+O4 tetrahedral layers with Kagomé (6c site) and adjacent triangular (2a site) lattices along the crystallographic c-axis.51 The oxygen framework can be labelled as hexagonal “ABCB” stacking of close-packed alternating O4 and BaO3 layers.52 Barium and yttrium cations have 12- and 6-fold coordination with oxygen atoms. In addition, the yttrium cation is likely to be substituted by another cation easily, whereas cobalt is not readily replaced by other transition metal ions so we see the formation of unwanted impurity phases, as can be seen in Fig. 1a and b.53 In order to provide further insight into the impact of dopants on the YBC structure, the trends in cell parameters of the primary YBC phase for the various samples were analyzed using the XRD data (Tables 1 and 2).
| Materials (space group: P63mc, hexagonal) | Lattice parameter | Electro-negativity (dopants) | ASR at 600 °C (Ω cm2) | ASR at 650 °C (Ω cm2) | Activation energy (eV) | |
|---|---|---|---|---|---|---|
| a (Å) | C (Å) | |||||
| YBaCo4O7+δ | 6.270 ± 0.001 | 10.218 ± 0.001 | 1.22 (Y) | 5.588 | 2.513 | 1.28 |
| Y0.9Yb0.1BaCo4O7+δ | 6.258 ± 0.001 | 10.244 ± 0.001 | 1.10 (Yb) | 7.783 | 2.687 | 1.40 |
| Y0.9Ce0.1BaCo4O7+δ | 6.261 ± 0.002 | 10.243 ± 0.001 | 1.12 (Ce) | 5.937 | 2.400 | 1.28 |
| Y0.9Nd0.1BaCo4O7+δ | 6.279 ± 0.004 | 10.238 ± 0.002 | 1.14 (Nd) | 6.001 | 2.364 | 1.30 |
| Y0.9Sm0.1BaCo4O7+δ | 6.273 ± 0.003 | 10.238 ± 0.002 | 1.17 (Sm) | 6.532 | 2.546 | 1.34 |
| Y0.9Gd0.1BaCo4O7+δ | 6.287 ± 0.002 | 10.245 ± 0.001 | 1.20 (Gd) | 4.846 | 2.143 | 1.23 |
| Y 0.9 Er 0.1 BaCo 4 O 7+δ | 6.265 ± 0.002 | 10.254 ± 0.002 | 1.24 (Er) | 4.004 | 1.649 | 1.24 |
| Y0.9La0.1BaCo4O7+δ | 6.276 ± 0.001 | 10.246 ± 0.001 | 1.10 (La) | — | — | — |
| Materials (space group: P63mc, hexagonal) | Lattice parameter | Electro-negativity (dopants) | ASR at 600 °C (Ω cm2) | ASR at 650 °C (Ω cm2) | Activation energy (eV) | |
|---|---|---|---|---|---|---|
| a (Å) | c (Å) | |||||
| YBaCo4O7+δ | 6.270 ± 0.001 | 10.218 ± 0.001 | 1.88 (Co) | 5.588 | 2.513 | 1.28 |
| YBaCo3.6Fe0.4O7+δ | 6.267 ± 0.003 | 10.209 ± 0.001 | 1.83 (Fe) | 12.773 | 4.618 | 1.46 |
| YBaCo 3.6 Ga 0.4 O 7+δ | 6.290 ± 0.001 | 10.251 ± 0.001 | 1.81 (Ga) | 2.967 | 1.447 | 0.98 |
| YBaCo3.6Ni0.4O7+δ | 6.267 ± 0.001 | 10.235 ± 0.001 | 1.83 (Ni) | — | — | — |
| YBaCo3.6Mn0.4O7+δ | 6.280 ± 0.001 | 10.245 ± 0.001 | 1.55 (Mn) | — | — | |
| YBaCo3.6Cu0.4O7+δ | 6.279 ± 0.001 | 10.248 ± 0.004 | 1.9 (Cu) | — | — | |
| YBaCo3.6Cr0.4O7+δ | 6.282 ± 0.002 | 10.253 ± 0.001 | 1.66 (Cr) | — | — | |
| YBaCo3.6Al0.4O7+δ | 6.270 ± 0.002 | 10.227 ± 0.001 | 1.61 (Al) | — | — | |
The introduction of new atoms to YBC is expected to lead to changes in cell parameters. As seen in Fig. 2b, the lattice constant of YBC-based materials is linearly increased as the radius of the dopant increases. Lattice parameters a and c are 6.270 and 10.218 Å for YBC. After Y-site doping into YBC, a and c are in the range of 6.258–6.287 and 10.238–10.254 Å for YLBC, respectively, depending on the relative radius of dopants to the Y cation. That is, Y-site doping by rare-earth metals leads to a significant increase in the c-axis lattice parameter (Fig. 2c). In contrast, there is no clear correlation between the transition metal dopants in the Co-site and structural parameters of YBCT (data is shown here). Impurity phases in the structure and various oxidation states of the transition metals in YBCT may result in deviations from the linear relationship between the cell parameters and the atomic radius of dopants. Dopant atoms in YBC-based crystal may alter not only the overall lattice parameter with lattice contraction and expansion but also the local crystal lattice surrounding the dopant atoms (e.g. lattice distortions). The changes in the interatomic spacings due to the local lattice distortions that are a result of alterations related to the ionic radii of the doping atoms in the YBC are eventually reflected by changes in the electronic states and bonding states of Co–O, this then influences the electrocatalytic activity of cathode materials in IT-SOFCs.54 This will be discussed further in the next section.
Electrical conductivity of YBC-based materials
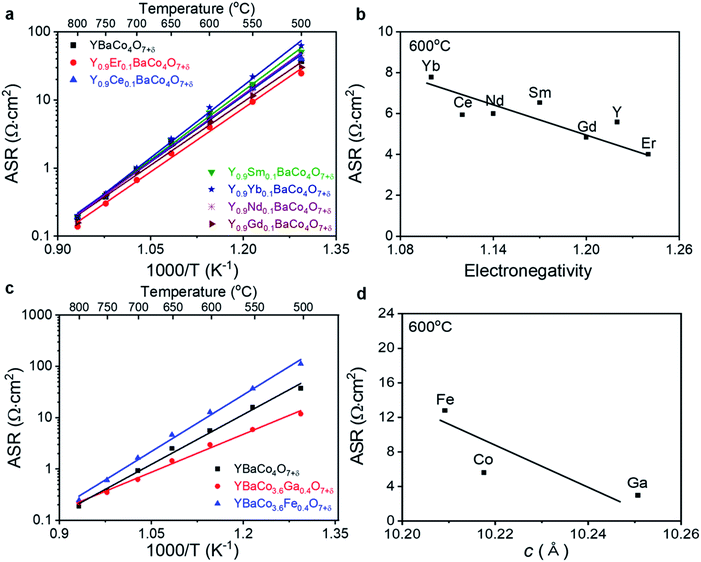
In order to evaluate the electrocatalytic activity of YBC-based materials for oxygen electrode reactions, electrochemical impedance spectroscopy (EIS) analysis was performed under open-circuit voltage (OCV) conditions in dry air at 500–800 °C. ASRs of the YBC-based materials were measured in symmetrical cells with SDC electrolyte. Fig. 3 shows the Arrhenius plot of ASR versus temperature for the YBC-based materials. In the case of Y-site doping in the YBC (Fig. 3a), the Y0.9Er0.1BaCo4O7+δ cell substituted with Er3+ exhibited lower ASR than that of YBC and other doped YBC materials in all temperature ranges. In addition, using Gd3+ and Er3+ as dopants for the Y-site was very effective in decreasing the ASR of YBC at IT, whereas the Y0.9Sm0.1BaCo4O7+δ (YSBC) and Y0.9Yb0.1BaCo4O7+δ (YYBC) with Sm3+ and Yb3+ dopants showed higher ASRs than YBC.It is interesting to note that the ASR of YLBC materials tends to increase with the electronegativity of cations at 600 °C, as shown in Fig. 3b. In particular, Er, which possesses a higher electronegativity (1.24) than other rare-earth metals, exhibits the lowest ASR with the lowest activation of 1.24 eV, among the various rare-earth oxide dopants (Table 1). These results may come from the fact that the doping of high electronegativity cations in the Y-site is likely to increase the covalent character of YLBC. That is, the electronegativity of Er strongly attracts nearby electrons in the crystal structure due to the significantly high electronegativity of Co (1.88) and O (3.44), allowing more electrons to share with the Co–O bond. In addition, Suntivich et al.55 and Shao-Horn et al.56 reported that increased covalency in the M–O bond facilitates charge transfer between surface cations and absorbates such as O2− and O22− for ORR, which can result in lower ASRs of cathode materials. In contrast, it was not easy to find any correlation between the electrical conductivity and cell parameters (Fig. S1†).
To investigate the doping of transition and post-transition metal oxides in to the Co-sites of YBC, the ASRs of YBCF- and YBCG-based symmetrical cells with SDC electrolyte were also determined by EIS measurements under OCV conditions in dry air at 500–800 °C and the results are presented in Fig. 3c (Fig. S2†). The EIS results for the other transition and post-transition metals oxide cells are not presented here because of the impurity phases that accompanied the doping process. In the case of Co-site doping into the YBC, the YBaCo3.6Ga0.4O7+δ (YBCG) cell substituted with Ga3+ exhibited lower ASRs than YBC or YBaCo3.6Fe0.4O7+δ (YBCF) at IT. Interestingly, the ASRs of YBCT materials decreased linearly with the increase in the cell parameters at 600 °C, as shown in Fig. 3d. In particular, Ga (YBCG), which has a larger structural parameter than other transition metal oxides (YBCT), demonstrates the lowest ASRs (Table 2). This increase is simply due to the large cell parameters of YBCG; the wider the lattice and the larger the spaces in a structure, the faster it is for oxide ions to transport through the lattice. In addition, the different oxidation states of the substituents may contribute to improve the ASR of YBCT with the modification of the average Co valence, this would affect the mixed ionic and electronic conductivity of YBC-based materials.
Er and Ga doped YBC materials
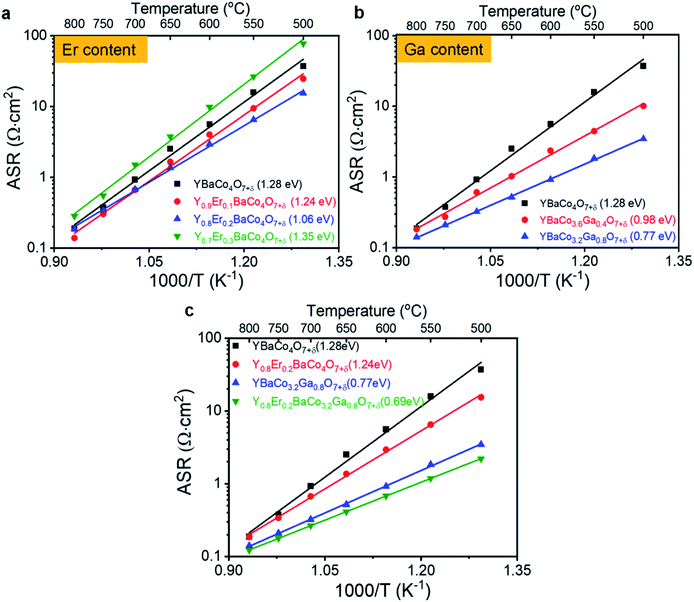
We investigate the synergistic effects of two dopants (Er and Ga) in the Y- and Co-sites on the performance of a swedenborgite structured YBC cathode. First, to optimize the concentration of Er-doping in Y1−xErxBaCo4O7+δ (YEBC, x = 0.1, 0.2, and 0.3), the YEBC was synthesized using the solid-state reaction method. The diffraction peaks from the Y-site substituted YEBC with the different concentrations of Er appeared as a single phase without any secondary phase, as shown in Fig. 1c. A slight shift in the XRD peaks towards a higher 2θ for YEBC was observed with increasing Er content (inset of Fig. 1c), indicating that the lattice parameter does decrease slightly, this is because the Er3+ (0.89 Å) has a smaller ionic radius than Y3+ (0.90 Å).ASRs of the YEBC materials with various Er concentration were determined by EIS and the results are presented in Fig. 4a. 20 mol% Er-doped YBC (Y0.8Er0.2BaCo4O7+δ) reveals the lowest ASRs in the intermediate temperature range (500–700 °C) among the Er-doped YBC materials. In addition, the activation energies of YBC, Y0.9Er0.1BaCo4O7+δ, Y0.8Er0.2BaCo4O7+δ, and Y0.7Er0.3BaCo4O7+δ, calculated from Arrhenius equation, were 1.28, 1.24, 1.06, and 1.35 eV, respectively, in the SDC electrolyte symmetrical cell. That is, the oxygen electrode activity increases with increasing Er in the YEBC materials. However, the ASR of the Y0.7Er0.3BaCo4O7+δ cathode material is lower than that of Y0.8Er0.2BaCo4O7+δ with significantly higher activation energy. The decreased oxygen electrode activity for high concentrations of Er doping might result from a slight phase instability or from defect interactions due to different dopant cations.
Second, the Ga-doped YBC materials (YBaCo4−yGayO7+δ, YBCG) were fabricated with various Ga contents (y = 0, 0.4, 0.8, and 1.2) to find the best composition for ASRs when used as a cathode material. The XRD patterns of the YBaCo3.6Ga0.4O7+δ, YBaCo3.2Ga0.8O7+δ, and YBaCo2.8Ga1.2O7+δ powders with the Rietveld refinement results are shown in Fig. 1d. Both the YBaCo3.6Ga0.4O7+δ and YBaCo3.2Ga0.8O7+δ formed single swedenborgite phases, whereas the YBaCo2.8Ga1.2O7+δ powder exhibited a minor secondary phase of yttrium oxides, such as Ba3Y4O9 and Y2O3, implying the solubility limit of Ga dopant in YBCG was reached. Interestingly, in contrast to the peak shift in the XRD patterns for the Y-site substituted YBCs with different Er concentrations, the YBCG exhibited a shift towards a lower 2θ angle with increasing Ga concentration (inset of Fig. 1d), indicating the increasing of cell parameters with the Ga doping. The electrochemical activity of YBCG towards oxygen electrode reactions was measured by EIS in ambient air, the ASRs are presented in Fig. 4b. The activity increases with increasing levels of Gd dopant, this is accompanied by decreasing activation energy for oxygen electrode reactions. The activation energies of YBC, YBaCo3.6Ga0.4O7+δ, and YBaCo3.2Ga0.8O7+δ were also calculated as 1.28, 0.98, and 0.77 eV, indicating that Ga doping facilitates the kinetics for ORR/OER really well as a result of the increased cell parameters, as mentioned in the previous section.
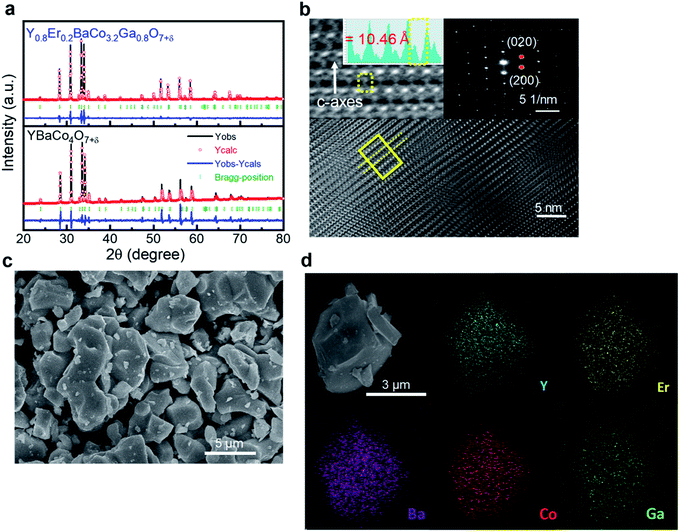
Third, we investigate the impact of co-doping of Er3+ and Ga3+ in YBC materials on oxygen electrode activity. Er- and Ga-doped YBC materials (Y0.8Er0.2BaCo3.2Ga0.8O7+δ, YEBCG) were synthesized using the solid-state reaction method. Fig. 5a shows the XRD results for the YEBCG powders with Rietveld refinement to obtain accurate structural information. The low reliability factors (e.g. Rwp = 2.37%, Rp = 1.24%) for YEBCG indicate good fitting between the experimental and calculated XRD patterns with a goodness-of-fit (χ2) of 6.16. The XRD patterns confirm the crystallization into a swedenborgite structure after calcinations. Table 3 lists the Rietveld analysis of YEBCG. The Rietveld refinement shows that all compounds crystallized in to a hexagonal structure with the space group P63mc, indicating that the space group symmetry of YBC does not change with the substitution of Er and Co.49,50 However, an increase in the structural parameters (a and c) was observed, a went from 6.270 to 6.304 Å and c went from 10.218 to 10.263 Å. This led to an increase in the unit cell volume from 347.897 Å3 (for YBC) to 353.223 Å3 (for YEBCG).
| Materials (space group: P63mc, hexagonal) | Lattice parameter | ASR at 600 °C (Ω cm2) | ASR at 650 °C (Ω cm2) | Activation energy (eV) | |
|---|---|---|---|---|---|
| a (Å) | c (Å) | ||||
| Y0.8Er0.2BaCo4O7+δ | 6.254 ± 0.001 | 10.235 ± 0.001 | 2.933 | 1.366 | 1.06 |
| Y0.7Er0.3BaCo4O7+δ | 6.248 ± 0.001 | 10.212 ± 0.002 | 9.792 | 3.745 | 1.35 |
| YBaCo3.2O0.8GaO7+δ | 6.303 ± 0.001 | 10.262 ± 0.001 | 0.917 | 0.517 | 0.77 |
| YBaCo2.8O1.2GaO7+δ | 6.307 ± 0.001 | 10.266 ± 0.001 | — | — | — |
| Y 0.8 Er 0.2 BaCo 3.2 Ga 0.8 O 7+δ | 6.304 ± 0.003 | 10.263 ± 0.002 | 0.680 | 0.413 | 0.69 |
In order to further understand the structure of YEBCG, HRTEM and FESEM examinations were performed. The swedenborgite structure in the YEBCG was confirmed with the selected area electron diffraction (SAED) pattern from the HRTEM image (Fig. 5b). Inset of Fig. 5b shows the representative points in the SAED pattern, which can be indexed as (020) and (200) reflections along the [001] zone axis of YEBCG, this is in agreement with the results obtained from Rietveld refinement (Table 3). Fig. 5c and d display the FESEM morphology of the YEBCG powders with HRTEM/EDX elemental mapping. The YEBCG shows an agglomerated spherical particle morphology 1–4 μm in size because of the high calcination temperature (1200 °C for 12 h) used in the conventional solid-state reaction method. Compositional mapping images of YEBCG confirmed that the all the constituent cations, Y, Er, Ba, Co, and Ga, are homogeneously distributed without any observable elemental segregation in the EDX analysis.
To look into the effects of co-doping in the YEBCG materials on electrocatalytic activity for oxygen electrode reactions, the ASR was measured by EIS under OCV in ambient air with a symmetrical cell. Fig. S3a† exhibits the Nyquist complex plane of YEBCG at 500–800 °C. Two parallel R–Q circuits were used to fit the EIS data, where Rohmic, RHF, and RLF represent the ohmic, charge transfer (HF; high frequency), and mass transfer (LF; low frequency) resistances of the cells, respectively, where Q2 and Q3 represent the constant phase elements. As shown in Fig. 4c, YEBCG exhibits lower ASRs in all measured temperature ranges compared to YBC, Y0.8Er0.2BaCo4O7+δ, and YBaCo3.2Ga0.8O7+δ, suggesting the co-doping of Er and Ga effectively enhanced the ORR electrocatalytic activity. In addition, the activation energy of YEBCG was considerably decreased from 1.28, 1.06, and 0.77 eV for YBC, Y0.8Er0.2BaCo4O7+δ, YBaCo3.2Ga0.8O7+δ, respectively, to 0.69 eV, indicating that the co-doping of Er and Co impressively expedites the kinetics for ORR/OER (Table 3). Furthermore, the YEBCG outperformed the other perovskite structured cathode materials at intermediate temperatures. For example, the ASR of YEBCG was 0.413 Ω cm2 at 650 °C, and it was much lower than that of LSCF57 and SSC,58 implying its appropriateness for triggering oxygen electrode reactions at intermediate temperatures (Fig. S3b†). Fig. S3c† shows the electrical conductivity of the YEBCG in the temperature range of 300–800 °C. The YEBCG demonstrated excellent electrical conductivity (1.4–2.7 S cm−1) at intermediate temperatures (500–700 °C).
Oxygen electrode reaction activity of YEBCG
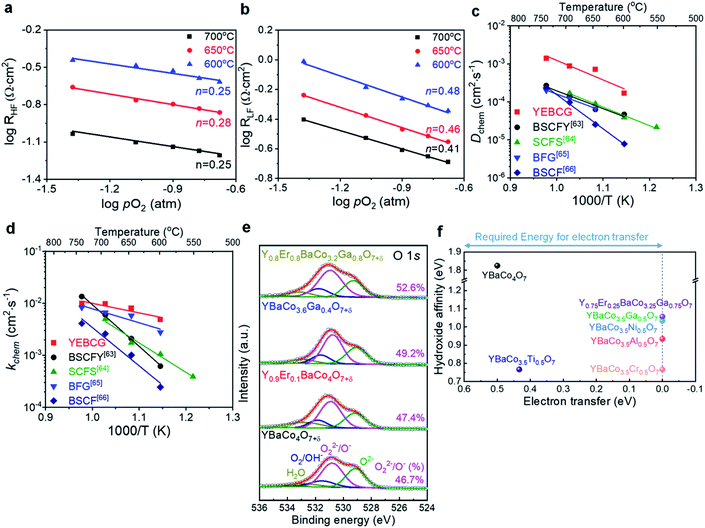
To analyze the oxygen electrode reaction step and understand electrochemical kinetics of YEBCG, EIS measurements of the symmetric cell were performed at various temperatures and with the pO2 as seen in Fig. 6. It can be seen that the polarization resistance (Rp) decreases linearly with increasing pO2 on a logarithmic scale, indicating that the ORR process is strongly affected by the oxygen concentration. The slope of the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Rp for YEBCG was calculated as a function of log
Rp for YEBCG was calculated as a function of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pO2 (in the relation Rp ∝ pO2n−) to estimate the rate-determining step (RDS) of the ORR process on the cathode.59 The oxygen activity for the cathode involves oxygen adsorption, dissociation, surface diffusion, and charge-transfer processes, how these processes occur is closely related to both the cathode's surface and bulk properties.60 For n = 1/4, the RDS is generally attributed to the charge transfer process at the cathode interface
pO2 (in the relation Rp ∝ pO2n−) to estimate the rate-determining step (RDS) of the ORR process on the cathode.59 The oxygen activity for the cathode involves oxygen adsorption, dissociation, surface diffusion, and charge-transfer processes, how these processes occur is closely related to both the cathode's surface and bulk properties.60 For n = 1/4, the RDS is generally attributed to the charge transfer process at the cathode interface 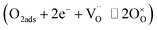 , while n = 1/2 is typically associated with the mass transfer processes such as surface oxygen adsorption and dissociation (O2ads ⇌ 2Oads). As shown in Fig. 6a and b, the n slopes of RHF and RLF turn up at around n = 1/4 and 1/2, respectively, indicating that the ORR at high frequency corresponds to the charge transfer process, whereas the RDS at low frequency is related to the oxygen adsorption and dissociation process. It should be noted that RHF is always smaller than RLF for various pO2 at 600–700 °C, this specifies that the RDS for the ORR is the diffusion process, on the other hand, the charge transfer of YEBCG is greatly improved.
, while n = 1/2 is typically associated with the mass transfer processes such as surface oxygen adsorption and dissociation (O2ads ⇌ 2Oads). As shown in Fig. 6a and b, the n slopes of RHF and RLF turn up at around n = 1/4 and 1/2, respectively, indicating that the ORR at high frequency corresponds to the charge transfer process, whereas the RDS at low frequency is related to the oxygen adsorption and dissociation process. It should be noted that RHF is always smaller than RLF for various pO2 at 600–700 °C, this specifies that the RDS for the ORR is the diffusion process, on the other hand, the charge transfer of YEBCG is greatly improved.
Furthermore, the kinetic properties for ORR/OER can be related to the bulk diffusion and surface exchange properties of oxygen.61 The chemical bulk diffusion (Dchem) and surface exchange coefficients (kchem) of YEBCG were determined from the measurement of the transient response to a step change in pO2 by the electrical conductivity relaxation (ECR) method (Fig. S4†).62 Both Dchem and kchem values for YEBCG were much faster than those for other perovskite structured materials in the temperature range studied (600–750 °C), as can be seen in Fig. 6c and d. As an example, Dchem and kchem of YEBCG were 7.12 × 10−4 and 8.01 × 10−3 cm2 s−1, respectively, at 650 °C, this is much higher than those of Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF, 2.52 × 10−5 and 1.01 × 10−3 cm2 s−1), Ba0.5Sr0.5Co0.8Fe0.175Y0.025O3−δ (BSCFY, 6.30 × 10−5 and 2.10 × 10−3 cm2 s−1), BaFe0.975Gd0.25O3−δ (BFG, 6.67 × 10−5 and 5.81 × 10−3 cm2 s−1), and SrCo0.6Fe0.3Sn0.1O3−δ (SCFS, 9.0 × 10−5 and 1.75 × 10−3 cm2 s−1 for Dchem and kchem, respectively at 650 °C).63-66
Upon doping YBC with atoms of different oxidation states and ionic radii, changes in the interatomic spacings due to local lattice distortions are eventually related to modifications in the electronic and bonding states of Co–O, this influences the electrocatalytic activity of the air electrode materials for SOFCs and RPCCs, as mentioned earlier. The population of mobile oxygen defects has been also found to profoundly influence the oxygen electrode reaction kinetics associated with oxygen bulk diffusion and surface exchange of cathode materials. There are different kinds of oxygen species existing on the cathode surface, which is important to the ORR/OER process. As shown in Fig. 6e, the four strong peaks centering at approximately 529.1, 530.8, 531.7, and 532.9 eV can be designated as 4 lattice oxygen species (O2−), highly oxidative oxygen species (O22−/O−), surface absorbed oxygen (O2/OH−), and molecular water absorbed on the surface (H2O), respectively.67 The formation of a highly active oxy(hydroxide) surface layer, which is correlated with the surface oxygen defects of the materials, can be favorable to the OH− adsorption kinetics and electron transport properties for ORR/OER.68 The relative content of highly oxidative oxygen species on the YEBCG surface (52.6%) was estimated from the integrated area ratios of the sub-peaks, this is obviously higher than in YBC (46.7%), YEBC (47.4%), and YBCG (49.2%), demonstrating that YEBCG is highly electrocatalytically active for ORR/OER.
The co-doping effect of YBC-based materials was further investigated using the first-principles calculation. In oxygen electrode process for ORR/OER, electron-transfer energy refers to the required energy value when electrons move from the redox potential of electrolyte to the conduction band of the oxide, and is commonly known as a Schottky barrier. As the electron-transfer energy decreases, the energy required for electrons to diffuse at the oxide/electrolyte interface also decreases, which results in enhanced ORR/OER activity.69 Moreover, hydroxide-affinity is related to the Fermi level (Ef) of oxide.70 When the Fermi level of the oxide is lower than the redox potential of the electrolyte, the surface of the oxide is negatively charged to balance the electrolyte interface. These charged oxides can attract hydroxide ions from the electrolyte, and when these potential differences are large, the chemical interaction of ORR/OER also increases. Thus, theoretical ORR/OER properties of the oxide, such as electron transfer and hydroxide affinity, can be derived from their projected DOS (pDOS) of oxygen and transition metal ions.70 As presented in Fig. 6f, we calculated the pDOS of Y1−xErxBaCo4−yTryO7+δ (x = 0.25; y = 0.5 and 0.75; Tr = Al, Cr, Ga, Ni and Ti) structures. Their full DOS are illustrated in Fig. S5†. It was predicted that pristine YBC exhibits high hydroxide affinity but high electron-transfer, which implies that pristine YBC is not suitable for oxygen electrode process for ORR/OER. Most of Co-site doped YBCT, on the other hand, delivered negligible Schottky barrier compared to pristine YBC, which indicates facile electron diffusion from electrolyte to oxide at Co-site doped YBCT compared to pristine YBC. In particular, YEBCG exhibited excellent oxygen catalytic properties compared to the other samples, indicating that the calculated results were consistent with experimental results.
Electrochemical performances and durability of the YEBCG cell
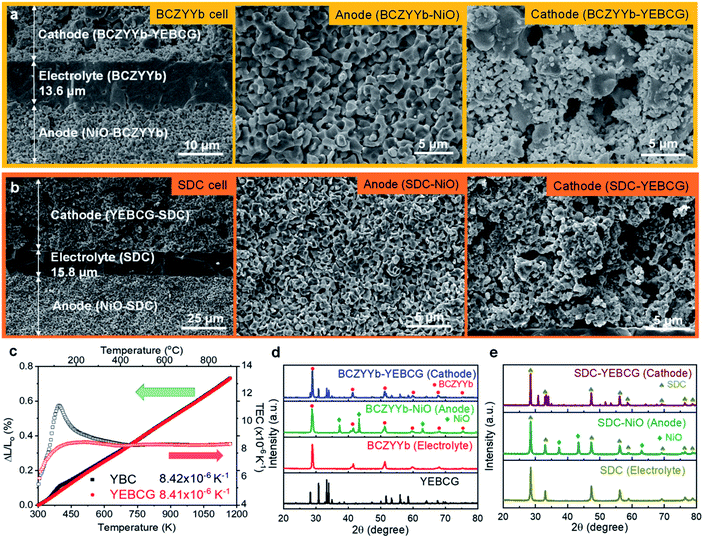
To verify the excellent performance of YEBCG for ORR/OER, NiO-BCZYYb and NiO-SDC anode-supported cells were fabricated using the tape-casting method. The NiO-BCZYYb (SDC) anode-supported cells mainly consist of anode substrate, electrolyte (BCZYYb, SDC), and composite cathode (YEBCG-BCZYYb, YEBCG-SDC), as characterized using FESEM in Fig. 7a and b. The dense BCZYYb (SDC) electrolyte was deposited on the porous anode substrate with a thickness of ∼13.6 (15.8) μm. The composite YEBCG-BCZYYb (SDC) cathode was attached to the electrolyte in a uniform manner with sufficiently porous fine particles. The good wettability of the cathode layer with the electrolyte may be due to the similar thermal expansion coefficient (TEC) values between YEBCG and BCZYYb (SDC). As shown in Fig. 7c, the TEC values for the YEBCG is 8.41 × 10−6 K−1, which is relatively close to that of BCZYYb (9.5 × 10−6 K−1) and SDC electrolyte (12.8 × 10−6 K−1).25,71 Furthermore, the YEBCG has advantages in terms of long-term thermal stability since large thermal mismatch of cell components can create critical mechanical stress between interfaces.The phase purities of the cell components were investigated by XRD (Fig. 7d and e). The tape-casted NiO-BCZYYb (SDC) anode substrate show the pure NiO and cubic perovskite (fluorite) structure of BCZYYb (SDC) phases with no unwanted impurities. The XRD diffractograms for the BCZYYb (SDC) electrolyte clearly demonstrate that the powders are comprised of the perovskite phase. The XRD patterns for the composite YEBCG-BCZYYb (SDC) reveal all the major characteristic peaks of YEBCG swedenborgite and BCZYYb perovskite (SDC fluorite) structures without any detectable impurity peaks after annealing at a high temperature of 950 °C for 2 h.
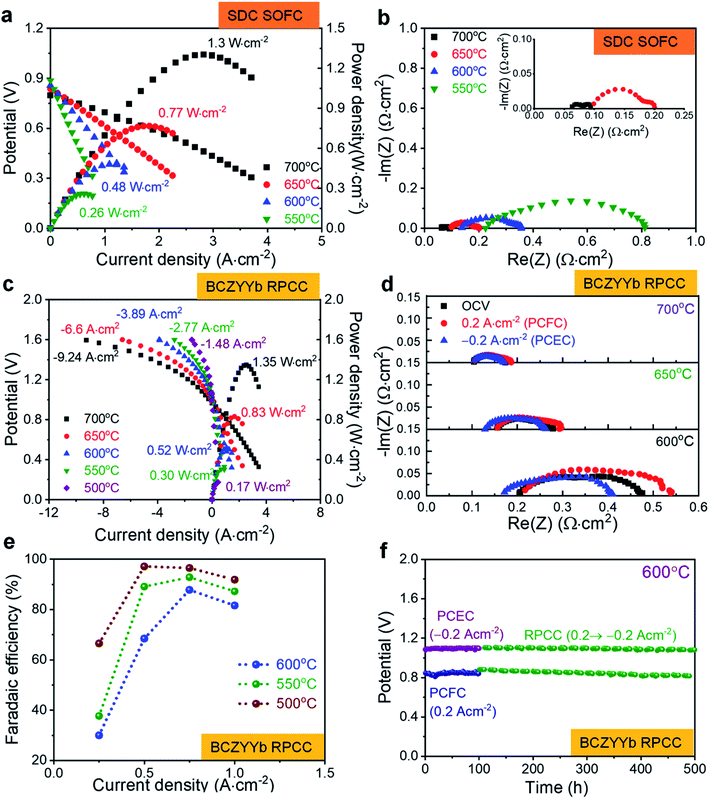
The electrochemical performance of the YEBCG cells was measured at 500–700 °C using humidified hydrogen (3 vol%) on the anode side and ambient air on the cathode side for SOFCs and PCFCs. I–V–P curves of the YEBCG cell (with SDC electrolyte) in an SOFC are presented in Fig. 8a. The open-circuit voltage (OCV) values of the YEBCG cell were 0.799–0.887 V at 550–700 °C. The low OCV of the cells is because of the reduction of the thin SDC electrolyte under a reducing atmosphere, despite the fact the SDC layers were dense without any pinholes. The maximum power densities (MPDs) of the YEBCG cell were 0.48, 0.77, 1.30 W cm−2 at 600, 650, and 700 °C, respectively. At 550 °C, the MPD of the YEBCG cell was 0.26 W cm−2. Fig. 8b displays the EIS plots of YEBCG cell under OCV at 550–700 °C. The Rohmic and Rp of YEBCG cell decreased with increasing temperature from 550 to 700 °C due to the thermally activated processes that occur. The ohmic ASR was 0.064–0.225 Ω cm2 at 700–550 °C; the electrode ASR was 0.033–0.587 Ω cm2 at these temperatures.
In PCFC mode under RPCC operation, the high OCVs of the cells (1.05 and 1.02 V at 550 and 650 °C, respectively) can be seen in Fig. 8c, this demonstrates that the electrolyte is dense. The MPDs of the cell were 0.30, 0.52, 0.83, and 1.35 W cm−2 at 550, 600, 650, and 700 °C, respectively. The ohmic and electrode ASRs were 0.152–0.289 and 0.07–0.592 Ω cm2, respectively, under OCV at 700–550 °C, according to the EIS results shown in Fig. 8d and S6.† The YEBCG RPCCs were also tested in PCEC mode at 500–700 °C. 20% steam in ambient air and 10% H2 in a wet N2 stream were supplied to the steam and fuel electrode, respectively. The YEBCG RPCC achieved remarkably high current densities of −0.61, −1.75 and −4.42 A cm−2 at 500, 600 and 700 °C, respectively, at an applied voltage of 1.4 V. The electrode ASRs under −0.2 A cm−2 in PCEC mode were 0.066, 0.128 and 0.239 Ω cm2 at 700, 650 and 600 °C, respectively, these are considerably lower than those obtained in PCFC mode. Moreover, faradaic efficiency of YEBCG cell was measured at 500, 550, and 600 °C, respectively, at an applied current range of 0.25–1 A cm−2. As shown in Fig. 8e, the faradaic efficiencies of YEBCG cell were 96.5, 92.9, and 87.8% at 500, 550, and 600 °C at 0.75 A cm−2. As the operation temperature increased, the faradaic efficiency of the YEBCG cell decreased because the electronic charge carrier contribution increases with temperature (Fig. S7†).9,11
We evaluated the long-term durability of cells under a constant current density of 0.2 (PCFC mode) and −0.2 A cm−2 (PCEC mode) at 600 °C to investigate the feasibility of the swedenborgite structured YEBCG electrode materials under RPCC conditions. As shown in Fig. 8f, no degradation was observed during the entire 100 h PCFC, 100 h PCEC, and 800 h RPPC mode (cycles of PCFC for 10 h and PCEC for 10 h) operation. This result further demonstrates the effectiveness of the Er and Ga co-doped YBC electrode material for the high performance of RPCCs with excellent durability at intermediate temperatures.
Conclusions
A systematic examination to gain a fundamental understanding of YBC-based swedenborgites oxides was carried out in order to boost the ORR/OER catalytic activity and phase stability of air electrode materials used in SOFCs and RPCCs at reduced temperatures. YBC (Y0.8Er0.2BaCo3.2Ga0.8O7+δ) with Er- and Ga-doped into Y- and Co-sites, respectively, presented lower ASRs with a lower activation energy of 0.69 eV in a symmetric cell when compared with other doped YBC materials. Both Dchem and kchem values of YEBCG indicated much faster processes than those in other perovskite structured materials in the intermediate temperature range. Upon doping atoms with different oxidation states and ionic radii, and high electronegativity into YBC, changes in interatomic spacings due to local lattice distortions occurred, these changes eventually lead to modifications in electronic states and covalent bonding characters of Co–O that boost the electrocatalytic activity of YEBCG. In addition, the high content of highly oxidative oxygen species on the YEBCG surface contributed to improve its OH− adsorption kinetics and electron transport properties for ORR/OER. The YEBCG cells achieved notably high power densities of 1.30 and 1.35 W cm−2 for SOFCs and PCFCs, respectively, at 700 °C. In addition, the YEBCG cells reached a current density of −1.75 A cm−2 at 600 °C under an applied voltage of 1.4 V in PCEC mode. Furthermore, the faradaic efficiency of the YEBCG cell was measured to be 97.2% under −0.5 A cm−2 at 500 °C, this clearly demonstrates that the YEBCG cell produces the correct amount of H2 to match with theory.Author contributions
J.-S. Shin and J.-Y. Park designed all the experiments. J.-S. Shin, K. Park, M. Saqib, and M. Jo performed the synthesis of materials for the cell components and physicochemical and electrochemical analyses. J.-S. Shin, K. Park, and M. Saqib measured the cell performances under SOFC and RPCC conditions. J.-S. Shin, M. Jo, H.-T. Lim, J. H. Kim analyzed the crystalline and electronic structures of the materials. H. Park and J. Kim carried out the DFT calculation. J.-S. Shin, H.-T. Lim, J. H. Kim, and J. Kim wrote the paper. All authors contributed to writing and editing the document.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research was supported by the Mid-career Researcher and Next-generation Engineering Researchers Development Program through the National Research Foundation of Korea (NRF-2017R1A2A2A05069812 and NRF-2019H1D8A2106002) and by the Industrial Technology Innovation R&D Program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), which has been granted financial resources from the Ministry of Trade, Industry & Energy, Republic of Korea (No. 20173010032290).References
- E. P. Murray, T. Tsai and S. A. Barnett, Nature, 1999, 400, 649 CrossRef CAS.
- K. Bae, H. S. Noh, D. Y. Jang, J. Hong, H. Kim, K. J. Yoon, J. H. Lee, B. K. Kim, J. H. Shim and J. W. Son, J. Mater. Chem. A, 2016, 4, 6395 RSC.
- Y. Choi, S. K. Cha, H. Ha, S. Lee, H. K. Seo, J. Y. Lee, H. Y. Kim, S. O. Kim and W. Jung, Nat. Nanotechnol., 2019, 14, 245 CrossRef CAS PubMed.
- Y. D. Kim, J. Yang, J. I. Lee, M. Saqib, J. S. Shin, K. Park, M. Jo, S. J. Song and J. Y. Park, J. Power Sources, 2020, 452, 227758 CrossRef CAS.
- Y. J. Meng, X. Y. Wang, W. Zhang, C. Xia, Y. N. Liu, M. H. Yuan, B. Zhu and Y. Ji, J. Power Sources, 2019, 421, 33 CrossRef CAS.
- W. P. Sun, M. F. Liu and W. Liu, Adv. Energy Mater., 2013, 3, 1041 CrossRef CAS.
- E. Fabbri, L. Bi, D. Pergolesi and E. Traversa, Adv. Mater., 2012, 24, 195 CrossRef CAS PubMed.
- C. C. Duan, R. J. Kee, H. Y. Zhu, C. Karakaya, Y. C. Chen, S. Ricote, A. Jarry, E. J. Crumlin, D. Hook, R. Braun, N. P. Sullivan and R. O'Hayre, Nature, 2018, 557, 217 CrossRef CAS PubMed.
- S. Choi, C. J. Kucharczyk, Y. G. Liang, X. H. Zhang, I. Takeuchi, H. I. Ji and S. M. Haile, Nat. Energy, 2018, 3, 202 CrossRef CAS.
- M. Saqib, J. I. Lee, J. S. Shin, K. Park, Y. D. Kim, K. B. Kim, J. H. Kim, H. T. Lim and J. Y. Park, J. Electrochem. Soc., 2019, 166, F746 CrossRef CAS.
- C. C. Duan, R. Kee, H. Y. Zhu, N. Sullivan, L. Z. Zhu, L. Z. Bian, D. Jennings and R. O'Hayre, Nat. Energy, 2019, 4, 230 CrossRef CAS.
- Y. Zhang, R. Knibbe, J. Sunarso, Y. J. Zhong, W. Zhou, Z. P. Shao and Z. H. Zhu, Adv. Mater., 2017, 29, 1700132 CrossRef PubMed.
- E. D. Wachsman and K. T. Lee, Science, 2011, 334, 935 CrossRef CAS PubMed.
- C. C. Duan, D. Hook, Y. C. Chen, J. H. Tong and R. O'Hayre, Energy Environ. Sci., 2017, 10, 176 RSC.
- Y. Chen, S. Yoo, K. Pei, D. C. Chen, L. Zhang, B. deGlee, R. Murphy, B. T. Zhao, Y. X. Zhang, Y. Chen and M. L. Liu, Adv. Funct. Mater., 2018, 28, 2458 Search PubMed.
- D. Ding, X. X. Li, S. Y. Lai, K. Gerdes and M. L. Liu, Energy Environ. Sci., 2014, 7, 552 RSC.
- K. Y. Park, Y. D. Kim, J. I. Lee, M. Saqib, J. S. Shin, Y. Seo, J. H. Kim, H. T. Lim and J. Y. Park, ACS Appl. Mater. Interfaces, 2019, 11, 457 CrossRef CAS PubMed.
- S. Park, S. Choi, J. Shin and G. Kim, RSC Adv., 2014, 4, 1775 RSC.
- J. Kim, S. Sengodan, G. Kwon, D. Ding, J. Shin, M. L. Liu and G. Kim, ChemSusChem, 2014, 7, 2811 CrossRef CAS.
- R. Zohourian, R. Merkle, G. Raimondi and J. Maier, Adv. Funct. Mater., 2018, 28, 1801241 CrossRef.
- F. Brieuc, G. Dezanneau, M. Hayoun and H. Dammak, Solid State Ionics, 2017, 309, 187 CrossRef CAS.
- L. Li, F. J. Jin, Y. Shen and T. M. He, Electrochim. Acta, 2015, 182, 682 CrossRef CAS.
- A. S. Harvey, F. J. Litterst, Z. Yang, J. L. M. Rupp, A. Infortuna and L. J. Gauckler, Phys. Chem. Chem. Phys., 2009, 11, 3090 RSC.
- K. Świerczek, K. Zheng and A. Klimkowicz, ECS Trans., 2013, 57, 1993 CrossRef.
- L. M. Zhang, S. Y. Yang, S. Z. Zhang and Y. X. Yang, Int. J. Hydrogen Energy, 2019, 44, 27921 CrossRef CAS.
- S. Y. Istomin, O. A. Tyablikov, S. M. Kazakov, E. V. Antipov, A. I. Kurbakov, A. A. Tsirlin, N. Hollmann, Y. Y. Chin, H. J. Lin, C. T. Chen, A. Tanaka, L. H. Tjeng and Z. Hu, Dalton Trans., 2015, 44, 10708 RSC.
- R. Pelosato, G. Cordaro, D. Stucchi, C. Cristiani and G. Dotelli, J. Power Sources, 2015, 298, 46 CrossRef CAS.
- J. H. Kim, Y. N. Kim, S. M. Cho, H. Wang and A. Manthiram, Electrochim. Acta, 2010, 55, 5312 CrossRef CAS.
- D. Medvedev, J. Lyagaeva, G. Vdovin, S. Beresnev, A. Demin and P. Tsiakaras, Electrochim. Acta, 2016, 210, 681 CrossRef CAS.
- M. A. Bhat, R. A. Zargar, A. Modi, M. Arora and N. K. Gaur, Mater. Sci., 2016, 34, 786 CAS.
- G. K. Tian, H. Chen, C. X. Lu, Y. Xin, Q. Li, J. A. Anderson and Z. L. Zhang, Catal. Sci. Technol., 2016, 6, 4511 RSC.
- N. Hollmann, Z. Hu, M. Valldor, A. Maignan, A. Tanaka, H. H. Hsieh, H. J. Lin, C. T. Chen and L. H. Tjeng, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 085111 CrossRef.
- A. Maignan, S. Hebert, V. Caignaert, V. Pralong and D. Pelloquin, Solid State Commun., 2008, 147, 470 CrossRef CAS.
- D. S. Tsvetkov, V. Pralong, N. S. Tsvetkova and A. Y. Zuev, Solid State Ionics, 2015, 278, 1 CrossRef CAS.
- K. Y. Lai and A. Manthiram, Chem. Mater., 2016, 28, 9077 CrossRef CAS.
- N. A. Danilov, A. P. Tarutin, J. G. Lyagaeva, E. Y. Pikalova, A. A. Murashkina, D. A. Medvedev, M. V. Patrakeev and A. K. Demin, Ceram. Int., 2017, 43, 15418 CrossRef CAS.
- D. K. Lim, S. Y. Jeon, B. Singh, J. Y. Park and S. J. Song, J. Alloys Compd., 2014, 610, 301 CrossRef CAS.
- I. Yasuda and T. Hikita, J. Electrochem. Soc., 1994, 141, 1268 CrossRef CAS.
- F. Ciucci, Solid State Ionics, 2013, 239, 28 CrossRef CAS.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15 CrossRef CAS.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953 CrossRef PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- V. I. Anisimov, F. Aryasetiawan and A. I. Lichtenstein, J. Phys.: Condens. Matter, 1997, 9, 767 CrossRef CAS.
- D.-H. Seo, H. Gwon, S.-W. Kim, J. Kim and K. Kang, Chem. Mater., 2010, 22, 518 CrossRef CAS.
- B. Sabir, G. Murtaza and R. M. A. Khalil, J. Mol. Graphics Modell., 2020, 94, 107482 CrossRef CAS PubMed.
- A. Jain, G. Hautier, S. P. Ong, C. J. Moore, C. C. Fischer, K. A. Persson and G. Ceder, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 045115 CrossRef.
- B. J. Morgan, D. O. Scanlon and G. W. Watson, J. Mater. Chem., 2009, 19, 5175 RSC.
- W. Wong-Ng, W. Xie, Y. Yan, G. Liu, J. Kaduk, E. Thomas and T. Tritt, J. Appl. Phys., 2011, 110, 113706 CrossRef.
- A. Gomez, D. Fuchs and O. Moran, Thin Solid Films, 2013, 545, 140 CrossRef CAS.
- L. M. Hou, Q. B. Yu, K. Wang, T. Wang, F. Yang and S. Zhang, J. Therm. Anal. Calorim., 2019, 137, 317 CrossRef CAS.
- M. Iakovleva, S. Zimmermann, J. Zeisner, A. Alfonsov, H. J. Grafe, M. Valldor, E. Vavilova, B. Buchner and V. Kataev, Phys. Rev. B, 2017, 96, 064417 CrossRef.
- B. Raveau, V. Caignaert, V. Pralong and A. Maignan, Z. Anorg. Allg. Chem., 2009, 635, 1869 CrossRef CAS.
- O. Parkkima and M. Karppinen, Eur. J. Inorg. Chem., 2014, 2014, 4056 CrossRef CAS.
- X. F. Ding, Z. P. Gao, D. Ding, X. Y. Zhao, H. Y. Hou, S. H. Zhang and G. L. Yuan, Appl. Catal., B, 2019, 243, 546 CrossRef CAS.
- J. Suntivich, H. A. Gasteiger, N. Yabuuchi, H. Nakanishi, J. B. Goodenough and Y. Shao-Horn, Nat. Chem., 2011, 3, 647 CrossRef CAS.
- J. Park, M. Risch, G. Nam, M. Park, T. J. Shin, S. Park, M. G. Kim, Y. Shao-Horn and J. Cho, Energy Environ. Sci., 2017, 10, 129 RSC.
- T. Hong, K. S. Brinkman and C. R. Xia, ChemElectroChem, 2016, 3, 805 CrossRef CAS.
- W. He, X. L. Wu, G. M. Yang, H. G. Shi, F. F. Dong and M. Ni, ACS Energy Lett., 2017, 2, 301 CrossRef CAS.
- P. Z. Li, Z. H. Wang, X. Q. Huang, L. Zhu, Z. Q. Cao, Y. H. Zhang, B. Wei, X. B. Zhu and Z. Lu, J. Alloys Compd., 2017, 705, 105 CrossRef CAS.
- J. Wang, M. Saccoccio, D. J. Chen, Y. Gao, C. Chen and F. Ciucci, J. Power Sources, 2015, 297, 511 CrossRef CAS.
- Y. L. Zhu, W. Zhou, R. Ran, Y. B. Chen, Z. P. Shao and M. L. Liu, Nano Lett., 2016, 16, 512 CrossRef CAS PubMed.
- D. Rupasov, T. Makarenko and A. J. Jacobson, Solid State Ionics, 2014, 265, 68 CrossRef CAS.
- P. F. Haworth, S. Smart, J. M. Serra and J. C. D. da Costa, Phys. Chem. Chem. Phys., 2012, 14, 9104 RSC.
- Y. B. Chen, B. M. Qian and Z. P. Shao, J. Power Sources, 2015, 294, 339 CrossRef CAS.
- Y. Lu, H. L. Zhao, X. W. Chang, X. F. Du, K. Li, Y. H. Ma, S. Yi, Z. H. Du, K. Zheng and K. Swierczek, J. Mater. Chem. A, 2016, 4, 10454 RSC.
- D. J. Chen and Z. P. Shao, Int. J. Hydrogen Energy, 2011, 36, 6948 CrossRef CAS.
- S. X. She, Y. L. Zhu, Y. B. Chen, Q. Lu, W. Zhou and Z. P. Shao, Adv. Energy Mater., 2019, 9, 1970071 CrossRef.
- J. Dai, Y. L. Zhu, Y. C. Yin, H. A. Tahini, D. Q. Guan, F. F. Dong, Q. Lu, S. C. Smith, X. W. Zhang, H. T. Wang, W. Zhou and Z. P. Shao, Small, 2019, 15, 1903120 CrossRef PubMed.
- W. T. Hong, K. A. Stoerzinger, Y.-L. Lee, L. Giordano, A. Grimaud, A. M. Johnson, J. Hwang, E. J. Crumlin, W. Yang and Y. Shao-Horn, Energy Environ. Sci., 2017, 10, 2190 RSC.
- J. Hwang, R. R. Rao, L. Giordano, Y. Katayama, Y. Yu and Y. Shao-Horn, Science, 2017, 358, 751 CrossRef CAS PubMed.
- Q. Xu, D. P. Huang, F. Zhang, W. Chen, M. Chen and H. X. Liu, J. Alloys Compd., 2008, 454, 460 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ta11000k |
| This journal is © The Royal Society of Chemistry 2021 |