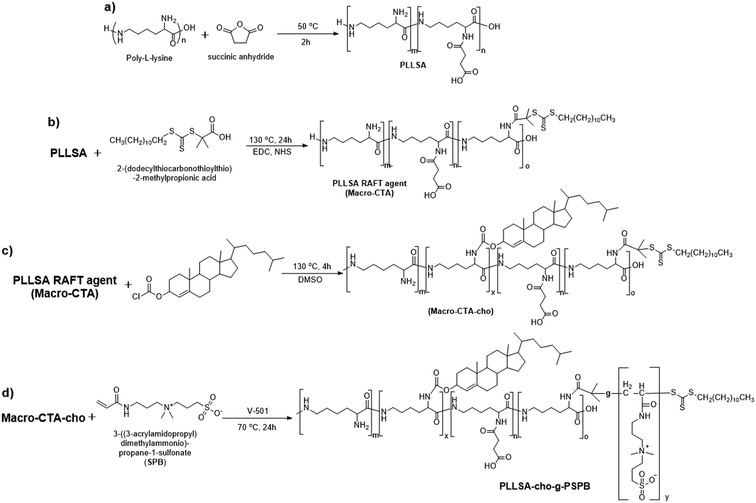
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development and structural analysis of dual-thermo-responsive self-assembled zwitterionic micelles†
Dandan
Zhao
a,
Robin
Rajan
 a,
Shin-ichi
Yusa
a,
Shin-ichi
Yusa
 b,
Masaru
Nakada
c and
Kazuaki
Matsumura
b,
Masaru
Nakada
c and
Kazuaki
Matsumura
 *a
*a
aSchool of Materials Science, Japan Advanced Institute of Science and Technology, 1-1 Asahidai, Nomi, Ishikawa 923-1292, Japan. E-mail: mkazuaki@jaist.ac.jp
bDepartment of Applied Chemistry, Graduate School of Engineering, University of Hyogo, 2167 Shosha, Himeji, Hyogo 671-2280, Japan
cToray Research Center, Inc., 3-7 Sonoyama 3-chome, Otsu, Shiga 520-8567, Japan
First published on 8th April 2022
Abstract
Multi-stimuli-responsive materials may dominate next-generation drug delivery systems. Herein, dual-thermo-responsive micelles were prepared by introducing cholesterol chloroformate to facilitate the spontaneous self-assembly of graft polymers prepared by combining two charged polymers, poly-sulfobetaine and carboxylated ε-poly-L-lysine. This polymerization was controlled by reversible addition fragmentation chain transfer polymerization. Turbidimetry measurements and temperature-dependent 1H NMR spectroscopy were used to investigate the phase transition behaviors; transmission electron microscopy and atomic force microscopy were used to determine the morphology of the micelles. The dependence of self-assembled structures on temperature was investigated through ultra-small-angle X-ray scattering (USAXS). The micelles formed spherical shapes in water which was confirmed by TEM and AFM. Interestingly, different, temperature-dependent micelle size change behaviors were observed through dynamic light scattering, Ultraviolet-visible (UV-Vis) spectroscopy, and USAXS; this might be due to the concentration-dependent hierarchical phase transition. This study provides crucial information on the mesoscopic structure of the micelles, and will enable greater control over their transition temperatures for numerous biomaterial applications.
Introduction
Micelles that can be employed as a water-insoluble drug delivery system and simultaneously enhance disease diagnostics have attracted considerable attention.1–3 Amphiphilic polymers can form micelles in aqueous solutions by self-assembly; the hydrophobic core of the micelles can load water-insoluble drugs,4,5 thereby enhancing the solubility of hydrophobic drugs, and the presence of a hydrophilic shell can increase the stability of the micelles in an aqueous environment and prolong the metabolism time.6,7 Most antitumor drugs, such as paclitaxel, camptothecin, and cabazitaxel are water-insoluble, which limits their applicability; this issue can be solved by the use of micelles to deliver such drugs.8–10 Additionally, employing micelles may also suppress the side effects of the drugs and increase the efficacy of the active pharmaceutical ingredient. Although some micelle formulations, such as Genexol PM, have been applied in clinical treatment,11 several challenges hinder the widespread use of micelles, with the main limitation being the absence of disease specificity.12To overcome these limitations, stimuli-responsive micelle systems that can change their physical or chemical properties with changes in the environment, such as pH, light, and temperature, are being investigated extensively.13–15 In particular, multi-stimuli systems are interesting, owing to the presence of additional properties that are capable of switching states under two different conditions, and have been employed in various applications,16–19 including delivering more than two drugs using a single system.20–22 Among them, temperature-responsive micelle systems have been preferred.23 The temperature of tumor tissues is reported to be slightly higher than that of the normal tissues, owing to faster metabolism.24–28 Moreover, tissues can withstand temperatures of up to 43 °C for long periods of time, without any irreversible consequences.29 Therefore, micelles of temperature-responsive polymers may have the ability of targeted release at the delivery site.30
Poly(N-isopropylacrylamide) (PNIPAAM) is one of the most studied thermo-responsive polymers, and in an aqueous solution, this polymer undergoes a coil–globule transition with an increase in temperature.31 Moreover, its transition temperature is around 32 °C, which is close to the human physiological temperature.32–36 Our group previously reported polyampholytes exhibiting a novel lower critical solution temperature (LCST) property, and these were synthesized by modifying the free amino groups in ε-poly-L-lysine (PLL) with succinic anhydride (PLLSA) for cryopreservation applications.37,38 The LCST of these polymers was found to depend significantly on their hydrophobicity, degree of substitution of the anhydride, and concentration of the polymer solution. Interestingly, unlike other LCST-type polymers, the LCST in PLLSA is accompanied by liquid–liquid phase separation and coacervate formation in the aqueous phase, differing from the conventional coil–globule transition.39
In contrast, polymers that tend to be insoluble at low temperatures and soluble at high temperatures are said to exhibit upper critical solution temperature (UCST) properties. Polymers such as poly(N-acryloyl glycinamide),40,41 poly(acrylamide-co-acrylonitrile) (P(AAm-co-AN))42 and poly(sulfobetaine) (PSPB)43 have been found to exhibit UCST behavior. Among the UCST-type polymers, PSPB polymers have attracted the most attention.44 Their protein-like structures provide high biocompatibility, and they exhibit several biomedical properties such as anti-biofouling,45,46 cryopreservation,47 and protein aggregation inhibition.48–52
A few thermo-responsive micelles have been reported, such as poly(ethylene glycol)-co-poly(acrylamide-co-acrylonitrile) (PEG-co-P(AAm-co-AN)),42 poly(sulfobetaine methacrylate)-block-poly(N,N-dimethylaminoethyl methacrylate) (poly(SBMA)-b-poly(DMEMA)),53 and poly(2-(N-morpholino)ethyl methacrylate)-b-poly(2-(diethylamino)ethyl methacrylate.54 Notably, despite the great potential of these micelles for use in biomedical applications, their corresponding studies did not transition into in vivo or clinical studies, thereby suggesting the need for further development of these types of systems. Moreover, insufficient data are available on the microstructures of these micelles. Compared with PNIPAM, more factors affect the LCST of PLLSA and these parameters may provide information on a more complex mesoscopic structure of the systems that contain PLLSA blocks in the micelles. Therefore, detailed structural information studies are necessary not only for the PLLSA homopolymer, but also for complex polymer systems containing PLLSA.
Micellization has often been triggered by the introduction of cholesterol,20 which is a highly hydrophobic polycyclic molecule, an essential component of mammalian cells, and is responsible for membrane fluidity and permeability, intracellular transport, signal transduction, and cell trafficking.55–57 As cholesterol is associated with many membrane-related bioprocesses, the incorporation of cholesterol not only promotes polymer self-assembly in aqueous media but also enables easier crossing of the cellular membrane by micelles,58,59 while enhancing the encapsulation of polycyclic drugs.60 Meanwhile, the degradability of cholesterol also suppresses the damage caused by the micelles.
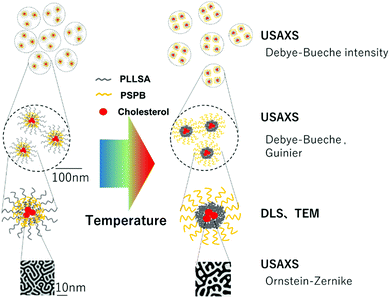
In our previous work, we successfully synthesized graft polymers by combining PLLSA and PSPB (PLLSA-g-PSPB).61 These polymers exhibit UCST-LCST type dual-thermo-responsive property, and we found that they exhibited a suppression of protein aggregation and loaded proteins electrostatically and subsequently released them upon changing the pH. However, their linear structure did not exhibit a strong bonding between the polymer chain and the therapeutic agent, such as drugs or proteins, because electrostatic interactions are not sufficiently strong and cannot be used under all conditions. Therefore, in the present study, we optimized PLLSA-g-PSPB linear systems by modifying them with cholesterol to obtain micellar structures formed by self-assembly in an aqueous solution. These micelles exhibited dual-thermo-responsive properties owing to the presence of two different thermo-responsive polyampholyte blocks and hence can be a strong candidate in the field of thermo-responsive drug delivery systems. Structural analysis and soft matter characterization have been widely studied using synchrotron radiation X-rays because of their light which is brighter than conventional laboratory X-ray sources.62 Ultra-small angle X-ray scattering (USAXS) has particularly been used to elucidate the structural information of the micelles in detail, and it revealed interesting contrasting phase separation behaviors between the two charged segments in the micelle. To the best of our knowledge, this is the first study to show the formation of dual thermo-responsive micelles made by the combination of two different polyampholytes.
Experimental
Materials
A 25% (w/w) PLL (molecular weight 4000) aqueous solution was purchased from JNC Corp. (Tokyo, Japan). The sulfobetaine monomer was donated by Osaka Organic Chemical Ind., Ltd. (Osaka, Japan) and used without further purification. Succinic anhydride (SA), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride, and N-hydroxysuccinimide (NHS) were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan). 2-(Dodecylthiocarbonothioylthio)-2-methylpropionic acid (reversible addition fragmentation chain transfer (RAFT) agent), 4,4′-azobis-(4-cyanovaleric acid) (V-501, initiator), pyrene, and cholesterol were purchased from Sigma-Aldrich (St. Louis, MO) and used as received.Carboxylated poly-L-lysine (PLLSA) and macro-chain transfer agent (macro-CTA; PLLSA-RAFT agent) were prepared according to a previously reported synthetic method.61
Synthesis of cholesterol chloroformate-modified Macro-CTA (PLLSA-cho-RAFT agent)
0.33 mmol PLLSA50 or PLLSA65 (numbers represent the degree of succinylation of amino groups of PLL by SA) was dissolved in 10 mL DMSO at 130 °C. After complete dissolution, 1 mL dichloromethane (DCM) containing 0.03 mmol cholesterol chloroformate (cho) solution was added to the DMSO solution. After 6 h, the solution was transferred to a dialysis membrane (MWCO 3.5 KDa, Repligen Corp. Waltham, MA, US) and dialyzed against water for 3 days. After dialysis, the polymer was washed with DCM, and the aqueous phase was dried in an oven, followed by vacuum drying to obtain the desired polymer.RAFT Polymerization of PLLSA-cho-PSPB
SPB monomer (6.6 mmol) and V-501 (6.6 μmol) were dissolved in water (45 mL). In another vial, macro-cho-CTA (PLLSA50-cho-RAFT and PLLSA65-cho-RAFT agents, respectively) (33 μmol) were dissolved in DMSO. Then, the DMSO solution was added to the water solution, and the mixture was purged with nitrogen gas for 1 h and subsequently polymerized at 90 °C for 24 h. The ratio of [SPB monomer]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [V-501]
[V-501]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [macro-cho-CTA] was 1000
[macro-cho-CTA] was 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The polymer was then purified by dialysis against water for 3 days using a dialysis membrane (MWCO 14 kDa). After dialysis, the polymer was obtained by lyophilization.
5. The polymer was then purified by dialysis against water for 3 days using a dialysis membrane (MWCO 14 kDa). After dialysis, the polymer was obtained by lyophilization.
Polymer characterization
All NMR spectra were recorded on a Bruker AV400 Avance III spectrometer operating at 400 MHz. The temperature-dependent 1H NMR experiment of M1 in D2O was performed in the temperature range of 15–75 °C in 10 °C intervals. The NMR data were analyzed using Topspin 3.5 software. The number of repeating units of the graft copolymers was estimated by 1H NMR spectroscopy, and the number of free NH2 groups was determined by a 2,4,6-trinitrobenzene sulfonic acid (TNBS) assay.63Determination of critical micelles concentration (CMC)
The CMC of the self-assembled micelles was estimated using pyrene as a fluorescent probe. Four microliters of pyrene (1.0 mM in acetone) were transferred to a 10 mL test tube, followed by complete drying under a stream of nitrogen gas. Micelle solutions of varying concentrations were added to each tube (4 mL). Then, the solutions were sonicated for 30 min, followed by incubation at 60 °C for 3 h. After 3 h, the samples were allowed to cool overnight. The fluorescence of the solubilized pyrene was measured using a JASCO FP-8500 spectrofluorometer at room temperature from 350 to 450 nm with an excitation wavelength of 339 nm. The ratio of the intensities of the peaks at 394 and 373 nm was plotted against the concentration of micelles to obtain the CMC.Particle size and zeta potential
The diameter and zeta potential of the micelles were determined using a Zetasizer 300 system (Malvern Instruments, Worcestershire, UK) equipped with a He–Ne laser with a wavelength of 633 nm and a scattering angle of 173°. Sample solutions were prepared at 0.1% (w/w) in distilled water.Atomic force Microscopy (AFM)
AFM images were obtained using an AFM5000II SPA-400 (HITACHI) with a Si probe (SI-DF20, Seiko). The sample solution (0.1% w/w) was dropped onto a freshly cleaved mica surface and air-dried overnight prior to measurement at room temperature in the dynamic force microscope (DFM) mode.Transmission electron microscopy (TEM)
Transmission electron micrographs (H-7650 Hitachi, Tokyo, Japan) were obtained to observe the morphology of the micelles. The samples were prepared by adding a drop of the polymer solution (0.1% w/v) on a copper grid (NS-C15 Cu150P; Stem, Tokyo, Japan). After drying at 10, 45, and 70 °C, the grid was negatively stained with 1% phosphotungstic acid (Sigma Aldrich, Steinheim, Germany) for 30 s, washed with a drop of distilled water, and air-dried.Turbidimetry
The cloud points of the micelles were determined using UV-visible spectroscopy (UV-Vis; UV-1800, Shimadzu Corp., Kyoto, Japan). Each transmittance value was obtained at different temperatures during heating runs in steps of 2 °C at a wavelength of 550 nm, and the equilibration time at each temperature was 12 min.Ultra-small-angle X-ray scattering
USAXS measurements were carried out at the BL08B2, SPring-8 (Harima, Japan) using an X-ray with a wavelength of λ = 0.10 nm and the distance from sample to detector of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mm.
000 mm.
The 2-dimensional USAXS data measured by Pilaus3-S-1M detector (172 μm square pixel size, DECTRIS) were converted into the one-dimensional intensity I(q) as a function of the scattering vector q (q = 4π sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/λ, where 2θ is the scattering angle, beam width: 0.2 × 0.3 mm2) by circular averaging. The covered range of q was 0.008–0.2 nm−1.
θ/λ, where 2θ is the scattering angle, beam width: 0.2 × 0.3 mm2) by circular averaging. The covered range of q was 0.008–0.2 nm−1.
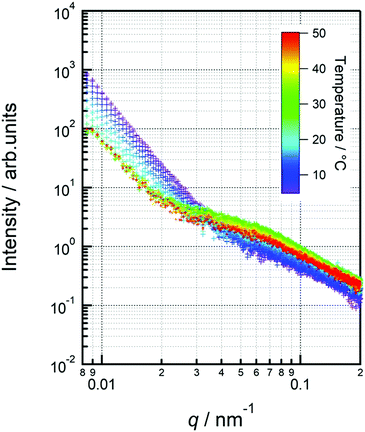
PLLSA50-cho-PSPB was dissolved in double distilled water to 5% w/w and 10 μL of the solution was placed in a silicone mold (ϕ = 2 nm, thickness = 1 mm) and covered with Kapton film and loaded onto the sample chamber. Samples were measured in the temperature range of 5–50 °C at a heating speed of 5 °C min−1 by attaching a temperature-controlling plate (Linkam 10002L Cooling Stage, Linkam Scientific Instruments, UK). The exposure time was 30 s for each temperature.
The SAXS data can be analysed by following structure model.
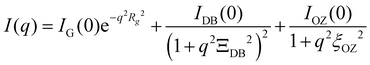 | (1) |
The first term represents the Guinier raw, which can estimate the radius of gyration (Rg) of micelles and the intensity of Guinier raw at q = 0 (IG(0)). The second and third terms account for the scattering originating from the inhomogeneous micelle assembly structure and the fluctuation intra-micelle, respectively. In the equation, ΞDB indicates the size of micelle assembly and ξOZ denotes the correlation length of thermal fluctuation.64,65
Cytotoxicity study
The cytotoxicity of the micelles was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium bromide (MTT) assay. L929 (American Type Culture Collection, Manassas, VA, USA) cells were cultured and treated with different concentrations of micelle solutions. First, 1 × 103 cells in 0.1 mL of culture medium were seeded on a 96-well plate and incubated for 72 h. Then, 0.1 mL of the culture medium with different concentrations of micelles was added to the 96-well plate. After re-incubation for 24 h, 0.1 mL MTT (3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide) solution (300 μg mL−1) was added to each well, and the cells were incubated for an additional 3 h. After removing the medium, (DMSO, 0.1 mL) was added to dissolve the purple formazan crystals formed by the living cells. Cells without micelle treatment were used as controls. The absorbance at 540 nm was measured using a microplate reader (versa max, Molecular Devices Co., CA, USA). Dulbecco's modified Eagle's medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% heat-inactivated fetal bovine serum was used as the culture medium.Results and discussion
Characteristics of copolymers
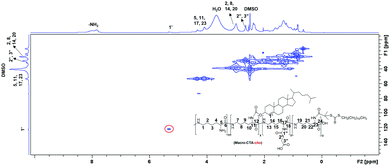
Dual-thermo-responsive polymers with cholesterol were synthesized by RAFT polymerization. As shown in Scheme 1, in the first step, carboxylated PLL was synthesized by adding different amounts of SA to ε-PLL.37 The degree of substitution was determined by 1H NMR spectroscopy (Fig. S1 and S2, ESI†) and was close to the feed ratio. (Table 1). In the next step, macro-CTA was synthesized by adding a controlled amount of RAFT agent to PLLSA. The incorporation of the RAFT agent was again evaluated using 1H NMR spectroscopy, and the results showed that nearly 1 RAFT agent was introduced per chain of PLLSA (Fig. S3 and S4, ESI†), which is in accordance with the desired synthetic strategy (Table 1). Subsequently, a cholesterol-modified chain transfer agent was synthesized on the PLLSA backbone by reacting the macro-CTA with cholesterol chloroformate. A TNBS assay was used to determine the amount of cholesterol incorporated by estimating the free amino group before and after the reaction (Table 1). Fig. 1 shows the 2D 1H–13C heteronuclear single quantum coherence (HSQC) spectrum of Macro-CTA-cho. The addition of cholesterol increased the hydrophobicity of the polymer; thus, this NMR spectrum was obtained in DMSO-d6. HSQC provides information on the correlation of two different nuclei separated by a single bond.66 From this spectrum, the small peak that appears at δH = 5.2 ppm can be correlated with δC = 125 ppm (indicated by red circle in Fig. 1), which clearly shows that this peak represents the cyclic alkene group in the cholesterol moiety. This result indicates that a cholesterol modified-macro-chain transfer agent was successfully synthesized.| Entry | Polymeric micelles | Degree of substitution of SA into PLL | Number of RAFT agents substituted per chainb | Number of cho substituted per chainc | Number of repeating units | M w × 10−3 | Zeta potential | ||
|---|---|---|---|---|---|---|---|---|---|
| In feed | In polymera | PLLSA | PSPB | ||||||
| a Determined using 1H NMR. b Determined by subtracting the number of NH2 groups in PLLSA from the RAFT agent substituted PLLSA. c Calculated using the TNBS assay. | |||||||||
| M1 | PLLSA50-cho-PSPB | 50% | 47% | 1.2 | 3.6 | 31.25 | 172.8 | 54.5 | 3.53 ± 0.142 |
| M2 | PLLSA65-cho-PSPB | 65% | 63% | 0.9 | 1.9 | 31.25 | 132.8 | 43.8 | −14.9 ± 0.872 |
The macro-CTA was then used to polymerize SPB to yield copolymers of PLLSA-cho-PSPB. The number of repeating units of SPB in the feed was fixed at 200 for each polymer. To obtain micelles of different charges, PLLSA65 and PLLSA50, with two different degrees of substitution of SA in PLL, were synthesized to impart negative and neutral charges to micelles, respectively. The structure of the polymers was characterized by 1H NMR and 13C NMR spectroscopy, and the results are shown in the Supplementary Information (Fig. S5–S8, ESI†). The characteristics of the micelles are presented in Table 1. M1 indicates the neutral charged micelle PLLSA50-cho-PSPB, and M2 indicates the negatively charged micelle PLLSA65-cho-PSPB.
Critical micelles concentration (CMC)
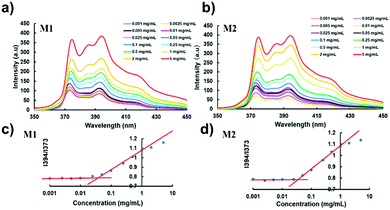
Micelle formation by the self-assembly of PLLSA-cho-PSPB was determined using pyrene as a fluorescent probe. Pyrene is highly hydrophobic and preferentially migrates into the core of the micelle in the aqueous solution.67 The fluorescence of pyrene is quenched below the CMC of micelles. The fluorescence spectra of pyrene at different concentrations of M1 or M2 are shown in Fig. 2. An increase in the emission intensity indicates the transformation of a linear or random polymer structure into a micelle-like structure. Fig. 2(a) and (b) clearly show that the fluorescence intensity increases as the polymer concentration increases, indicating the formation of micelles.68 The CMC was estimated from the intensity ratio of the peaks at 394 and 373 nm. The CMC of M1 and M2 was estimated to be approximately 6 μg mL−1 (Fig. 3(c) and (d)).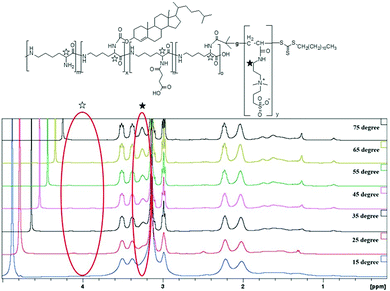 | ||
| Fig. 3 Temperature dependent 1H NMR spectra of M1 in D2O. Temperature increases from 15 °C (bottom curve) to 75 °C (top curve) in steps of 10 °C. | ||
Thermo-responsive property
| Micelles | Temperature/°C | Diameter/nm |
|---|---|---|
| M1 | 10 | 103.9 ± 9.62 |
| 45 | 81.4 ± 2.18 | |
| 70 | 80.7 ± 2.26 | |
| M2 | 10 | 129.1 ± 12.1 |
| 45 | 101.3 ± 1.68 | |
| 70 | 109.1 ± 4.91 |
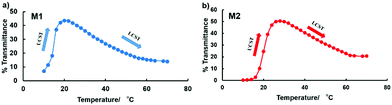
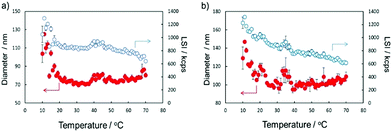
The temperature responsiveness of both micelles also showed a similar behavior. At 10 °C, their micelle diameter was the largest, and their size decreased with increase in temperature and showed a small local maximum at approximately 45 and 35 °C for M1 and M2, respectively, and subsequently increased slightly with increasing temperature. Interestingly, the light scattering intensity (LSI) of M2 showed a continuous decrease. This indicates that the density of the micelles decreased. Since M2 has a small degree of substitution of cholesterol and excess anionic groups, loss of the molecules from the micelles might occur at higher temperatures because of weak packing and higher repulsion. This trend agrees well with the turbidimetry results.
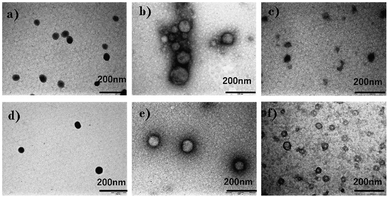
Fig. S9 (ESI†) shows AFM images of M1 and M2. The concentration of the samples was 0.1% (w/w) in water. From the images, it is apparent that the size of each micelle was not uniform. This may be due to van der Waals interactions between the micelle structures. Also, we can observe that the size of M1 is larger than that of M2. All TEM and AFM results correspond to the DLS results. The size of micelles obtained by DLS was larger than the sizes obtained by TEM and AFM owing to the extension of polymer chains in solution.
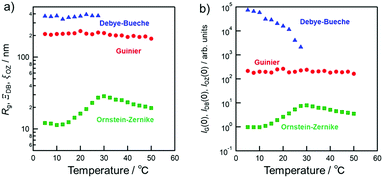
The separation size (Ξ) at the low q region calculated using the Debye–Bueche function was approximately 350 nm (Fig. 8(a)). This can be explained by the large aggregation of micelles73 which was diminished by the coil globule transition of the PSPB part at approximately 30 °C. In the high q region, the intensity of the q value increased with increasing temperature. This is due to the LCST-type phase separation of the PLL part. Then, we evaluated the Ornstein–Zernike approximation and calculated the correlation length (Fig. 8(a)). Although this value is smaller than Ξ, molecular-level fluctuations occurred in the LCST. In addition, the LCST was observed much earlier by USAXS analysis, as compared with UV-vis spectroscopy (Fig. 4); hence, USAXS analysis arguably detected the region of separation earlier. At the microscopic view, phase transition begins around 30 °C, and it might be correlated with the diminishing Ξ calculated using the Debye–Bueche function74 in Fig. 8(a). O’Reilly and co-workers employed SAXS technique to demonstrate that temperature responsive transition from micelle to unimer takes place, and they revealed the critical temperature of micelle deformation detected by SAXS was lower than that detected by DLS.75 Furthermore, the Guinier terms (Rg and IG(0)) were invariant with respect to temperature change, which indicates that the average size and amount of the micelle are invariant. An example of fitted curves using Guimier, Denye–Bueche, and Ornstein–Zernike functions at 5 °C is shown in Fig. S11 (ESI†).
Interestingly, the USAXS results suggest that the UCST and LCST phenomena occurred at entirely different scales in terms of size. For UCST phenomenon in PSPB, a large change in particle size was observed at around ΞDB = 350 nm. This is likely due to the coil–globule transition in the PSPB. This is supported by the observation of spherical structures in TEM and AFM images. The actual difference in size might be owing to the high concentration of polymer in the SAXS analysis. The decrease in the Debye–Bueche intensity (IDB(0)) during the SAXS analysis upon heating (due to UCST) indicates that the electron density contrast between particle and solution decreases because of the globule-to-coil transition. The disappearance of the intensity at 30 °C clearly indicates that some of the polymer chains could be dissolved in the solvent. This also agreed with the results of the light scattering intensity (LCI) decrease in DLS (Fig. 5(b)). In contrast, the LCST phenomenon in PLLSA was observed with a much smaller change in the size of the correlation length between the two phases with higher and lower densities. This might be due to the liquid–liquid phase transition of PLLSA.39 This was supported by the result that the value of this correlation length (ξOZ) was in the range of approximately 1–4 nm, which was similar to the values in the PLLSA molecules (Fig. S10(b), ESI†). From the results of UV-vis, TEM, AFM, DLS, and USAXS, it is possible that PLLSA-cho-PSPB formed micelles by self-assembly and the UCST type transition at lower temperature was caused by the PSPB chain coil–globule transition,76 and the LCTS-type transition was caused by the PLLSA part through liquid–liquid phase transition with different size scales (Fig. 9).
Cytotoxicity
The cytotoxicity of polymeric micelles towards L929 cells was evaluated by the MTT assay, as shown in Fig. 10. The cell viability slightly decreased with the increase in the concentration of polymeric micelles, while the cell viability was still around 90% even at 1% concentration of polymeric micelles. This agrees well with the previously obtained low toxicity of PLLSA.37,77 The negligible toxicity of the polymeric micelles shows that these micelles have great potential for use in delivering drugs or other cargoes into mammalian tissues.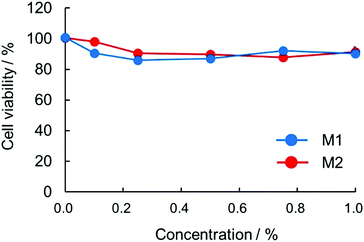 | ||
| Fig. 10 Cytotoxicity of the micelles. L929 cells were treated with different concentrations of polymers: M1 (blue filled circles) and M2 (red filled circles). | ||
Conclusions
Cholesterol-modified self-assembled micelles exhibiting UCST and LCST behavior in aqueous solutions were successfully synthesized. The spherical micelles were observed by TEM and AFM. Turbidimetric measurements showed that the structure of the micelles shrank and aggregated at low temperatures and high temperatures, respectively, and the chain of micelles extended and remained in the dissolved state at intermediate temperatures. Owing to the presence of PLLSA, the structure of micelles formed by self-assembly became complex. Both liquid–liquid phase separation and coil–globule transition were observed in our polymeric micelles. At low temperatures, the coil–globule transition of PSPB played an important role, whereas at high temperatures, liquid–liquid phase separation led to a decrease in transmittance. USAXS was employed to study the change in micelle structure with temperature. Interestingly, the phase transition temperature measured by USAXS was different from the temperature measured by UV-Vis spectroscopy. It was also found that the size scale of each phase transition of the UCST and LCST behaviors was completely different. This study provides detailed information on the structure of micelles containing the PLLSA segment. These micelles have potential applications in water-insoluble drug delivery systems and can be used for the protection of proteins owing to the presence of protein-stabilizing poly-SPB and PLLSA. Further studies are in progress to introduce degradability into such micelles, which will enable these micelles to successfully deliver therapeutic proteins inside the body.Author contributions
Z. D.: writing – original draft, investigation, visualization; R. R.: conceptualization, methodology, project administration, supervision, writing – review & editing; S. Y.: investigation, visualization, funding acquisition; M. N.: investigation, visualization; K. M.: conceptualization, funding acquisition, methodology, project administration, resources, supervision, validation, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by Grants-in-Aid for Scientific Research (21H05535 (S. Y.), 21H05516 (K. M.)) from the Japan Society for the Promotion of Science (JSPS). The synchrotron radiation experiments were performed at the BL08B2 of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2018B3337 and 2019A3337).References
- C. Lu, L. Jiang, W. Xu, F. Yu, W. Xia, M. Pan, W. Zhou, X. Pan, C. Wu and D. Liu, Colloids Surf., B, 2019, 182, 110384 CrossRef CAS PubMed.
- K. Jelonek, S. Li, X. Wu, J. Kasperczyk and A. Marcinkowski, Int. J. Pharm., 2015, 485, 357–364 CrossRef CAS PubMed.
- K. Rajagopal, A. Mahmud, D. A. Christian, J. D. Pajerowski, A. E. X. Brown, S. M. Loverde and D. E. Discher, Macromolecules, 2010, 43, 9736–9746 CrossRef CAS PubMed.
- R. Savić, A. Eisenberg and D. Maysinger, J. Drug Targeting, 2006, 14, 343–355 CrossRef PubMed.
- H. Lee, F. Zeng, M. Dunne and C. Allen, Biomacromolecules, 2005, 6, 3119–3128 CrossRef CAS.
- D. E. Owens and N. A. Peppas, Int. J. Pharm., 2006, 307, 93–102 CrossRef CAS PubMed.
- P. J. Photos, L. Bacakova, B. Discher, F. S. Bates and D. E. Discher, J. Controlled Release, 2003, 90, 323–334 CrossRef CAS.
- T. Y. Kim, D. W. Kim, J. Y. Chung, S. G. Shin, S. C. Kim, D. S. Heo, N. K. Kim and Y. J. Bang, Clin. Cancer Res., 2004, 10, 3708–3716 CrossRef CAS PubMed.
- A. L. Z. Lee, S. Venkataraman, S. B. M. Sirat, S. Gao, J. L. Hedrick and Y. Y. Yang, Biomaterials, 2012, 33, 1921–1928 CrossRef CAS PubMed.
- P. Laskar, S. Samanta, S. K. Ghosh and J. Dey, J. Colloid Interface Sci., 2014, 430, 305–314 CrossRef CAS PubMed.
- S. C. Kim, D. W. Kim, Y. H. Shim, J. S. Bang, H. S. Oh, S. W. Kim and M. H. Seo, J. Controlled Release, 2001, 72, 191–202 CrossRef CAS PubMed.
- W. R. Sanhai, J. H. Sakamoto, R. Canady and M. Ferrari, Nat. Nanotechnol., 2008, 3, 242–244 CrossRef CAS PubMed.
- S. Mura, J. Nicolas and P. Couvreur, Nat. Mater., 2013, 12, 991 CrossRef CAS PubMed.
- C. J. F. Rijcken, O. Soga, W. E. Hennink and C. F. v. Nostrum, J. Controlled Release, 2007, 120, 131–148 CrossRef CAS PubMed.
- D. Schmaljohann, Adv. Drug Delivery Rev., 2006, 58, 1655–1670 CrossRef CAS PubMed.
- P. Schattling, F. D. Jochum and P. Theato, Polym. Chem., 2014, 5, 25–36 RSC.
- M. Joglekar and B. G. Trewyn, Biotechnol. J., 2013, 8, 931–945 CrossRef CAS PubMed.
- Y. Kotsuchibashi, Polym. J., 2020, 52, 681–689 CrossRef CAS.
- J. Zhao, V. E. Lee, R. Liu and R. D. Priestley, Annu. Rev. Chem. Biomol. Eng., 2019, 10, 361–382 CrossRef CAS PubMed.
- G. Ma, W. Lin, Z. Yuan, J. Wu, H. Qian, L. Xu and S. Chen, J. Mater. Chem. B, 2017, 5, 935–943 RSC.
- M. Patel, T. Kaneko and K. Matsumura, J. Mater. Chem. B, 2017, 5, 3488–3497 RSC.
- M. Patel, T. Nakaji-Hirabayashi and K. Matsumura, J. Biomed. Mater. Res., Part A, 2019, 107, 1094–1106 CrossRef CAS PubMed.
- M. Karimi, P. Sahandi Zangabad, A. Ghasemi, M. Amiri, M. Bahrami, H. Malekzad, H. Ghahramanzadeh Asl, Z. Mahdieh, M. Bozorgomid, A. Ghasemi, M. Reza Rahmani Taji Boyuk and M. R. Hamblin, ACS Appl. Mater. Interfaces, 2016, 8, 21107–21133 CrossRef CAS PubMed.
- S. Gammas, K. Suzuki, C. Sone, Y. Sakurai, K. Kataoka and T. Okano, J. Controlled Release, 1997, 48, 157–164 CrossRef.
- I. S. Kim, Y. Il Jeong, C. S. Cho and S. H. Kim, Int. J. Pharm., 2000, 205, 165–172 CrossRef CAS PubMed.
- A. Chilkoti, M. R. Dreher, D. E. Meyer and D. Raucher, Adv. Drug Delivery Rev., 2002, 54, 613–630 CrossRef CAS.
- C. De Las Heras Alarcón, S. Pennadam and C. Alexander, Chem. Soc. Rev., 2005, 34, 276–285 RSC.
- S. Ganta, H. Devalapally, A. Shahiwala and M. Amiji, J. Controlled Release, 2008, 126, 187–204 CrossRef CAS PubMed.
- A. Bordat, T. Boissenot, J. Nicolas and N. Tsapis, Adv. Drug Delivery Rev., 2019, 138, 167–192 CrossRef CAS PubMed.
- Y. Hu, V. Darcos, S. Monge and S. Li, Int. J. Pharm., 2015, 491, 152–161 CrossRef CAS PubMed.
- M. Heskins and J. E. Guillet, J. Macromol. Sci., Chem., 1968, 2, 1441–1455 CAS.
- M. Sahn, T. Yildirim, M. Dirauf, C. Weber, P. Sungur, S. Hoeppener and U. S. Schubert, Macromolecules, 2016, 49, 7257–7267 CrossRef CAS.
- R. Hoogenboom, Angew. Chem., Int. Ed., 2009, 48, 7978–7994 CrossRef CAS PubMed.
- S. Nayak, H. Lee, J. Chmielewski and L. A. Lyon, J. Am. Chem. Soc., 2004, 126, 10258–10259 CrossRef CAS PubMed.
- G. Pasparakis, A. Cockayne and C. Alexander, J. Am. Chem. Soc., 2007, 129, 11014–11015 CrossRef CAS PubMed.
- T. Wolf, T. Rheinberger, J. Simon and F. R. Wurm, J. Am. Chem. Soc., 2017, 139, 11064–11072 CrossRef CAS PubMed.
- K. Matsumura and S.-H. Hyon, Biomaterials, 2009, 30, 4842–4849 CrossRef CAS PubMed.
- K. Matsumura, F. Hayashi, T. Nagashima, R. Rajan and S.-H. Hyon, Commun. Mater., 2021, 2, 15 CrossRef CAS.
- E. Das and K. Matsumura, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 876–884 CrossRef CAS.
- K. Zhang, M. Chen, K. J. Drummey, S. J. Talley, L. J. Anderson, R. B. Moore and T. E. Long, Polym. Chem., 2016, 7, 6671–6681 RSC.
- F. Käfer, A. Lerch and S. Agarwal, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 274–279 CrossRef.
- F. Käfer, F. Liu, U. Stahlschmidt, V. Jérôme, R. Freitag, M. Karg and S. Agarwal, Langmuir, 2015, 31, 8940–8946 CrossRef PubMed.
- P. Mary, D. D. Bendejacq, M. P. Labeau and P. Dupuis, J. Phys. Chem. B, 2007, 111, 7767–7777 CrossRef CAS PubMed.
- N. Morimoto, Y. Oishi and M. Yamamoto, Macromol. Chem. Phys., 2020, 221, 1900429 CrossRef CAS.
- H. Kitano, T. Mori, Y. Takeuchi, S. Tada, M. Gemmei-Ide, Y. Yokoyama and M. Tanaka, Macromol. Biosci., 2005, 5, 314–321 CrossRef CAS PubMed.
- H. Kitano, T. Kondo, T. Kamada, S. Iwanaga, M. Nakamura and K. Ohno, Colloids Surf., B, 2011, 88, 455–462 CrossRef CAS PubMed.
- R. Rajan, F. Hayashi, T. Nagashima and K. Matsumura, Biomacromolecules, 2016, 17, 1882–1893 CrossRef CAS PubMed.
- R. Rajan, S. Ahmed, N. Sharma, N. Kumar, A. Debas and K. Matsumura, Mater. Adv., 2021, 2, 1139–1176 RSC.
- K. Matsumura, R. Rajan, S. Ahmed and M. Jain, in Biopolymers for Medical Applications, CRC Press, Boca Raton, FL, 2016, pp. 165–182 Search PubMed.
- R. Rajan, Y. Suzuki and K. Matsumura, Macromol. Biosci., 2018, 18, 1800016 CrossRef PubMed.
- R. Rajan and K. Matsumura, Sci. Rep., 2017, 7, 45777 CrossRef CAS PubMed.
- R. Rajan and K. Matsumura, J. Mater. Chem. B, 2015, 3, 5683–5689 RSC.
- Z. Dong, J. Mao, D. Wang, M. Yang, W. Wang, S. Bo and X. Ji, Macromol. Chem. Phys., 2014, 215, 111–120 CrossRef CAS.
- V. Butun, N. C. Billingham and S. P. Armes, J. Am. Chem. Soc., 1998, 120, 11818–11819 CrossRef.
- P. L. Yeagle, Biochimie, 1991, 73, 1303–1310 CrossRef CAS PubMed.
- Frederick R. Maxfield and Ira Tabas, Nature, 2005, 438, 612–621 CrossRef CAS PubMed.
- P. L. Yeagle, Biochim. Biophys. Acta, Biomembr., 1985, 822, 267–287 CrossRef CAS.
- L. Hosta-Rigau, Y. Zhang, B. M. Teo, A. Postma and B. Städler, Nanoscale, 2013, 5, 89–109 RSC.
- Y. Wang, H. Wang, G. Liu, X. Liu, Q. Jin and J. Ji, Macromol. Biosci., 2013, 13, 1084–1091 CrossRef CAS PubMed.
- S. Zheng, Y. Xie, Y. Li, L. Li, N. Tian, W. Zhu, G. Yan, C. Wu and H. Hu, Int. J. Nanomed., 2013, 9, 55–66 CrossRef PubMed.
- D. Zhao, R. Rajan and K. Matsumura, ACS Appl. Mater. Interfaces, 2019, 11, 39459–39469 CrossRef CAS PubMed.
- A. Takahara, Y. Higaki, T. Hirai and R. Ishige, Polymers, 2020, 12(7), 1624 CrossRef CAS PubMed.
- A. F. S. A. Habeeb, Anal. Biochem., 1966, 14, 328–336 CrossRef CAS PubMed.
- P. Debye, A. M. Bueche, P. Debye and A. M. Bueche, J. Appl. Phys., 1949, 20, 518–525 CrossRef CAS.
- E. Di Cola, C. Lefebvre, A. Deffieux, T. Narayanan and R. Borsali, Soft Matter, 2009, 5, 1081–1090 RSC.
- M. Elyashberg, TrAC, Trends Anal. Chem., 2015, 69, 88–97 CrossRef CAS.
- F. Zhou, H. Xu, Z. Song, L. Zhu, S. Feng and R. Feng, New J. Chem., 2019, 43, 444–453 RSC.
- S. Ahmed, F. Hayashi, T. Nagashima and K. Matsumura, Biomaterials, 2014, 35, 6508–6518 CrossRef CAS PubMed.
- N. S. Vishnevetskaya, V. Hildebrand, B. Niebuur, I. Grillo, S. K. Filippov, A. Laschewsky, P. Mu and C. M. Papadakis, Macromolecules, 2017, 50, 3985–3999 CrossRef CAS.
- Y. Maeda, H. Mochiduki and I. Ikeda, Macromol. Rapid Commun., 2004, 25, 1330–1334 CrossRef CAS.
- F. R. Cheng, Y. J. Yang, Y. Liang, J. Q. Yan, J. Cao, T. Su, L. Jiang, B. He, X. L. Luo and Z. W. Gu, RSC Adv., 2014, 4, 62708–62716 RSC.
- M. A. Subhan, S. S. K. Yalamarty, N. Filipczak, F. Parveen and V. P. Torchilin, J. Pers. Med., 2021, 11(6), 571 CrossRef PubMed.
- S. C. Liao, C. S. Lai, D. Di Yeh, M. Habibur Rahman, C. S. Hsu, H. L. Chen and S. A. Chen, React. Funct. Polym., 2009, 69, 498–506 CrossRef CAS.
- M. Amann, J. S. Diget, J. Lyngsø, J. S. Pedersen, T. Narayanan and R. Lund, Macromolecules, 2019, 52, 8227–8237 CrossRef CAS.
- K. E. B. Doncom, A. Pitto-Barry, H. Willcock, A. Lu, B. E. McKenzie, N. Kirby and R. K. O’Reilly, Soft Matter, 2015, 11, 3666–3676 RSC.
- Y. Higaki, M. Kobayashi and A. Takahara, Langmuir, 2020, 36, 9015–9024 CrossRef CAS PubMed.
- K. Matsumura, S. Hatakeyama, T. Naka, H. Ueda, R. Rajan, D. Tanaka and S.-H. Hyon, Biomacromolecules, 2020, 21, 3017–3025 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d1ma01189h |
| This journal is © The Royal Society of Chemistry 2022 |