Effect of lithium salt on fluorescence quenching in glycerol: a comparison with ionic liquid/deep eutectic solvent†
Manish
Kumar
,
Anjali
,
Divya
Dhingra
,
Ankit
Yadav
and
Siddharth
Pandey
 *
*
Department of Chemistry, Indian Institute of Technology Delhi, Hauz Khas, New Delhi – 110016, India. E-mail: sipandey@chemistry.iitd.ac.in; Fax: +91-11-26581102; Tel: +91-11-26596503
First published on 26th November 2021
Abstract
It was reported earlier that the addition of LiCl to the deep eutectic solvent (DES) ChCl:Urea (composed of the salt choline chloride and the H-bond donor urea in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio) and the addition of LiTf2N [Tf2N:(CF3SO2)2N] to the ionic liquid (IL) [C2C1im][Tf2N] ([C2C1im]:1-ethyl-3-methylimidazolium), respectively, results in an increase in the dynamic viscosity of the medium. However, as the concentration of the Li salt is increased, instead of decreasing, the bimolecular quenching rate constant (kq) for the quenching of pyrene fluorescence by nitromethane is observed to first increase and only then decreases within both media. This unusual initial increase in quenching is hypothesized to be due to structural changes in the DES ChCl:Urea and the IL [C2C1im][Tf2N], respectively, as the Li salt is added. We tested this hypothesis by comparing the physical properties and fluorescence quenching behavior between 1 wt% water in glycerol solution which has similar viscosity to that of the DES ChCl:Urea with the aforementioned DES and IL in the presence of lithium salt as media. In complete contrast, irrespective of the temperature, kq is found to decrease monotonically with increasing concentration of LiCl within 1 wt% water in glycerol media. These findings therefore highlight the unusual characteristics of ILs and DESs as solubilizing media. The ionic nature of the IL and the high concentration of ions in the DES are deemed responsible for these outcomes.
2 molar ratio) and the addition of LiTf2N [Tf2N:(CF3SO2)2N] to the ionic liquid (IL) [C2C1im][Tf2N] ([C2C1im]:1-ethyl-3-methylimidazolium), respectively, results in an increase in the dynamic viscosity of the medium. However, as the concentration of the Li salt is increased, instead of decreasing, the bimolecular quenching rate constant (kq) for the quenching of pyrene fluorescence by nitromethane is observed to first increase and only then decreases within both media. This unusual initial increase in quenching is hypothesized to be due to structural changes in the DES ChCl:Urea and the IL [C2C1im][Tf2N], respectively, as the Li salt is added. We tested this hypothesis by comparing the physical properties and fluorescence quenching behavior between 1 wt% water in glycerol solution which has similar viscosity to that of the DES ChCl:Urea with the aforementioned DES and IL in the presence of lithium salt as media. In complete contrast, irrespective of the temperature, kq is found to decrease monotonically with increasing concentration of LiCl within 1 wt% water in glycerol media. These findings therefore highlight the unusual characteristics of ILs and DESs as solubilizing media. The ionic nature of the IL and the high concentration of ions in the DES are deemed responsible for these outcomes.
1. Introduction
Ionic liquids (ILs) and deep eutectic solvents (DESs) have emerged as novel and alternative solubilizing media in recent times.1–3 Both ILs and DESs have low vapour pressure, relatively wide liquid range and thermal stability.2,3 However, depending upon the anionic group and side chain, some ILs are reported to be hazardous, especially for aquatic and terrestrial eco-systems.4 DESs, on the other hand, have emerged as novel media for various chemical and electrochemical processes. Their environmental benefits are well appreciated due to their non-hazardous natural components, such as glycerol and choline chloride.5–7Due to the completely ionic nature of ILs and extensive H-bonding nature of DESs, unexpected and sometimes unusual outcomes have emerged during investigation of these solubilizing media.8–11 In one such set of studies, an IL 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([C2C1im][Tf2N]) and a DES (termed ChCl:Urea, which is constituted of the organic salt choline chloride and the H-bond donor urea mixed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio) were investigated for their potential with reference to electrolytes for Li-ion batteries.10,11 Among other physical and physicochemical properties, solute diffusion within LiTf2N-added IL [C2C1im][Tf2N] and LiCl-added DES ChCl:Urea was explored via a detailed fluorescence quenching investigation using a common and popular fluorophore pyrene and a well-established fluorescence quencher nitromethane.10,11 The fluorescence quenching of the pyrene–nitromethane fluorophore–quencher pair takes place via an electron/charge transfer mechanism where nitromethane acts as an electron/charge acceptor and excited pyrene as an electron/charge donor.12–18
2 molar ratio) were investigated for their potential with reference to electrolytes for Li-ion batteries.10,11 Among other physical and physicochemical properties, solute diffusion within LiTf2N-added IL [C2C1im][Tf2N] and LiCl-added DES ChCl:Urea was explored via a detailed fluorescence quenching investigation using a common and popular fluorophore pyrene and a well-established fluorescence quencher nitromethane.10,11 The fluorescence quenching of the pyrene–nitromethane fluorophore–quencher pair takes place via an electron/charge transfer mechanism where nitromethane acts as an electron/charge acceptor and excited pyrene as an electron/charge donor.12–18
It is reported that the bulk dynamic viscosity of both IL [C2C1im][Tf2N] and DES ChCl:Urea increases exponentially with an increase in the mole fraction of LiTf2N (xLiTf2N) and the molality of LiCl (mLiCl), respectively.19,20 It was envisaged that the diffusion would be hampered within the IL and DES upon Li salt addition to these media due to such a dramatic increase in dynamic viscosity.19,20 It was observed, however, that, on the contrary, as the Li salt is incrementally added, the bimolecular quenching rate constant (kq) for the pyrene–nitromethane fluorophore–quencher pair, instead of decreasing monotonically, first increases, reaches a maximum and only then decreases.10,11 This highly unusual trend observed in both IL and DES was attributed to the molecular-level structural re-organization originating within these unusual media due to the presence of the salt.10,11,21–23
Herein, we report pertinent physicochemical properties along with the fluorescence quenching of pyrene by nitromethane within LiCl-added 1 wt% water in glycerol solution; a system possessing extensive H-bonding and having a dynamic viscosity similar to that of the DES ChCl:Urea. We compare and contrast the trends in physicochemical properties, especially kq, reported within Li-salt-added IL [C2C1im][Tf2N] and DES ChCl:Urea to those in LiCl-added 1 wt% water in glycerol solution. The results reported here confirm that IL/DES undergoes significant molecular/ionic re-organization upon Li salt addition due to their inherent structural features as such behavior is found to be either absent or of no consequence in 1 wt% water in glycerol solution.
2. Experimental
The acquisition, characterization, and purification of all materials, except glycerol, were described earlier.11 Glycerol (≥99.5%) was purchased from Sigma-Aldrich and stored in an Auto Secador desiccator cabinet. Glycerol was rigorously dried under vacuum for at least 24 hours before use. A Karl Fisher titrator was subsequently used to measure the water content of glycerol prior to its use and it was found to be <100 ppm. HPLC-grade water from Merck was used to prepare the 1 wt% water in glycerol solutions. A calculated amount of dried glycerol was transferred to a glass vial and weighed using an analytical balance with a precision of ±0.1 mg. A pre-calculated amount of HPLC-grade water was added to the vial to obtain a water-added glycerol solution. The components were mixed thoroughly to obtain a homogeneous solution. As per the requirement, a pre-calculated amount of LiCl was added to this solution and mixed over a magnetic stirrer at 60 °C until all of the LiCl had dissolved, and a homogeneous solution was obtained. The methodology, including associated precision, for the density (ρ) and dynamic viscosity (η) measurements along with the sample preparation and data acquisition/collection are described in detail elsewhere.193. Results and discussion
As fluorescence quenching is known to depend strongly on the viscosity of the medium, we first aimed to create an aqueous glycerol system with a dynamic viscosity similar to that of the DES ChCl:Urea within the investigated temperature regime. We found that the dynamic viscosity of just 1 wt% water in glycerol solution is similar to that of ChCl:Urea in the investigated temperature regime (Table 1).24 It is also noteworthy that our dynamic viscosity values for 1 wt% water in glycerol compare well with those reported in the literature (Table 2).25,26| T (K) | Dynamic viscosity (mPa s) | ||
|---|---|---|---|
| This work | Ref. 25 | Ref. 26 | |
| a Standard uncertainty: u(p) = ±0.005 MPa. b Standard uncertainty: u(T) = ±0.05 K. | |||
| 293.15 | 1169.8 | 1194.0 | 1150.0 |
| 298.15 | 754.2 | 772.0 | — |
| 303.15 | 500.0 | 509.0 | 500.0 |
| 313.15 | 238.5 | — | 235.0 |
| 323.15 | 118.2 | — | 122.0 |
| 333.15 | 67.6 | — | 69.1 |
| 343.15 | 41.7 | — | 43.6 |
| 353.15 | 27.7 | — | 27.8 |
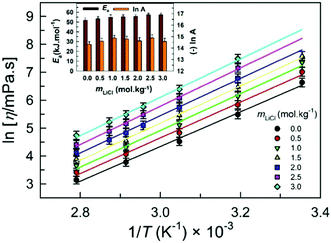
It is convenient to note that the temperature dependence of the dynamic viscosity of 1 wt% water in glycerol solution and ChCl:Urea both adhere to Arrhenius-type behavior with statistically the same pre-exponential factors (values of A) and activation energies of viscous flow (values of Ea,η) (Fig. 1).24 The dynamic viscosity dependence on temperature for LiTf2N-added [C2C1im][Tf2N], however, is reported to follow the Vogel–Fulcher–Tammann (VFT) relation rather than the Arrhenius relation.20
3.1. Density and dynamic viscosity
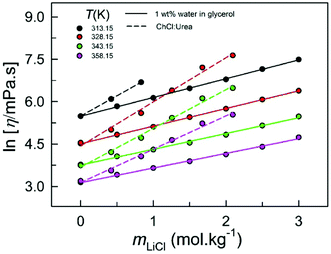
While the density values of 1 wt% water in glycerol solution are between those of IL [C2C1im][Tf2N] and DES ChCl:Urea (density trend: [C2C1im][Tf2N] > 1 wt% water in glycerol > ChCl:Urea), the variation of density with temperature is linear in all three systems (Fig. S1A, ESI†).20,24 The extent of the decrease in density with increasing temperature, represented by the slope of the density–temperature best-fit straight lines (inset of Fig. S1A, ESI†), are very similar for 1 wt% water in glycerol and ChCl:Urea; it is comparatively higher for [C2C1im][Tf2N].20,24 (Table S1, ESI†). Furthermore, at a given temperature, similar to that observed for LiCl-added DES ChCl:Urea, the density of 1 wt% water in glycerol also varies linearly with the molality of added LiCl (mLiCl) (Fig. S1B, ESI†).19 Irrespective of temperature, the extent of the increase in density with mLiCl, represented by the slope of the density–mLiCl best-fit straight lines (inset of Fig. S1B, ESI†), is statistically the same for LiCl-added ChCl:Urea and 1 wt% water in glycerol19 (Table S2, ESI†). It may be noted that a quadratic dependence of density on mole fraction of LiTf2N (xLiTf2N) was reported for LiTf2N-added IL [C2C1im][Tf2N]20 (Table S3, ESI†).At a given temperature, ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η of the (1 wt% water in glycerol + LiCl) media increases linearly with increasing mLiCl (Fig. 2). This trend in (1 wt% water in glycerol + LiCl) is similar to that reported earlier for (DES ChCl:Urea + LiCl) media.19 The increase in dynamic viscosity upon LiCl addition at higher temperatures is not as dramatic as that at lower temperatures. Most importantly, on addition of LiCl, the dynamic viscosity increases much more rapidly for ChCl:Urea compared to that for 1 wt% water in glycerol; at each LiCl addition, the increase in dynamic viscosity is much more for the DES ChCl:Urea than that for 1 wt% water in glycerol.19 Also, Ea,η for ChCl:Urea is higher than that for 1 wt% water in glycerol at similar additions of LiCl. These observations hint at the fact that structural changes are more pronounced in LiCl-added ChCl:Urea compared to those in LiCl-added 1 wt% water in glycerol (vide infra).19 It should be noted that the dynamic viscosity of the IL [C2C1im][Tf2N] is also reported to increase exponentially with increasing xLiTf2N.20
η of the (1 wt% water in glycerol + LiCl) media increases linearly with increasing mLiCl (Fig. 2). This trend in (1 wt% water in glycerol + LiCl) is similar to that reported earlier for (DES ChCl:Urea + LiCl) media.19 The increase in dynamic viscosity upon LiCl addition at higher temperatures is not as dramatic as that at lower temperatures. Most importantly, on addition of LiCl, the dynamic viscosity increases much more rapidly for ChCl:Urea compared to that for 1 wt% water in glycerol; at each LiCl addition, the increase in dynamic viscosity is much more for the DES ChCl:Urea than that for 1 wt% water in glycerol.19 Also, Ea,η for ChCl:Urea is higher than that for 1 wt% water in glycerol at similar additions of LiCl. These observations hint at the fact that structural changes are more pronounced in LiCl-added ChCl:Urea compared to those in LiCl-added 1 wt% water in glycerol (vide infra).19 It should be noted that the dynamic viscosity of the IL [C2C1im][Tf2N] is also reported to increase exponentially with increasing xLiTf2N.20
3.2. Pyrene I1/I3 and excited-state intensity decay
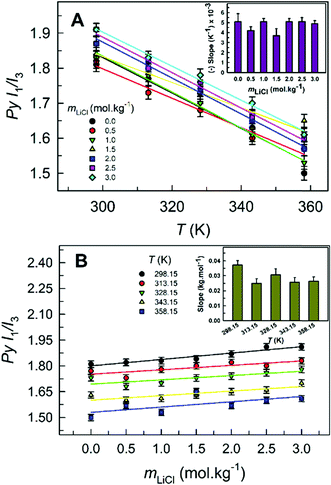
Pyrene is a commonly used neutral fluorescent probe.27–32 In our work we have employed the unique features of pyrene to explore the solvent parameters of LiCl-added 1 wt% water in glycerol solution in the temperature range 303.15–358.15 K.As reported earlier,11 pyrene band-1-to-band-3 emission intensity ratios (Py I1/I3) were estimated at different concentrations of LiCl dissolved in 1 wt% water in glycerol solution in the range 298.15–358.15 K (Fig. 3). The Py I1/I3 in 1 wt% water in glycerol solution (1.81 ± 0.02 at 298.15 K) is considerably lower than those reported in ChCl:Urea (2.81 ± 0.04 at 298.15 K)11 or [C2C1im][Tf2N] (2.10 ± 0.02 at 298.15 K).10 This indicates the relatively lower dipolarity of the pyrene cybotactic region as sensed within 1 wt% water in glycerol solution as compared to that in ChCl:Urea and [C2C1im][Tf2N]. The Py I1/I3 at a fixed LiCl concentration is also found to decrease linearly with increasing temperature (Fig. 3A), implying a decrease in the dipolarity of the LiCl-added 1 wt% water in glycerol solution with increasing temperature. This observation is in line with the reported decrease in static dielectric constants (ε) of several liquids, including DESs and ILs, as the temperature is increased.29,33 Also, similar observations were reported for the LiCl-added ChCl:Urea and LiTf2N-added [C2C1im][Tf2N], respectively, at similar temperatures.10,11 It is noteworthy, however, that the extent of the decrease in Py I1/I3 with temperature, represented by the slope of the Py I1/I3–temperature best-fit straight line (inset of Fig. 3A), is greater for ChCl:Urea [(8–12) × 10−3 K−1] compared to that for 1 wt% water in glycerol solution (5 × 10−3 K−1).11
The slope of Py I1/I3versus temperature for 1 wt% water in glycerol solution is found to be nearly constant for different LiCl concentrations. Therefore, the temperature sensitivity of Py I1/I3 is independent of the concentration of LiCl. However, Py I1/I3, in general, increases with increasing concentration of LiCl (Fig. 3B), suggesting that the pyrene cybotactic region is becoming more polar. Therefore, the dipolarity of the 1 wt% water in glycerol solution increases with increasing concentration of LiCl. At a given temperature, no systematic trend in Py I1/I3 with changing LiCl concentration is reported for ChCl:Urea, hinting at the consistency in the dipolarity of the pyrene cybotactic region within ChCl:Urea irrespective of the presence of LiCl.11 This is similar to the observation for LiTf2N-added [C2C1im][Tf2N] at similar temperatures.10
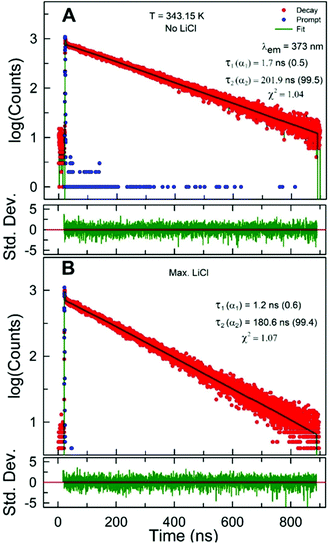
Excited-state intensity decays of pyrene dissolved in LiCl-added 1 wt% water in glycerol solution were collected for varying concentrations of LiCl at different temperatures in the range 298.15–358.15 K. Similar to that observed for LiCl-added ChCl:Urea,11 the excited-state emission intensity decay, I(t), of pyrene was found to fit best to a double-exponential decay expression irrespective of the temperature or the concentration of LiCl:
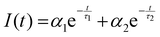 | (1) |
As reported for LiCl-added ChCl:Urea,11 the recovered decay data reveals the presence of a short along with a long decay time at each temperature and LiCl concentration. The excited-state intensity decay of pyrene is known to depend on the milieu; depending upon the solvation environment, single-, multi-, and non-exponential decays have been reported for this probe (e.g., pyrene decays were found to fit best to a single-exponential model in LiTf2N-added [C2C1im][Tf2N]10).12,13 At 298.15 K, the value of the longer of the decay times in 1 wt% water in glycerol solution is found to be higher than the corresponding value in ChCl:Urea (240 ns versus 160 ns).11 This may be attributed to the presence of Cl− in ChCl:Urea; as Cl− is known to mildly quench the fluorescence of several probes.34
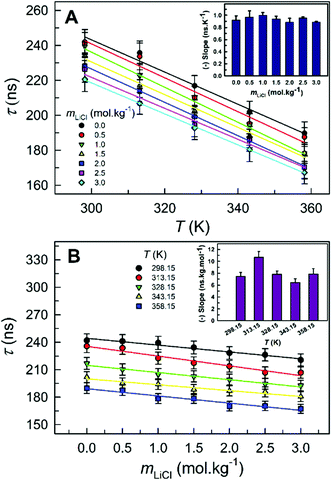
As expected and reported previously in the DES ChCl:Urea and IL [C2C1im][Tf2N],10,11 the longer of the decay times of pyrene is found to decrease with an increase in temperature within the 1 wt% water in glycerol solution (Fig. 5A) due to the increased rate of non-radiative decay pathways (i.e., thermal quenching due to the increased rate of collisional deactivation of the excited probe with solvent molecules/ions).12,13 The decrease in the longer decay time (τ) with increasing temperature at all LiCl concentrations investigated shows an acceptable linear behavior. However, the extent of the decrease in decay time with increasing temperature, represented by the slope of the decay time–temperature best-fit straight line (inset of Fig. 5A), is found to be similar irrespective of the concentration of LiCl. More importantly, the extent of the decrease in decay time with increasing temperature is greater for 1 wt% water in glycerol solution than for the DES ChCl:Urea (slopes of −0.9 ns K−1versus −0.6 ns K−1).11
Similar to that observed for LiCl-added ChCl:Urea,11 the decay time of pyrene in 1 wt% water in glycerol solution is found to decrease with increasing LiCl concentration (Fig. 4 and 5B). The decay time at each temperature decreases by ∼20–25 ns on the addition of up to 3.5mLiCl. The decrease in decay time as LiCl is added is believed to be due to chloride (Cl−) ions. Heavier halide ions, such as Br− and I−, are known to reduce the fluorescence for different probes.12,13,35 The relatively lighter Cl− has also been reported to reduce the fluorescence of some long-lived species but to smaller extents.12,13 Plots of τ versus mLiCl at different temperatures reveal the decrease in τ with the concentration of LiCl to be linear at all temperatures investigated. The recovered slopes at different temperatures (inset of Fig. 5B) represent the efficiency of Cl− in reducing the pyrene decay time within LiCl-added 1 wt% water in glycerol solution. It is clear that the efficiency of Cl− in reducing the pyrene decay time is almost the same irrespective of the temperature. It is important to note that this efficiency is similar to that observed for LiCl-added ChCl:Urea, indicating similar diffusion behavior of the pyrene–Cl− pair within the two systems due perhaps to their similar dynamic viscosities.11
3.3. Fluorescence quenching of pyrene by nitromethane
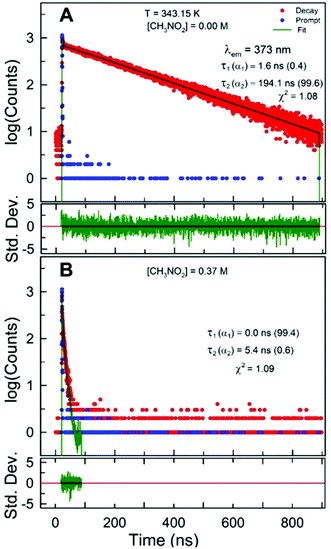
From the information obtained in previous sections, it is clear that 1 wt% water in glycerol solution has similar physical properties, especially dynamic viscosity, to those of the DES ChCl:Urea. Furthermore, changes in pyrene fluorescence with temperature and with the addition of LiCl within 1 wt% water in glycerol solution also compare well with those within ChCl:Urea. We now analyze the results of our investigation regarding the effect of LiCl on the quenching of pyrene fluorescence by an electron/charge transfer quencher nitromethane within LiCl-added 1 wt% water in glycerol solution and compare the outcomes with those observed within the corresponding LiCl- and LiTf2N-added DES ChCl:Urea and IL [C2C1im][Tf2N], respectively.10,11The excited-state intensity decay of pyrene dissolved in LiCl-added 1 wt% water in glycerol solution in the absence and presence of varying concentrations of quencher CH3NO2 was collected at different temperatures (K). The excited-state intensity decay of pyrene in the presence of CH3NO2 was again found to fit best to a double-exponential decay expression irrespective of the temperature, concentration of LiCl, or concentration of CH3NO2 (recovered decay parameters, τ1, τ2, α1, α2, and goodness-of-fit in terms of χ2 are provided in Table S5 (ESI†)).
The longer decay time of pyrene was found to decrease considerably with increasing concentration of quencher CH3NO2 at a fixed temperature and LiCl concentration (representative fits are presented in Fig. 6). Also, the shorter decay time did not show any trend with changing concentration of quencher. The decrease in decay time up to 0.37 M CH3NO2 addition is fairly significant and confirms the effective quenching of pyrene fluorescence by CH3NO2 within LiCl-added 1 wt% water in glycerol solution.
The τ0/τ versus [CH3NO2] were plotted for pyrene dissolved in LiCl-added 1 wt% water in glycerol solution at varying concentrations of LiCl and different temperatures (Fig. 7). Here, τ0 and τ are the longer pyrene decay times in the absence and presence of nitromethane, respectively. The data fit to the simplistic Stern–Volmer formulation irrespective of the temperature and the concentration of LiCl is clearly apparent:
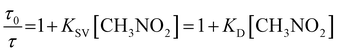 | (2) |
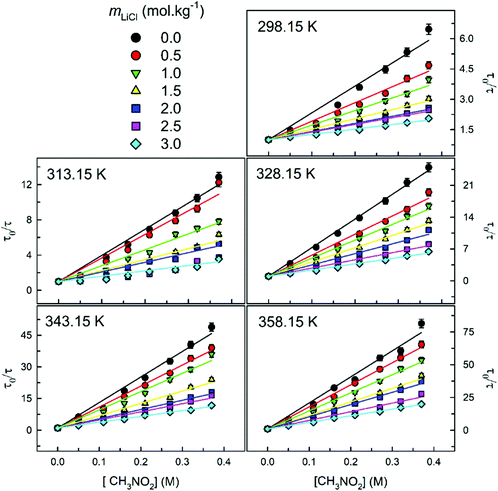 | ||
| Fig. 7 Plots of τ0/τ vs. [CH3NO2] for LiCl-added 1 wt% water in glycerol at a given temperature. Solid lines show the fit to the simple Stern–Volmer equation (eqn (2)). Estimated values of kq (eqn (3)) from the linear regression analysis at each temperature and mLiCl are listed in Table 3. The error associated with τ0/τ is ≤±5%. | ||
It is clear that for the excited-state electron-donor fluorophore (pyrene)–electron-acceptor quencher (nitromethane) pair Stern–Volmer linearity prevails within a wide range of temperature, quencher concentration, and concentrations of LiCl. The bimolecular quenching rate constant (kq) was estimated using
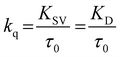 | (3) |
| T (K) | |||||
|---|---|---|---|---|---|
| m LiCl (mol kg−1) | 298.15 | 313.15 | 328.15 | 343.15 | 358.15 |
| 0.0 | 0.55 ± 0.05 | 1.26 ± 0.10 | 2.90 ± 0.18 | 5.99 ± 0.46 | 10.45 ± 0.02 |
| 0.5 | 0.38 ± 0.03 | 1.14 ± 0.10 | 2.20 ± 0.16 | 5.09 ± 0.02 | 8.96 ± 0.03 |
| 1.0 | 0.30 ± 0.03 | 0.81 ± 0.07 | 1.94 ± 0.13 | 4.45 ± 0.03 | 7.71 ± 0.03 |
| 1.5 | 0.22 ± 0.02 | 0.57 ± 0.06 | 1.54 ± 0.11 | 3.10 ± 0.04 | 5.78 ± 0.03 |
| 2.0 | 0.18 ± 0.01 | 0.51 ± 0.05 | 1.25 ± 0.10 | 2.34 ± 0.04 | 5.46 ± 0.03 |
| 2.5 | 0.17 ± 0.01 | 0.29 ± 0.04 | 0.93 ± 0.06 | 2.07 ± 0.03 | 3.85 ± 0.03 |
| 3.0 | 0.12 ± 0.01 | 0.26 ± 0.03 | 0.71 ± 0.05 | 1.53 ± 0.10 | 3.01 ± 0.70 |
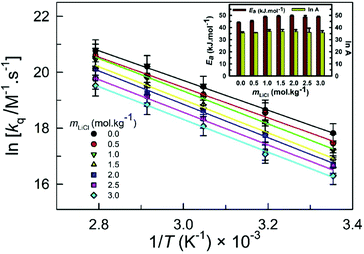
The kq in 1 wt% water in glycerol solution is in the range 0.55(±0.05) × 108 M−1 s−1 (at 298.15 K) to 10.45(±0.02) × 108 M−1 s−1 (at 358.15 K). These values of kq are somewhat higher than the corresponding values in the similar-viscosity DES ChCl:Urea [0.38(±0.04) × 108 M−1 s−1 at 298.15 K to 6.59(±0.38) × 108 M−1 s−1 at 358.15 K];11 however, they are understandably significantly lower than the values observed in the low-viscosity IL [C2C1im][Tf2N] [7.2(±0.2) × 108 M−1 s−1 at 298.15 K to 30.7(±1.00) × 108 M−1 s−1 at 358.15 K].10 As expected, at a fixed LiCl concentration, the quenching rate constant kq is observed to increase with increasing temperature. The reason is usually two-fold: increasing the temperature leads to a lowering in viscosity, leading to faster diffusion and increasing temperature, leading to faster translational motion, resulting in faster kinetics and subsequently higher kq. The linear relation between ln kq and 1/T (Fig. 8) implies Arrhenius-type behavior with Ea in the range 44.17 kJ mol−1 (in 0.0mLiCl) to 49.49 kJ mol−1 (in 2.0mLiCl).
In comparison, the Ea in LiCl-added ChCl:Urea varies from 40.55 kJ mol−1 (in 0.83mLiCl) to 48.23 kJ mol−1 (in 0.42mLiCl)11 and in LiTf2N-added [C2C1im][Tf2N] from 19.60 kJ mol−1 (in 0.05xLiTf2N) to 36.30 kJ mol−1 (in 0.40xLiTf2N).10 It is noteworthy that Ea is similar in LiCl-added 1 wt% water in glycerol and LiCl-added ChCl:Urea and is relatively higher than that in LiTf2N-added [C2C1im][Tf2N]. This can be related to the activation energy of the viscous flow, Ea,η, in LiCl-added ChCl:Urea; Ea,η varies from 51.34 kJ mol−1 (in 0.0mLiCl) to 68.42 kJ mol−1 (in 2.03mLiCl)19 and that in LiCl-added 1 wt% water in glycerol varies from 51.72 kJ mol−1 (in 0.0mLiCl) to 57.29 kJ mol−1 (in 3.0mLiCl), hinting at the similar temperature response of the dynamic viscosity and thus of the quenching behavior in both solvent systems.
The starkest difference in the quenching behavior of the pyrene–nitromethane fluorophore–quencher pair between 1 wt% water in glycerol solution versus DES ChCl:Urea/IL [C2C1im][Tf2N] as Li salt is added is observed with respect to the trend in bimolecular quenching rate constant (kq) with the concentration of added Li salt. For 1 wt% water in glycerol, kq is plotted against mLiCl at different investigated temperatures (Fig. 9A). It is clear that the quenching rate constant (kq) decreases monotonically with an increase in LiCl concentration within 1 wt% water in glycerol media. This is attributed to the increased dynamic viscosity of 1 wt% water in glycerol as more and more LiCl is added, rendering it difficult for the quencher CH3NO2 to diffuse to the excited pyrene. Interestingly, kq exhibits excellent linear correlation with mLiCl (Fig. 9A). The inset shows the extent of the decrease in kq with increasing LiCl concentration, represented by the slope of the kqvs. mLiCl plot, at different temperatures. The slope increases rapidly with increase in temperature; in fact, the slope nearly doubles with every 15 K increment in temperature. This can be accounted for by the fact that the dynamic viscosity of the LiCl-added 1 wt% water in glycerol solution decreases exponentially with increasing temperature (vide supra). The monotonous linear decrease in kq with mLiCl within LiCl-added 1 wt% water in glycerol solution is in stark contrast with the trend observed in LiCl-added DES ChCl:Urea and LiTf2N-added [C2C1im][Tf2N], respectively – in the latter two, as the concentration of Li salt is increased, kq first increases, reaches a maximum, and then decreases (Fig. 9B).10,11
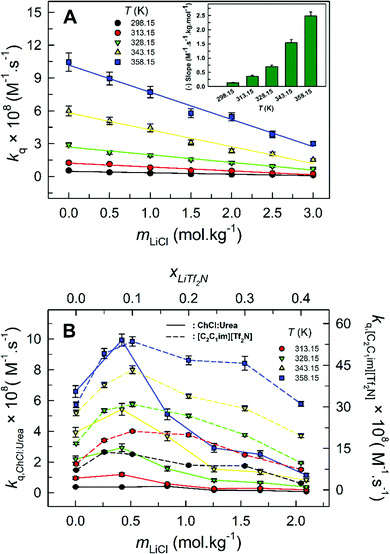 | ||
| Fig. 9 Bimolecular quenching rate constant (kq) plotted as a function of mLiCl at different temperatures for 1 wt% water in glycerol (panel A) and that for ChCl:Urea and [C2C1im][Tf2N] (panel B). kq (ChCl:Urea) vs. mLiCl and kq ([C2C1im][Tf2N]) vs. xLiTf2N are plotted in the same graph for comparison.10,11 | ||
It is implied from our observations that in LiCl-added 1 wt% water in glycerol solution, the increased viscosity as more and more Li salt is added controls the diffusion behavior, leading to a rather monotonic linear decrease in kq with an increase in the amount of Li salt. The unusual increase in kq at initial additions of Li salt within LiCl-added ChCl:Urea and LiTf2N-added [C2C1im][Tf2N] is attributed to the higher “effective” concentration of the anions Cl− and Tf2N−, respectively, since a substantial amount of Cl− is already present in bulk DES ChCl:Urea (due to its component choline chloride) and of Tf2N− in bulk IL [C2C1im][Tf2N] (Tf2NCl− is the anion of the IL). We thus believe that the anomalous behavior, where kq increases even when the viscosity of the medium increases, originates from the unique structural features of the DES ChCl:Urea and IL [C2C1im][Tf2N], respectively, in the presence of Li salt. The novelty in structural features of the DESs and ILs over those of glycerol as molecular solvents in the presence of Li salt is clearly established.
4. Conclusions
The dynamic viscosity of 1 wt% water in glycerol is close to that of the DES ChCl:Urea, and variations in key physicochemical properties as Li salt is added to 1 wt% water in glycerol are also similar to those observed in Li-salt-added DES ChCl:Urea and IL [C2C1im][Tf2N], respectively. Specifically, as Li salt is added to each of the three systems, the dynamic viscosity of the media increases exponentially. Understandably, kq for the pyrene–nitromethane fluorophore–quencher pair within 1 wt% water in glycerol decreases monotonically with incremental addition of Li salt. This observation, however, is in contrast to that observed in Li-salt-added DES ChCl:Urea and IL [C2C1im][Tf2N], where kq first increases, reaches a maximum, and only then starts to decrease. The effect of the presence of excess anions within Li-salt-added ChCl:Urea and [C2C1im][Tf2N] overcomes the increased viscosity effects and subsequently leads to an initial increase in kq. However, in 1 wt% water in glycerol solution, the concentration of anions is not as high as that in the aforementioned ionic liquid and DES systems, and thus is unable to overcome the effect of the increasing viscosity of the medium. The ionic nature of ILs and the abundance of ions in DESs, as opposed to a molecular solvent, are deemed responsible for this contrasting behavior. This is evident in the higher polarity reported for pyrene in the aforementioned IL/DES as compared to that in 1 wt% glycerol in water. These outcomes further highlight the unusual characteristics of ILs and DESs as solubilizing media and may help open up new avenues for extended applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is generously supported by the Department of Science and Technology – Science & Engineering Research Board (DST-SERB), Government of India through a grant to Siddharth Pandey (grant number EMR/2016/005053).References
- Z. Lei, B. Chen, Y.-M. Koo and D. R. MacFarlane, Chem. Rev., 2017, 117, 6633–6635 CrossRef PubMed.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232–1285 CrossRef CAS PubMed.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- T. Phuong, C.-W. Cho and Y.-S. Yun, Water Res., 2010, 44, 352–372 CrossRef PubMed.
- M. K. AlOmar, M. Hayyan, M. A. Alsaadi, S. Akib, A. Hayyan and M. A. Hashim, J. Mol. Liq., 2016, 215, 98–103 CrossRef CAS.
- K. Biernacki, H. K. S. Souza, C. M. R. Almeida, A. L. Magalhães and M. P. Gonçalves, ACS Sustainable Chem. Eng., 2020, 8, 18712–18728 CrossRef CAS.
- A. Banerjee, K. Ibsen, T. Brown, R. Chen, C. Agatemor and S. Mitragotri, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, E11000–E11001 CrossRef CAS PubMed.
- J. M. S. S. Esperanca, M. Tariq, A. B. Pereiro, J. M. M. Araújo, K. R. Seddon and L. P. N. Rebelo, Front. Chem., 2019, 7, 450 CrossRef CAS PubMed.
- L. J. B. M. Kollau, M. Vis, A. van den Bruinhorst, A. C. C. Esteves and R. Tuinier, Chem. Commun., 2018, 54, 13351–13354 RSC.
- A. Kadyan and S. Pandey, J. Phys. Chem. B, 2018, 122, 5106–5113 CrossRef CAS PubMed.
- D. Dhingra, Bhawna, A. Pandey and S. Pandey, J. Phys. Chem. B, 2019, 123, 3103–3111 CrossRef CAS PubMed.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, Boston, MA, USA, 2006 Search PubMed.
- B. Valeur and M. N. Berberan-Santos, Molecular Fluorescence Principles and Applications, Wiley-VCH, Weinheim, Germany, 2013 Search PubMed.
- A. Pandey, A. Yadav, Bhawna and S. Pandey, J. Lumin., 2017, 183, 494–506 CrossRef CAS.
- S. Trivedi and S. Pandey, J. Phys. Chem. C, 2013, 117, 1818–1826 CrossRef CAS.
- S. Pandey, W. E. Acree and J. C. Fetzer, Anal. Chim. Acta, 1996, 324, 175–181 CrossRef CAS.
- P. Bhavya, R. Melavanki, R. Kusanur, K. Sharma, V. T. Muttannavar and L. R. Naik, Luminescence, 2018, 33, 933–940 CrossRef CAS PubMed.
- S. A. El-Daly, E.-Z. M-Ebeid, A. S. Babaqi and S. M. Al-Hazmy, J. Photochem. Photobiol., A, 2004, 163, 497–501 CrossRef CAS.
- D. Dhingra, Bhawna and S. Pandey, J. Chem. Thermodyn., 2019, 130, 166–172 CrossRef CAS.
- A. Kadyan and S. Pandey, J. Chem. Thermodyn., 2018, 116, 159–165 CrossRef CAS.
- S. Duluard, J. Grondin, J.-L. Bruneel, I. Pianet, A. Grélard, G. Campet, M.-H. Delville and J.-C. Lasségues, J. Raman Spectrosc., 2008, 39, 627–632 CrossRef CAS.
- Y. Umebayashi, T. Mitsugi, S. Fukuda, T. Fujimori, K. Fujii, R. Kanzaki, M. Takeuchi and S.-I. I. Ishiguro, J. Phys. Chem. B, 2007, 111, 13028–13032 CrossRef CAS PubMed.
- J.-C. Lassegues, J. Grondin and D. Talaga, Phys. Chem. Chem. Phys., 2006, 8, 5629–5632 RSC.
- A. Yadav and S. Pandey, J. Chem. Eng. Data, 2014, 59, 2221–2229 CrossRef CAS.
- J. B. Segur, H. E. Helen and E. Oberstar, Ind. Eng. Chem., 1951, 43, 2117–2120 CrossRef CAS.
-
J. A. Dean, Lange's Handbook of Chemistry, McGraw-Hill,
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) New York, 15th edn, 1999 Search PubMed.
New York, 15th edn, 1999 Search PubMed. - K. Ayyavoo and P. Velusamy, New J. Chem., 2021, 45, 10997–11017 RSC.
- D. S. Karpovich and G. J. Blanchard, J. Phys. Chem., 1995, 99, 3951–3958 CrossRef CAS.
- D. C. Dong and M. A. Winnik, Can. J. Chem., 1984, 62, 2560–2565 CrossRef CAS.
- G. Bains, A. B. Patel and V. Narayanaswami, Molecules, 2011, 16, 7909–7935 CrossRef CAS PubMed.
- A. Pandey, R. Rai, M. Pal and S. Pandey, Phys. Chem. Chem. Phys., 2014, 16, 1559–1568 RSC.
- A. Pandey and S. Pandey, J. Phys. Chem. B, 2014, 118, 14652–14661 CrossRef CAS PubMed.
- J. Hunger, A. Stoppa, S. Schrödle, G. Hefter and R. Buchner, ChemPhysChem, 2009, 10, 723–733 CrossRef CAS PubMed.
- J. H. Gutow, J. Chem. Educ., 2005, 82, 302 CrossRef CAS.
- A. Chmyrov, T. Sandén and J. Widengren, J. Phys. Chem. B, 2010, 114, 11282–11291 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1cp03678e |
| This journal is © the Owner Societies 2022 |