Free-standing lamellar 3D architectures assembled from chitosan as a reusable titanium-immobilized affinity membrane for efficiently capturing phosphopeptides†
Lei
Pan
ab,
Shujuan
Ma
b,
Ruizhi
Tang
b,
Wenrui
Wu
ab,
Junjie
Ou
 *bc,
Cong
Li
*bc,
Cong
Li
 *a and
Yehua
Shen
a
*a and
Yehua
Shen
a
aKey Laboratory of Synthetic and Natural Functional Molecule of Ministry of Education, College of Chemistry and Materials Science, National Demonstration Center for Experimental Chemistry Education, Northwest University, Xi'an, 710127, China. E-mail: licong@nwu.edu.cn; Fax: +86-29-88302635; Tel: +86-13571961479
bKey Laboratory of Separation Science for Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences (CAS), Dalian, 116023, China. E-mail: junjieou@dicp.ac.cn; Fax: +86-411-84379620; Tel: +86-411-84379576
cUniversity of Chinese Academy of Sciences, Beijing, 100049, China
First published on 26th November 2021
Abstract
Protein phosphorylation is involved in many biological processes and associated with some diseases. However, because of the low abundance and large dynamic changes of phosphopeptides in biological samples, it is necessary to enrich phosphopeptides before mass spectrometry detection. Many kinds of materials have been developed for phosphopeptide enrichment, but most of them are disposable and their preparation processes are toxic. In this study, a green strategy was presented to fabricate immobilized metal affinity chromatography (IMAC) materials. Using chitosan as a precursor, polyethylene glycol diglycidyl ether as a cross-linking agent and 2,3,4-trihydroxybenzaldehyde as a functional monomer, a series of chitosan membranes (CMs) with free-standing lamellar 3D architectures were prepared via a freeze-casting technique and after chelating with titanium ions (Ti4+) could serve as IMAC adsorbents for phosphopeptide enrichment in biological samples. Due to the stronger chelating force between Ti4+ and the pyrogallol ligand than that between Ti4+ and the phosphate groups, the IMAC material is reusable for the enrichment of phosphopeptides, exhibiting an excellent enrichment performance and a higher capacity than other IMAC materials such as organosilica hybrid monolith and carbonaceous spheres. Besides, four endogenous phosphopeptides and two dephosphorylated fragments were captured by Ti4+-CM from the human serum. Therefore, Ti4+-CM demonstrates potential commercialization prospects because of its green preparation process and reusability.
1. Introduction
Nature has become an important source of bioinspiration in the design and construction of high-performance engineering materials.1,2 Many natural phenomena related to wettability, such as the self-cleaning effect of a lotus leaf and the anisotropic dehumidification behavior of a rice leaf, are intimately related to the unique micro- and nanostructures on their surfaces.3 Both plants and animals possess analogous tissues containing networks of hierarchical pores to deal with mass transport and rates of reactions.4 The design of bioinspired porous materials mimicking such hierarchical cellular structures has emerged as an important approach for the fabrication of high-performance structural and functional materials in diverse application fields.5,6 The presence of anisotropic structures across multiple scales is one of the remarkable features of these materials. The most common fabrication approaches include the template replica method, sacrificial template method and direct foaming method without using templates.7 For example, Yu and co-workers assembled 1D silver nanowires into free-standing complex macroscopic hierarchical 3D architectures8 and fabricated bioinspired polymeric woods with wood-like cellular microstructures by the ice-templating assembly approach.9 Although porous materials have received considerable attention as a new class of materials with a wide range of applications, such as artificial bone materials, drug delivery carriers, catalyst carriers, and even parts of an automobile,7 to date, it is still urgently necessary to construct novel porous materials with 3D architectures and apply them in bioseparation sciences.Protein phosphorylation, as a kind of reversible post-translational modification, implicates in many cellular processes including growth, metabolism, proliferation and apoptosis.10 Irregularity in phosphorylation is associated with many diseases, such as cancer.11 So far, mass spectrometry (MS) technology has become a powerful tool for the detection of phosphopeptides due to its great sensitivity in proteomics.12 However, it always faces a great challenge because of the low abundance, high dynamic range of phosphopeptides and serious interference of non-phosphopeptides in the protein digests.13 So, a pretreatment process of efficient separation and enrichment is essential prior to MS analysis.14 To date, several strategies have been developed for the enrichment of phosphopeptides, such as immobilized metal affinity chromatography (IMAC),15 metal oxide affinity chromatography16 and boronate affinity chromatography.17 Among them, IMAC is commonly exploited in virtue of the simplicity of its operational process, excellent specificity and reproducibility, as well as high specificity to phosphopeptides.18 In this strategy, the negatively charged phosphopeptides were captured by various metal ions, such as Fe3+, Ga3+, Zr4+ and Ti4+, immobilized nanomaterials,19 polymeric materials,20 magnetic metal oxides21 and so on. However, the synthesis process of these IMAC materials was troublesome and time-consuming, and usually required several procedures to modify their surfaces, inevitably using toxic organic solvents.19–21 Additionally, the immobilized metal ions generally suffered from dissociation in the process of releasing the bound phosphopeptides, owing to the equal interaction of metal ions with both phosphopeptides and the matrix. Thus, these IMAC materials could not be reused, leading to a serious waste of resources. As a result, it is urgently necessary to develop a simple and robust approach to fabricate recyclable IMAC materials, achieving the green goal of energy saving and consumption reduction.
Chitosan, as the only basic polysaccharide in natural polysaccharides, has been selected as a precursor to form a hydrophilic macroporous biomimetic membrane for the enrichment of glycosylated peptides.22 It offers us a hint to further develop the biomimetic IMAC membrane for the enrichment of phosphopeptides. In particular, polyethylene glycol and the class of glycerol-based glycidyl ethers have been reviewed to be nontoxic in animals and approved by the Food and Drug Administration in USA. Polyethylene glycol diglycidyl ether (PEGDGE), as a kind of non-toxic and inexpensive polymer, was utilized in biomedical research and green processes.23–26 Kono prepared novel hydrogels by cross-linking PEGDGE with carboxymethyl cellulose sodium salt, which served as a carrier for the drug delivery system of protein-based drugs.27 Zhang et al. reported a reusable PEGDGE/ZnO system to produce dimethyl carbonate.28 In this case, 2,3,4-trihydroxybenzaldehyde (THBA) was first utilized as a new chelating ligand to construct free-standing lamellar 3D architectures assembled from chitosan and PEGDGE via a freeze-casting technique. The resulting chitosan membranes (CMs) could serve as a novel IMAC sorbent to enrich phosphopeptides from biological samples after chelating with tetravalent titanium ions (Ti4+). Owing to the strong chelating force between Ti4+ and the pyrogallol ligand, Ti4+ would not be dissociated from CMs even after the adsorbed phosphopeptides were eluted using alkaline ammonia solution. So, a facile and “green” strategy for the construction of free-standing lamellar 3D architectures assembled from non-toxic chitosan and PEGDGE in an aqueous system was successfully developed, and the as-synthesized Ti4+-CM as an IMAC material was reusable, which conformed to the sustainable development.
2. Experimental section
2.1 Chemicals and materials
Chitosan (CS, ≥95%, deacetylated) was purchased from Macklin Biochemical Co., Ltd (Shanghai, China). Polyethylene glycol diglycidyl ether (PEGDGE, Mn 500) was obtained from Aladdin (Shanghai, China). 2,3,4-Trihydroxybenzaldehyde (THBA, ≥98.0%) was bought from Tokyo Chemical Industry Co., Ltd (Tokyo, Japan). Ammonium bicarbonate (NH4HCO3), sodium chloride (NaCl), immunoglobulin G (IgG), β-casein, bovine serum albumin (BSA), urea, 1,4-dithiothreitol (DTT), iodoacetamide (IAA), trypsin and trifluoroacetic acid (TFA) were purchased from Sigma-Aldrich (St Louis, MO, USA). Ammonium hydroxide (NH3·H2O, 25%) and sodium borohydride (NaBH4) were acquired from Damao Chemical Reagent Factory (Tianjin, China). Peptide-N-glycosidase F (PNGase F) was bought from New England Biolabs Inc. (Ipswich, USA). Acetonitrile (ACN) was purchased from Merck (Darmstadt, Germany). Titanium(IV) sulfate and 2,5-dihydroxybenzoic acid (DHB) were bought from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). The human serum of a healthy person was provided by the Second Affiliated Hospital of Dalian Medical University (Dalian, China), the utilization of which complied with the guidelines of the Ethics Committee of the hospital. A written consent from all volunteers was obtained before the research. A titanium(IV)-immobilized monodisperse polymeric microsphere was fabricated according to a previous protocol,29 which was recently commercialized by J&K Scientific Ltd (Beijing, China). The chemical reagents used were of pure analytical grade.2.2 Preparation and modification of composite CMs
The CMs were prepared via a freeze-casting method referring to our previous report,22 the preparation diagram of which is shown in Fig. 1a. Take the preparation of CM-I as an example. First, 250 mg of chitosan, 233 mg of PEGDGE and 24 mg of THBA were dissolved in 25 mL of 0.4% (v%) acetic acid solution and transferred into a metal mold with a flat bottom. Next, the mixture was frozen in liquid nitrogen for 5–8 min followed by lyophilization in a freeze-dryer for 16–24 h to form a membrane. After curing at 60 °C for 2 h, the membrane was immersed in NaBH4 solution (2 mg mL−1) for 4 h to reduce the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H groups to –N–H groups in the structure. Subsequently, the resulting membrane was washed three times with water and freeze-dried before use (assigned CM-I). Finally, CM-I was incubated in 100 mg mL−1 of titanium sulfate solution to chelate Ti4+ with the pyrogallol groups, and the as-synthesized chitosan membrane was assigned Ti4+-CM-I.
H groups to –N–H groups in the structure. Subsequently, the resulting membrane was washed three times with water and freeze-dried before use (assigned CM-I). Finally, CM-I was incubated in 100 mg mL−1 of titanium sulfate solution to chelate Ti4+ with the pyrogallol groups, and the as-synthesized chitosan membrane was assigned Ti4+-CM-I.
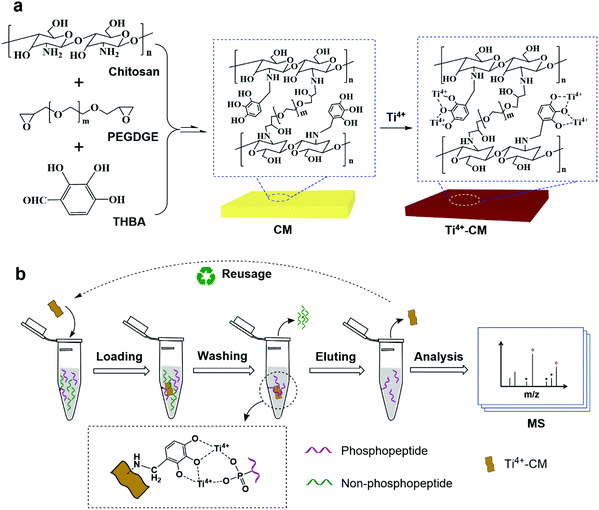 | ||
| Fig. 1 (a) Schematic preparation of the CM and Ti4+-CM and (b) the reusage of Ti4+-CM for the enrichment of phosphopeptides. | ||
2.3 Characterization of the materials
Helium Ion Microscopy (HIM) images were collected using an Orion NanoFab scanning helium ion microscope (Carl Zeiss, Oberkochen, Germany) with ZEN Intellesis. Attenuated total reflection-Fourier transform infrared spectroscopy (ATR-FTIR) spectra were measured using a Thermo Nicolet IS50 spectrometer (Nicolet, Wisconsin, USA). X-ray photoelectron spectroscopy (XPS) was performed using an ESCALAB 250XI XPS spectrometer (Thermo Scientific, USA). Water contact angles were measured using a DSA 100 machine (KRUSS, Hamburg, Germany). Nitrogen adsorption/desorption experiments were performed using an ASAP 2460 Physisorption Analyzer (Micrometitics, USA). The specific surface area was measured using the Brunauer–Emmett–Teller (BET) method. The element content of titanium in the eluent was determined using an ICP OES 7300D coupled plasma-optical emission spectrometer (PerkinElmer, USA). Limit of detection was 0.01 ppm and limit of quantitation was 0.03 ppm.Matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) analysis was performed using a 5800 MALDI-TOF/TOF instrument (AB Sciex, CA). All mass spectra were obtained in the linear positive ion reflector mode. 0.5 μL of the eluent and 0.5 μL of DHB solution (25 g L−1 DHB in 70/29/1, ACN/H2O/H3PO4, v/v/v) were spotted on the MALDI plate for the MALDI-TOF MS analysis.
2.4 Protein digestion
In this case, β-casein, IgG and BSA were digested by trypsin. Specifically, 2 mg of β-casein was dissolved in 200 μL of the denaturant solution containing 6 mol L−1 guanidine hydrochloride and 100 mmol L−1 NH4HCO3. Then Tris-HCl buffer (pH 8.0) was then added to the solution in order to dilute the urea concentration to 1.0 mol L−1 and maintained at 37 °C for 18 h with trypsin at an enzyme-to-protein mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25. Tryptic digests of β-casein were lyophilized and stored in a refrigerator at −20 °C for further use. Then, 2 mg of the protein (IgG or BSA) was dissolved in 1.0 mL of the denaturant solution containing 8 mol L−1 urea and 100 mmol L−1 NH4HCO3. Then, 20 μL of DTT aqueous solution (20 mmol L−1) was added to the above solution and incubated at 37 °C for 2 h. Next, 7.4 mg of IAA was added to the solution, and the mixture was incubated for 35 min at room temperature without light. Tris-HCl buffer (pH 8.0) was then added to the solution in order to dilute the urea concentration to 1.0 mol L−1. Trypsin was added according to the enzyme–protein mass ratio of 1
25. Tryptic digests of β-casein were lyophilized and stored in a refrigerator at −20 °C for further use. Then, 2 mg of the protein (IgG or BSA) was dissolved in 1.0 mL of the denaturant solution containing 8 mol L−1 urea and 100 mmol L−1 NH4HCO3. Then, 20 μL of DTT aqueous solution (20 mmol L−1) was added to the above solution and incubated at 37 °C for 2 h. Next, 7.4 mg of IAA was added to the solution, and the mixture was incubated for 35 min at room temperature without light. Tris-HCl buffer (pH 8.0) was then added to the solution in order to dilute the urea concentration to 1.0 mol L−1. Trypsin was added according to the enzyme–protein mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, and reacted at 37 °C for 18 h. Next, the enzymatic hydrolysate was desalted using an SPE column packed with C18 particles. Finally, the obtained peptide solution was lyophilized and stored in a refrigerator at −20 °C before use.
25, and reacted at 37 °C for 18 h. Next, the enzymatic hydrolysate was desalted using an SPE column packed with C18 particles. Finally, the obtained peptide solution was lyophilized and stored in a refrigerator at −20 °C before use.
2.5 Enrichment of phosphopeptides and glycopeptides
The enrichment process of phosphopeptides includes loading, washing, eluting and MS analysis. As shown in Fig. 1b, 2 mg of Ti4+-CM were soaked in 200 μL of the loading solution (ACN/H2O/TFA, 80/14/6, v/v/v) twice for equilibrating. Then, it was immersed in the loading solution containing the protein digest at 25 °C for 45 min. The supernatant was removed by centrifugation. Next, the membrane was successively washed with a solution of ACN/H2O/TFA (50/44/6, v/v/v) containing 200 mmol L−1 NaCl solution, and another solution of ACN/H2O/TFA (30/69/1, v/v/v) three times to remove the non-phosphopeptides. Thereafter, the adsorbed phosphopeptides were eluted from the sorbent with 100 μL of 10% (w/w) NH3·H2O solution. Finally, the eluent was directly analyzed by MALDI-TOF MS. After enrichment, the Ti4+-CM was equilibrated with the loading solution (ACN/H2O/TFA, 80/14/6, v/v/v) for the next enrichment experiment.The enrichment process of glycopeptides was similar to that of phosphopeptides. Two milligrams of the CM were firstly equilibrated with 200 μL of the loading solution (ACN/H2O/TFA, 85/14/1, 87/12/1, 89/10/1 or 91/8/1, v/v/v) 3 times. Afterward, 10 μg of IgG digests were dissolved in 200 μL of the loading solution, and incubated at 25 °C for 30 min with CM. After the removal of the supernatant by centrifugation, the materials were washed with the loading solution to remove the adsorbed non-glycopeptides 3 times. The captured N-glycopeptides were finally released from the CM with 100 μL of the eluent (ACN/H2O/TFA, 30/69/1, v/v/v) for a direct analysis by MALDI-TOF MS.
2.6 Dynamic and static adsorption experiments
Pyridoxal 5′-phosphate, a kind of multifunctional coenzyme, was utilized to simulate the phosphopeptides and investigate the enrichment behavior of Ti4+-CMs via dynamic and static experiments referring to our report.30For the kinetic adsorption experiment, 1.0 mL of pyridoxal 5′-phosphate aqueous solution (2 mg mL−1) was used as the sample and 2 mg of Ti4+-CM as the sorbent. The UV detection wavelength was set at 295 nm. The sample concentration was detected after adsorption for different time periods. The adsorption capacity was obtained using the following equation:
| Qe = (C0 − Ce) V/m, | (1) |
The results were treated with pseudo-first-order and pseudo-second-order kinetic models, according to the following equation:
Pseudo-first-order:
ln (Qe − Qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − k1t. Qe − k1t. | (2) |
Pseudo-second-order:
| t/Qt = 1/k2Qe2 + t/Qe, | (3) |
For the static adsorption experiment, pyridoxal 5′-phosphate was firstly dissolved in the aqueous solution and configured to the concentrations of 0.8, 1.2, 1.4, 1.6, 1.8 and 2.0 mg mL−1, respectively. Next, 2.0 mg of Ti4+-CM were added into these solutions (1.0 mL) separately and incubated for 30 min. Finally, the concentration of the adsorbed solution at equilibrium was measured to calculate the adsorption capacity of Ti4+-CM for pyridoxal 5′-phosphate. In order to investigate the adsorption type, the results were fitted according to the following equations:
Freundlich isotherm:
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe = log Qe = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KF + log KF + log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce/n. Ce/n. | (4) |
Langmuir isotherm:
| Ce/Qe = 1/(QmKL) + Ce/Qm, | (5) |
2.7 DFT theoretical calculations
All structures were fully optimized at the B3LYP-D3/6-311G (d,p) level using the Gaussian 09 package.44 Frequency calculations were performed at the same level to ensure that all reported structures are true minima. Moreover, the bonding energy of all structures were calculated at the B3LYP-D3/cc-pVTZ level, in which the solvent effect was considered.3. Results and discussion
3.1 Design and fabrication of lamellar 3D architectures assembled from chitosan
The IMAC material was commonly composed of a matrix, a chelator and metal ions, in which nitrilotriacetic acid (NTA) and iminodiacetic acid (IDA) generally serve as chelators and further coordinate with certain metal ions such as Fe3+ and Ga3+.31 Actually, the specificity of them is not too high due to the binding between some acidic non-phosphopeptides and sorbents. Although blocking the acidic residues (carboxylic groups of peptides) with methyl esterification before enrichment could improve the specificity towards phosphopeptides, the tedious derivation procedures limit their application on a large scale. Our group has selected phosphate groups to chelate Zr4+/Ti4+ for the design and fabrication of a variety of IMAC materials, which displayed better selectivity for phosphopeptides than NTA/IDA-based IMAC sorbents.32–35 Furthermore, a kind of monodisperse macroporous adsorption resin (MAR)-based IMAC material has been fabricated and commercialized.29 Although these materials are highly specific for phosphopeptides owing to the interaction of metal ions with the phosphate groups of phosphopeptides, it is an encumbered problem that metal ions immobilized on the surface of materials must suffer a dissociation during the release of phosphopeptides. As a result, these Zr4+/Ti4+-phosphate modified IMAC materials were disposable and could not be reused, leading to a waste of resources and not conforming to the purpose of green chemistry. However, chitosan and PEGDGE are widely used in many research fields because they are cheap and non-toxic, such as medical dressings, surface functionalization, drug deliveries and biomedical research. Therefore, we initially tried to select THBA, containing a pyrogallol group, as a chelator to construct a more stable and reusable IMAC material based on chitosan and PEGDGE after immobilizing with Ti4+ ions.Membrane technology is a dynamic field of separation science. It spurred researchers to seek suitable green strategies for constructing sustainable materials.36,37 Membrane materials were considered in many areas because of their low-cost, easy large-scale fabrication and relatively high environmental safety.38 Moreover, membrane materials have excellent physical properties, such as small footprint and adjustable pore structure.39 However, to date, membrane materials have rarely been reported for the enrichment of phosphopeptides. In recent years, chitosan, as a natural non-toxic material, has been widely applied in the biological field owing to its good biocompatibility, biodegradation and antibacterial properties.40 Herein, a variety of 3D membranes containing the pyrogallol groups were synthesized with chitosan, THBA as a functional monomer and PEGDGE as a crosslinker, as shown in Fig. 1a. The preparation conditions were carefully investigated, and the detailed information is given in Table 1.
| Membrane | Chitosan concentration (mg mL−1) | Chitosan/PEGDGE/THBA (mol/mol/mol) | Distance between synapsesa (μm) | Height of synapsesa (μm) | Densityb (mg cm−3) |
|---|---|---|---|---|---|
| a The distance between the synapses and the height of synapses in the image were measured using ZEN Intellesis. b The densities of the CMs were calculated from the mass and volume of the square CMs with a side length of 5 cm. | |||||
| CM-I | 10 | 10/3/1 | 14–20 | 9–12 | 19.0 |
| CM-II | 10 | 10/3/2 | 16–18 | 4–6 | 20.1 |
| CM-III | 10 | 10/3/4 | 12–18 | 3–5 | 21.7 |
| CM-IV | 10 | 10/2/2 | 12–16 | 9–12 | 17.3 |
| CM-V | 10 | 10/4/2 | 25–35 | 5–7 | 22.9 |
| CM-VI | 5 | 10/3/1 | 15–22 | 4–6 | 9.5 |
| CM-VII | 15 | 10/3/1 | 13–20 | 11–15 | 29.0 |
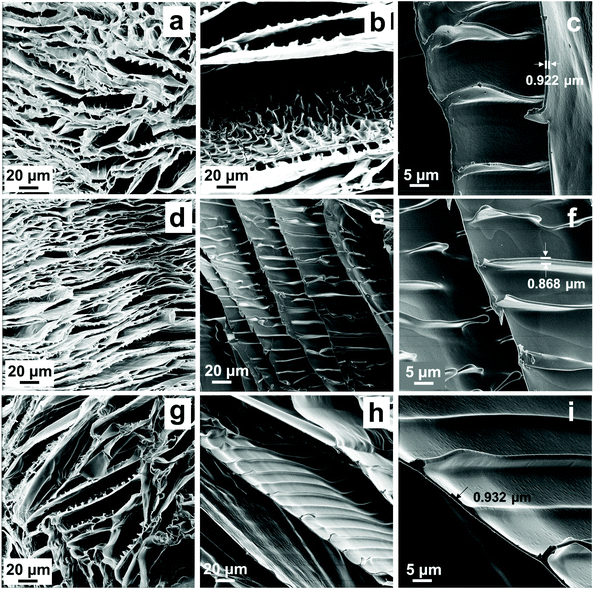
HIM was performed to characterize the morphology of CMs. As shown in Fig. 2 and S1 and S2,† the free-standing lamellar 3D architecture could be clearly observed, which might be considered as a “brick-bridge-mortar” (BBM) microstructure. The top surface of the microstructures of CM-I-VII is shown in Fig. S1.† The distance between the synapses and the height of synapses in the image were obtained using ZEN Intellesis. Particularly, the thickness of each layer was 0.718–1.034 μm (in Fig. 2c, f and i and S2c, f, i and l†). The formation of such a highly ordered 3D structure was related to the oriented growth of ice crystals in the chitosan solution.8 During the solidification process, the chitosan chains were pushed by the growing ice fingers to form a long-range lamellar architecture.41 As described in the previous report, the formation of bridge could be regulated by solution viscosity, which mainly depended on the chitosan concentration in this study. Due to the limitation of the solubility of precursors, three kinds of CMs (VI, I and VII) were fabricated with the concentration of 5, 10 and 15 mg mL−1 chitosan, respectively. Fig. 2a–c and S2g–l† show their microstructures, and a slight difference could be observed. When the chitosan concentration was low (5 mg mL−1), the bridge structure was not obvious and only some tiny synapses with 4–6 μm height are found in Fig. S2i.† However, as shown in Fig. S2l,† regular and dense synapses with 11–15 μm height emerged, and the bridges were formed when the concentration of chitosan increased to 15 mg mL−1. Because of the lower viscosity of the chitosan solution and little restriction to particle movement, almost all of the particles were expelled to the gaps at a relatively high speed by the growing ice and only tiny synapses were formed. With the increase of the viscosity of the chitosan solution, the height of the synapses gradually increased and even bridges appeared. Furthermore, the influence of the amount of PEGDGE on the 3D structure was then investigated (CM-IV, -II and -V). As shown in Fig. 2e, S2b and S2e,† the distance between the synapses in CM-IV was 12–16 μm, 16–18 μm in CM-II, and even 25–35 μm in CM-V. Obviously, the distance between the synapses increased with an increase of PEGDGE content. It is speculated that this phenomenon might be related to the increase of the chain length of chitosan crosslinked by PEGDGE. The BET surface areas of CMs and Ti4+-CMs were only 2–6 m2 g−1. Additionally, all the resulting CMs were soft, and CM-VI with a low concentration of chitosan was fingerprinted on the surface when was touched. The weight and volume of CMs with the same size were measured, and the density of CMs was further calculated. Thus, ultra-light CMs were acquired with an ultra-low density of 9.5–29.0 mg cm−3.
3.2 Characterization of membranes
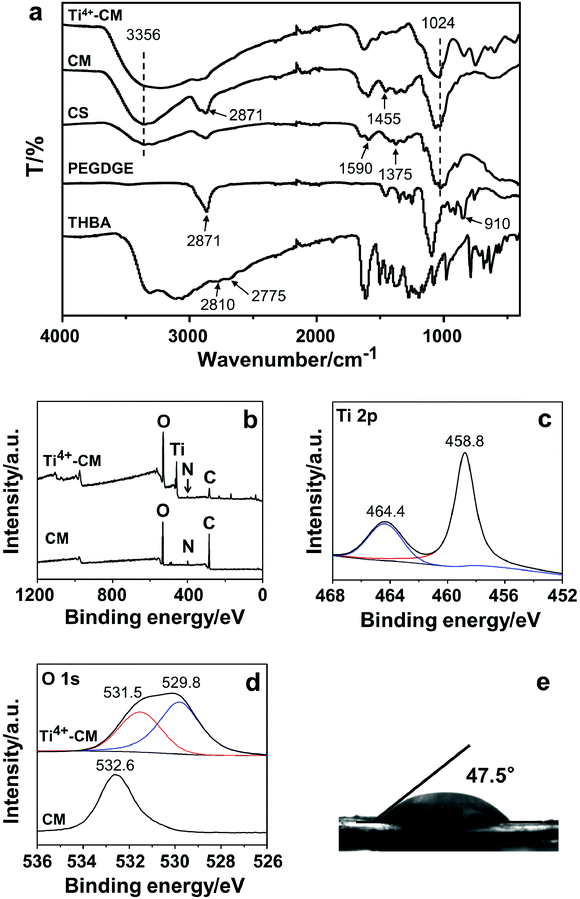
In order to determine the reaction mechanism, ATR-FTIR was employed to characterize both CMs and the three precursors (chitosan, PEGDGE and THBA). CM-III with the highest THBA content was selected as the representative for characterization. As presented in Fig. 3a, the absorption peaks at 3356 cm−1 (–OH and –NH stretching), 2871 cm−1 (–CH stretching), 1590 cm−1 (N–H bending amide II), 1375 cm−1 (C–N stretching amide III) and 1024 cm−1 (C–O–C stretching) were observed in the spectra of both chitosan and CM-III. In addition, the peak at 2871 cm−1 (–CH stretching) of CM-III was significantly enhanced, but the characteristic peak of epoxy at 910 cm−1 could not be observed in the spectrum of CM-III, which indicated the introduction of PEGDGE via an epoxy-amine ring-opening reaction. Meanwhile, two peaks at 2775 cm−1 and 2810 cm−1, which were assigned to the –CH stretching from the aldehyde group of THBA, disappeared in the spectrum of CM-III. A new band at 1455 cm−1 might be attributed to the aromatic hydroxyl stretching, indicating the chemical modification of CM-III by THBA. The peaks at 500–700 cm−1 in the spectrum of Ti4+-CM-III were found to be related to the Ti–O vibration. Based on the above analysis, it is speculated that the ring-opening reaction between chitosan and PEGDGE and the condensation reaction of chitosan and THBA simultaneously occurred in the formation of CM-III.XPS analysis was performed to characterize the surface chemical composition. The full spectra of CM-III and Ti4+-CM-III are presented in Fig. 3b–d. The strong peaks of C 1s (284 eV), N 1s (400 eV) and O 1s (529 eV) could be detected in both two spectra, while the peak of Ti 2p (458 eV) was only discovered in the spectrum of Ti4+-CM-III (Fig. 3b). In the high-resolution XPS spectra of Ti4+-CM-III (Fig. 3c), the peaks at 464.4 and 458.8 eV belonged to the 1/2p and 3/2p orbitals of titanium(IV), respectively. Only one peak of O 1s at 532.6 eV, which came from the –C–OH group or –O–C– group, emerged in the high-resolution XPS spectrum of CM. Additionally, another peak at 529.8 eV also appeared in the spectrum of Ti4+-CM-III, which belonged to the –O–Ti groups. Therefore, it was reasonable that Ti4+-CM-III was prepared by chelating Ti4+ with the pyrogallol groups on the surface of CM-III.
Meanwhile, the hydrophilicity of CMs was characterized by water contact angle analysis. Three kinds of CMs (CM-I-III) were fabricated by increasing the pyrogallol groups in their structure and measured. As shown in Fig. 3e and S3,† their contact angles were below 58°, indicating excellent hydrophilicity for all of them. The content of THBA had little effect on the hydrophilicity of CMs.
Due to the hydrophilicity, CMs could be applied in the enrichment of glycopeptides as a hydrophilic interaction chromatography (HILIC) sorbent. The enrichment protocol was similar to that of phosphopeptide enrichment. CM-III was employed as a representative. The tryptic digest of IgG was utilized as the sample. The loading solution was optimized by regulating the ACN concentration since it has been proved to have a significant influence on the enrichment efficiency.42 The ACN/H2O/TFA (89/10/1, v/v/v) solution was finally chosen owing to the satisfying enrichment performance (Fig. S4 and S5†). As shown in Fig. S5a,† no signals of glycopeptides could be observed in the MS spectrum of IgG digest before enrichment. However, glycopeptide signals dominated the spectrum and non-glycopeptide signals almost disappeared (Fig. S5b†), demonstrating that CM-III exhibited great enrichment performance to glycopeptides. Similar results were acquired for CM-I (Fig. S6a†) and CM-II (Fig. S6b†).
3.3 Enrichment performance and reusability of Ti4+-CMs to phosphopeptides
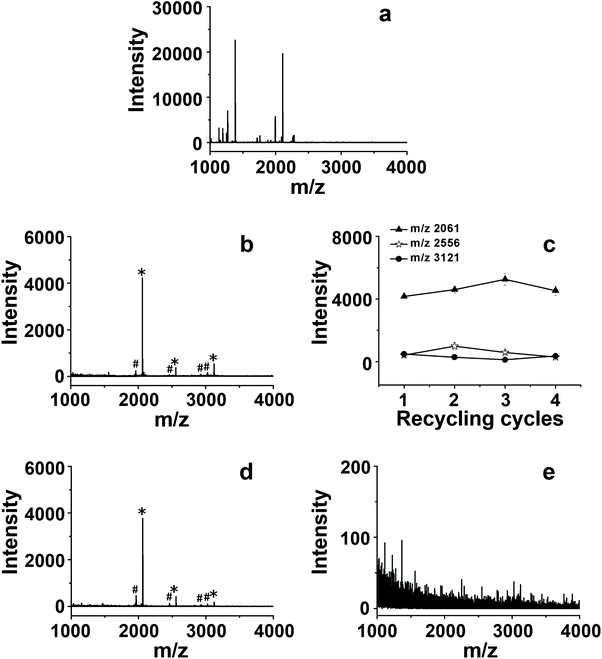
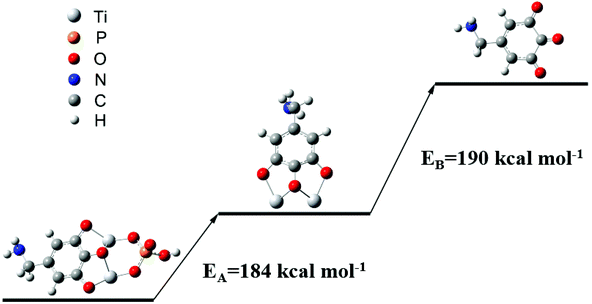
It was necessary to enrich phosphopeptides before MS analysis, due to the difficulty of direct detection of low abundance phosphopeptides in biological samples. The CMs could be utilized as IMAC sorbents to enrich phosphopeptides after chelating with Ti4+. The enrichment abilities of three kinds of Ti4+-CM-I-III were firstly evaluated by employing tryptic digest of β-casein as a representative sample. The enrichment protocol was referred to from the report by our group,29 which includes four steps: sample loading, washing, eluting and MS analysis, as shown in Fig. 1b. The result is presented in Fig. 4 and S7.† The signals of non-phosphopeptides dominated the spectrum, and phosphopeptide signals were not observed before enrichment (Fig. 4a). Obviously, 3 characteristic phosphopeptide signals (2061, 2556 and 3121, m/z) and their dephosphorylated fragments (1964, 2458, 2926 and 3023, m/z) were clearly observed in Fig. S7,† and the signals of non-phosphopeptides almost disappeared after enrichment with CMs, indicating their great enrichment ability to phosphopeptides. In comparison, it was found that the highest intensity of the enriched phosphopeptides (2061, m/z) reached 4179, which was obtained by Ti4+-CM-III (Fig. S7c†), while the intensities were only 872 and 2611 for Ti4+-CM-I (Fig. S7a†) and II (Fig. S7b†), respectively. These results indicated that Ti4+-CM-III possessed the best enrichment efficiency.The reusability of Ti4+-CM-III as the IMAC sorbent was investigated in detail through four successive cycles of phosphopeptide enrichment, and the results are shown in Fig. 4. The intensities of 3 characteristic phosphopeptides in the fourth run (Fig. 4b) were 4222 (2061, m/z), 362 (2556, m/z) and 527 (3121, m/z), respectively, which were still similar to those in the first run (Fig. S7c†). Fig. 4c shows Ti4+-CM-III could be reused 4 times and the enrichment efficiency was not remarkably decreased. The enrichment efficiency of Ti4+-CM-III began to degrade after performing the enrichment processes four times due to the destruction of CM. However, a commercial Ti4+-IMAC material based on a phosphate ligand could not capture any phosphopeptides in the second run under the same conditions (Fig. 4d and e). The loss of enrichment ability to phosphopeptides after only one use indicated that the commercial Ti4+-IMAC material was disposable and could not be reused. For comparison, as shown in Table 2, a few IMAC materials utilizing phosphate or carboxylic acids as the ligands have proven their great enrichment efficiency for phosphopeptides, but they were not reusable due to the dissociation of Ti4+ from the sorbents in the process of eluting the captured phosphopeptides with alkaline solution. It was reported that the chelating force between Zr4+ and the pyrogallol groups in a Zr4+-based metal–organic framework, namely MIL-163, was stronger than that between Zr4+ and the phosphate groups.43 So, it was deduced that the same phenomenon would be observed for Ti4+. The bonding energy of both Ti4+-phosphate group and Ti4+-pyrogallol group was calculated at the B3LYP-D3/cc-pVTZ level using the Gaussian 09 package,44 in which the solvent effect was considered. As shown in Fig. 5, the bonding energy between Ti4+ and the phosphate ligand was 184 kcal mol−1, while the bonding energy between Ti4+ and the pyrogallol ligand was 190 kcal mol−1. As for the phosphate-based IMAC materials, Ti4+ was immobilized on the surface of the sorbents by the chelating force between Ti4+ and the phosphate groups, which was almost same as that between Ti4+ and the phosphopeptides. Once this chelating force was broken in the alkaline solution (such as NH3·H2O), the Ti4+ ions would be easily dissociated, leading to the failure of reusability. Obviously, the bonding energy of Ti4+-trihydroxybenzene was higher than that of Ti4+ and the phosphopeptides, therefore, it is possible to elute the adsorbed phosphopeptides from the sorbent without the dissociation of Ti4+ by employing a pyrogallol-based IMAC material. The content of titanium in the eluent of NH3·H2O solution was determined using ICP, which could directly reflect the Ti4+ content change of the adsorbent after recycling. The result displayed that about 1.0 ppm of titanium emerged in the eluent with the commercial Ti4+-IMAC material, but titanium ions were not detected in the eluent with Ti4+-CM-III. Herein, it is the first case to fabricate a reusable Ti4+-immobilized IMAC material, which proved that the strong chelation of Ti4+-trihydroxybenzene could not be easily broken by alkaline solution when the chelation of Ti4+-phosphates was broken.
| Material | Coupling linker | Limit of detection | Adsorption capacitya (mg g−1) | Bifunctionalb | Reusage | Ref. |
|---|---|---|---|---|---|---|
| a Pyridoxal 5′-phosphate was utilized to simulate the phosphopeptides. b Bifunctional enrichment of glycopeptides and phosphopeptides; –: not mentioned; √: enrichment of glycopeptides and phosphopeptides. | ||||||
| Zwitterion coating chitosan nanomaterials | Iminodiacetic acid (–COO–Ti4+) | 4000 fmol | — | √ | — | 19 |
| Magnetite/ceria-codecorated titanoniobate nanosheet | CeO2 | 40 nmol | — | — | √ | 46 |
| Fe3O4-embedded titanoniobate nanosheets | Fe3O4 | 16 fmol | — | — | — | 47 |
| Magnetic mesoporous nanocomposite | Polydopamine (–ph–O2–Ti4+) | 10 fmol | — | — | √ | 48 |
| Chitosan composite material | Aminomethyl phosphonic acid (–PO3–Ti4+) | 0.4 fmol | — | — | — | 49 |
| Hierarchically porous hybrid monolith | Vinylphosphonic acid (–PO3–Ti4+) | 5 fmol | 63.6 | — | — | 30 |
| Organosilica hybrid monolith | Vinylphosphonic acid (–PO3–Ti4+) | 10 fmol | 82.6 | — | — | 45 |
| Carbonaceous spheres | Vinylphosphonic acid (–PO3–Ti4+) | 5 fmol | 0.7 | — | — | 34 |
| Macroporous adsorption resin microspheres | O-Phospho-L-serine (–PO3–Ti4+) | 1 fmol | — | √ | — | 35 |
| Ti3AlC2 nanolayered material | Ti | 5 fmol | — | — | √ | 50 |
| CM | THBA (–ph–O3–Ti4+2) | 100 fmol | 369 | √ | √ | This work |
The sensitivity of Ti4+-CM-III to phosphopeptides was evaluated by decreasing the amount of β-casein digest. As shown in Fig. S8,† the phosphopeptide signal at 2061 m/z could be distinctly observed after enrichment with Ti4+-CMs when the sample amount was as low as 100 fmol, demonstrating high sensitivity of these materials. In order to investigate the enrichment specificity, the mixed digests of β-casein and BSA with different mass ratios were employed as the sample. The results are presented in Fig. S9.† As shown in Fig. S9a, e and i,† none of the signals of phosphopeptides could be observed before enrichment at different mass ratios of BSA to β-casein digests. The signals of three characteristic phosphopeptides (2061, 2556 and 3121, m/z) and their dephosphorylated fragments (1964, 2458, 2926 and 3023, m/z) were clearly identified after enrichment by CM-I-III, which are respectively shown in Fig. S9b–d.† The highest abundances of enriched phosphopeptides (2061, m/z) were 2643, 2689 and 2787 for Ti4+-CM-I, -II and –III, respectively. Besides, it was found that the phosphopeptides enriched by CM-III suffered the least interference compared to those by CM-I and CM-II. The same is especially true when more BSA digest was added to the β-casein digest at a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (Fig. S9f–h†). As shown in Fig. S9j–l,† three characteristic phosphopeptides could also be enriched and identified even if 100 times the amount of BSA digest was added into the β-casein digest. In Fig. S9l,† the highest intensity of phosphopeptide enriched by CM-III was 156 at 2061, m/z, and this spectrum had minimal interference of non-phosphopeptides, which indicated that Ti4+-CM-III had the highest specificity to phosphopeptides. This could be attributed to the fact that more pyrogallol ligands existed in the structure of CM-III than the other two CMs and it could chelate more Ti4+ to offer more action sites and thus had better specificity for the enrichment of phosphopeptides.
50 (Fig. S9f–h†). As shown in Fig. S9j–l,† three characteristic phosphopeptides could also be enriched and identified even if 100 times the amount of BSA digest was added into the β-casein digest. In Fig. S9l,† the highest intensity of phosphopeptide enriched by CM-III was 156 at 2061, m/z, and this spectrum had minimal interference of non-phosphopeptides, which indicated that Ti4+-CM-III had the highest specificity to phosphopeptides. This could be attributed to the fact that more pyrogallol ligands existed in the structure of CM-III than the other two CMs and it could chelate more Ti4+ to offer more action sites and thus had better specificity for the enrichment of phosphopeptides.
Pyridoxal 5′-phosphate, a multifunctional coenzyme, was utilized to simulate the phosphopeptides to investigate the adsorption behavior of Ti4+-CMs via dynamic and static experiments, referring to our previous report.30 Ti4+-CM-III was chosen as a representative of CMs due to its excellent enrichment performance to phosphopeptides. As shown in Fig. S10a,† the kinetic curve of Ti4+-CM-III indicated that the adsorption amount continuously increased within 240 min and finally reached the adsorption equilibrium within 480 min. The data were further treated with pseudo-first-order and pseudo-second-order kinetic models and are shown in Fig. S10b and c and Table S1.† Obviously, the kinetic fitting curve of the pseudo-first-order was linear and the correlation coefficient (r1) was 0.9962, while the fitting curve of the pseudo-second-order model was nonlinear. It was assumed that the diffusion rate played a decisive role in the adsorption rate when the dynamic fitting curve fitted the pseudo-first-order model, while the pseudo-second-order model assumed that the chemical force was dominant. Therefore, it was a reasonable corollary that the adsorption rate of Ti4+-CM was controlled by a diffusion rate, which was probably related to the layered porous skeleton structure of Ti4+-CM-III. Fig. S10d† presents the static adsorption curve. The maximum adsorption capacity of Ti4+-CM-III was 369 mg g−1 when the concentration of pyridoxal 5′-phosphate was 1.8 mg mL−1, which was remarkably higher than the other IMAC adsorbents, such as hierarchically porous hybrid monolith (63.6 mg g−1),30 organosilica hybrid monolith (82.6 mg g−1)45 and carbonaceous spheres (0.7 mg g−1)34 under the same conditions. The adsorption isotherm curves were drawn and fitted with the Freundlich isotherm and Langmuir isotherm. As shown in Fig. S10e and f and Table S2,† the values of the correlation coefficient of the Freundlich isotherm and Langmuir isotherm were 0.9595 and 0.9280, respectively. As a result, the adsorption process was more consistent with the Freundlich adsorption model, indicating that the active site was not uniform on the surface. It might be attributed to the uneven surface of the active site formed by the titanium ions of Ti4+-CM-III.
3.4 Enrichment of phosphopeptides from the biological samples
Encouraged by the above results, Ti4+-CM-III was employed to enrich endogenous phosphopeptides from the human serum, which is often used to discover the biomarkers associated with disease diagnosis as a common clinical specimen. Fig. 6 shows the MALDI-TOF MS spectra of the human serum before and after enrichment. As illustrated in Fig. 6a, the signals of phosphopeptides were heavily masked by the signals of non-phosphopeptides and only one endogenous phosphopeptide (1545, m/z) was observed before enrichment, while the signals of four endogenous phosphopeptides (Table S3†) and two dephosphorylated fragments were unequivocally identified after enrichment with Ti4+-CM-III shown in Fig. 6b, which was comparable with the enrichment of Ti4+-IMAC carbonaceous spheres.34 These results suggested the potential prospect of Ti4+-CM in the enrichment of phosphopeptides from complicated biological samples.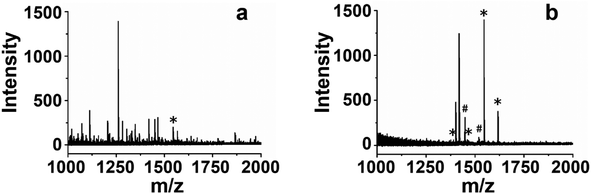 | ||
| Fig. 6 MALDI-TOF MS spectra of the serum (a) before enrichment and (b) after enrichment by Ti4+-CM-III. The asterisk (*) indicates phosphopeptides and (#) indicates dephosphorylated fragments. | ||
4. Conclusions
A facile and “green” strategy for the construction of free-standing lamellar 3D architectures assembled from chitosan was successfully developed via a freeze-casting technique, in which water was the sole solvent employed in this whole process, which is environmentally friendly. The resulting membranes had a layered structure and wrinkled skeleton, which provided a large number of active sites. To the best of our knowledge, a novel chelator, 2,3,4-trihydroxybenzaldehyde, was first utilized to prepare hydrophilic Ti4+-IMAC composite membranes, exhibiting excellent enrichment performance to phosphopeptides. Unlike the traditional Ti4+-IMAC materials based on a phosphate ligand, Ti4+-CM was reusable due to the strong chelation between Ti4+ and the pyrogallol ligand and could be used four times without the loss of enrichment efficiency. Moreover, the intermediates before chelating titanium ions could be employed as HILIC sorbents to capture glycopeptides in the samples because of their good hydrophilicity. As a result, this kind of reusable IMAC material was considered to follow green and sustainable development, remarkably reducing the cost of preparation compared to the traditional IMAC materials. Furthermore, with the industrialization of freeze-drying technology, it also offered a new idea for the large-scale preparation of IMAC materials and promoted the commercialization process of IMAC materials. The successful use of this novel chelator would provide an inspiration to construct more sustainable materials and save more energy and resources.Author contributions
Lei Pan: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing – original draft, and writing – review and editing. Shujuan Ma: data curation, formal analysis, investigation, methodology, and writing – original draft. Ruizhi Tang: data curation, formal analysis and investigation. Wenrui Wu: data curation, formal analysis, investigation and methodology. Junjie Ou: conceptualization, data curation, funding acquisition, methodology, investigation, project administration, resources, supervision, writing – original draft, and writing – review and editing. Cong Li: funding acquisition, methodology, investigation, project administration, resources, supervision, writing – original draft, and writing – review and editing. Yehua Shen: funding acquisition, methodology, investigation, project administration, resources and supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21675125) to Y. Shen and the National Natural Science Foundation of China (No. 21974137), the CAS-Weigao Research & Development Program ([2017]-009) and the Innovation Program of Science and Research from the Dalian Institute of Chemical Physics (DICPI202005) to J. Ou.References
- F. Bouville, E. Maire, S. Meille, B. Moortele, A. J. Stevenson and S. Deville, Nat. Mater., 2014, 13, 508–514 CrossRef CAS PubMed.
- Y. Cui, H. Gong, Y. Wang, D. Li and H. Bai, Adv. Mater., 2018, 30, 1706807 CrossRef.
- T. Sun, L. Feng, X. Gao and L. Jiang, Acc. Chem. Res., 2005, 38, 644–652 CrossRef CAS PubMed.
- G. X. Y. Zheng, J. M. Terry, P. Belgrader, P. Ryvkin, Z. W. Bent, R. Wilson, S. B. Ziraldo, T. D. Wheeler, G. P. McDermott, J. Zhu, M. T. Gregory, J. Shuga, L. Montesclaros, J. G. Underwood, D. A. Masquelier, S. Y. Nishimura, M. Schnall-Levin, P. W. Wyatt, C. M. Hindson, R. Bharadwaj, A. Wong, K. D. Ness, L. W. Beppu, H. J. Deeg, C. McFarland, K. R. Loeb, W. J. Valente, N. G. Ericson, E. A. Stevens, J. P. Radich, T. S. Mikkelsen, B. J. Hindson and J. H. Bielas, Nat. Commun., 2017, 8, 14049 CrossRef CAS PubMed.
- L. Cseri, R. Hardian, S. Anan, H. Vovusha, U. Schwingenschlogl, P. M. Budd, K. Sada, K. Kokado and G. Szekely, J. Mater. Chem. A, 2021, 9, 23793–23801 RSC.
- D. Zhao, J. F. Kim, G. Ignacz, P. Pogany, Y. M. Lee and G. Szekely, ACS Nano, 2019, 13, 125–133 CrossRef CAS PubMed.
- J. Lee and Y. Deng, Soft Matter, 2011, 7, 6034–6040 RSC.
- H. Gao, Y. Lu, L. Mao, D. An, L. Xu, J. Gu, F. Long and S. Yu, Mater. Horiz., 2014, 1, 69–73 RSC.
- Z. L. Yu, N. Yang, L. C. Zhou, Z. Y. Ma, Y. B. Zhu, Y. Y. Lu, B. Qin, W. Y. Xing, T. Ma, S. C. Li, H. L. Gao, H. G. Wu and S. H. Yu, Sci. Adv., 2018, 4, 7223 CrossRef.
- J. V. Olsen, B. Blagoev, F. Gnad, B. Macek, C. Kumar, P. Mortensen and M. Mann, Cell, 2006, 127, 635–648 CrossRef CAS PubMed.
- I. H. Chen, L. Xue, C. C. Hsu, J. S. Paez, L. Pan, H. Andaluz, M. K. Wendt, A. B. Iliuk, J. K. Zhu and W. A. Tao, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 3175–3180 CrossRef CAS PubMed.
- H. Steen, J. A. Jebanathirajah, J. Rush, N. Morrice and M. W. Kirschner, Mol. Cell. Proteomics, 2006, 5, 172–181 CrossRef CAS PubMed.
- A. Schmidt, E. Csaszar, G. Ammerer and K. Mechtler, Proteomics, 2008, 8, 4577–4592 CrossRef CAS.
- J. Peng, H. Niu, H. Zhang, Y. Yao, X. Zhao, X. Zhou, L. Wan, X. Kang and R. Wu, ACS Appl. Mater. Interfaces, 2018, 10, 32613–32621 CrossRef CAS.
- J. Huang, X. Liu, D. Wang, Y. Cui, X. Shi, J. Dong, M. Ye and L. Li, Anal. Chem., 2021, 93, 8568–8576 CrossRef CAS PubMed.
- M. Wang, C. Deng, Y. Li and X. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 11775–11782 CrossRef CAS.
- Z. Liu, Y. Wang, Y. Yao, Z. Fang, Q. R. Miao and M. Ye, J. Proteomics, 2019, 208, 103501 CrossRef CAS.
- T. E. Thingholm, O. N. Jensen, P. J. Robinson and M. R. Larsen, Mol. Cell. Proteomics, 2008, 7, 661–671 CrossRef CAS.
- X. Zou, J. Jie and B. Yang, Anal. Chem., 2017, 89, 7520–7526 CrossRef CAS.
- M. Najam-ul-Haq, A. Saeed, F. Jabeen, F. Maya, M. N. Ashiq and A. Sharif, ACS Appl. Mater. Interfaces, 2014, 6, 3536–3545 CrossRef CAS.
- A. I. Diez, I. Govender, P. Naicker, S. Stoychev, J. Jordaan and O. N. Jensen, J. Proteome Res., 2021, 20, 453–462 CrossRef.
- L. Zhang, S. Ma, Y. Chen, Y. Wang, J. Ou, H. Uyama and M. Ye, Anal. Chem., 2019, 91, 2985–2993 CrossRef CAS PubMed.
- L. C. Cesteros, Green Chem., 2011, 13, 197–206 RSC.
- B. Grignard, J. M. Thomassin, S. Gennen, L. Poussard, L. Bonnaud, J. M. Raquez, P. Dubois, M. P. Tran, C. B. Park, C. Jerome and C. Detrembleur, Green Chem., 2016, 18, 2206–2215 RSC.
- R. J. Li, J. Gutierrez, Y. Chung, C. W. Frank, S. L. Billingtonc and E. S. Sattely, Green Chem., 2018, 20, 1459–1466 RSC.
- R. Dinu and A. Mija, Green Chem., 2019, 21, 6277–6289 RSC.
- H. Kono, Carbohydr. Polym., 2014, 106, 84–93 CrossRef CAS.
- Z. Zhang, L. Zhang, C. Wu, Q. Qian, Q. Zhu and B. Han, Green Chem., 2016, 18, 798–801 RSC.
- H. Zhou, M. Ye, J. Dong, E. Corradini, A. Cristobal, A. J. Heck, H. Zou and S. Mohammed, Nat. Protoc., 2013, 8, 461–480 CrossRef CAS.
- H. Zhang, J. Ou, Y. Yao, H. Wang, Z. Liu, Y. Wei and M. Ye, Anal. Chem., 2017, 89, 4655–4662 CrossRef CAS PubMed.
- T. S. Nuhse, A. Stensballe, O. N. Jensen and S. C. Peck, Mol. Cell. Proteomics, 2003, 2, 1234–1243 CrossRef.
- Y. Yao, J. Dong, M. Dong, F. Liu, Y. Wang, J. Mao, M. Ye and H. Zou, J. Chromatogr. A, 2017, 1498, 22–28 CrossRef CAS.
- Y. Bian, L. Li, M. Dong, X. Liu, T. Kaneko, K. Cheng, H. Liu, C. Voss, X. Cao, Y. Wang, D. Litchfield, M. Ye, S. S. Li and H. Zou, Nat. Chem. Biol., 2016, 12, 959–966 CrossRef CAS PubMed.
- H. Zhang, X. Li, S. Ma, J. Ou, Y. Wei and M. Ye, Green Chem., 2019, 21, 2052–2060 RSC.
- R. Tang, Y. Yu, J. Dong, Y. Yao, S. Ma, J. Ou and M. Ye, Anal. Chim. Acta, 2021, 1144, 111–120 CrossRef CAS PubMed.
- Y. L. Wu, M. Yan, X. L. Liu, P. Lv, J. Y. Cui, M. J. Meng, J. D. Dai, Y. S. Yan and C. X. Li, Green Chem., 2015, 17, 3338–3349 RSC.
- J. K. Gao, J. Q. Wang, Q. Y. Xu, S. B. Wu and Y. Chen, Green Chem., 2021, 23, 5633–5646 RSC.
- M. G. Buonomenna, RSC Adv., 2013, 3, 5694–5740 RSC.
- S. J. Garcia-Vergara, P. Skeldon, G. E. Thompson and H. Habazaki, Electrochim. Acta, 2006, 52, 681–687 CrossRef CAS.
- X. M. He, X. C. Liang, X. Chen, B. F. Yuan, P. Zhou, L. N. Zhang and Y. Q. Feng, Anal. Chem., 2017, 89, 9712–9721 CrossRef CAS PubMed.
- H. Zhao, Y. Yue, L. Guo, J. Wu, Y. Zhang, X. Li, S. Mao and X. Han, Adv. Mater., 2016, 28, 5099–5105 CrossRef CAS PubMed.
- X. Li, H. Zhang, N. Zhang, S. Ma, J. Ou and M. Ye, ACS Sustainable Chem. Eng., 2019, 7, 11511–11520 CrossRef CAS.
- G. Mouchaham, L. Cooper, N. Guillou, C. Martineau, E. Elkaim, S. Bourrelly, P. L. Llewellyn, C. Allain, G. Clavier, C. Serre and T. Devic, Angew. Chem., Int. Ed., 2015, 54, 13297–13301 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, revision D.01, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- H. Zhang, X. Li, Y. Yao, S. Ma, Z. Liu, J. Ou, Y. Wei and M. Ye, Anal. Chim. Acta, 2019, 1046, 199–207 CrossRef CAS PubMed.
- Q. Min, S. Li, X. Chen, E. S. Abdel-Halim, L. P. Jiang and J. J. Zhu, ACS Appl. Mater. Interfaces, 2015, 7, 9563–9572 CrossRef CAS PubMed.
- X. Chen, S. Li, X. Zhang, Q. Min and J. J. Zhu, Nanoscale, 2015, 7, 5815–5825 RSC.
- Z. Xu, Y. Wu, H. Wu, N. Sun and C. Deng, Anal. Chim. Acta, 2021, 1146, 53–60 CrossRef CAS PubMed.
- C. Zhu, J. Wu, X. Jin, Y. Yan, C.-F. Ding, K. Tang and D. Zhang, J. Chromatogr. A, 2021, 1643, 462072 CrossRef CAS.
- X. Li, N. Zhang, R. Tang, J. Lyu, Z. Liu, S. Ma, J. Ou and M. Ye, Nanoscale, 2021, 13, 2923–2930 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1gc03290a |
| This journal is © The Royal Society of Chemistry 2022 |