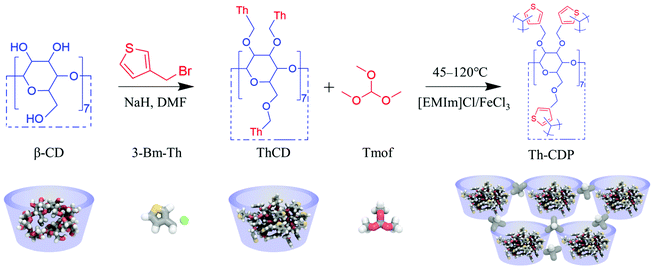
A porous thienyl cyclodextrin polymer synthesized in a homogeneous ionic liquid catalytic system for the rapid removal of pharmaceuticals and personal care products (PPCPs)†
Yizhou
Tu
a,
Huimin
Wang
a,
Pingping
Yang
a,
Guizhou
Xu
a,
Xuejiao
Hu
a,
Aimin
Li
ac and
Xianchuan
Xie
 *abd
*abd
aState Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing, P. R. China. E-mail: xchxie@nju.edu.cn; 24255542@qq.com; Fax: +086-25-83594492; Tel: +086-25-83594492
bKey Laboratory of Poyang Lake Environment and Resource Utilization, Ministry of Education, School of Resources Environmental & Chemical Engineering, Nanchang University, Nanchang 330031, P. R. China
cQuanzhou Institute for Environmental Protection Industry, Nanjing University, Quanzhou, 362008, P. R. China
dJiangxi Nanxin Environmental Protection Technology Co. LTD, Jiujiang City, Jiangxi Province 330300, P. R. China
First published on 19th November 2021
Abstract
Pharmaceuticals and personal care products (PPCPs) are widely distributed in aquatic environments due to their large consumption and low biodegradability, causing ecological risks. In this study, a porous thienyl cyclodextrin polymer (Th-CDP) with a specific surface area of 730 m2 g−1 was synthesized in an ionic liquid system for the first time to remove PPCPs. The green and recyclable 1-ethyl-3-methylimidazolium chloride/ferric chloride ([EMIm]Cl/FeCl3) ionic liquid was synthesized as a solvent and catalyst, superior to traditional toxic organic solvents and heterogeneous catalysts. In Th-CDP synthesis, thienyl cyclodextrin (ThCD) was synthesized, followed by polymerization with the three-dimensional cross-linking agent trimethyl orthoformate (Tmof) in [EMIm]Cl/FeCl3via the Friedel–Crafts alkylation reaction. Th-CDP shows excellent adsorption towards the studied PPCPs (diclofenac, ibuprofen, carbamazepine, and sulfamethoxazole) due to the rich pore structure. In particular, Th-CDP achieved 90% removal of hydrophobic diclofenac and ibuprofen in 5 min, and the rate constants were 16.7 and 10.5 times higher than those of activated carbon, respectively. The adsorption capacity of Th-CDP is related to the molecular weight and pKa of the compound, and is about twice that of activated carbon. Th-CDP has more pronounced adsorption advantages over activated carbon for acidic compounds due to its positive charge under neutral conditions. In addition, multiple mechanisms, including hydrophobic interaction caused by thiophene and cyclodextrin, electrostatic interaction, and heteroatom interaction (24.8% S content), enable Th-CDP to efficiently remove PPCPs. Moreover, Th-CDP has great application potential due to its excellent regenerability, anti-interference ability, and green synthesis process.
1. Introduction
In recent years, pharmaceuticals and personal care products (PPCPs) have been widely used in production and consumption, whose persistence, however, enables them to continuously accumulate and enrich in the aquatic environment through the wastewater treatment process. Consequently, the resulting ecological health risks have attracted widespread attention.1The rigid demand of PPCPs makes it difficult to control the source by prohibiting certain types of compounds like other pollutants. In 2015, the total global antibiotic consumption was estimated to be 42.3 billion defined daily doses.2 However, Luo et al. reported that the average removal of most PPCPs in the wastewater treatment plant is less than 60%. The anticonvulsant carbamazepine (CBZ) is the most persistent, being removed by an average of only 32.7%.1 Refractory compounds generally share common characteristics, such as highly branched, complex polycyclic structures or electron-withdrawing functional groups.3 Consequently, PPCPs are widely found in surface water and even in drinking water.4,5 Among them, ibuprofen (IBU) is present at high levels in surface water, with the highest concentration reaching 36.8 μg L−1.1 Unfortunately, environmental standards for PPCPs are still scarce, although, in 2015, diclofenac (DCF) and estrogen were placed on the watch list by the European Union (EU Commission, 2015).6 Based on the risk level, Bu et al. identified 6 PPCPs as priority controls, including DCF, IBU, and sulfamethoxazole (SMX).7 Studies have shown that DCF poses a major threat to wildlife (causing mortality in condors) and potential hazards to aquatic life.8 In addition, continuous exposure to PPCPs leads to ecological health risks such as resistance genes and endocrine interference. Therefore, effective methods are in increasingly pressing need.8,9
Due to the general antibacterial properties and slow degradation rate of refractory pharmaceuticals, traditional biological methods are difficult to implement or inefficient to degrade them.1 Adsorption is an effective method to remove PPCPs. Activated carbon is the most commonly used adsorption material in water treatment, with the main adsorption mechanism of hydrophobic interaction and electrostatic interaction.3,10 However, the uncharged or negatively charged surface of activated carbon under neutral conditions is detrimental to the removal of hydrophilic and acidic compounds by activated carbon.11 Furthermore, refractory PPCPs generally have low pKa and lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow values, coupled with the slow adsorption and poor regeneration of activated carbon, indicating that activated carbon is not a suitable adsorbent for removing PPCPs.
Kow values, coupled with the slow adsorption and poor regeneration of activated carbon, indicating that activated carbon is not a suitable adsorbent for removing PPCPs.
Cyclodextrin (CD) is extracted from starch with a unique hydrophobic cavity that can encapsulate organic matter.12 The synthesized polymer has a high absorption rate and good recyclability. In our previous research, a porous CD polymer was synthesized by introducing many rigid molecules, one of the reported CD materials with the highest specific surface area.13 However, previous hyper-crosslinked porous polymers were realized from traditional organic solvents.14,15 These volatile chlorinated organic solvents used in the reaction have increased the risk of cardiovascular disease and cancer.16 Besides, the metal chloride catalyst used is difficult to recycle due to the heterogeneous reaction. Therefore, finding alternatives to harmful solvents and heterogeneous metal chloride catalysts is a top priority. An ionic liquid, a compound composed entirely of ions, has the advantages of good solubility, non-volatility, and easy recovery as a green solvent.17 By combining imidazole salt with metal chloride and adjusting to a certain ratio, a Lewis acid ionic liquid can be easily obtained, where [EMIm]Cl/FeCl3 exhibits good solubility and catalytic activity in organic reactions.18,19
Inspired by the above description, thienyl cyclodextrin (ThCD) was first synthesized and then polymerized in the 1-ethyl-3-methylimidazolium chloride/ferric chloride ([EMIm]Cl/FeCl3) ionic liquid system with the three-dimensional cross-linking agent trimethyl orthoformate (Tmof) to obtain the porous thienyl cyclodextrin polymer (Th-CDP). The thiophene ring is introduced to provide hydrophobic interaction and heteroatom interaction. In addition, the three-dimensional cross-linking agent can increase the cross-linking density. It is worth noting that the composite ionic liquid can be used as both catalyst and solvent for the Friedel–Crafts alkylation reaction. To our knowledge, this is the first porous CD polymer synthesized in an ionic liquid catalyst system. It is hoped that its porous structure and multiple adsorption mechanisms can be utilized for the rapid removal of refractory PPCPs in water.
2. Experimental
2.1. Reagents and material
β-Cyclodextrin (β-CD, dried under vacuum at 120 °C before use), anhydrous N,N-dimethylformamide (DMF, 99.8%), 1-ethyl-3-methylimidazolium chloride ([EMIm]Cl, 98%), anhydrous sodium sulfate, anhydrous trimethyl orthoformate (Tmof), acetic acid (HPLC grade, 99.9%), and diclofenac sodium (DCF, 98%) were purchased from Aladdin. Anhydrous ferric chloride was obtained from Alfa Aesar. Anhydrous sodium hydride (NaH, 60%) and granular activated carbon (GAC, DARCO, SBET = 612 m2 g−1, 12–20 mesh) were purchased from Sigma-Aldrich. 3-Bromomethylthiophene (3-Bm-Th) was purchased from ACCELA. Deuterated acetone (99.9%) was purchased from Macklin. Ibuprofen, carbamazepine, and sulfamethoxazole (IBU, CBZ, and SMX, 98%) were purchased from J&K. The standard products of DCF, IBU, CBZ, and SMX were purchased from TMstandard. HPLC grade methanol and acetonitrile and LC-MS grade ammonium acetate were purchased from Merck. Fulvic acid (FA, 95%) was purchased from NJDULY. The reagents dichloromethane, chloroform, benzene and NaCl were purchased from Nanjing Chemical Reagent Co., Ltd. Ultrapure water was prepared by using a Milli-Q ultrapure water machine.2.2. Synthesis of [EMIm]Cl/FeCl3 and a porous thienyl CD polymer
2.3. Characterization of Th-CDP
The moisture content was measured by using a halogen moisture analyzer HB43-S (METTLER TOLEDO, Switzerland). Liquid and solid-state nuclear magnetic resonance (NMR) spectra were obtained by using AVANCE III HD 600 and Bruker AVANCE III 400 (BRUKER, Germany), respectively. Fourier-transform infrared (FT-IR) spectra were obtained with TENSOR 27 (BRUKER, Germany). Elemental analysis was performed by using an organic elemental analyzer (Elementar, Germany). The N2 adsorption isotherm was determined by using ASAP2020 (Micromeritics, USA). Thermogravimetric analysis (TGA) was performed using Pyris 1 DSC (PerkinElmer, USA) at a heating rate of 20 °C min−1. Scanning electron microscopy (SEM) was performed using FEI QUANTA 250 FEG (FEI, USA), and the sample was sprayed with gold. Transmission electron microscopy (TEM) was performed with JEM-2100F (JEOL, Japan). The zeta potential was measured by using Zetasizer Nano (Malvern, UK).2.4. Adsorption experiments
The adsorption performance and mechanism of Th-CDP were studied using GAC as a comparative material. Four refractory PPCPs (DCF, IBU, CBZ, and SMX) were selected for adsorption in water. Besides, the adsorbents used were ground to 60–100 mesh prior to adsorption. The samples were filtered with an Agilent 0.45 μm PTFE-Q filter membrane and tested by HPLC with an Agilent EC C18 (4 μm, 4.6 × 250 mm) chromatographic column. The mobile phase used 30% water (containing 0.1% acetic acid and 0.1 mmol L−1 ammonium acetate) and 70% acetonitrile. The detection wavelengths of the pollutants (DCF, IBU, CBZ, and SMX) were 280, 220, 286, and 270 nm, respectively; the limits of quantification were 0.1650, 0.1330, 0.1444, and 0.0477 mg L−1, respectively. In terms of the environmentally relevant concentration experiments, the sample was filtered with an Agilent 0.2 μm PTFE-Q filter membrane and measured by LC-MS (Agilent 1260 in series AB SCIEX API4000) with an Agilent HPH C18 (2.1 × 100 mm, 2.7 μm) chromatographic column. The mobile phase is the same as described above, while the water ratio to the organic phase is 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45. The mass spectrometry method is shown in Tables S1 and S2.†
45. The mass spectrometry method is shown in Tables S1 and S2.†
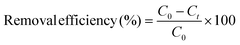 | (1) |
The kinetic data were fitted with pseudo-first-order kinetics and pseudo-second-order kinetics models. The formulas are as follows:
| qt = qe(1 − e−k1t) | (2) |
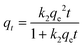 | (3) |
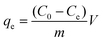 | (4) |
The isotherm data were fitted with the Freundlich and Langmuir models to evaluate the adsorption capacity. The model equation is as follows.
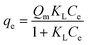 | (5) |
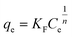 | (6) |
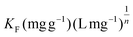 represents the Freundlich constant, and
represents the Freundlich constant, and 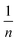 (dimensionless; 0 < n < 10) is the Freundlich intensity parameter.
(dimensionless; 0 < n < 10) is the Freundlich intensity parameter.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 1
methanol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) and desorbed at the same temperature for 15 min.22 A total of 5 adsorption and desorption cycles were performed.
9) and desorbed at the same temperature for 15 min.22 A total of 5 adsorption and desorption cycles were performed.
3. Results and discussion
3.1. [EMIm]Cl/FeCl3 preparation
Ionic liquids, considered a green solvent, are widely used in organic reactions, because of their wide solubility and non-volatility.18 The Lewis acid ionic liquid can be obtained by combining imidazole salt and metal chloride, which also applies to the Friedel–Crafts alkylation reaction.19,21,23 Accordingly, the [EMIm]Cl/FeCl3 ionic liquid was prepared (Fig. 1a), where the molar ratio of ferric chloride to imidazole is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the ionic liquid is acidic (the water content is less than 0.1%). The FT-IR spectra of [EMIm]Cl and [EMIm]Cl/FeCl3 are shown in Fig. S1.† The peaks at 3096 cm−1 and 2989 cm−1 are the C–H stretching vibrations of imidazole and alkanes, respectively, 1573 cm−1, the N–C bond stretching vibration of the imidazole ring, and 1462 cm−1 and 1170 cm−1, the alkane and the imidazole ring C–H bending vibration, respectively.24 These results indicate the successful preparation of [EMIm]Cl/FeCl3.
1, and the ionic liquid is acidic (the water content is less than 0.1%). The FT-IR spectra of [EMIm]Cl and [EMIm]Cl/FeCl3 are shown in Fig. S1.† The peaks at 3096 cm−1 and 2989 cm−1 are the C–H stretching vibrations of imidazole and alkanes, respectively, 1573 cm−1, the N–C bond stretching vibration of the imidazole ring, and 1462 cm−1 and 1170 cm−1, the alkane and the imidazole ring C–H bending vibration, respectively.24 These results indicate the successful preparation of [EMIm]Cl/FeCl3.
 | ||
| Fig. 1 (a) Physical pictures of the [EMIm]Cl/FeCl3 ionic liquid (left), ThCD (middle) and Th-CDP (right); (b) structural diagram of the studied PPCP pollutants. | ||
3.2. Synthesis of the porous thienyl CD polymer (Th-CDP)
As shown in Fig. 2, ThCD (Fig. 1a) was first synthesized by reacting β-CD with a thienyl reagent. Then, [EMIm]Cl/FeCl3 acts as a solvent and catalyst for the Friedel–Crafts alkylation reaction simultaneously, and ThCD and the three-dimensional cross-linking agent Tmof, were polymerized in a homogeneous system to obtain Th-CDP (Fig. 1a). The reaction is exothermic during the reactant dosing and mixing stage, but overheating was avoided by using a cooling bath. In the optimization process, it was found that using a mild temperature (45 °C) at the beginning of the reaction, and then programming it to the final temperature were conducive to obtaining a higher specific surface area. When the final temperature is 60 °C, the specific surface area is only 56 m2 g−1 (Fig. S2†). When the final temperature is 120 °C, the material has a specific surface area of 730 m2 g−1, where the specific surface area of the micropores reaches 193 m2 g−1 (Fig. 3a). The product yield is 0.42 g. Therefore, the reaction temperature greatly influences the polymerization reaction, especially the initial process, probably because the intermolecular cross-linking occurred mainly at 45 °C, which was favorable to form a better polymerization network for ThCD.25 The intramolecular cross-linking at elevated temperatures resulted in a significant increase in the degree of cross-linking. The higher reactivity brought by high temperature is probably an important reason for the rapid increase in the specific surface area.26 Therefore, the moisture resistance (compared to AlCl3) and suitable catalytic activity of [EMIm]Cl/FeCl3 proved it to be a suitable catalyst for polymerization reactions.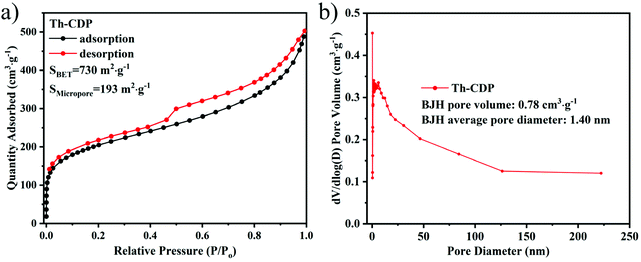 | ||
| Fig. 3 (a) Nitrogen adsorption isotherm of Th-CDP measured at 77 K (activated in a vacuum at 170 °C for 12 h in advance); (b) pore size distribution diagram of Th-CDP. | ||
As shown in Fig. 3a, the N2 adsorption isotherm of Th-CDP showed a type IV isotherm, and the adsorption capacity increased rapidly due to the existence of micropores at low pressure. The presence of desorption hysteresis in a wide area of relatively high pressure indicated a complex pore network and a slightly narrow pore size distribution.27 Besides, Th-CDP had a rich pore structure with an average BJH pore diameter of 1.4 nm (Fig. 3b). The introduction of many thiophene rings with rigid structures is of great significance for pore formation and provides abundant cross-linking sites. Furthermore, the high degree of cross-linking obtained by the three-dimensional cross-linking agent significantly increases the specific surface area of the material.13,15,28,29
3.3. Characterization of Th-CDP
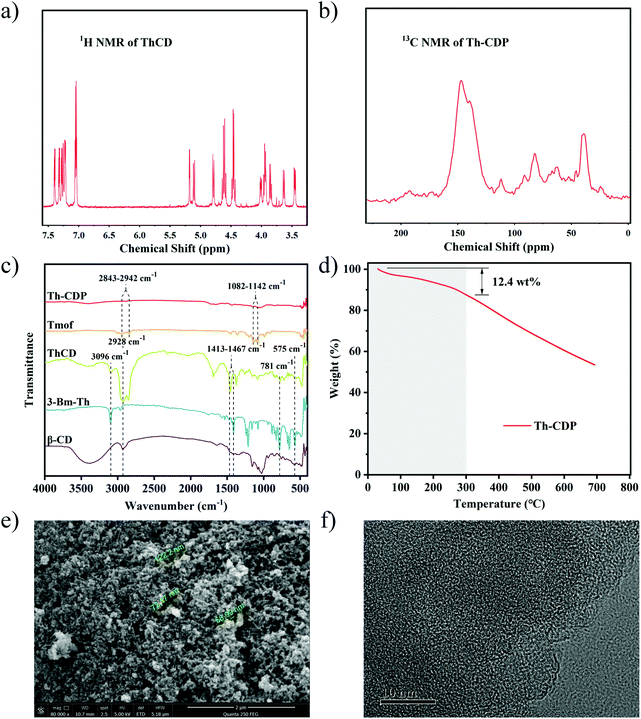
The structure of ThCD was determined by NMR. In the 1H NMR spectrum (Fig. 4a), the peaks in the range of 7.01–7.41 ppm belong to the thiophene rings substituted at the hydroxyl positions on β-CD, and the peaks in the range of 3.4–5.2 ppm belong to β-CD and the methylene groups of the thiophene rings. 1H NMR (600 MHz, acetone) δ 7.41–7.20 (m, 42H), 7.08–7.01 (m, 21H), 5.18 (d, J = 3.5 Hz, 7H), 5.11 (d, J = 11.2 Hz, 7H), 4.79 (d, J = 11.3 Hz, 7H), 4.61 (q, J = 12.3 Hz, 14H), 4.46 (q, J = 12.1 Hz, 14H), 4.03–3.83 (m, 28H), 3.63 (d, J = 10.1 Hz, 7H), 3.45 (dd, J = 9.6, 3.6 Hz, 7H). In the 13C NMR spectrum (Fig. S3†), the peaks in the range of 121–142 ppm belong to the thiophene rings and the rest of the peaks belong to β-CD and the methylene groups of the thiophene rings. The structure of Th-CDP was characterized by solid-state 13C NMR. As shown in Fig. 4b, the peaks around 147 ppm correspond to the thiophene rings, while the peaks around 60–85 ppm and 39 ppm belong to β-CD and the crosslinkers.The structures of the obtained samples were characterized by FT-IR, as shown in Fig. 4c. In terms of ThCD, the hydroxyl peak intensity of β-CD is significantly reduced at 3400 cm−1, and the corresponding C–H stretching vibration of the thiophene ring alkene appears at 3096 cm−1. The peak at 2928 cm−1 is the alkane C–H stretching vibration of β-CD. The peaks in the range of 1413–1476 cm−1 indicate the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in the thiophene ring. In addition, the C–S–C bond (781 cm−1) appears, and the bromomethyl peak (575 cm−1) disappears.15,26 For Th-CDP, the polymer peak is significantly weaker; a methine peak (2843–2942 cm−1) appears; the peak intensity of the ether bond (1082–1142 cm−1) is sharply reduced, which all indicate the polymerization of the material.
C bond in the thiophene ring. In addition, the C–S–C bond (781 cm−1) appears, and the bromomethyl peak (575 cm−1) disappears.15,26 For Th-CDP, the polymer peak is significantly weaker; a methine peak (2843–2942 cm−1) appears; the peak intensity of the ether bond (1082–1142 cm−1) is sharply reduced, which all indicate the polymerization of the material.
The elemental analysis results (Table S3†) show that the O and S content of Th-CDP are 12.7 wt% and 24.8 wt%, respectively, where the S content is higher than those of other heteroatom-doped materials.30,31 According to the TGA analysis (Fig. 4d), Th-CDP exhibits good thermal stability with a mass loss of only 12.4% at 300 °C.
As shown in Fig. 4e, it can be observed from the SEM diagram that the particles of Th-CDP are relatively uniform with an obvious macroporous structure. From the TEM diagram (Fig. 4f), the microporous structure of Th-CDP can be seen.
The zeta potential of the materials in different pH ranges was measured to study the charge characteristics (Fig. S4†). At pH 6 and 8, Th-CDP is positively charged with a zeta potential of 17.17 mV and 14.80 mV, respectively, against the comparative values of −9.88 mV and −11.37 mV of GAC.
3.4. Adsorption experiments
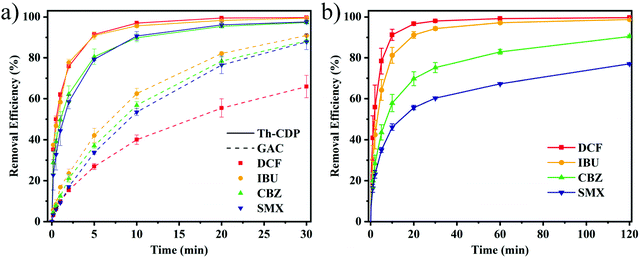
Four different hydrophilic and hydrophobic PPCPs (DCF, IBU, CBZ, and SMX) were selected as target pollutants, whose physical properties and structures are shown in Fig. 1b and Table S4.† The pKa values of the selected PPCPs are all less than 6, except for CBZ (13.94). Adsorption experiments were carried out to study the performance and mechanism of Th-CDP, and GAC was used as the comparative material.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow > 3.5) reached 90% in 5 min and 99.5% in 30 min, and for the removal of CBZ and SMX (log
Kow > 3.5) reached 90% in 5 min and 99.5% in 30 min, and for the removal of CBZ and SMX (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow < 2.5), it reached 80% in 5 min, and 97.4% in 30 min. The removal of the four compounds by GAC did not reach equilibrium within 30 min, while the removal efficiency of DCF was only 66.0%, and the remaining three compounds were between 87.8% and 91.0%. The kinetic data were fitted by pseudo-first-order and second-order kinetic models (Table 1 and Fig. S5†). Th-CDP is more in line with the pseudo-second-order kinetic model (R2 > 0.97).32 The correlation coefficient of the pseudo-first-order kinetic model is greater than 0.91, while the GAC correlated well for both models (R2 > 0.95). In terms of the four compounds (DCF, IBU, CBZ, and SMX), the adsorption rate constants of Th-CDP are 16.7, 10.5, 7.9, and 6.3 times higher than those of GAC. The results show that the adsorption rate of Th-CDP was positively correlated with the hydrophobicity of the compounds.33 The cyclodextrin cavity and the thiophene ring conferred a strong hydrophobic interaction on Th-CDP. The abundantly accessible pore structure, especially the external pores, promoted the diffusion process of adsorption.15,34 Moreover, GAC showed a poor removal rate for DCF, while Th-CDP performed well on it, indicating the existence of other adsorption mechanisms. DCF showed the lowest pKa value (4.18) among the four compounds. Besides, the negative charge on the surface in the neutral range of GAC produced electrostatic repulsion, which affected the adsorption process. However, Th-CDP was positively charged, and its electrostatic interaction made it better to remove DCF.26,35,36 In addition, a large number of thiophene rings and heteroatoms (24.8 wt%) in Th-CDP can form hydrogen bonds or interaction mechanisms with the compounds, which makes a significant contribution to adsorption.15,31,37,38
Kow < 2.5), it reached 80% in 5 min, and 97.4% in 30 min. The removal of the four compounds by GAC did not reach equilibrium within 30 min, while the removal efficiency of DCF was only 66.0%, and the remaining three compounds were between 87.8% and 91.0%. The kinetic data were fitted by pseudo-first-order and second-order kinetic models (Table 1 and Fig. S5†). Th-CDP is more in line with the pseudo-second-order kinetic model (R2 > 0.97).32 The correlation coefficient of the pseudo-first-order kinetic model is greater than 0.91, while the GAC correlated well for both models (R2 > 0.95). In terms of the four compounds (DCF, IBU, CBZ, and SMX), the adsorption rate constants of Th-CDP are 16.7, 10.5, 7.9, and 6.3 times higher than those of GAC. The results show that the adsorption rate of Th-CDP was positively correlated with the hydrophobicity of the compounds.33 The cyclodextrin cavity and the thiophene ring conferred a strong hydrophobic interaction on Th-CDP. The abundantly accessible pore structure, especially the external pores, promoted the diffusion process of adsorption.15,34 Moreover, GAC showed a poor removal rate for DCF, while Th-CDP performed well on it, indicating the existence of other adsorption mechanisms. DCF showed the lowest pKa value (4.18) among the four compounds. Besides, the negative charge on the surface in the neutral range of GAC produced electrostatic repulsion, which affected the adsorption process. However, Th-CDP was positively charged, and its electrostatic interaction made it better to remove DCF.26,35,36 In addition, a large number of thiophene rings and heteroatoms (24.8 wt%) in Th-CDP can form hydrogen bonds or interaction mechanisms with the compounds, which makes a significant contribution to adsorption.15,31,37,38
| Pollutants | Absorbents | Pseudo-first-order kinetic | Pseudo-second-order kinetic | |||
|---|---|---|---|---|---|---|
| q e (mg g−1) | k 1 (min−1) | R 2 | k 2 (g mg−1 min−1) | R 2 | ||
| DCF | Th-CDP | 32.43 | 1.1765 | 0.93 | 0.0679 | 0.98 |
| GAC | 27.41 | 0.0651 | 0.95 | 0.0041 | 0.99 | |
| IBU | Th-CDP | 20.50 | 1.0560 | 0.92 | 0.0961 | 0.98 |
| GAC | 20.52 | 0.1084 | 0.99 | 0.0092 | 0.99 | |
| CBZ | Th-CDP | 23.07 | 0.6825 | 0.91 | 0.0520 | 0.97 |
| GAC | 23.28 | 0.0899 | 0.99 | 0.0066 | 0.99 | |
| SMX | Th-CDP | 26.11 | 0.5376 | 0.95 | 0.0358 | 0.98 |
| GAC | 25.40 | 0.0870 | 1.00 | 0.0057 | 0.97 | |
| Pollutants | Absorbents | Freundlich | Langmuir | ||||
|---|---|---|---|---|---|---|---|
| K F | 1/n | R 2 | Q m (mg g−1) | K L | R 2 | ||
| DCF | Th-CDP | 218.8 | 0.150 | 0.97 | 211.9 | 65.1 | 0.88 |
| GAC | 105.8 | 0.111 | 0.98 | 99.9 | 244.9 | 0.79 | |
| IBU | Th-CDP | 551.3 | 0.233 | 0.99 | 432.2 | 181.9 | 0.91 |
| GAC | 235.8 | 0.231 | 0.98 | 218.6 | 32.4 | 0.81 | |
| CBZ | Th-CDP | 288.9 | 0.218 | 0.97 | 273.1 | 49.1 | 0.95 |
| GAC | 139.1 | 0.132 | 0.95 | 131.7 | 207.4 | 0.89 | |
| SMX | Th-CDP | 374.2 | 0.245 | 0.99 | 350.4 | 37.9 | 0.94 |
| GAC | 181.8 | 0.156 | 0.96 | 171.8 | 144.3 | 0.94 | |
The results demonstrated a higher adsorption capacity of Th-CDP than that of GAC, which is closely related to the suitable porous structure and surface chemistry of Th-CDP. The difference in the adsorption capacity of the compound shows a significant correlation with its molecular weight and pKa (Table S4†), which is probably attributed to the fact that the diffusion of larger molecules in the pore size of the material is probably affected within a certain range.39 In addition, due to electrostatic repulsion, there is a significant decrease in the adsorption of GAC on the anionic compounds, especially on DCF.40 However, Th-CDP has a more pronounced advantage in the DCF adsorption because of its positive charge and the heteroatoms.
3.4.3.1. Competitive adsorption. Mixed solutions of the above four PPCPs were prepared for the kinetic experiment of Th-CDP. As shown in Fig. 5b, Th-CDP exhibits preferential adsorption in the order of DCF, IBU, CBZ, and SMX, which is positively correlated with log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow (Table S4†). These results indicate that the hydrophobic interaction plays a dominant role in the adsorption process, which is achieved by the cyclodextrin cavity itself, many introduced thiophene rings, and a high degree of cross-linking. The strong hydrophobic interaction of Th-CDP enables it to remove PPCPs efficiently.
Kow (Table S4†). These results indicate that the hydrophobic interaction plays a dominant role in the adsorption process, which is achieved by the cyclodextrin cavity itself, many introduced thiophene rings, and a high degree of cross-linking. The strong hydrophobic interaction of Th-CDP enables it to remove PPCPs efficiently.
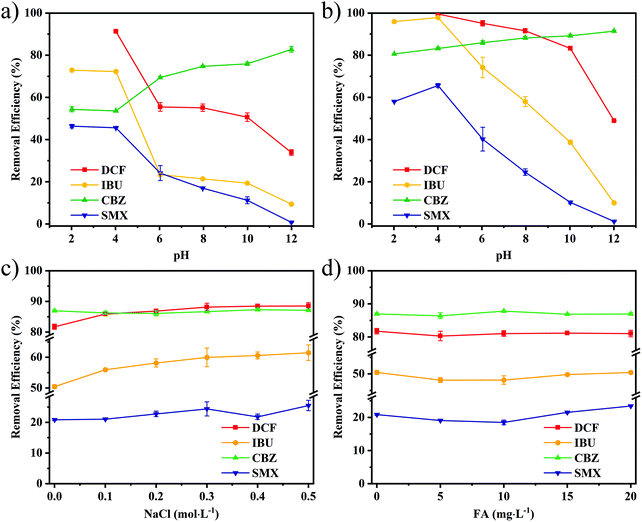
3.4.3.2. The effect of pH on adsorption. As shown in Fig. 6a and b, the effects of pH on the adsorption efficiency are all related to the pKa value of the compounds. The pKa value of the four target compounds is different, of which DCF, IBU, and SMX are in the range from 4 to 6, while CBZ is at 13.94. The removal efficiency of the acidic compounds decreases significantly near the pKa value, while the CBZ keeps increasing as the pH value increases. GAC has a good removal efficiency for alkaline CBZ because of its negatively charged surface under neutral conditions. Furthermore, as the pH increases, the hydroxyl groups of the acidic compound are deprotonated, thus making them negatively charged and generating electrostatic repulsion. As a result, the adsorption efficiency is significantly reduced. Specifically for the hydrophilic substance SMX, GAC is mainly adsorbed by electrostatic interaction, thus almost no adsorption under strong alkali conditions.40 Th-CDP also showed the same trend. With the increase of pH, the removal efficiency of the hydrophilic compounds decreases more significantly due to the electrostatic repulsion and the suppression of hydrogen bonds,35,36 which also proved that the hydrophilic compounds are more affected by static electricity and heteroatoms. For DCF, there is still a certain removal efficiency under strong alkali conditions, indicating the strong hydrophobic interaction of Th-CDP.
3.4.3.3. The influence of salt and fulvic acid on adsorption. The influence of salt and fulvic acid on Th-CDP adsorption was investigated because they tend to affect the adsorption in a real water environment. As shown in Fig. 6c, the adsorption efficiency of Th-CDP did not decrease as the concentration of NaCl increased from 0 to 0.5 mol L−1, and the acidic compound DCF, IBU, and SMX increased by 7.64%, 17.94%, and 18.18%, respectively. The reason is that the salt leads to a decrease in the solubility of the compound, thus promoting the hydrophobic interaction. In addition, the influence of fulvic acid on adsorption was considered. The results (Fig. 6d) showed almost no decrease in the adsorption efficiency and an increase of 10.99% in the removal efficiency of SMX, which could be attributed to the increase of acidity in the solution. In summary, Th-CDP shows stable performance under different water chemical conditions and good potential for practical environmental applications.
3.4.4.1. Adsorption of Th-CDP at environmentally relevant concentrations. Adsorption experiments were performed with 1 L of the four PPCP (50 μg L−1) mixed solution to investigate the adsorption of Th-CDP at environmentally relevant concentrations. As shown in Fig. 7, Th-CDP was still able to remove PPCPs well at microgram concentration, and the removal efficiency of the four PPCPs reached above 97.4%. The excellent adsorption performance of Th-CDP proved its application potential to remove PPCPs in the real environment.
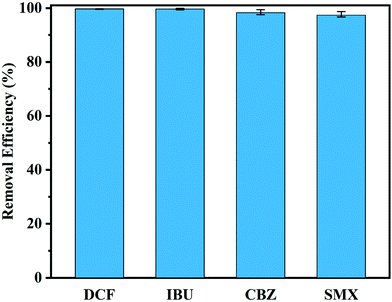 | ||
| Fig. 7 Adsorption of four PPCP (50 μg L−1) mixed solutions by Th-CDP at environmentally relevant concentrations. | ||
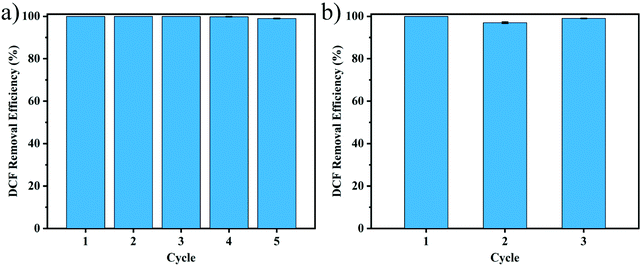
3.4.4.2. Regeneration of Th-CDP. Regeneration performance is an important evaluation index for adsorbents. Due to the high cost of regeneration, the further application of activated carbon is limited. Therefore, the regeneration performance of Th-CDP was studied. 0.1 mmol L−1 DCF was selected as the target compound due to its strong adsorption affinity. In each cycle, Th-CDP was adsorbed at 25 °C for 45 min and then eluted with an acetic acid methanol solution at the same temperature for 15 min. As shown in Fig. 8a, Th-CDP can be quickly regenerated at room temperature. The removal efficiency of Th-CDP on DCF still reached 99% after 5 cycles of adsorption and desorption, proving its excellent regeneration performance and potential for application.
3.4.4.3. Regeneration of [EMIm]Cl/FeCl3. In order to evaluate the regeneration performance of the ionic liquid, [EMIm]Cl/FeCl3 was recycled, and adsorption experiments were conducted on the synthesized Th-CDP each time. The mixture resulting from the Th-CDP synthesis includes the product and the ionic liquid phase. [EMIm]Cl/FeCl3 can be collected by using methanol, and separated by filtration, and the collected ionic liquid was concentrated and can be reused after sufficient drying under vacuum at 80 °C for 18 h. As shown in Fig. 8b, after [EMIm]Cl/FeCl3 was reused three times, the adsorption efficiency of the synthesized Th-CDP on DCF still reached 98.7%. The recyclable characteristics give the [EMIm]Cl/FeCl3 ionic liquid good potential to replace traditional volatile solvents.20,21
4. Conclusions
In this study, porous Th-CDP was synthesized in an ionic liquid system for the first time. As a green solvent, the recyclable [EMIm]Cl/FeCl3 ionic liquid can be used both as a solvent and catalyst for the Friedel–Crafts alkylation reaction with good potential to replace traditional organic solvents in polymerization reactions. The temperature has a great influence on the polymerization reaction, especially in the early stage of the reaction. Moreover, the introduction of thiophene rings and the three-dimensional cross-linking agent Tmof makes Th-CDP have a high specific surface area. The hydrophobic interaction and porous structure endow Th-CDP with a high adsorption rate for PPCPs, especially for hydrophobic compounds whose adsorption rate constant was 10.5–16.7 times that of activated carbon. In addition, the surface of Th-CDP is positively charged, having obvious advantages over activated carbon in the adsorption of acidic compounds. Th-CDP has a high content of heteroatoms (24.8%); thus, it can interact with PPCPs to promote adsorption. The multiple adsorption mechanisms of Th-CDP, including hydrophobic, electrostatic, and heteroatom interactions, can efficiently remove refractory PPCPs, which is of great significance for reducing ecological risks in the aquatic environment. In addition, the easy regeneration of the material coupled with its good hydrochemical stability and efficient adsorption ability at low concentrations proved its good application potential.Abbreviations
| PPCPs | Pharmaceuticals and personal care products |
| β-CD | β-Cyclodextrin |
| ThCD | Thienyl cyclodextrin |
| [EMIm]Cl | 1-Ethyl-3-methylimidazolium chloride |
| FeCl3 | Ferric chloride |
| Tmof | Trimethyl orthoformate |
| Th-CDP | Porous thienyl cyclodextrin polymer |
| DMF | N,N-Dimethylformamide |
| NaH | Sodium hydride |
| DCF | Diclofenac sodium |
| GAC | Granular activated carbon |
| 3-Bm-Th | 3-Bromomethylthiophene |
| IBU | Ibuprofen |
| CBZ | Carbamazepine |
| SMX | Sulfamethoxazole |
| FA | Fulvic acid |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (52070094), the Major Science and Technology Program for Water Pollution Control and Treatment of China (2017ZX07602), and the High-Level Talent Team Project of Quanzhou City (2018CT006).References
- Y. L. Luo, W. S. Guo, H. H. Ngo, L. D. Nghiem, F. I. Hai, J. Zhang, S. Liang and X. C. C. Wang, Sci. Total Environ., 2014, 473, 619–641 CrossRef PubMed.
- E. Y. Klein, T. P. Van Boeckel, E. M. Martinez, S. Pant, S. Gandra, S. A. Levin, H. Goossens and R. Laxminarayan, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, E3463–E3470 CrossRef CAS PubMed.
- P. Westerhoff, Y. Yoon, S. Snyder and E. Wert, Environ. Sci. Technol., 2005, 39, 6649–6663 CrossRef CAS PubMed.
- M. J. Benotti, R. A. Trenholm, B. J. Vanderford, J. C. Holady, B. D. Stanford and S. A. Snyder, Environ. Sci. Technol., 2009, 43, 597–603 CrossRef CAS.
- D. W. Kolpin, E. T. Furlong, M. T. Meyer, E. M. Thurman, S. D. Zaugg, L. B. Barber and H. T. Buxton, Environ. Sci. Technol., 2002, 36, 1202–1211 CrossRef CAS.
- EU Commission, COMMISSION IMPLEMENTING DECISION (EU) 2015/495 of 20 March 2015 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council, 2015.
- Q. W. Bu, B. Wang, J. Huang, S. B. Deng and G. Yu, J. Hazard. Mater., 2013, 262, 189–211 CrossRef CAS.
- L. Lonappan, S. K. Brar, R. K. Das, M. Verma and R. Y. Surampalli, Environ. Int., 2016, 96, 127–138 CrossRef CAS.
- J.-L. Liu and M.-H. Wong, Environ. Int., 2013, 59, 208–224 CrossRef CAS.
- Y. Ling, M. J. Klemes, S. Steinschneider, W. R. Dichtel and D. E. Helbling, Water Res., 2019, 154, 217–226 CrossRef CAS PubMed.
- J. Qian, M. Shen, P. Wang, C. Wang, K. Li, J. Liu, B. Lu and X. Tian, Chemosphere, 2017, 182, 215–222 CrossRef CAS PubMed.
- G. Crini, Chem. Rev., 2014, 114, 10940–10975 CrossRef CAS PubMed.
- Y. Tu, G. Xu, L. Jiang, X. Hu, J. Xu, X. Xie and A. Li, Chem. Eng. J., 2020, 382, 123015 CrossRef CAS.
- H. Li, B. Meng, S.-H. Chai, H. Liu and S. Dai, Chem. Sci., 2016, 7, 905–909 RSC.
- Y. L. Luo, B. Y. Li, W. Wang, K. B. Wu and B. Tan, Adv. Mater., 2012, 24, 5703–5707 CrossRef CAS PubMed.
- A. M. Ruder, in Living in a Chemical World: Framing the Future in Light of the Past, ed. M. A. Mehlman, M. Soffritti, P. Landrigan, E. Bingham and F. Belpoggi, 2006/11/23 edn, 2006, vol. 1076, pp. 207–227 Search PubMed.
- T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef CAS.
- C. Yue, D. Fang, L. Liu and T.-F. Yi, J. Mol. Liq., 2011, 163, 99–121 CrossRef CAS.
- T. Schoetz, C. P. de Leon, A. Bund and M. Ueda, Electrochim. Acta, 2018, 263, 176–183 CrossRef CAS.
- S. J. Ji, M. F. Zhou, D. G. Gu, Z. Q. Jiang and T. P. Loh, Eur. J. Org. Chem., 2004, 2004, 1584–1587 CrossRef.
- M. Chen, D. Li, Y. Luo, M. He, J. Xie, H. Li and X. Yuan, J. Ind. Eng. Chem., 2011, 17, 14–17 CrossRef CAS.
- N. Prasetya and K. Li, Chem. Eng. J., 2021, 417, 129216 CrossRef CAS.
- M. Chen, Y. Luo, G. Li, M. He, J. Xie, H. Li and X. Yuan, Korean J. Chem. Eng., 2010, 26, 1563–1567 CrossRef.
- T. I. Morrow and E. J. Maginn, J. Phys. Chem. B, 2002, 106, 12807–12813 CrossRef CAS.
- F. Maya and F. Svec, Polymer, 2014, 55, 340–346 CrossRef CAS.
- Y. Wang, Y. Cao, X. Zeng, J. Huang and Y.-N. Liu, Ind. Eng. Chem. Res., 2021, 60, 931–938 CrossRef CAS.
- M. Thommes and K. A. Cychosz, Adsorption, 2014, 20, 233–250 CrossRef CAS.
- C. D. Wood, B. Tan, A. Trewin, F. Su, M. J. Rosseinsky, D. Bradshaw, Y. Sun, L. Zhou and A. I. Cooper, Adv. Mater., 2008, 20, 1916–1921 CrossRef CAS.
- G. Xu, X. Xie, L. Qin, X. Hu, D. Zhang, J. Xu, D. Li, X. Ji, Y. Huang, Y. Tu, L. Jiang and D. Wei, Green Chem., 2019, 21, 6062–6072 RSC.
- W. Tian, H. Zhang, H. Sun, A. Suvorova, M. Saunders, M. Tade and S. Wang, Adv. Funct. Mater., 2016, 26, 8651–8661 CrossRef CAS.
- Y. Shi, G. Liu, L. Wang and X. Zhang, Chem. Eng. J., 2015, 259, 771–778 CrossRef CAS.
- Y. S. Ho and G. McKay, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- T. X. Bui, V. H. Pham, S. T. Le and H. Choi, J. Hazard. Mater., 2013, 254–255, 345–353 CrossRef CAS PubMed.
- N. Morin-Crini, P. Winterton, S. Fourmentin, L. D. Wilson, E. Fenyvesi and G. Crini, Prog. Polym. Sci., 2018, 78, 1–23 CrossRef CAS.
- C. Cheng, Y. Cai, G. Guan, L. Yeo and D. Wang, Angew. Chem., Int. Ed., 2018, 57, 11177–11181 CrossRef CAS.
- C. Cheng, Y. Cai, G. Guan, L. Yeo and D. Wang, Angew. Chem., 2018, 130, 11347–11351 CrossRef.
- A. R. Millward and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 17998–17999 CrossRef CAS.
- K. Wang, Y. Tang, Q. Jiang, Y. Lan, H. Huang, D. Liu and C. Zhong, J. Energy Chem., 2017, 26, 902–908 CrossRef.
- D. Eeshwarasinghe, P. Loganathan, M. Kalaruban, D. P. Sounthararajah, J. Kandasamy and S. Vigneswaran, Environ. Sci. Pollut. Res., 2018, 25, 13511–13524 CrossRef CAS.
- S.-W. Nam, D.-J. Choi, S.-K. Kim, N. Her and K.-D. Zoh, J. Hazard. Mater., 2014, 270, 144–152 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1gc03141d |
| This journal is © The Royal Society of Chemistry 2022 |