Effects of protecting groups on luminescent metal nanoclusters: spectroscopic signatures and applications
Subhajit
Chakraborty
and
Saptarshi
Mukherjee
 *
*
Department of Chemistry, Indian Institute of Science Education and Research Bhopal, Bhopal Bypass Road, Bhopal 462 066, Madhya Pradesh, India. E-mail: saptarshi@iiserb.ac.in
First published on 12th November 2021
Abstract
Luminescent metal nanoclusters (NCs) have been established as next-generation fluorophores. Their biocompatible and non-toxic nature, along with excellent chemical- and photo-stability, enables them to find applications in multi-disciplinary areas. However, preparing NCs which are stable is always challenging, primarily owing to their small size and propensity to self-aggregate. In this review, we highlight a holistic approach as to how ligands and templates can monitor the stability of NCs, tune their spectroscopic signatures, and alter their applications. The role of small molecules of a large ligand in the preparation of NCs and their associated limitations are also discussed. We have summarized how these NCs can be utilized in sensing several metal ions, pH, viscosity and temperature of many systems which have biological relevance. Additionally, these luminescent metal NCs find usage in cell-imaging, discriminating between cancerous and non-cancerous cell lines and also targeting specific organelles within the cellular environment.
Introduction
Recently, ultra-small-sized metal nanoclusters (NCs) have drawn considerable research interest owing to their unique size-dependent characteristics consequent to the quantum confinement effect.1–14 These metal NCs are characterized by easy synthetic protocols, high photostability, water solubility, catalytic properties, biocompatibility, non-toxicity, etc., which enable them as next-generation fluorophores.15–30 Generally, metal NCs are very robust to photon irradiation; they are much more photostable as compared to commercial organic dyes.13,15 This thereby enables them to be used in single-molecule spectroscopic investigations where photobleaching is one of the challenges that need to be overcome. Additionally, these metal NCs are generally non-toxic and biocompatible.13,19,29 This feature of metal NCs differentiates them from analogous quantum dots (QDs) which are generally far more toxic. Due to these interesting features, metal NCs can be utilized for a plethora of applications in interdisciplinary research fields and thereby can act as a replacement for organic dyes and quantum dots (QDs). However, despite these advantages, there are some bottlenecks related to metal NCs which require further exploration and refinements. One of the major challenges is related to the stability of these metal NCs after their formation. Lack of proper surface protection can lead to the oxidation of metal atoms present in the core of NCs which results in their degradation. Often, larger nanoparticles are generated along with NCs which are mostly non-fluorescent. Consequently, an inherent heterogeneity appears within the system. For this reason, the selection of capping ligands and the optimization of synthetic protocols must always be properly addressed. Another shortcoming of these metal NCs is their low quantum yields (QY) as compared to those of QDs and conventional fluorophores. Although investigations are going on to circumvent this aspect, it requires more exploration so that these metal NCs can be used in applications that require greater photoluminescence intensities.The optical properties of larger metal nanoparticles (NPs) are strongly dependent on the free electron density and their sizes, which can be explained by the Mie theory.31–33 The Mie theory can still provide an understanding for the particles having a size comparable to the electron mean free path.31,34 However, interestingly, when the particle size approaches the Fermi wavelength of the conduction electron (i.e., formation of NCs), the optical properties become drastically different from the larger metal NPs as the continuous electronic band structure is converted into quantized discrete energy levels.35–39 Consequently, these NCs become luminescent and act like molecular species. According to the Drude model,1 the free electrons of the metal atoms (valence s-orbital for noble metals) become delocalized over the cluster core. The total Hamiltonian of the metal NCs may be expressed as a combination of the noninteracting Hamiltonians of the free electrons.1 This concept is based on the “one-particle approximation” and is related to the particle-in-a-box model which explains the behaviour of free electrons in metals.1 According to Hooke's law, a linear restoring force when applied on free electrons makes the quantized energy levels behave as three-dimensional harmonic oscillators.40–42 Using the concept of free electrons based on the Drude theory, the Jellium model has been introduced to estimate the cluster–core composition which actually governs the luminescence properties of the said metal NC.16 A mathematical expression of the most accepted Jellium model, which connects the Fermi energy of the particular metal (EFermi), the corresponding emission energy (Eem) and the number of atoms in a given cluster–core (N), is as follows:16
| Eem= EFermi/N0.3 | (1) |
The most unique feature of metal NCs is their size-dependent luminescence properties. By tuning the size, one can regulate the photophysical characteristics of metal NCs.43 However, various other factors like capping ligand and the environment around the cluster core have an enormous influence on the photophysical properties of metal NCs (will be discussed later).43 Thus, the optical properties of metal NCs do not follow a linear relationship with the size of the NC alone.43,44 Due to the presence of free electrons and their collective oscillations, NPs display characteristic surface plasmon resonance (SPR) bands in the UV-Vis absorption profile. This feature differentiates them from metal NCs. The formation of discrete energy levels due to the reduction in size during the formation of metal NCs facilitates the one-electron transition in between quantized energy states. Consequently, a stepwise optical absorption results in a continuous absorption signature. Interestingly, the concept of enhancement of luminescence intensity through aggregation has been explained in recent times. In 2012, Xie and co-workers reported the formation of bright emitting AuNCs and their luminescence properties have been ascribed to the concept of aggregation-induced emission (AIE).45 In contrast to larger sized non-luminating NPs, AIE provides the attachment of the neighbouring protecting ligands of the individual cluster–cores with one another.7,46 Thus, the AIE phenomenon is associated with the large Stokes shift and long excited-state lifetime decay components.46
There are various ways by which NCs can be synthesized, and such protocols are well-documented.47,48 Generally, two such standard approaches are followed: (i) top-down approach and (ii) bottom-up approach (Scheme 1). The top-down approach involves some dry and wet grinding/etching processes. Additionally, the yields of the final products are generally low which thereby limits this approach toward the formation of stable and cost-effective NCs. Hence, the favoured approach is the bottom-up approach, which is a combination of reduction of metal ions to their zero-valent oxidation states followed by the aggregation of the reduced metal atoms and encapsulation by ligands which leads to the formation of stable metal NCs.12 In general, this process involves two steps: (i) bio-mineralization;12,49 reduction of metal ions in the presence of reducing agent(s) (sometimes the ligand itself can serve this purpose) which produces polydispersed cluster–cores50 and (ii) subsequent nucleation; formation of molecularly pure cluster–cores. Very recently, Xie's and Ying's groups well described the 2e− reduction process in a stepwise manner for the growth of cluster–cores of AuNCs containing Au0.51–53 Sometimes the pH of the medium activates the ligand molecule or particularly, the functional group of the ligand molecule by deprotonation to exhibit the reducing ability.13,15
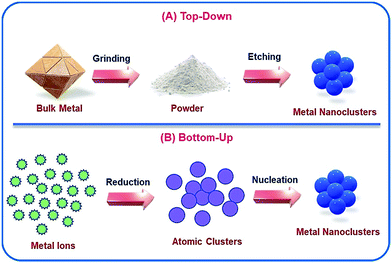 | ||
| Scheme 1 Schematic representation of the two different ways by which NCs can be prepared: (A) top-down approach and (B) bottom-up approach. | ||
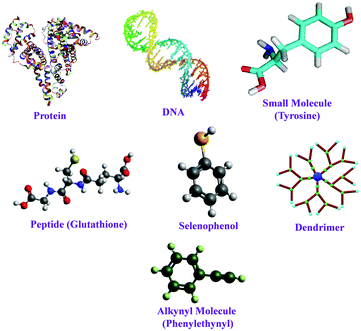
To avoid the aggregation of tiny clusters once formed and the subsequent formation of bigger-sized NPs, the ligands play a pivotal role by protecting the zero-valent cluster cores.54,55 Not only the protection and surface stabilization of the cluster–core, but also the photophysical and morphological properties of NCs are also highly influenced by the capping agents.56,57 To date, a large variety of ligands have been used for the facile preparation of noble metal (Au, Ag and Cu) NCs, such as protein, peptides, polymers, DNA, small molecules, etc. (Scheme 2).8
In 1978, Bartlett and co-workers prepared 0.8 nm sized gold nanoclusters (AuNCs) using an amino-substituted triarylphosphine ligand (Ar3P)7Au11X3).58 They observed that when the substituent, “X” was iodine, the clusters so formed were unstable and had a tendency to undergo oxidative decomposition.58 However, Hutchison and co-workers demonstrated that stable AuNCs having a similar core composition (i.e., Au11) can be prepared when alkanethiols are used as a capping agent instead of triarylphosphine ligands.59 In a seminal work, Yang and Chen demonstrated that the increment of particle band gap energy (from 1.4 to 1.8 eV) can be achieved for gold particles by altering the capping ligand from triphenylphosphines to alkanethiolates.60 Zhu and co-workers very nicely demonstrated that selenolate capped Au25(SePh)18− NCs exhibited totally different electronic distribution and optical properties in comparison to its thiolate counterpart (Fig. 1), Au25(SCH2CH2Ph)18−, despite having a similar icosahedral Au13 core and semi-ring motifs (Au2(SeR)3 or Au2(SR)3) (Fig. 2).61 Thus, the rational use of ligands can alter the HOMO–LUMO energy gap of the molecule.
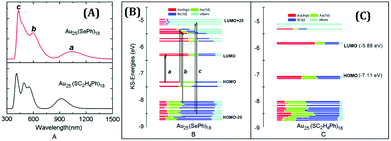 | ||
| Fig. 1 (A) Theoretical optical curves of Au25(SePh)18 and Au25(SC2H4Ph)18 nanoclusters as marked in the figure. Comparison of the component of KS orbitals of Au25(SePh)18 (B) and Au25(SC2H4Ph)18 (C) nanoclusters. Reproduced with permission from ref. 61. Copyright 2014, The Royal Society of Chemistry. | ||
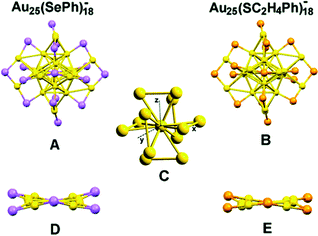 | ||
| Fig. 2 Crystal structures of Au25(SePh)18− (A) and Au25(SC2H4Ph)18− (B) nanoclusters. (C) The Au13 core of Au25(SePh)18−. (D) and (E) The distortions of Au2Se3 and Au2S3 motifs, respectively, in the σh plane along x–y (color labels: yellow = Au, violet = Se, orange = S, for clarity C and H atoms are omitted). Reproduced with permission from ref. 61. Copyright 2014, The Royal Society of Chemistry. | ||
Also, the facile binding affinity (between the electron-donating atom of the ligand and metal ions/atoms) leads to the selectivity of ligands toward the formation of NCs. For example, the soft–soft interaction between the S atom and Au atom leads to the formation of highly stable AuNCs.62,63 Using Raman spectroscopy, Duguid et al. monitored the interaction of DNA and a series of divalent metal atoms and demonstrated that the nitrogen centers of the nucleobases are more favourable toward bond formation with metal ions.64 In two separate studies, Ono et al. and Ritchie et al. had opined that the cytosine-rich (C-rich) sequence of DNA promotes the easy formation of AgNCs as cytosine has a high affinity to bind with Ag+ ions.65,66
In a recent study, Chevrier et al. investigated how the template influences the structure and properties of Au18(SR)14 NCs (where, –SR = cyclohexanethiolate ligand and glutathione ligand) using synchrotron X-ray spectroscopy and quantum mechanics/molecular mechanics (QM/MM) simulations (Fig. 3).57 This report clearly demonstrated that encapsulation by glutathione can provide more stability through compact surface construction with suitable rigidity (Fig. 4). Also, the enhanced photoluminescence and biocompatibility of these AuNCs have been explained based on the above-mentioned concept.57
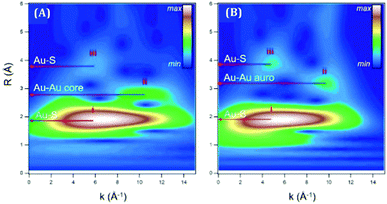 | ||
| Fig. 3 The plots represent the Au L3-edge WT-EXAFS for (A) Au18(SC6H11)14 in toluene and (B) Au18(SG)14 in water. Reproduced with permission from ref. 57. Copyright 2018, American Chemical Society. | ||
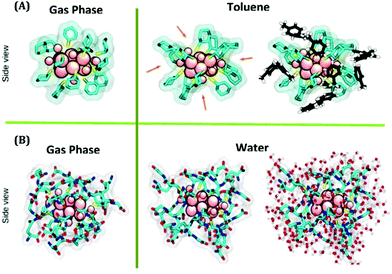 | ||
| Fig. 4 (A) Simulated structures of Au18(SC6H11)14 in the gas phase and toluene phase, respectively, obtained from QM and QM/MM simulations. (B) Simulated structures of Au18(SG)14 in the gas phase and water phase, respectively obtained from QM and QM/MM simulations. Color labels: pink atoms = Au, yellow sticks = S, and cyan sticks = C. In (A), the toluene molecules of the first solvation shell are represented in black (carbon) and white (hydrogen). In (B), the water molecules of the first solvation shell are shown in red (oxygen) and white (hydrogen). Reproduced with permission from ref. 57. Copyright 2018, American Chemical Society. | ||
Noble metal NCs have been widely used in sensing applications due to their selective and sensitive detection ability towards various chemical entities.63,67–74 This property of these next-generation fluorophores enables them to be used for both in vitro and in vivo sensing. It is always challenging to sense chemical entities inside complex biological systems as they have direct relevance to biomedical investigations.67 Discriminating cancerous and non-cancerous cells is extremely important for the early detection of diseases. It has been reported that AuNCs, templated by the globular protein (human serum albumin, HSA; bovine serum albumin, BSA) can be effectively utilized to differentiate between cancerous and non-cancerous cell lines.62,63 The uptake of these AuNCs is much more in the case of cancerous cells as compared to the non-cancerous ones. Additionally, targeting specific organelles within a cell is also important from the perspective of drug delivery and subsequent release. The associated dynamics inside a cell are primarily controlled by the microenvironment viscosity, polarity, pH, etc. To have an in-depth understanding of the dynamics inside a cell and probe the microenvironment in and around an organelle, luminescent metal NCs are widely used.75–78 The fact that these NCs are photostable, non-toxic and biocompatible in nature enables them to be efficiently used in single-molecule spectroscopic studies and for bioimaging investigations.62,63,79–81 For most of the cases, specific targeting of organelles within a cell can be realized by rational use of ligands.67 It is pertinent to be mentioned that the translational diffusion of NCs is not similar in every cell due to the different microenvironment and system-specific localization of NCs inside the cell lines.62,81 Bhattacharyya and co-workers studied the translational relaxation of AuNCs using fluorescence correlation spectroscopy (FCS) (Fig. 5).62 They demonstrated that the AuNCs follow 2D diffusion in the non-cancerous MCF10A cell lines (i.e., diffusion through the cell membrane only), whereas the same set of NCs experienced a combination of 2D and 3D diffusion in the cancerous MCF7 cell lines (i.e., localization both in the membrane and the cytoplasm, Fig. 5).62
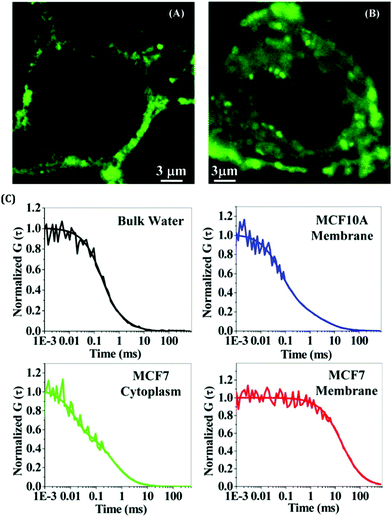 | ||
| Fig. 5 Confocal images of breast cells stained with BSA-templated AuNCs. The degree of staining is much more for cancerous cells as compared to non-cancerous cells. (A) Normal breast cell (MCF10A) and (B) breast cancer cell (MCF7). (C) Normalized FCS traces of these AuNCs in bulk water (black), inside the cell membrane of MCF10A (blue), inside the cytoplasm of MCF7 (green), and in the cell membrane of MCF7 (red). Reproduced with permission from ref. 62. Copyright 2014, American Chemical Society. | ||
Additionally, NCs are the suitable choice for the sensitive detection of pH,82–84 various biomolecules such as proteins,85 nucleic acids,86,87 small biomolecules67 and even viruses.74,88 Triulzi et al. were the first to report the detection of human IgG using PAMAM coated AuNCs which were used as a fluorescence probe.89 In another study, Chen et al. observed the rapid detection ability of GSH templated AuNCs for the detection of glutathione S-transferase (GST)-tagged proteins.90 Using DNA-templated AgNCs, Martinez and co-workers reported the detection of a specific protein, thrombin, with an impressive detecting limit (1 nM).91 Digestion of proteins is mainly controlled by the protease enzyme which basically breaks the peptide bonds of the proteins. Wang et al. developed AuNCs using BSA as a capping ligand and demonstrated the sensitive nanoscale detection of protease (detection limit of 1 ng mL−1) by turn-off emission signal phenomena.92 In another work, Zhu and co-workers prepared DNA-protected AgNCs which can act as a sentient towards the determination of adenosine deaminase (ADA).93
Detecting toxins within biological systems and that too at low concentrations will certainly be useful. Nair et al. strategically prepared urease encapsulated NIR emitting AuNCs and demonstrated that these AuNCs can be utilized to selectively detect urea in human blood and cow milk samples.94 Xie et al. developed GSH stabilized AgNCs for the sensitive and specific detection of cysteine (Cys), a sulfur-containing amino acid with the detection limit of <3 nM.95 It is already established that a certain level of reactive oxygen species (ROS) is important for the normal functioning of a cell. However, the generation of excessive ROS inside the cell can easily affect this severely which may, in turn, lead to permanent damage of the cell. We have recently developed a potential antimicrobial agent (tyrosine templated silver nanomaterials, AgNMs) which can show the prompt killing of cell lines by creating a high level of ROS inside the cells.96 On the other hand, detection and quantification of ROS is equally important for therapeutic applications. Chen et al. developed a dual emissive AuNC decorated silica nanosensor for the efficient detection of highly ROS (hROS) inside the live HeLa cell lines (Fig. 6).97
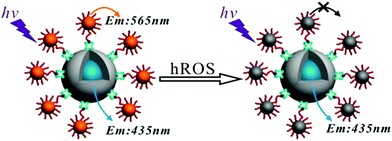 | ||
| Fig. 6 Detection of hROS using the crown nanoparticle assembly. The system consists of a core dye encapsulated silica particle and multiple satellite glutathione-templated AuNCs. Reproduced with permission from ref. 97. Copyright 2013, American Chemical Society. | ||
Additionally, NCs can be utilized for the efficient detection of various inorganic cations and anions. In a recent work, Nain et al. synthesized AuNCs, AgNCs and CuNCs using a mixture of BSA and thiosalicylic acid (TSA) as the encapsulating ligands.98 Interestingly, they have shown that each of these NCs can selectively detect a metal ion(s); AuNCs, AgNCs, and CuNCs can separately detect Hg2+, As3+ and Cr6+ ions, respectively.98 We have demonstrated that HSA templated AuNCs can show ultra-sensitive and selective detection of Hg2+ ions in vitro as well as in vivo environments.63 In a previous study from our group, Ghosh et al. demonstrated that HSA-templated Ag9:HSA NCs can sense Co2+ ions by a turn-off luminescence mechanism.99 Interestingly, these quenched NCs can again detect Zn2+ ions in solution by restoring the luminescence intensity (turn-on luminescence mechanism).99 Chang and co-workers100 developed DNA-templated AgNCs for the selective detection of Cu2+ ions in Montana soil (SRM 2710) and pond water. GSH-capped CuNCs displayed the selective sensing ability towards Fe3+ ions with a low limit of detection (LOD) of ∼25 nM.101 Cytidine-stabilized AuNCs have the ability for the selective detection of Ag+ ions in solution by turn-on fluorescence,102 whereas Pb2+ ions can also be detected by GSH-templated AgNCs with a LOD of ∼0.2 nM.103 Additionally, metal NCs can serve as efficient biomarkers.19,63,81 The easy encapsulation of NCs inside the cell lines and their bio-imaging abilities have been well-documented.19,63,81,93 Sun et al. fabricated NIR luminescent AuNCs using GSH as a capping and reducing agent and demonstrated that further conjugation of folic acid rendered these NCs as efficient biomarkers through encapsulation inside HeLa cell lines.104 A ligand comprising 18 peptides has been used to prepare AuNCs characterized by bright emission at the NIR region (1100–1350 nm). These NCs were subsequently utilized for in vivo brain vessel imaging and tumour metastasis.105 Recently, Xie and co-workers developed cyclodextrin (CD) templated AuNCs exhibiting NIR-II emission (λem = 1050 nm) which have thereby been used as a NIR-II imaging probe for in vivo investiogations.106
In our research work, we have used various kinds of ligands, such as proteins (human serum albumin, HSA), tripeptides (reduced glutathione, GSH), small molecules (tyrosine, Tyr), etc., to prepare several metal NCs.63,72,80,96,99,107 We have prepared AgNCs having two different compositions (Ag9:HSA and Ag14:HSA) using HSA as a template and for the first time demonstrated that NCs can be toggled/interconverted using simple redox chemistry.107,108 Using the same template HSA, we have also reported that CuNCs can exhibit thermal sensitivity and act as a nanothermometer. Additionally, these CuNCs serve as a FRET pair with the standard dye Coumarin-153, characterized by an efficiency of energy transfer of ∼85%.109 We have also prepared CuNCs using GSH as a capping agent.104 These CuNCs act as a biomarker for cancerous cells and also selectively detected Fe3+ ions in solution and the protein haemoglobin, which in turn can lead to the early detection of cyanosis.104 In our investigations on HSA-capped AgNCs and CuNCs, no external reducing agent was used. This was achieved by keeping the pH of the medium greater than the pKa of tyrosine.15 In these studies, we had proposed that the 18 tyrosine amino acid residues inherently present within the protein matrix provide the necessary reducing environment (at a pH ∼ 11, i.e., greater than 10.46, which is the pKa of tyrosine15,107) and result in the formation of stable NCs. To substantiate our proposal, we developed a unique strategy of using bare L-tyrosine as a capping agent outside the protein environment to prepare stable NCs.110 These investigations not only highlight the role of a small molecule of a large ligand in preparing stable NCs, but also clearly demonstrate the advantages of having larger templates as compared to smaller ones as the former reduces the extent of heterogeneity within the system.110 This feature article highlights the various fundamental aspects and related applications of luminescent metal NCs. The field of cluster chemistry is developing extremely rapidly and encompasses various other interdisciplinary research objectives. Since the properties of luminescent metal NCs greatly depend on the choice of ligands used, we have in detail delineated the effect of ligands on the spectroscopic signatures of these NCs. Besides the fundamental physics/chemistry involved with their preparation, metal NCs have been established as next-generation fluorophores owing to their tunable size-dependent luminescence properties. Herein, we have discussed how these NCs can be used in bio-medics, sensing metal ions with unprecedented limits of detection, sensing the pH and viscosity of cellular environments, and serving as bio-markers in differentiating both cancerous and non-cancerous cell lines.
Effects of protecting ligands
As mentioned earlier, for the preparation of stable metal NCs, generally, the “bottom-up” approach is preferred. In this process, reduction of metal ions to zero-valent metal atoms followed by cluster–core formation and encapsulation by ligands are the key factors that lead to the formation of NCs. Generally, the cluster–core, comprising metal atoms in their zero-valent oxidation states, has a strong propensity to self-aggregate owing to a very high surface energy. This in turn leads to the formation of larger-sized nanoparticles. However, to restrict further aggregation (i.e., to prevent the formation of nanoparticles from NCs), ligands play a vital role and offer the necessary surface stability.54,55 Not only the formation, but also the long-time stability, photophysical properties, etc. can be regulated by capping agents.63,109,110 In general, the compactness around the cluster–core can provide extra surface stability to NCs.63,109 Recently, small molecules (like thiolates, amino acids, etc.) have been widely used for the preparation of stable NCs.101,110 However, macromolecular ligands generally provide extra surface rigidity that is required to ensure the formation of a NC, which is more robust and exhibits greater photo-, thermal- and chemical stability.63,109We have prepared blue light-emitting CuNCs using the globular protein HSA as a capping as well as reducing agent.109 HSA is the most abundant unglycosylated monomeric circulatory protein found in the human blood plasma and is produced in the liver.111,112 The average molecular mass of HSA is 66.4 kDa and this protein consists of 585 amino acid residues in a single poly-peptide chain.113 Ghosh et al. proposed that the Tyr amino acid residues which are present in the HSA scaffolds are actually responsible for the formation of NCs.109 Bao and co-workers also reported that the Tyr act at the final step in reducing Au+ ions to Au atoms during the preparation of AuNCs using BSA as a protective ligand.114 In these synthetic protocols, the pH of the reaction medium is maintained at ∼11 which renders the Tyr to behave as a reducing agent as the pKa of Tyr is 10.46.15,107 These CuNCs exhibited a strong luminescence intensity centered at ∼414 nm upon excitation at 330 nm. However, this spectroscopic signature was almost completely lost when these CuNCs were treated with a strong oxidant, H2O2. Here, H2O2 oxidizes the Cu0 from the cluster–core to Cu+ or Cu2+ which eventually results in the diminution of luminescence intensity.109 The time-resolved decay transients of these CuNCs exhibited a bi-exponential decay [1.10 ns (30%) and 3.40 ns (70%)]. This bi-exponential nature of the decay transient has been attributed to the electronic transitions involved between the filled d orbitals and the vacant sp conduction band. The longer component of 3.40 ns has been ascribed to the transition between Cu and the ligand (here HSA), while Cu–Cu interactions account for the shorter component of 1.10 ns.109 Through time-dependent emission profiles, it has been demonstrated that the formation of stable CuNCs is a slow process with the entrapped Cu2+ ions getting stabilized through electrostatic interactions involving several functional groups and the bulky structure of the protein. Besides showing thermal sensitivity and reversibility, these CuNCs demonstrated temperature-dependent FRET. The CuNCs served as the donor while the acceptor was Coumarin153, non-covalently attached to the protein matrix (Scheme 3). The protein, HSA, is a heart-shaped molecule having three domains, I, II and III, each having two sub-domains, A and B.113 Molecular docking study suggested that the probe, Coumarin153, gets attached at the subdomain IB of HSA (probable location being near the Cys-34 amino acid residue of the protein) and attains stabilization through hydrophobic and electrostatic interactions.109 The distance between the donor (CuNCs) and the acceptor (Coumarin153) estimated from molecular docking was ∼20 Å, while that estimated from FRET-based assay was ∼ 19 Å (Fig. 7).109
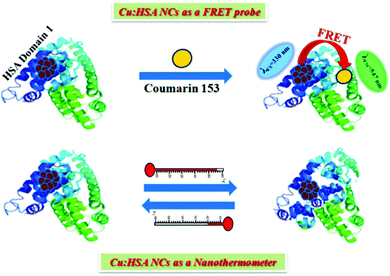 | ||
| Scheme 3 (A) Schematic representation displaying the phenomenon of FRET (donor: CuNCs; acceptor: Coumarin153) and nanothermometric behaviour exhibited by HSA-templated CuNCs. Reproduced with permission from ref. 109. Copyright 2015, American Chemical Society. | ||
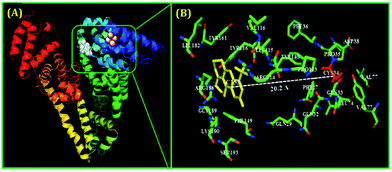 | ||
| Fig. 7 (A) Molecular docking results representing the minimum-energy conformations of HSA upon binding with Coumarin153 (non-covalently attached to subdomain IB of HSA) and the plausible location of the binding of the fluorophore, Coumarin153. (B) Zoomed-in view displaying the several amino acid residues which are present in the immediate vicinity of Coumarin153 and the estimated distance between the acceptor (Coumarin153) and Cys-34 (20.2 Å). Reproduced with permission from ref. 109. Copyright 2015, American Chemical Society. | ||
In a different work, Anand et al. prepared two AgNCs having different compositions, but using the common capping agent/template, i.e., HSA.107 For the preparation of both these AgNCs, the pH of the medium was maintained greater than 10.46, i.e., the pKa of Tyr,107 and the temperature was maintained at 37 °C. However, the time taken for synthesis vastly differed; Ag9:HSA was prepared in 10 hrs and no external reducing agent was used, while the other NC, Ag14:HSA, was prepared within 2–3 minutes as in this case, and a strong reductant, NaBH4, was used (Scheme 4). Since the composition differed, so did their spectroscopic signatures; while Ag9:HSA (QY ∼ 16%) emitted at 460 nm, Ag14:HSA (QY ∼ 11%) exhibited a strong luminescence at 620 nm.107 The most interesting aspect of this investigation was the interconvertibility among these NCs (Fig. 8). Using simple redox chemistry, it has been demonstrated that these NCs can be toggled retaining almost all their photophysical properties (Scheme 4). These results highlight the role played by a bulky ligand. HSA provides the necessary protective environment as the cluster–cores do not get destroyed when a strong reductant (NaBH4) and an oxidant (H2O2) were used to carry out the toggling between the two NCs.107
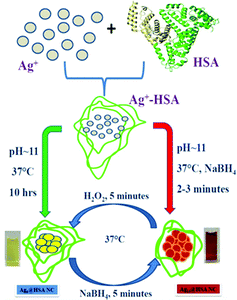 | ||
| Scheme 4 Schematic representation showing the formation and interconversion of the Ag9:HSA and Ag14:HSA NCs using redox chemistry. The two NCs were prepared independently using two different synthetic protocols. Reproduced with permission from ref. 107. Copyright 2012, American Chemical Society. | ||
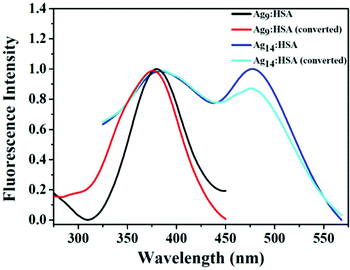 | ||
| Fig. 8 Normalized fluorescence excitation spectra of the two HSA-templated AgNCs. The similar nature of these excitation spectra corroborates the fact that even after inter-conversion (from Ag9:HSA to Ag14:HSA and vice-versa), both the NCs retain their spectroscopic signatures signifying that upon inter-conversion, there are no major structural alterations. Reproduced with permission from ref. 107. Copyright 2012, American Chemical Society. | ||
Besides surface stabilization, ligands can also influence the photophysical properties of NCs. Zhang and co-workers prepared carboxylate-protected AgNCs which showed excitation-dependent emission (Fig. 9A).115 Similar observation has been reported for DNA-coated AgNCs by Shao and co-workers (Fig. 9B).116 Previously, this phenomenon was ascribed to the formation of heterogeneity (i.e., the presence of NCs with different sizes) in the system.117,118 However, Zhang and co-workers first explained this observation based on the surface plasmon and emitter coupling.115 In these AgNCs, the luminescence was controlled by the coupling between ligand-to-metal−metal charge transfer (LMMCT), i.e., from the complex Ag(I)–carboxylate to the core Ag atoms (Fig. 9C). Coupling between the surface plasmon and emitter can generate a new energy state which can be mathematically expressed as follows:116
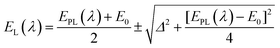 | (2) |
 | ||
| Fig. 9 (A) Excitation-dependent emission exhibited by carboxylate-templated AgNCs using PMAA as a scaffold, owing to a strong coupling between the surface plasmon and the emitter. (B) Excitation-dependent emission profiles of the DNA-templated AgNCs. The samples were excited from 440 nm to 650 nm, with an interval of 10 or 20 nm. (C) Structural illustrations of luminescent Ag–carboxylate with Ag+–carboxylate complex shell. (D) Emission spectra of Tyr-templated AgNCs as a function of excitation wavelengths as marked in the figure. (A) and (C) Reproduced with permission from ref. 115. Copyright 2014, American Chemical Society. (B) Reproduced with permission from ref. 116. Copyright 2015, American Chemical Society. (D) Reproduced with permission from ref. 96. Copyright 2021, American Chemical Society. | ||
Here, EPL(λ) represents the uncoupled surface plasmon energy and λ is the excitation wavelength, with EPL and λ expressed in eV and nm, respectively. E0 denotes the transition energy of the ligand to metal charge transfer (LMCT) and Δ is the energy related to the interactions between the photons and electrons.116 This coupling process can also influence the QY and excited-state lifetime of NCs. On the other hand, when we prepared AgNCs using L-Tyr as a template, no such excitation-dependent emission profiles were observed (Fig. 9D).96 In this case, the emission wavelength did not alter with a change in excitation wavelength, and only a change in emission intensity was obtained, as expected. Thus, it can be rationalized that the template can play a pivotal role in facilitating the plasmon-emitter coupling phenomenon.
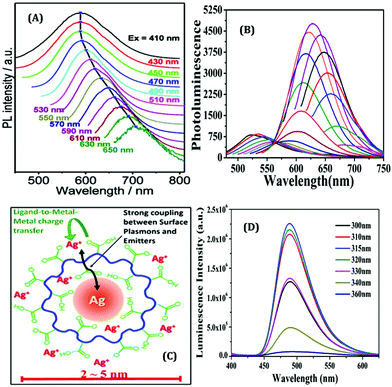
The surface charge of any particular ligand also has a profound influence on the properties of metal NCs.119 Shang and co-workers strategically engineered the surface charged groups of BSA molecules for the preparation of different AuNCs.119 They designed two different types of BSA molecules from normal BSA (nBSA): one having more negative net charges (aBSA) and another one having more positive net charges (cBSA). Zeta-potential study confirmed the successful transformation of these two types of BSA molecules.119 Interestingly, the spectroscopic signatures of these (a/n/c)-BSA-templated AuNCs were very different (Fig. 10A). The following order was observed both in terms of luminescence intensities (all being red-emitting) and excited-state lifetimes: (cBSA capped AuNCs) > (nBSA capped AuNCs) > (aBSA capped AuNCs) (Fig. 10B).
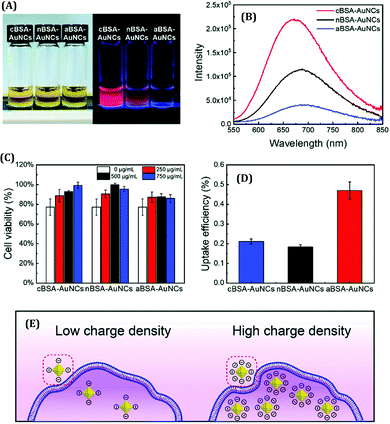 | ||
| Fig. 10 (A) Photographic images of (c/n/a)-BSA-templated AuNCs. The left panel represents the photographs when viewed under ordinary daylight while the right panel represents the same when viewed under a 365 nm UV lamp. (B) Luminescence profiles of different BSA-templated AuNCs as marked in the figure. (C) Cell viability assay of HeLa cells when incubated with (0 mg mL−1, 250 mg mL−1, 500 mg mL−1 and 750 mg mL−1) (c/n/a)-BSA-templated AuNCs for 4 hours. (D) Uptake efficiency of (c/n/a)-BSA-templated AuNCs after incubation with HeLa for 4 hours. (E) Pictorial representation of the cellular uptake of BSA-templated AuNCs with low and high charge densities. Reproduced with permission from ref. 119. Copyright 2020, The Royal Society of Chemistry. | ||
Generally, the formation of AuNCs is initiated by the complex formation between Au(I) and the ligand (in this case, ligands are cBSA and aBSA molecules). It is expected that this complex formation could be more facile for the positively charged amino groups as the salt of Au is in AuCl4− form in the solution phase. Also, the emission maximum for cBSA capped AuNCs was observed to be more blue-shifted as compared to the aBSA capped AuNCs (Fig. 10B). Based on the Jellium model, one can expect that the number of metal atoms comprising one NC will be more in the case of aBSA capped AuNCs as compared to the cBSA capped AuNCs. In fact, the TEM micrographs reported by Shang and co-workers also support the same conjecture.119 Additionally, these two AuNCs demonstrated different cellular uptake and viabilities (Fig. 10C–E).119 Inductively coupled plasma-optical emission spectrometry (ICP-OES) suggested that the encapsulation of these AuNCs inside cell lines was around 2.5-fold higher for the aBSA capped AuNCs compared to the other two AuNCs (Fig. 10D). In this regard, the non-specific interaction of negatively charged (ζ = −31.6 mV) AuNCs with positive sites of the cell membrane favoured the endocytosis of AuNCs (Fig. 10E).119
In another work, Song et al. demonstrated that the capping ligand can also regulate the electrochemical properties of NCs despite their similar core crystal structures.61 Crystal structure determination revealed that the icosahedral Au13 core and semi-ring Au2(XR)3 motifs (where, X = Se and S) are common for both AuNCs. They have determined the electrochemical properties of selenolate-templated Au25 clusters using square wave voltammogram (SWV) experiments and outlined the differences with the previously reported thiolate-capped Au25 clusters.61 While the Au25(SePh)18 NCs displayed four reduction peaks (R1, R2, R3 and R4), the Au25(SCH2CH2Ph)18 NCs exhibited only two reduction peaks (R1 and R2) (Fig. 11).61 Also, thiolate protected AuNCs were characterized by a greater electrochemical HOMO–LUMO energy gap (∼1.62 V) as compared to selenolate protected AuNCs (∼1.24 V). Furthermore, the gap between two successive oxidation states (O1 and O2) was calculated to be ∼0.70 V for the Au25(SePh)18 NCs and ∼0.30 V for the Au25(SC2H4Ph)18 NCs.61 This difference was explained based on the different dielectric environments experienced by both the cluster–cores owing to the different templates used. Thus again, the role played by a ligand in controlling the electrochemical properties of a NC is exemplified.
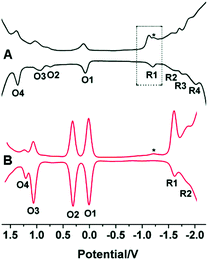 | ||
| Fig. 11 Square-wave voltammograms (SWVs) of (A) Au25(SePh)18 and (B) Au25(SCH2CH2Ph)18 NCs. The experiment was carried out in CH2Cl2 containing 0.1 M Bu4NPF6 having a concentration of NCs as 3 mg mL−1. Here the asterisk (*) indicates the wave for incompletely removed O2. Reproduced with permission from ref. 61. Copyright 2014, The Royal Society of Chemistry. | ||
So far, we have discussed how NCs can be prepared using relatively large molecules as templates. However, the situation becomes far more challenging when small molecules are used instead to prepare stable NCs. Although difficult in terms of synthetic protocols, and many-a-times stability after formation becomes an issue when such small molecules are used, recently, this topic has garnered enough scientific interest.101,120 We have already discussed that the small moiety of large molecules may be responsible for the formation of NCs, such as the Tyr molecules present inside the serum albumin proteins act as a reducing agent of metal ions.63,107,109,114 Thus, to understand the role of small ligands and the subsequent fundamental aspects related to the preparation of NCs, in two different independent studies, we have recently investigated the role played by the common template, Tyr, in the generation of AgNCs and CuNCs.96,110 For both cases, we have developed optimized and easy one-pot synthetic protocols. We have thoroughly investigated the role of Tyr in the formation of stable CuNCs using various experimental techniques as well as theoretical approaches.110 The as-prepared CuNCs displayed blue luminescence (at 415 nm) upon excitation at 315 nm. The spectroscopic signatures of these CuNCs were in excellent agreement with those reported for the other CuNCs prepared using the large molecule, HSA, as the template.76,87 This observation supported the argument/conjecture regarding the role of Tyr towards the formation of CuNCs. However, the luminescence signal obtained from NCs when HSA was used as a template was far more intense than what was observed when Tyr was used instead (Fig. 12).109,110 Additionally, the cluster–core comprised 12 Cu atoms (using HSA as a template)109 against 9 Cu atoms (when Tyr was used as the template).110 These minor discrepancies highlight the fact that the macromolecular structure of HSA provided extra surface stability and compactness in contrast to the small molecule, Tyr. Hence, it can be rationalized that besides the role played in the formation of NCs, large molecules can provide extra surface stability to the cluster–core.
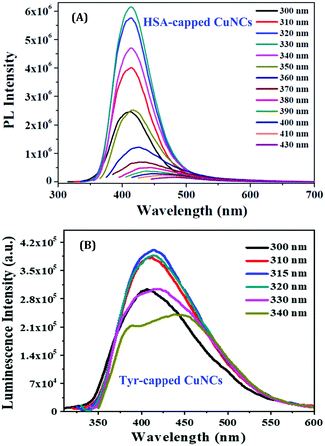 | ||
| Fig. 12 Excitation-dependent emission profiles of (A) HSA-capped CuNCs and (B) Tyr-capped CuNCs. The greater luminescence intensities obtained for HSA-capped CuNCs can be readily seen from the figures. Reproduced with permission from ref. 109 and 110. Copyright 2015 and 2021, American Chemical Society. | ||
Although only Tyr can lead to the formation of NCs, it must be mentioned that such systems are not devoid of heterogeneity. When HSA or BSA is used for the generation of NCs, generally, a narrow distribution in terms of cluster size is obtained.63,109,114 However, when we synthesized CuNCs using Tyr as the template, a Tamarix dioica leaf-like fibrillar pattern was observed from the TEM imaging experiments (Fig. 13A).110 Based on several control experiments, we concluded that the origin of fibrillar-like structures was the Tyr molecule itself (Fig. 13B). The TEM images displayed that the as-synthesized system consists of an aggregated fibrillar structure along with tiny CuNCs.110 It is already reported that in an alkaline medium, Tyr exists as a di-Tyr molecule.121,122 Our results suggested that during synthesis, the formation of di-Tyr is very rapid in nature (as compared to the formation of CuNCs) as the pH of the reaction medium was alkaline. This rapid formation of di-Tyr in an alkaline medium was well-corroborated by our UV-Vis absorption kinetics study.110
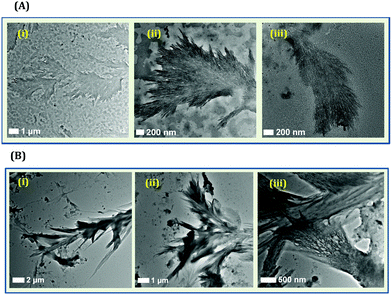 | ||
| Fig. 13 (A): (i–iii) TEM images of the fibrillar-like pattern formed by Tyr ligands during the preparation of Tyr-templated CuNCs. These fibrillar structures were formed along with smaller sized CuNCs. (B): (iv–vi) TEM images displaying the aggregated Tyr molecules (due to the formation of di-Tyr) in the presence of NaOH and in the absence of Cu2+. Reproduced with permission from ref. 110. Copyright 2021, American Chemical Society. | ||
The associated thermodynamics due to this transformation (from Tyr to di-Tyr) was also studied using the isothermal titration calorimetry (ITC) technique. From the ITC results, we obtained the evidence of di-Tyr formation in the presence of the added alkali (NaOH), along with the generation of hydrophobic interactions (characterized by ΔH > 0, ΔS > 0).110,123 To support this result, we have also performed molecular docking studies and found that the distance and angle between two π clouds satisfied the pre-requisites for π–π interactions (distance: <5 Å and angle: <45°) (Fig. 14A–D).124 It is also well known that this hydrophobic interaction is one of the major factors for fibril formation.110 Thus, we concluded that the π–π interaction of the Tyr or di-Tyr molecules in an alkaline medium facilitated fibrillar-like structure formation during the synthesis of CuNCs. Su and co-workers also reported the presence of di-Tyr inside the BSA molecule after the preparation of AuNCs (Fig. 14E).122 Although the di-Tyr molecules were also present within the system, the bulky nature of the BSA molecule provided the necessary encapsulation to AuNCs once formed, and hence, the aspect of heterogeneity has not been reported.122 Using the same template Tyr, we have observed a different type of heterogeneity in the case of AgNCs.96 The time required for the formation of AgNCs was less than that required for CuNCs. Interestingly, in this study, besides the stable Tyr-templated AgNCs, the system was having larger-sized nanoparticles which were non-luminescent.96 Once smaller-sized NCs were formed within a very short period, they were not protected instantly by the small template and hence self-aggregated to form larger-sized nanoparticles. Thus, the role played by a ligand is again established as in this case we obtained a dual-component system using a small molecule as a template.76
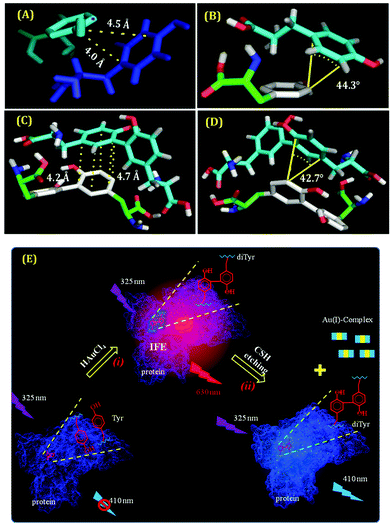 | ||
| Fig. 14 (A) and (C) The best-docked structures displaying the distance of one Tyr molecule bound to another Tyr molecule and one di-Tyr molecule interacting with another di-Tyr molecule, respectively. (B) and (D) The best-docked structures demonstrating the angle of Tyr bound to another Tyr molecule and di-Tyr interacting with another di-Tyr molecule, respectively. All these data suffice the pre-requisites for obtaining π–π interactions present in these systems. Reproduced with permission from ref. 110. Copyright 2021, American Chemical Society. (E) Schematic representation of the formation of di-Tyr residues inside the protein matrix. Step (i) represents the protein oxidation via the formation of AuNCs inside the protein scaffolds. Step (ii) represents the subsequent removal of AuNCs by the cysteamine-induced etching process, thereby leading to the luminescence properties of di-Tyr. Reproduced with permission from ref. 122. Copyright 2015, American Chemical Society. | ||
The effect of surface rigidity on the luminescence properties of AuNCs was demonstrated by Pyo et al.125 In this study, the surface of the already prepared AuNCs using glutathione as a template was more rigidified by using tetraoctylammonium (TOA) cations (Fig. 15A(i–ii)). This led to further enhancement of the QY of glutathione-capped AuNCs as compared to what was observed without the addition of TOA (Fig. 15A(iii)).125 Similarly, by monitoring the luminescence intensities of HSA-templated AuNCs, we observed that with a decrease in temperature, the luminescence intensity of AuNCs increases and vice-versa (Fig. 15B(i)).63 Upon decreasing the temperature, the protein achieves thermal compactness, and hence, the wrapping of the ligand around the cluster–core gets augmented. A blue shift in the emission maximum, which reflects a higher order of hydrophobic environment in and around the fluorophore (here AuNCs), was also observed (Fig. 15B(ii)), which further delineates our temperature-dependent observation.63
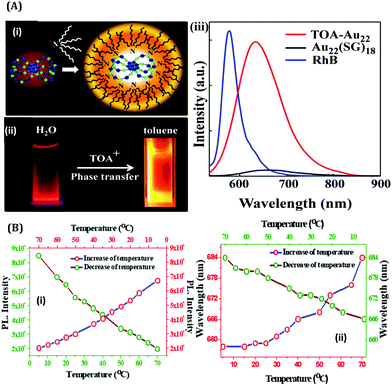 | ||
| Fig. 15 (A): (i) Schematic illustration of the binding of TOA to Au22(SG)18 NCs (color label: Au = blue; S = green). (ii) Photographic images of Au22(SG)18 in water and TOA–Au22 NCs in toluene when viewed under a 365 nm irradiation. (iii) Luminescence spectral profiles of Au22(SG)18 NCs in water and TOA–Au22 in toluene. The luminescence intensities have been compared with a standard fluorophore, Rhodamine B (RhB, QY = 31%), with the same optical density. Reproduced with permission from ref. 125. Copyright 2015, American Chemical Society (B): (i) Variation in the maximum luminescence intensity as a function of temperature for the HSA-templated AuNCs, as marked in the figure. (ii) Variation in the emission wavelength maximum as a function of temperature for the HSA-templated AuNCs, as marked in the figure. Reproduced with permission from ref. 63. Copyright 2021, The Royal Society of Chemistry. | ||
Owing to their photostable and biocompatible nature, luminescent metal NCs are widely used for bio-marking studies.62,63,67,126 For targeting the point of interest within a cell, intercellular localization studies are extremely important. Convertible organelle-targeting properties can be achieved by a controlled design of ligand exchange method.127 Recently, Yang et al. efficiently demonstrated such a ligand exchange strategy that empowers AuNCs to specifically target mitochondria instead of lysosomes which were initially targeted prior to ligand exchange.75 This study demonstrated that hydrosoluble glutathione (–SG) templated Au18SG14 NCs can accumulate mainly in lysosomes inside the cell lines. Using a suitable ligand exchange engineering, Au18SG12MTPB2 NCs were prepared (where MTPB = (4-mercaptobutyl)triphenylphosphonium bromide).75 Interestingly, these Au18SG12MTPB2 NCs were more inclined to encapsulate in the mitochondrial site within the cell lines investigated.75 Additionally, it was further demonstrated that upon photoexcitation at 638 nm, the Au18SG12MTPB2 NCs can induce reduced cytotoxicity (due to the generation of lesser singlet oxygen, 1O2) compared to Au18SG14 NCs (Scheme 5).
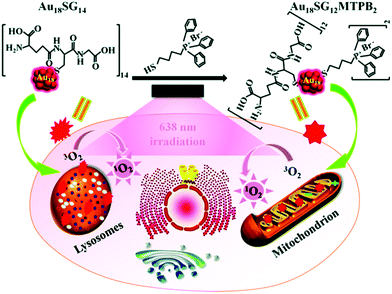 | ||
| Scheme 5 Switching of intracellular targeting organelles (from lysosomes to mitochondria) by Au18SG12MTPB2 NCs and demonstration of photodynamic therapy (PDT) toward cancer cells. Reproduced with permission from ref. 75. Copyright 2018, The Royal Society of Chemistry. | ||
Sensing
Due to their high QYs, organic dyes and quantum dots (QDs) were the most popular fluorescence probes for sensing applications.128–136 However, tedious synthetic protocols, environmentally hazardous nature, low biocompatibility, poor water solubility, lack of cost-effective production and, most importantly, toxicity limit their usage and hence alternative solutions to circumvent these limitations are surely needed. Hence, owing to their remarkable properties as discussed earlier, luminescent metal NCs are widely used for sensing purposes.62,63,79–81 These NCs, which thereby serve as next-generation fluorophores, have been utilized for sensing applications in various inter-disciplinary research fields like in vitro and in vivo sensing of cations, anions, pH, temperature, viscosity, biomolecules, etc.19,63,67,80,86,106Inorganic ion sensing
From environmental pollution to industrial purposes, the detection of ions (especially metal ions) is of utmost importance as it has direct relevance to health issues. Although some instrumental techniques (e.g., ICP-OES, ICP-MS, AAS, AFS, and some X-ray based techniques) are being used extensively for the detection of elements, such approaches are not user-friendly because of the long analysis period, high power consumption, expensive instrumentation, complex analysis procedures, need for large-scale samples, limitation in in vivo detection, etc.137,138 In this context, metal NCs become a natural choice, especially for the detection of ions based on their luminescent signatures. Metal NCs serve as optical sensors and can be categorized as “turn-on” and “turn-off” luminescence probes. The efficiency of detection, which is normally quantified from the limit of detection (LOD), categorizes these NCs accordingly.Xie et al. first reported the detection of Hg2+ ions in solution using red emissive AuNCs.139 Recently, we have reported HSA templated AuNCs as a selective sensor for Hg2+ ions with very high sensitivity.63 These AuNCs were robust in nature against thermal and chemical perturbations except for Hg2+ ions, whereby the luminescence intensity gets severely quenched in the presence of these Hg2+ ions (Fig. 16A).63 The optimum QY (>10%) and very high photostability (the AuNCs were stable more than a year) make these NCs suitable sensors (LOD ∼ 1 nM). This is the first ever approach to sense Hg2+ ions with HSA-templated AuNCs using the FCS technique.63 This single molecular study demonstrated the ultra-sensitive nature of AuNCs towards Hg2+ detection with an extremely low LOD value (∼0.01 nM) which is much lower than the international standards set for Hg2+ levels in drinking water (10 nM) according to the U.S. EPA.140 We also studied the effect of interaction of AuNCs with Hg2+ when this ion was bound to a biomolecule, insulin. To this effect, we have fabricated a model system by mixing insulin and Hg2+ forming the insulin–Hg (I–H) complex. We have concluded that these AuNCs can also sense Hg2+ with almost similar efficiency as observed in an aqueous medium.63 From our FCS investigations, the step-wise decrement of correlation signal and the diffusion time were demonstrated which correlate our observation based on the quenching of luminescence intensity owing to the gradual destruction of the cluster–core (Fig. 16B). The selectivity toward Hg2+ ion detection and the visible color change of the AuNCs upon interaction with the Hg2+ ions are represented in Fig. 16C and D, respectively. This interaction of AuNCs and Hg2+ can be rationalized by the concept of metallophillic interaction (d10–d10) phenomenon.63,139,141 Also, the higher reduction potential of Hg2+/Hg0 (Ered = 0.86 V) as compared to other heavy metals was the important factor that led to the interaction of Hg2+ with Au cluster–cores. Most interestingly, bio-imaging study as a function of time showed that these AuNCs can also sense the Hg2+ ions inside the breast cancer cell lines (MDA-MB-231) (Fig. 16E).63
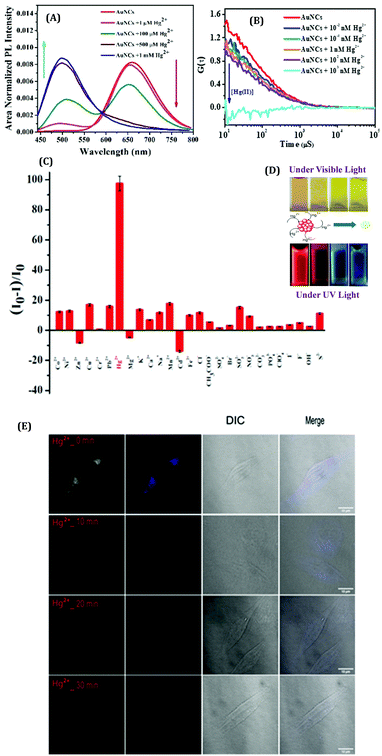 | ||
| Fig. 16 (A) Modulations in the emission spectra of the AuNCs with the increasing concentrations of Hg2+. The gradual quenching of the red emission peak (λem = 660 nm) is due to the step-wise dissociation of the parent NC and the simultaneous generation of blue-emitting NCs (λem ∼500 nm) of smaller size observed at the higher concentrations of Hg2+ ions. (B) Auto correlation curves obtained from the FCS measurements for AuNCs with the increasing concentrations of Hg2+ ions. (C) Histogram displaying the selectivity of these AuNCs in sensing Hg2+ ions among the several other ions which were also screened. (D) Photographic images displaying the change in color of the AuNC solutions in the presence of increasing concentrations of Hg2+ ions, when viewed under ordinary and UV light. (E) Confocal images of the MDA-MB-231 (breast cancer) cell lines when incubated with Hg2+ ions in the presence of AuNCs. Reproduced with permission from ref. 63. Copyright 2021, The Royal Society of Chemistry. | ||
In another work from our group, we demonstrated the photo-switch nature of HSA templated Ag9:HSA NCs for the label-free detection of Co2+ and Zn2+ ions in the aqueous phase.99 Ag9:HSA NCs showed a “turn-off’ signal in the presence of Co2+ ions; the luminescence intensity of Ag9:HSA NCs was reduced by 74% in the presence of 1 mM Co2+ ions (Fig. 17A). The LOD was calculated to be 100 nM. Most interestingly, these quenched NCs regained their luminescence intensity, i.e., exhibited a “turn-on” signal in the presence of Zn2+ ions, characterized by a LOD of 50 nM (Fig. 17A).99 This sensing mechanism of these Ag9:HSA NCs based on “turn-on” and “turn-off” luminescence intensities make them behave as a label-free photoswitch (Fig. 17B) which can be used to detect pollutants in water from several sources.99
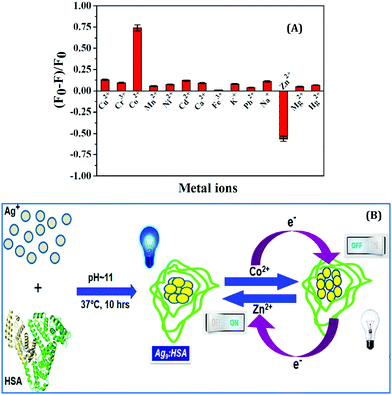 | ||
| Fig. 17 (A) Histograms demonstrating the selectivity of Ag9:HSA NCs in detecting Co2+ and Zn2+ ions in solution by “turn-off” and “turn-on” signals, respectively. (B) Schematic illustration of the mechanism of “on” and “off” luminescence features of the Ag9:HSA NCs which make them behave as a label-free photoswitch. Reproduced with permission from ref. 99. Copyright 2014, The American Chemical Society. | ||
He and co-workers developed thymine-rich (T-rich) DNA strand templated yellow luminating CuNCs which can efficiently detect Mn2+ ions in the aqueous phase by the “turn-on” signal with a detection limit of 10 μM.142 In an earlier study, Lan et al. demonstrated an interesting application of DNA-capped AgNCs which were used to detect Cu2+ ions in Montana soil (SRM 2710) and pond water with a very low LOD of 8 nM in water.100 In another study having direct application in pharmaceutical industries, Kim and co-workers143 successfully sensed the presence of Ag+ (as silver sulfadiazine) in a dermatological burn ointment, Silmazins®. In this study, Cytosine12 (Cyt12) DNA chain was used for the preparation of red luminating AgNCs which were induced to form a dimer in the presence of Ag+ ions (the monomeric AgNCs were anchored by a bridge formation) and the detection of Ag+ ions were demonstrated by a visual color change of the sample from red to green.143
Recently, Pradeep and co-workers studied the effect of heavy metal ions on the luminescence signatures of BSA-coated Au38 NCs.144 This report demonstrated that these AuNCs can act as a fluorescent chemodosimeter for selectively sensing Cd2+ and Pb2+ through the formation of cluster aggregates and subsequent augmentation of luminescence intensity (Fig. 18A).144 The DLS study showed that the aggregation phenomenon gets initiated beyond 500 ppb concentrations of both metal ions.144 Shen et al. designed nanospheres and nanovesicles containing self-assembled Ag6 NCs using polyethyleneimine (PEI) as a template.145 They examined the sensing ability of these nano-aggregates and reported their superior sensing ability towards the detection of Al3+ ions (by monitoring the quenching of emission signal of AgNCs, Fig. 18B). In a seminal work, Ngeontae and co-workers reported the detection of Al3+ ions in solution with a LOD of 26.7 nM using the concept of “turn-on” luminescence signal of cysteamine-capped CuNCs.146
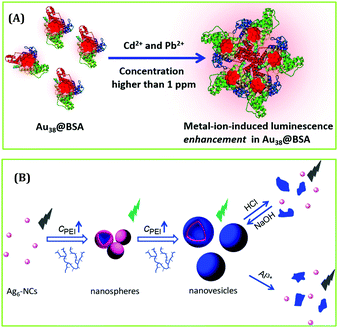 | ||
| Fig. 18 (A) Schematic representation demonstrating the augmentation in luminescence intensity of Au38 NCs in the presence of Cd2+ and Pb2+ ions. Reproduced with permission from ref. 144. Copyright 2019, The American Chemical Society. (B) Selective detection of Al3+ ions by Ag6 NCs. Reproduced with permission from ref. 145. Copyright 2018, The American Chemical Society. | ||
Das et al. prepared a relatively small molecule, tripeptide glutathione-templated blue-emissive CuNCs, as a selective sensor for the detection of Fe3+ ions and estimated the LOD to be ∼25 nM.101 Addition of 1 mM Fe3+ to CuNCs resulted in an 80% reduction of the luminescence signal of the CuNCs (Fig. 19). Low concentrations of Fe3+ ions led to complex formation with the CuNCs through the mechanism of static quenching. However, further addition of Fe3+ ions resulted in a drastic decrease in the luminescence intensity involving the dynamic quenching phenomenon followed by electron transfer between the CuNCs and Fe3+ ions.101 Additionally, this work also delineated how these CuNCs can sense Fe3+ ions present in the methemoglobin of human blood, thereby proving how these can be used for the early detection of cyanosis.101
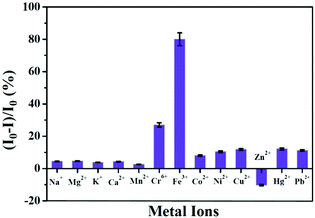 | ||
| Fig. 19 Selective detection of Fe3+ ions in solution by glutathione-templated CuNCs. The process was characterized by a LOD of ∼25 nM. Reproduced with permission from ref. 101. Copyright 2015, The American Chemical Society. | ||
Not only the cations, several reports also demonstrated the detection of anions by luminescent metal NCs. Chang and co-workers synthesized CuNCs using thiosalicylic acid (TA) as a capping agent.147 This system acts as a dual sensing probe for the selective detection of nitrite (NO2−) and cyanide (CN−) anions and interestingly, it has been demonstrated that the sensing was controlled by the pH of the medium. An alkaline medium favours the detection of CN−, whereas NO2− sensing was prominent only under acidic conditions.147 Tai et al. utilized cysteamine stabilized CuNCs for the selective detection of I− ions in the aqueous phase as well as in human urine.148 Li et al. fabricated CuNCs which displayed an elevation of luminescence signal through an aggregation-induced process in the presence of S2− ions with a detection limit of 42 nM.149
pH, viscosity and temperature sensing
pH, viscosity and temperature play significant roles in governing the dynamics and associated structure-function inter-relations in complex biological systems.150–152 Zhang and co-workers prepared stable CuNCs using natural silk fibroin (SF) as a ligand precursor and demonstrated the pH sensitivity and reversibility of these CuNCs displaying a linear relationship in the pH range of 6.08–10.05.153 The pH of sample water collected from different tap waters and rivers was examined using these CuNCs, and when compared with commercial pH meters, the results obtained were in good agreement (Table 1).153 Huang and co-workers154 synthesized trypsin-templated CuNCs and reported the linear nature of sensing over a pH range of 2.02–12.14. In another study, Bonanno et al. reported that β-nicotinamide adenine dinucleotide phosphate (NADP) templated dual emissive AuNCs can efficiently sense very acidic pH (0.6–2.7).155 As Helicobacter pylori (micro-organism) and several enteric pathogens favour acidic conditions and even gastric juice possesses a pH of ∼1.0 to 3.5, this investigation could be relevant for further applications in estimating the pH of such biological systems.| Sample | pHa | pHb | Relative error (%) | |
|---|---|---|---|---|
| Tap water 1 | 7.72 | 7.76 | −0.52 | |
| Tap water 2 | 7.77 | 7.84 | −0.89 | |
| Tap water 3 | 7.76 | 7.79 | −0.39 | |
| River water 1 | 8.06 | 8.00 | 0.75 | |
| River water 2 | 8.02 | 7.97 | 0.63 | |
| River water 3 | 8.10 | 8.04 | 0.74 |
To understand the microenvironmental viscosity, we have performed rotational and translational dynamics measurements of HSA-templated AuNCs inside two complex systems: a pluronic co-polymer hydrogel, F127 (Fig. 20A); living cervical cancerous cell lines, HeLa cells.80 Using the conventional method,132 we have prepared the F127 hydrogel with the incorporation of AuNCs inside the gel network.80 Anisotropy data revealed that the rotational time-scale inside the gel was almost similar to that of bulk water (Fig. 20B). From our translational dynamics measurements, we concluded that AuNCs were sensing ∼12 to 18 times more viscosity inside the F127 hydrogel compared to water.80 From our FCS and time-resolved anisotropy experiments, we concluded that although the AuNCs were actually localized at the corona region of the hydrogel network, they were more exposed to the void or “water pool” region (Fig. 20A).80 We have extended this work to more relevant applications by monitoring similar dynamics of the AuNCs inside the living HeLa cells and finally compared the results with the hydrogel system. We also concluded that the viscosity of the micro-environment of the HeLa cell lines around the localization site of AuNCs to be ∼23 cP (Fig. 20C), which is close to the value obtained inside the F127 hydrogel (∼12–18 cP).80 Previously, Bhattacharyya and co-workers also studied the translational dynamics of BSA-coated AuNCs inside the cancerous MCF7 and non-cancerous MCF10A cell lines.62 Feng and co-workers, using the concept of aggregation induced emission, demonstrated the “turn-on” sensing ability with an increase of system viscosity of GSH-protected AuNCs and also used these NCs for the inter-cellular imaging of the A549 (lung cancer) cell lines.156
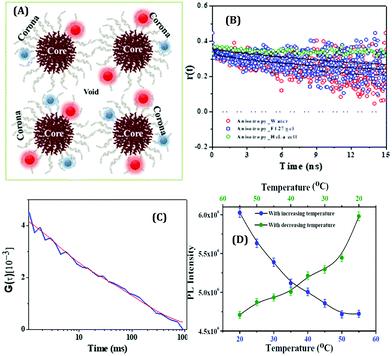 | ||
| Fig. 20 (A) Pictorial illustration of the micelle and gel network formed by a triblock copolymer, pluronic F127, and the localization of HSA-templated AuNCs inside the gel. The red spheres and the blue spheres denote the Au23 and Au9 NCs, respectively. (B) Fluorescence anisotropy decay of AuNCs in bulk water (Green), inside the F127 gel (red) and inside the HeLa cell (blue) (λex = 405 nm, λem = 473 nm for both water and F127 gel and λem = 510 nm for the HeLa cell). (C) FCS trace with best fit of AuNCs incorporated inside the HeLa cell lines, λem = 510 nm. Reproduced with permission from ref. 80. Copyright 2020, John Wiley and Sons. (D) PL reversibility exhibited by HSA-templated CuNCs as a function of temperature. The protein unfolds upon increasing the temperature and a reverse phenomenon is observed when the temperature is ramped down. Reproduced with permission from ref. 109. Copyright 2015, The American Chemical Society. | ||
In another study from our group, we have prepared CuNCs using HSA as a template (partially discussed earlier) and showed that these NCs can act as temperature sensors.109 With an increase in temperature, the luminescence intensity of CuNCs reduced gradually and vice-versa. The elevation of temperature resulted in the unfolding of the protein around the core of CuNCs, i.e., decrease of rigidity and thus, the stability of the surface diminishes. Furthermore, abatement of temperature enabled the cluster–core surface to gain stability through the refolding of the protein and consequently, led to an increase of emission intensity of CuNCs (Fig. 20D). Most interestingly, this temperature-dependent luminescence property was reversible in nature and thus, this feature rendered these CuNCs as efficient temperature detectors (served as nano-thermometric probes).109 Similarly, Tseng and co-workers demonstrated that BSA-coated AuNCs can display temperature sensing ability with reversibility in the range of 21–41 °C.157 Huang et al. also observed the reversible and linear relationship between temperature and emission intensity in the range of 20–65 °C using lipoic acid-protected Au/Ag bimetallic NCs.158
Sensing of biological systems
With the advancement of bio-imaging techniques and single molecular studies, the detection of biomolecular as well as biological systems became growing interest for researchers.Chattoraj et al. reported the in-situ generation of AuNCs inside live cells by incorporating HAuCl4 and the imidazolium-based room-temperature ionic liquid (RTIL, [pmim][Br]) conjugate inside the A549, MCF7, and HCT116 cancerous cell lines as well as WI38 and MCF10A normal cell lines (Fig. 21A).159 They observed that the localization of evolved AuNCs was 20–40 times higher in cancerous cell lines as compared to the normal cell lines (Fig. 21B).159 The higher concentration of GSH inside the cancerous cell lines facilitated the high number of AuNC generation. The emission signal, peaked at 490–530 nm, indicates the formation of the Au8-13 cluster–core composition inside the cell lines. This observation is highly corroborated with our data where we got the emission signal of HSA-templated AuNCs around 510 nm after incorporation inside the MDA-MB-231 cancerous cell lines, but our AuNCs is red-luminating in nature (λem = 660 nm, Au25) before endocytosis inside the cell lines.63 In contrast, we did not get any fluorescence image after the incubation of AuNCs with normal cell lines MCF10A.63 These results can thus help us in the discrimination of cell lines (cancerous and non-cancerous) by luminescent AuNCs (Fig. 21C and D). Besides this, the etching of relatively larger Au25 NCs (to relatively smaller Au13 NCs) during intercellular incorporation become understandable.
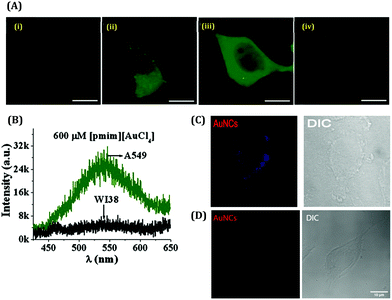 | ||
| Fig. 21 (A) Confocal images of live lung cells stained by in situ generated Au-NCs: (i) blank A549, (ii) 200 μM [pmim][AuCl4], (iii) 600 μM [pmim][AuCl4] and (iv) WI38 at 600 μM [pmim][AuCl4]. The scale bar corresponds to 4 μm. (B) Fluorescence spectra recorded by a spectrograph attached to the confocal microscope of the in situ generated AuNCs in live lung cells (λex = 405 nm): A549 (green) and WI38 (black) at 600 μm [pmim][AuCl4]. Reproduced with permission from ref. 136. Copyright 2016, John Wiley and Sons. (C) Confocal image of the MDA-MB-231 (breast cancer) cell line incubated with 30 μM AuNCs. (D) Confocal image of the MCF10A (normal breast cell) cell line treated with AuNCs. Reproduced with permission from ref. 63. Copyright 2021, The Royal Society of Chemistry. | ||
Xie and co-workers displayed the sensing ability of nucleic acid stabilized AgNCs towards adenosine as well as the RNA of let-7a.160 For both cases, the “turn-on” fluorescence signal strategy was used with the help of the photoinduced electron transfer (PET) mechanism.160 Recently, Pramanik et al. utilized GSH-stabilized CuNCs to discriminate single nucleotide mismatches (SNM) in a 20 base pair long double-stranded DNA.161 This report demonstrated that CuNCs could differentiate between well-matched DNA sequences and sequences containing single mismatched base pairs formed by cytosine.161 The calorimetric and spectroscopic results revealed that tripeptide functionalized CuNCs possess a stronger binding affinity towards cytosine-thymine mismatched (CT MM) DNA. They also showed that CuNCs could be utilized as luminescent probes to monitor the molecular diffusion of SNM DNA using FCS.161 The equilibrium binding constants derived from the association and dissociation rate constants suggested that CuNCs interact with CT MM DNA with a ∼3 times higher binding strength than that of the WM DNA-CuNC system at single molecular resolution.161 Hosseini and co-workers reported the “turn-on” detection of sequence-specific microRNA-155 (miRNA-155) by DNA-RNA heteroduplex formation.162 Luminescent CuNCs have also been used for the discrimination of nucleosides therein.162 Ni and co-workers prepared CuNCs in the presence of citric acid with four different nucleosides as templates (adenosine (A), cytidine (C), guanosine (G) and thymidine (T)) and demonstrated that T failed to produce CuNCs whereas C produced most intensely luminating CuNCs, but A-templated CuNCs possessed the highest QY (1.34%) as compared to other templates.120 In an interesting work, Bonis-O’Donnell and co-workers developed DNA-capped AgNCs for the selective detection of a neurotransmitter, dopamine, over other neurotransmitters under in vitro conditions.163 Verma and co-workers demonstrated the specificity of 8-mercapto-9-propyladenine stabilized AuNCs towards the nuclei of several cell lines via macropinocytosis pathway encapsulation.164 In a very recent review, Li et al. thoroughly discussed the role of AgNCs for being a potential sensor towards the detection of various viruses.74 For example, Zhang et al. demonstrated the prominent detection of H5N1 gene using two DNA strands165 and Shen et al. reported the possibility for the detection of Norovirus RNA by using GCC-loop containing DNA as the template.166 In an important study, Han et al. demonstrated the detection of three viral DNAs (HIV, H1N1 and H5N1) using AgNCs as a sensor with the detection limits of 3.53 nM, 0.12 nM, and 3.95 nM, respectively.167
Conclusions and future outlook
This review deals with the fundamental aspects of luminescent metal NCs. These small-sized metal-aggregates in their zero-valent oxidation states are non-toxic in nature and exhibit high cell viability. Thus, these can be safely used in deciphering dynamics inside biological self-assemblies.However, the propensity of these NCs to undergo oxidation and self-aggregation to form larger nanoparticles makes the synthetic protocols and optimization processes more tedious and challenging. On the other hand, since the associated ligand covers the core of the metal NCs, they can interact with the system and thus, their various applications can be regulated.168 Beside this, the stability, luminescence intensity and other photophysical properties of the metal NCs are also influenced by the compactness, surface coverage and functional groups inherently present within the ligands.168 The solubility of the NCs in the solvent is highly influenced by the capping ligands. The hydrophilic ligands (having groups such as hydroxyl, carboxyl, amine, etc.) facilitate the aqueous phase solubility of the NCs. This enables the development of newer templates that can be used for the preparation of water-soluble NCs which can have varied applications, especially related to biological systems. Thus, the role of ligands and templates becomes extremely critical in the development of NCs. Herein, we have systematically highlighted the role of such ligands/templates in developing NCs which are not only stable but also have characteristic spectroscopic signatures. These ligands help in tuning the morphological aspects of these NCs and this in turn control their applications. A major section of this review deals with the sensing capabilities of these NCs. Although there is a plethora of reports that deal with the modifications of ligands used in the preparation of NCs which in turn control the application aspects, yet a more cumulative and comprehensive understanding is required. In this feature article, we have summarized how and why these can be used to detect metal ions with selectivity and unprecedented limit of detection. Metal ions which are detrimental to human health (like heavy metals, mercury, etc.) can be easily detected by these NCs. The fact that these NCs are photostable enables them to be used in single molecular investigations. We have showed how they can be used to sense the pH, viscosity and temperature of several biological systems along with their ability to discriminate cancerous and non-cancerous cell lines and specifically target certain organelles within a cellular environment.
Although the field related to NCs has seen a huge upsurge recently, more in-depth investigations are needed to realize their applications in biomedical research. The future of these next-generation fluorophores will be directed towards the development of catalysis, solar cells, safe and pure drinking water, and investigations which will have direct physiological and biomedical relevance such as targeted drug delivery and early detection of diseases.
Author contributions
SC collated all the data; SM conceptualized the review; both SC and SM wrote the entire review article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
SC thanks IISER Bhopal for the fellowship and SM thanks SERB (CRG/2018/000760) for the financial support. SM thanks all the past and present members of the group. The support from IISER Bhopal, CIF IISER Bhopal and the Departmental TEM facility (FIST grant) is deeply acknowledged.References
- J. Zheng, P. R. Nicovich and R. M. Dickson, Annu. Rev. Phys. Chem., 2007, 58, 409 CrossRef CAS.
- L. A. Peyser, A. E. Vinson, A. P. Bartko and R. M. Dickson, Science, 2001, 291, 103 CrossRef CAS.
- C. T. Campbell, S. C. Parker and D. E. Starr, Science, 2002, 298, 811 CrossRef CAS.
- L. Shang, S. Dong and G. U. Nienhaus, Nano Today, 2011, 6, 401 CrossRef CAS.
- K. Zheng, V. Fung, X. Yuan, D. Jiang and J. Xie, J. Am. Chem. Soc., 2019, 141(48), 18977 CrossRef CAS PubMed.
- Z. Wu, Q. Yao, O. Jin, H. Chai, N. Ding, W. Xu, S. Zang and J. Xie, Angew. Chem., Int. Ed., 2020, 59, 9934 CrossRef CAS.
- T. Kawawaki, Y. Kataoka, M. Hirata, Y. Akinaga, R. Takahata, K. Wakamatsu, Y. Fujiki, M. Kataoka, S. Kikkawa, A. S. Alotabi, S. Hossain, D. J. Osborn, T. Teranishi, G. G. Andersson, G. F. Metha, S. Yamazoe and Y. Negishi, Angew. Chem., Int. Ed., 2021, 60, 21340 CrossRef CAS PubMed.
- H. Yu, B. Rao, W. Jiang, S. Yang and M. Zhu, Coord. Chem. Rev., 2019, 378, 595 CrossRef CAS.
- D. Bera and N. Goswami, J. Phys. Chem. Lett., 2021, 12, 9033 CrossRef CAS.
- W.-M. He, Z. Zhou, Z. Han, S. Li, Z. Zhou, L.-F. Ma and S.-Q. Zang, Angew. Chem., Int. Ed., 2021, 60, 8505 CrossRef CAS PubMed.
- M. R. Narouz, K. M. Osten, P. J. Unsworth, R. W. Y. Man, K. Salorinne, S. Takano, R. Tomihara, S. Kaappa, S. Malola, C.-T. Dinh, J. D. Padmos, K. Ayoo, P. J. Garrett, M. Nambo, J. H. Horton, E. H. Sargent, H. Häkkinen, T. Tsukuda and C. M. Crudden, Nat. Chem., 2019, 11, 419 CrossRef CAS.
- E. Khatun and T. Pradeep, ACS Omega, 2021, 6, 1 CrossRef CAS.
- K. Bhattacharyya and S. Mukherjee, Bull. Chem. Soc. Jpn., 2018, 91, 447 CrossRef.
- Y. Cao, S. Malola, M. F. Matus, T. Chen, Q. Yao, R. Shi, H. Häkkinen and J. Xie, Chem, 2021, 7, 2227 CAS.
- J. Xie, Y. Zheng and J. Y. Ying, J. Am. Chem. Soc., 2009, 131, 888 CrossRef CAS.
- J. Zheng, C. W. Zhang and R. M. Dickson, Phys. Rev. Lett., 2004, 93, 077402 CrossRef.
- A. Retnakumari, S. Setua, D. Menon, P. Ravindran, H. Muhammed, T. Pradeep, S. Nair and M. Koyakutty, Nanotechnology, 2010, 21, 055103 CrossRef PubMed.
- Q. Yao, Z. Wu, Z. Liu, Y. Lin, X. Yuan and J. Xie, Chem. Sci., 2021, 12, 99 RSC.
- I. Zare, D. M. Chevrier, A. Cifuentes-Rius, N. Moradi, Y. Xianyu, S. Ghosh, L. Trapiella-Alfonso, Y. Tian, A. Shourangiz-Haghighi, S. Mukherjee, K. Fan and M. R. Hamblin, Mater. Today, 2021 DOI:10.1016/j.mattod.2020.10.027.
- X.-D. Zhang, J. Chen, Z. Luo, D. Wu, X. Shen, S.-S. Song, Y.-M. Sun, P.-X. Liu, J. Zhao, S. Huo, S. Fan, F. Fan, X.-J. Liang and J. Xie, Adv. Healthcare Mater., 2014, 3, 133 CrossRef CAS PubMed.
- X. Yuan, Z. Luo, Y. Yu, Q. Yao and J. Xie, Chem. – Asian J., 2013, 8, 858 CrossRef CAS PubMed.
- S. Hasegawa, S. Takano, K. Harano and T. Tsukuda, JACS Au, 2021, 1, 660 CrossRef CAS PubMed.
- K. Koyasu and T. Tsukuda, J. Chem. Phys., 2021, 154, 140901 CrossRef CAS PubMed.
- Y. Song, H. Abroshan, J. Chai, X. Kang, H. Kim, M. Zhu and R. Jin, Chem. Mater., 2017, 29, 3055 CrossRef CAS.
- Q. Li, S. Wang, K. Kirschbaum, K. Lambright, A. Das and R. Jin, Chem. Commun., 2016, 52, 5194 RSC.
- T. Udayabhaskararao and T. Pradeep, J. Phys. Chem. Lett., 2013, 4, 1553 CrossRef CAS.
- Y. Du, H. Sheng, D. Astruc and M. Zhu, Chem. Rev., 2020, 120, 526 CrossRef CAS PubMed.
- R. Jin, G. Li, S. Sharma, Y. Li and X. Du, Chem. Rev., 2021, 121, 567 CrossRef CAS.
- H. Haidari, Z. Kopecki, R. Bright, A. J. Cowin, S. Garg, N. Goswami and K. Vasilev, ACS Appl. Mater. Interfaces, 2020, 12, 41011 CrossRef CAS PubMed.
- M. Cao, R. Pang, Q.-Y. Wang, Z. Han, Z.-Y. Wang, X.-Y. Dong, S.-F. Li, S.-Q. Zang and T. C. W. Mak, J. Am. Chem. Soc., 2019, 141, 14505 CrossRef CAS PubMed.
- U. Kreibig and M. Vollmer, Optical Properties of Metal Clusters, Springer, Berlin, 1995 Search PubMed.
- Y. A. Eremin, SCATTERING|Scattering Theory, Encyclopedia of Modern Optics, 2005, 326–330 Search PubMed.
- Y. Tang, X. Yu, J. Xu, B. Audit and S. Zhang, Noble Metal-Metal Oxide Hybrid Nanoparticles, 2019, 431–455 CAS.
- N. W. Ashcroft and N. D. Mermin, Solid State Physics, Holt/Rinehart & Winston, New York, 1976 Search PubMed.
- S. Link, A. Beeby, S. FitzGerald, M. A. El-Sayed, T. G. Schaaff and R. L. Whetten, J. Phys. Chem. B, 2002, 106, 3410 CrossRef CAS.
- C. Felix, C. Sieber, W. Harbich, J. Buttet, I. Rabin, W. Schulze and G. Ertl, Phys. Rev. Lett., 2001, 86, 2992 CrossRef CAS.
- X. Du and R. Jin, Dalton Trans., 2020, 49, 10701 RSC.
- R. S. Dhayal, W. E. van Zyl and C. W. Liu, Dalton Trans., 2019, 48, 3531 RSC.
- B. Nieto-Ortega and T. Bürgi, Acc. Chem. Res., 2018, 51, 2811 CrossRef CAS PubMed.
- W. A. de Heer, K. Selby, V. V. Kresin, J. Masui, M. Vollmer, A. Chatelain and W. D. Knight, Phys. Rev. Lett., 1987, 59, 1805 CrossRef CAS PubMed.
- W. A. de Heer, Rev. Mod. Phys., 1993, 65, 611 CrossRef CAS.
- R. L. Johnston, Atomic and Molecular Clusters, Taylor & Francis, London, 2002 Search PubMed.
- X. Kang and M. Zhu, Chem. Soc. Rev., 2019, 48, 2422 RSC.
- X. Yuan, N. Goswami, W. Chen, Q. Yao and J. Xie, Chem. Commun., 2016, 52, 5234 RSC.
- Z. Luo, X. Yuan, Y. Yu, Q. Zhang, D. T. Leong, J. Y. Lee and J. Xie, J. Am. Chem. Soc., 2012, 134, 16662 CrossRef CAS.
- Z. Wu, Q. Yao, S.-Q. Zang and J. Xie, Natl. Sci. Rev., 2021, 8, nwaa208 CrossRef PubMed.
- Z. Wang, X. Pan, S. Qian, G. Yang, F. Du and X. Yuan, Coord. Chem. Rev., 2021, 438, 213900 CrossRef CAS.
- L. Zhu, M. Gharib, C. Becker, Y. Zeng, A. R. Ziefuß, L. Chen, A. M. Alkilany, C. Rehbock, S. Barcikowski, W. J. Parak and I. Chakraborty, J. Chem. Educ., 2020, 97, 239 CrossRef CAS.
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346 CrossRef CAS.
- Z. Liu, Z. Wu, Q. Yao, Y. Cao, O. J. H. Chai and J. Xie, Nano Today, 2021, 36, 101053 CrossRef CAS.
- Q. Yao, X. Yuan, T. Chen, D. T. Leong and J. Xie, Adv. Mater., 2018, 30, 1802751 CrossRef.
- Q. Yao, T. Chen, X. Yuan and J. Xie, Acc. Chem. Res., 2018, 51, 1338 CrossRef CAS PubMed.
- X. Yuan, L. L. Chng, J. Yang and J. Y. Ying, Adv. Mater., 2020, 32, 1906063 CrossRef CAS.
- X. S. Shen, G. Z. Wang, X. Hong and W. Zhu, Phys. Chem. Chem. Phys., 2009, 11, 7450 RSC.
- P. Born and T. Kraus, Phys. Rev. E, 2013, 87, 062313 CrossRef PubMed.
- E. S. Shibu, M. A. H. Muhammed, T. Tsukuda and T. Pradeep, J. Phys. Chem. C, 2008, 112, 12168 CrossRef CAS.
- D. M. Chevrier, L. Raich, C. Rovira, A. Das, Z. Luo, Q. Yao, A. Chatt, J. Xie, R. Jin, J. Akola and P. Zhang, J. Am. Chem. Soc., 2018, 140, 15430 CrossRef CAS.
- P. A. Bartlett, B. Bauer and S. J. Singer, J. Am. Chem. Soc., 1978, 100, 5085 CrossRef CAS.
- G. H. Woehrle, M. G. Warner and J. E. Hutchison, J. Phys. Chem. B, 2002, 106, 9979 CrossRef.
- Y. Yang and S. Chen, Nano Lett., 2003, 3, 75 CrossRef CAS.
- Y. Song, J. Zhong, S. Yang, S. Wang, T. Cao, J. Zhang, P. Li, D. Hu, Y. Pei and M. Zhu, Nanoscale, 2014, 6, 13977 RSC.
- S. Chattoraj and K. Bhattacharyya, J. Phys. Chem. C, 2014, 118, 22339 CrossRef CAS.
- S. Chakraborty, A. Nandy, S. Ghosh, N. K. Das, S. Parveen, S. Datta and S. Mukherjee, Analyst, 2021, 146, 1455 RSC.
- J. Duguid, V. A. Bloomfield, J. Benevides and G. J. Thomas, Jr., Biophys. J., 1993, 65, 1916 CrossRef CAS.
- A. Ono, S. Cao, H. Togashi, M. Tashiro, T. Fujimoto, T. Machinami, S. Oda, Y. Miyake, I. Okamotoa and Y. Tanaka, Chem. Commun., 2008, 4825 RSC.
- C. M. Ritchie, K. R. Johnsen, J. R. Kiser, Y. Antoku, R. M. Dickson and J. T. Petty, J. Phys. Chem. C, 2007, 111, 175 CrossRef CAS.
- Y. Xiao, Z. Wu, Q. Yao and J. Xie, Aggregate, 2021, 2, 114 CrossRef.
- Y. Zhang, C. Zhang, C. Xu, X. Wang, C. Liu, G. I. N. Waterhouse, Y. Wang and H. Yin, Talanta, 2019, 200, 432 CrossRef CAS.
- H. Zhang, Z. Zhao, P. R. McGonigal, R. Ye, S. Liu, J. W. Y. Lam, R. T. K. Kwok, W. Z. Yuan, J. Xie, A. L. Rogach and B. Z. Tang, Mater. Today, 2020, 32, 275 CrossRef CAS.
- M. Shellaiah and K. Sun, Chemosensors, 2017, 5, 3667 CrossRef.
- I. Chakraborty and T. Pradeep, Chem. Rev., 2017, 117, 8208 CrossRef CAS PubMed.
- N. K. Das and S. Mukherjee, Phys. Sci. Rev., 2018, 3, 20170081, DOI:10.1515/psr-2017-0081.
- Q. Wang, S. Liu and Z. Tang, J. Mater. Chem. B, 2021, 9, 8174 RSC.
- D. Li, H. Chen, X. Gao, X. Mei and L. Yang, ACS Sens., 2021, 6, 613 CrossRef CAS.
- Y. Yang, S. Wang, S. Chen, Y. Shen and M. Zhu, Chem. Commun., 2018, 54, 9222 RSC.
- S. Kundu, M. Ghosh and N. Sarkar, Langmuir, 2021, 37, 9281 CrossRef CAS.
- S. Behzadi, V. Serpooshan, W. Tao, M. A. Hamaly, M. Y. Alkawareek, E. C. Dreaden, D. Brown, A. M. Alkilany, O. C. Farokhzad and M. Mahmoudi, Chem. Soc. Rev., 2017, 46, 4218 RSC.
- T. Pan, W. Song, H. Gao, T. Li, X. Cao, S. Zhong and Y. Wan, ACS Appl. Mater. Interfaces, 2016, 8, 19217 CrossRef CAS.
- N. K. Das, S. Chakraborty, M. Mukherjee and S. Mukherjee, Chem. Phys. Chem., 2018, 19, 2218 CrossRef.
- S. Chakraborty, S. Nandi, K. Bhattacharyya and S. Mukherjee, Chem. Phys. Chem., 2020, 21, 406 CrossRef CAS PubMed.
- S. Nandi, S. Ghosh and K. Bhattacharyya, J. Phys. Chem. B, 2018, 122, 3023 CrossRef CAS.
- M. Shamsipur, A. Barati and Z. Nematifar, J. Photochem. Photobio. C, 2019, 39, 76 CrossRef CAS.
- R. Ali, S. M. Saleh and S. M. Aly, Microchim. Acta, 2017, 184, 3309 CrossRef CAS.
- H. Xiong, W. Wang, J. Liang, W. Wen, X. Zhang and S. Wang, Sens. Actuators, B, 2017, 239, 988 CrossRef CAS.
- M. V. Romeo, E. Lopez-Martinez, J. Berganza-Granda, F. Goni-de-Cerio and A. L. Cortajarena, Nanoscale Adv., 2021, 3, 1331 RSC.
- L. Zhang and E. Wang, Nano Today, 2014, 9, 132 CrossRef CAS.
- B. Wang, M. Zhao, M. Mehdi, G. Wang, P. Gao and K.-Q. Zhang, Mater. Chem. Front., 2019, 3, 1722 RSC.
- Y. Zhang, C. Zhu, L. Zhang, C. Tan, J. Yang, B. Chen, L. Wang and H. Zhang, Small, 2015, 11, 1385 CrossRef CAS PubMed.
- R. C. Triulzi, M. Micic, S. Giordani, M. Serry, W.-A. Chiou and R. M. Leblanc, Chem. Commun., 2006, 5068 RSC.
- C.-T. Chen, W.-J. Chen, C.-Z. Liu, L.-Y. Chang and Y.-C. Chen, Chem. Commun., 2009, 7515 RSC.
- J. Sharma, H.-C. Yeh, H. Yoo, J. H. Werner and J. S. Martinez, Chem. Commun., 2011, 47, 2294 RSC.
- Y. Wang, Y. Wang, F. Zhou, P. Kim and Y. Xia, Small, 2012, 8, 3769 CrossRef CAS PubMed.
- K. Zhang, K. Wang, M. Xie, X. Zhu, L. Xu, R. Yang, B. Huang and X. Zhu, Biosens. Bioelectron., 2014, 52, 124 CrossRef CAS PubMed.
- L. V. Nair, D. S. Philips, R. S. Jayasree and A. Ajayaghosh, Small, 2013, 9, 2673 CrossRef CAS.
- X. Yuan, Y. Tay, X. Dou, Z. Luo, D. T. Leong and J. Xie, Anal. Chem., 2013, 85, 1913 CrossRef CAS PubMed.
- S. Chakraborty, P. Sagarika, S. Rai, C. Sahi and S. Mukherjee, ACS Appl. Mater. Interfaces, 2021, 13, 36938 CrossRef CAS PubMed.
- T. Chen, Y. Hu, Y. Cen, X. Chu and Y. Lu, J. Am. Chem. Soc., 2013, 135, 11595 CrossRef CAS PubMed.
- A. Nain, Y.-T. Tseng, Y.-S. Lin, S.-C. Wei, R. P. Mandal, B. Unnikrishnan, C.-C. Huang, F.-G. Tseng and H.-T. Chang, Sens. Actuators, B, 2020, 321, 128539 CrossRef CAS.
- S. Ghosh, U. Anand and S. Mukherjee, Anal. Chem., 2014, 86, 3188 CrossRef CAS.
- G.-Y. Lan, C.-C. Huang and H.-T. Chang, Chem. Commun., 2010, 46, 1257 RSC.
- N. K. Das, S. Ghosh, A. Priya, S. Datta and S. Mukherjee, J. Phys. Chem. C, 2015, 119, 24657 CrossRef CAS.
- Y. Zhang, H. Jiang and X. Wang, Anal. Chim. Acta, 2015, 870, 1 CrossRef CAS.
- C. Wanga, J. Wub, K. Jiangb, M. G. Humphrey and C. Zhang, Sens. Actuators, B, 2017, 238, 1136 CrossRef.
- Y. Sun, J. Wu, C. Wang, Y. Zhao and Q. Lin, New J. Chem., 2017, 41, 5412 RSC.
- H. Liu, G. Hong, Z. Luo, J. Chen, J. Chang, M. Gong, H. He, J. Yang, X. Yuan, L. Li, X. Mu, J. Wang, W. Mi, J. Luo, J. Xie and X.-D. Zhang, Adv. Mater., 2019, 31, 1901015 CrossRef CAS.
- X. Song, W. Zhu, X. Ge, R. Li, S. Li, X. Chen, J. Song, J. Xie, X. Chen and H. Yang, Angew. Chem., Int. Ed., 2021, 60, 1306 CrossRef CAS.
- U. Anand, S. Ghosh and S. Mukherjee, J. Phys. Chem. Lett., 2012, 3, 3605 CrossRef CAS PubMed.
- I. Chakraborty and T. Pradeep, Nanoscale, 2014, 6, 14190 RSC.
- S. Ghosh, N. K. Das, U. Anand and S. Mukherjee, J. Phys. Chem. Lett., 2015, 6, 1293 CrossRef.
- S. Chakraborty and S. Mukherjee, J. Phys. Chem. Lett., 2021, 12, 3266 CrossRef CAS.
- T. Peter Jr, All about Albumin: Biochemistry, Genetics and Medical Applications, 1996, Academic Press Search PubMed.
- S. Naveenraj and S. Anandan, J. Photochem. Photobiol., C, 2013, 14, 53 CrossRef CAS.
- X. He and D. C. Carter, Nature, 1992, 358, 209 CrossRef CAS.
- Y. Xu, S. Palchoudhury, Y. Qin, T. Macher and Y. Bao, Langmuir, 2012, 28, 8767 CrossRef CAS.
- Y. Chen, T. Yang, H. Pan, Y. Yuan, L. Chen, M. Liu, K. Zhang, S. Zhang, P. Wu and J. Xu, J. Am. Chem. Soc., 2014, 136, 1686 CrossRef CAS.
- X. Liu, R. Hu, Z. Gao and N. Shao, Langmuir, 2015, 31, 5859 CrossRef CAS.
- L. Shang and S. J. Dong, Chem. Commun., 2008, 1088 RSC.
- K. Morishita, J. L. MacLean, B. Liu, H. Jiang and J. Liu, Nanoscale, 2013, 5, 2840 RSC.
- W. Zhong, M. Wen, J. Xu, H. Wang, L.-L. Tana and L. Shang, Chem. Commun., 2020, 56, 11414 RSC.
- Y. Wang, T. Chen, Q. Zhuang and Y. Ni, ACS Appl. Mater. Interfaces, 2017, 9, 32135 CrossRef CAS.
- C. Giulivi, N. Traaseth and K. Davies, Amino Acids, 2003, 25, 227 CrossRef CAS.
- L. Su, T. Shu, J. Wang, Z. Zhang and X. Zhang, J. Phys. Chem. C, 2015, 119, 12065 CrossRef CAS.
- P. D. Ross and S. Subramanian, Biochemistry, 1981, 20, 3096 CrossRef CAS , and references therein.
- Y. An, J. W. G. Bloom and S. E. Wheeler, J. Phys. Chem. B, 2015, 119, 14441 CrossRef CAS.
- K. Pyo, V. D. Thanthirige, K. Kwak, P. Pandurangan, G. Ramakrishna and D. Lee, J. Am. Chem. Soc., 2015, 137, 8244 CrossRef CAS.
- R. R. Nasaruddin, T. Chen, Q. Yao, S. Zang and J. Xie, Coord. Chem. Rev., 2021, 426, 213540 CrossRef CAS.
- G. H. Woehrle and J. E. Hutchison, Inorg. Chem., 2005, 44, 6149 CrossRef CAS.
- M. Li, T. Chen, J. J. Gooding and J. Liu, ACS Sens., 2019, 4, 1732 CrossRef CAS.
- J. Goicoechea F. J. Arregui and I. R. Matias, in Quantum Dots for Sensing, ed. F. Arregui, Sensors Based on Nanostructured Materials, Springer, Boston, MA., 2009 Search PubMed.
- N. Chaniotakis and R. Buiculescu, Nanosensors for Chemical and Biological Applications, Woodhead publishing, 2014, 267 Search PubMed.
- M. Ganesan and P. Nagaraaj, Anal. Methods, 2020, 12, 4254 RSC.
- S. Chakraborty, S. Nandi, K. Bhattacharyya and S. Mukherjee, Chem. Phys. Chem., 2019, 20, 3221 CrossRef CAS.
- K. R. A. S. Sandanayake and I. O. Sutherland, Sens. Actuators, B, 1993, 11, 331 CrossRef CAS.
- N. K. Das, S. Ghosh, S. Jaiswal, A. Tewary and S. Mukherjee, Chem. Phys. Lett., 2017, 682, 147 CrossRef CAS.
- U. Pramanik, S. Chakraborty, K. Bhattacharyya and S. Mukherjee, Chem. Phys. Lett., 2021, 762, 138105 CrossRef CAS.
- S. Pramanik, A. Nandy, S. Chakraborty, U. Pramanik, S. Nandi and S. Mukherjee, J. Phys. Chem. Lett., 2020, 11, 2436 CrossRef CAS.
- B. Wei and L. Yang, Microchem. J., 2010, 94, 99 CrossRef CAS.
- E. Peňa-Vázquez, J. Villanueva-Alonso and P. Bermejo-Barrera, J. Anal. At. Spectrom., 2007, 22, 642 RSC.
- J. Xie, Y. Zheng and J. Y. Ying, Chem. Commun., 2010, 46, 961 RSC.
- L. Shang, L. Yang, F. Stockmar, R. Popescu, V. Trouillet, M. Bruns, D. Gerthsen and G. U. Nienhaus, Nanoscale, 2012, 4, 4155 RSC.
- C. Guo and J. Irudayaraj, Anal. Chem., 2011, 83, 2883 CrossRef CAS PubMed.
- B. Han, R. Xiang, X. Hou, M. Yu, T. Peng, Y. Li and G. He, Anal. Methods, 2017, 9, 2590 RSC.
- J. Lee, J. Park, H. H. Lee, H. Park, H. I. Kim and W. J. Kim, Biosens. Bioelectron., 2015, 68, 642 CrossRef CAS PubMed.
- J. S. Mohanty, K. Chaudhari, C. Sudhakar and T. Pradeep, J. Phys. Chem. C, 2019, 123, 28969 CrossRef.
- J. Shen, Z. Wang, D. Sun, C. Xia, S. Yuan, P. Sun and X. Xin, ACS Appl. Mater. Interfaces, 2018, 10, 3955 CrossRef CAS PubMed.
- C. Boonmee, V. Promarak, T. Tuntulani and W. Ngeontae, Talanta, 2018, 178, 796 CrossRef CAS PubMed.
- J. Cang, C.-W. Wang, P.-C. Chen, Y.-J. Lin, Y.-C. Li and H.-T. Chang, Anal. Methods, 2017, 9, 5254 RSC.
- Y.-T. Tai, T. Simon, Y.-Y. Chu and F.-H. Ko, Sens. Bio-Sens. Res., 2020, 27, 100319 CrossRef.
- Z. Li, S. Guo and C. Lu, Analyst, 2015, 140, 2719 RSC.
- E. Nader, S. Skinner, M. Romana, R. Fort, N. Lemonne, N. Guillot, A. Gauthier, S. Antoine-Jonville, C. Renoux, M.-D. Hardy-Dessources, E. Stauffer, P. Joly, Y. Bertrand and P. Connes, Front. Physiol., 2019, 10, 1329 CrossRef.
- A. Cuecas, J. Cruces, J. F. Galisteo-López, X. Peng and J. M. Gonzalez, Biophys. J., 2016, 111, 875 CrossRef CAS PubMed.
- A. Curnutt, K. Smith, E. Darrow and K. B. Walters, Sci. Rep., 2020, 10, 8760 CrossRef CAS PubMed.
- G. Zhang, T. Xu, H. Du, Y. Qiao, X. Guo, L. Shi, Y. Zhang, S. Shuang, C. Donga and H. Ma, J. Mater. Chem. C, 2016, 4, 3540 RSC.
- W. Wang, F. Leng, L. Zhan, Y. Chang, X. Xi Yang, J. Lana and C. Z. Huang, Analyst, 2014, 139, 2990 RSC.
- A. Bonanno, I. Pérez-Herráez, E. Zaballos-García and J. Pérez-Prieto, Chem. Commun., 2020, 56, 587 RSC.
- S. Pan, J. Zhou, W. Liu, Y. Ye, G. Chen, J. Xu, Z. Qian, J. Chen and H. Feng, Analyst, 2019, 144, 4483 RSC.
- Y.-T. Wu, C. Shanmugam, W.-B. Tseng, M.-M. Hiseh and W.-L. Tseng, Nanoscale, 2016, 8, 11210 RSC.
- H. Huang, H. Li, J.-J. Feng and A.-J. Wang, Sens. Actuators, B, 2016, 223, 550 CrossRef CAS.
- S. Chattoraj, Md. A. Amin, S. Mohapatra, S. Ghosh and K. Bhattacharyya, Chem. Phys. Chem., 2016, 17, 61 CrossRef CAS.
- K. Zhang, K. Wang, X. Zhu, Y. Gao and M. Xie, Chem. Commun., 2014, 50, 14221 RSC.
- S. Pramanik, L. Khamari, S. Nandi and S. Mukherjee, J. Phys. Chem. C, 2019, 123, 29047 CrossRef.
- Y.-S. Borgheia, M. Hosseinia and M. R. Ganjali, Clin. Chim. Acta, 2018, 483, 119 CrossRef PubMed.
- J. T. D. Bonis-O’Donnell, A. Thakrar, J. W. Hirschberg, D. Vong, B. N. Queenan, D. K. Fygenson and S. Pennathur, ACS Chem. Neurosci., 2018, 9, 849 CrossRef PubMed.
- V. Venkatesh, A. Shukla, S. Sivakumar and S. Verma, ACS Appl. Mater. Interfaces, 2014, 6, 2185 CrossRef CAS PubMed.
- Y. Zhang, F. Mu, Y. Duan, Q. Li, Y. Pan, H. Du, P. He, X. Shen, Z. Luo, C. Zhu and L. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 48357 CrossRef CAS PubMed.
- F. Shen, Y. Cheng, Y. Xie, H. Yu, W. Yao, H. W. Li, Y. Guo and H. Qian, Anal. Biochem., 2019, 583, 113365 CrossRef CAS.
- D. Han and C. Wei, Talanta, 2018, 181, 24 CrossRef CAS PubMed.
- B. Zhang, J. Chen, Y. Cao, O. J. H. Chai and J. Xie, Small, 2021, 17, 2004381 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |