Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mixed dimensionality of 2D/3D heterojunctions for improving charge transport and long-term stability in high-efficiency 1.63 eV bandgap perovskite solar cells†
Jinkun
Jiang
,
Congcong
Tian
,
Zhiang
Zhang
,
Xiao (Xiao)
Liu
,
Xin
Wang
,
Yiting
Zheng
,
Zhanfei
Zhang
,
Luyao
Wang
,
Xueyun
Wu
,
Jianghu
Liang
and
Chun-Chao
Chen
 *
*
School of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, P. R. China. E-mail: c3chen@sjtu.edu.cn
First published on 13th June 2022
Abstract
Developing 2D/3D heterojunctions on 3D perovskite surfaces is an attractive strategy to improve the photovoltaic efficiency and stability of perovskite solar cells (PSCs). Particularly, the mixed dimensionality (MD) of 2D phases is found to be beneficial to both interfacial charge transport and device stability. Nevertheless, the control of dimensionality in aromatic spacers for 2D perovskites is very difficult. In addition to that, the use of 2D/3D heterojunctions on wider (∼1.63 eV) bandgap perovskite solar cells is also uncommon. In this work, halogen para-substituted aromatic ligands based on R-α-methylbenzylammonium (R-α-MBA) were employed to form 2D capping layers on top of the wide-bandgap perovskite surface. The MD of the aromatic 2D capping layers (n = 1 and n = 2) was achieved through para-substitution with bromine (R-α-BrMBA), which increases the formation energy of the n = 1 phase. Thus, the interfacial charge transport was improved with the MD heterojunctions that had Type I band alignment, resulting in reduced energy loss. Furthermore, the introduction of aromatic ligands also flattens the surface morphologies and reduces the trap density. As a result, a champion power conversion efficiency (PCE) of 21.48% was delivered with an ultrahigh FF of 82.44% for 1.63 eV bandgap PSCs. Besides, long-term storage and thermal stabilities of the unencapsulated PSCs modified with R-α-BrMBA are substantially enhanced owing to the increased surface hydrophobicity and thermally stable MD passivation layers.
1. Introduction
Perovskite materials have become one of the most vital candidates in the photovoltaic field due to their excellent photoelectric properties, such as low exciton binding energy, long carrier lifetime and diffusion length, tunable band gap and high defect tolerance.1–3 The past decade has witnessed a leap in the PCEs of organic–inorganic hybrid perovskite solar cells (OIHPSCs): from 3.8% first published in 2009 to 25.7% certified recently, surpassing the widely marketed polycrystalline silicon solar cells.4 However, the PCE of single-junction PSCs still possesses tremendous space for improvement compared with the Shockley–Queisser (SQ) limit of 33.7%. Tandem solar cells based on wide-bandgap perovskites (bandgap >1.63 eV) are expected to be able to overcome the SQ limit and achieve higher PCEs in the future.5–9 Thus, it is important to continuously improve the performance of wider bandgap (∼1.63 eV) perovskite solar cells.At present, the interface problem is the main factor affecting the PCE and stability of PSCs, including wide-bandgap PSCs, prepared by a low-temperature solution method.10–14 Numerous defects such as dangling bonds, ion vacancies and grain boundaries (GBs) exist on the surface of the active layers due to the intrinsic ionic characteristics and polycrystalline properties of perovskites prepared by a solution method. These defect states eventually become the charge carrier capture centers, ion migration channels and the infiltration locations of water/oxygen, which can compromise the performance and stability of the devices. In order to optimize the device interfaces, surface modification strategies are commonly utilized to solve the interfacial problem.15–18 Among them, modifying the surface of three-dimensional (3D) perovskite absorbers with two-dimensional (2D) Ruddlesden–Popper layered perovskites to form a 2D/3D heterojunction has attracted extensive attention in recent years. 2D perovskites possess higher environmental stability than their 3D counterparts due to the introduction of hydrophobic organic spacer cations in their crystal structure.19 Besides, 2D perovskite capping layers do not only passivate the surface defects of 3D perovskite films, but also optimize the energy level arrangement at the perovskite/charge transport layer interface, which is conducive to improving the photovoltaic performance of PSCs.20 Furthermore, a 2D perovskite with larger n is preferential to grow vertically, which is beneficial to charge transfer.21 Thus, the dimensional regulation of 2D perovskite capping layers via their n-values is necessary to balance the PCE and stability.22–25 He et al. introduced isopentylammonium iodide (PNAI) to develop multiple n-value 2D capping layers by tuning the post-treatment temperature, which optimized the charge transfer performance and decreased the interfacial voltage loss.26 Yang et al. employed n-butylammonium bromide (BABr) to prepare stable and low-photovoltage-loss PSCs by covering bulk perovskites with mixed-dimensional 2D perovskite layers.27 Azmi et al. tailored the dimensionality of the interfacial 2D-perovskite fragments with n = 1 and n = 2 phases by applying oleylammonium-iodide (OLAI), which enables efficient top-contact passivation through 2D-perovskite passivation layers.28 However, it is evident that the dimensional regulation is more accessible for the 2D capping layers formed with alkyl ammonium spacers.
It was reported that the most widely used aromatic spacer ligand phenethylammonium (PEA) is not as functional as it should be on wide-bandgap PSCs and is more effective when it doesn’t form 2D passivation layers.13,15,29 Because of this, the control of dimensionality in 2D capping layers formed with aromatic ammonium spacers remains a difficult task. R-α-MBA is mainly adopted to prepare chiral 2D perovskites with unique spin-dependent properties, which have potential for use in advanced optical and spintronic devices.30–33 For example, Kim et al. demonstrated a spin-polarized light-emitting diode (spin-LED) via chiral 2D perovskite (R-α-MBA)2PbI4.34 Besides, Xu et al. introduced prepared (R-α-ClMBA)2PbI4, in which R-α-ClMBA is the chlorine-substituted analogue of R-α-MBA, into the 3D bulk perovskite recently.35 They found that the 2D perovskite additive promoted more effective charge separation and achieved 21.78% PCE compared with 18.28% PCE of the control. In fact, the great potential of the aromatic spacers for 2D perovskites capped on 3D perovskites is waiting to be explored, particularly for wide-bandgap PSCs.
Herein, we developed 2D/3D heterojunctions on top of 3D wide-bandgap perovskites via halogen para-substituted aromatic cations based on R-α-MBA. Since the properties of aromatic spacers can be tuned by replacing hydrogen atoms on the benzene ring with functional groups to influence the crystal structures and electronic properties of 2D perovskites, the MD of 2D capping layers (n = 1 and n = 2) prepared from aromatic spacers was successfully achieved through para-substitution with bromine (Br) owing to the higher formation energy of the n = 1 phase for 2D perovskites. The aromatic 2D capping layer further displayed the capability to smooth the perovskite surface with less defect density compared to that of the control film. Correspondingly, the MD heterojunctions with Type I band alignment improve the interfacial charge transport and reduce the energy loss. As a result, we realized a champion PCE of 21.48% with an ultrahigh fill factor (FF) of 82.44%, which is among the best results for 1.63 eV bandgap perovskite solar cells. In addition, shielding of water/oxygen with thermally stable MD layers was also demonstrated, which is beneficial to long-term storage and thermal stabilities.
2. Results and discussion
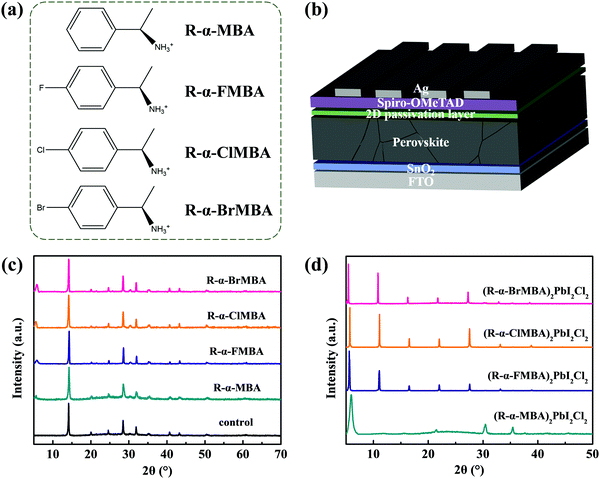
In this work, aromatic cation R-α-methylbenzylammonium (R-α-MBA) and its halogen-substituted analogues of chloride salts (R-α-methyl-4-fluorobenzylammonium (R-α-FMBA), R-α-methyl-4-chlorobenzylammonium (R-α-ClMBA) and R-α-methyl-4-bromobenzylammonium (R-α-BrMBA)) (Fig. 1a) were adopted to form 2D perovskite layers on the surface of 3D films. The 2D/3D device structure is shown as Fig. 1b, in which the 3D wide-bandgap perovskite system is Rb0.05Cs0.05(FAPbI3)0.83(MAPbBr3)0.17 (bandgap = 1.63 eV) prepared from a one-step anti-solvent method, following our previous reports.36,37In order to investigate the crystal structure of different 2D capping layers on 3D perovskites, X-ray diffraction (XRD) was carried out. As shown in Fig. 1c, all perovskite films present characteristic peaks of 3D perovskite at 2θ = 14.2°, 28.5° and 31.9°, corresponding to the (110), (220) and (310) crystal planes of perovskite crystals, respectively.38 In addition, new characteristic peaks appear at around 2θ = 5.7° for the perovskite films after surface modification, indicating the growth of 2D perovskites layers. To further understand the crystal structure of different 2D perovskites, the XRD patterns of (R-α-MBA)2PbI2Cl2, (R-α-MBA)2PbI2Cl2, (R-α-MBA)2PbI2Cl2 and (R-α-MBA)2PbI2Cl2 (2D films prepared by PbI2 and the chloride salts) were demonstrated in Fig. 1d. Apparently, the 2D perovskites incorporated with halogen-substituted ligands present evenly spaced peaks from 2θ = 5° to 2θ = 40°, which are ascribed to the crystallographic planes of (002), (004), (006), (008) and (0010). However, the diffraction peaks of the 2D perovskite incorporated with R-α-MBA (no halogen-substitution) is not spaced evenly. The more regular positions of the XRD peaks for 2D perovskites incorporated with halogen-substituted ligands imply that halogen atoms on the spacer ligands could improve the crystal orientation of the 2D perovskite phases due to hydrogen bonds between the halogen atoms and the hydrogen in adjacent benzene rings.39 Besides, the peak positions and intensities of the XRD spectra for 2D perovskites incorporated with halogen-substituted ligands are slightly varied, which could be derived from the different distortions within the 2D perovskites induced by different functional halogen atoms and thus play a variational impact on film surface states.40
A scanning electron microscope (SEM) and atomic force microscope (AFM) were utilized to meticulously reveal the changes in the perovskite films surface with different modification. Fig. S1 (ESI†) manifests that the films modified with R-α-MBA and R-α-FMBA present uneven morphologies. After modified with R-α-ClMBA and R-α-BrMBA, the surface morphology turned to be more uniform. This phenomenon could be ascribed to the different activation energies required for the interfacial reactions between halogen-substituted ligands and PbI2.41,42 Moreover, the root-mean-square (RMS) roughness decreases from 24.7 nm for the control film to 23.6 nm, 18.9 nm, 18.6 nm and 17.1 nm for the perovskite films modified with R-α-MBA, R-α-FMBA, R-α-ClMBA, and R-α-BrMBA, respectively. In other words, the surface of the perovskite/R-α-BrMBA film is the smoothest among all samples (Fig. S2, ESI†). It was reported that the transformation of the 3D perovskite to layered perovskite is progressive, and a slow transformation rate is much better for the quality and thickness control of 2D layers.43 Therefore, the variation in the film roughness is possibly related to the different dimensional reduction process. The improved flatness of the perovskite film surface can benefit the close contact between the absorber and Spiro-OMeTAD, which can facilitate carrier transfer and inhibit non-radiative recombination at the perovskite/hole transport layer (HTL) interface.44
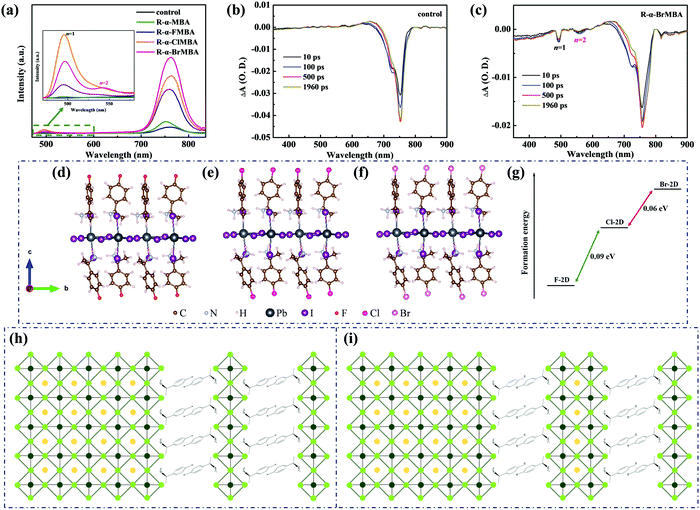
To further assess the effect of different treatments on perovskite films, steady-state photoluminescence (PL) and time-resolved photoluminescence (TRPL) measurements were implemented. As shown in Fig. 2a, PL peaks at 760 nm ascribed to bulk perovskites for 2D/3D films is significantly higher than that of the control film, indicating effectively reduced surface defects.45 In particular, the strongest PL intensity for the perovskite/R-α-BrMBA film demonstrates the optimal passivation effect of the Br-2D perovskite. Besides, the PL peaks at 495 nm for the modified films illustrate the formation of 2D perovskites (n = 1).46,47 Interestingly, the PL spectrum for the perovskite/R-α-BrMBA film presents another peak at 545 nm as shown in the inset of Fig. 2a, suggesting that the mixed dimensionality of n = 1 and n = 2 phases are formed for the Br-2D perovskite capping layer.48 The optimized dimensional structure is beneficial for charge transport and transfer.19 The TRPL decay curves for 2D/3D films are pictured in Fig. S3, (ESI†) and are fitted by a bi-exponential decay function: f(t) = A1exp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ((−t)/τ1) + A2exp
((−t)/τ1) + A2exp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ((−t)/τ2), where A1 and A2 are the corresponding decay amplitudes, τ1 is the fast decay lifetime and related to the bimolecular recombination of photo-generated carriers, τ2 is the slow decay lifetime and related to radiation recombination.49,50 The corresponding fitted lifetime parameters are summarized in Table S1, (ESI†) among which τavg is the average carrier lifetime: τavg = ((A1τ21+A2τ22))/((A1τ1 + A2τ2)).51 The τavg is 221.90 ns, 305.38 ns, 315.03 ns, 470.41 ns and 541.33 ns for the control, R-α-MBA-, R-α-FMBA-, R-α-ClMBA-, and R-α-BrMBA-modified films, respectively, indicating the prolonged charge carrier lifetime due to surface modification with covered 2D perovskites. Furthermore, the longest τavg for the R-α-BrMBA-modified film confirms the effective suppression of the surface trap states and inhibition of the non-radiative recombination, which is consistent with the above PL results.
((−t)/τ2), where A1 and A2 are the corresponding decay amplitudes, τ1 is the fast decay lifetime and related to the bimolecular recombination of photo-generated carriers, τ2 is the slow decay lifetime and related to radiation recombination.49,50 The corresponding fitted lifetime parameters are summarized in Table S1, (ESI†) among which τavg is the average carrier lifetime: τavg = ((A1τ21+A2τ22))/((A1τ1 + A2τ2)).51 The τavg is 221.90 ns, 305.38 ns, 315.03 ns, 470.41 ns and 541.33 ns for the control, R-α-MBA-, R-α-FMBA-, R-α-ClMBA-, and R-α-BrMBA-modified films, respectively, indicating the prolonged charge carrier lifetime due to surface modification with covered 2D perovskites. Furthermore, the longest τavg for the R-α-BrMBA-modified film confirms the effective suppression of the surface trap states and inhibition of the non-radiative recombination, which is consistent with the above PL results.
Furthermore, transient absorption (TA) spectra were measured to study the perovskite films with and without modification. It is found that only the perovskite film modified with R-α-BrMBA exhibits another two bleaching signals at 495 nm and 545 nm compared with the other films, which is assigned to the n = 1 and n = 2 phases of the 2D perovskite, respectively (Fig. 2b, c and Fig. S4, ESI†).52 Furthermore, it was reported that the bulky molecules penetrate and slice the 3D perovskite lattice to develop 2D perovskite onto the surface via forming discrete multilayer Pb–I slabs and then thinning down the Pb–I slabs.21,43,53 Therefore, the growth tendency of the 2D phases is closely related to their formation energy.28 Based on this, first principles density functional theory (DFT) calculations were implemented to calculate the formation energy of F-2D, Cl-2D and Br-2D perovskites (n = 1) to disclose the mechanism of the dimensional change under different modification (Fig. 2d–f). We found that the formation energy of the Br-2D perovskite (n = 1) is higher than that of F-2D and Cl-2D perovskites (n = 1), suggesting that R-α-BrMBA is more prone to form higher-n phases (Fig. 2g).43,54 Fig. S5 (ESI†) presents the calculated electrostatic surface potential (ESP) of R-α-FMBA, R-α-ClMBA and R-α-BrMBA molecules. The positive potential of the ammoniums gradually increases with the intensified electronegativity of bromine (Br), chlorine (Cl) and fluorine (F) atoms. Therefore, the activation energy required for reaction between R-α-BrMBA and perovskite is higher than that required for R-α-FMBA.55,56 This means that the formation rate of the 2D layered perovskite with R-α-BrMBA is slower, which leads to uniformly covered film morphology. As a result, the regulated dimensionality is achieved by substituting different halogen atoms in our case.
Structural diagrams of 2D perovskites formed via the reaction between the 3D perovskite and R-α-XMBA (X = −F, −Cl, −Br) are portrayed according to the above analyses. The 2D perovskites formed with R-α-XMBA (X = −F, −Cl) modification only possess the phase of n = 1 as shown in Fig. 2h. However, the 2D perovskites formed with R-α-BrMBA modification possess mixed phases of n = 1 and n = 2 as shown in Fig. 2i. The higher n-value 2D phases are more conducive to the charge transport process at the perovskite/HTL interface, which boosts the efficiency of the PSCs.
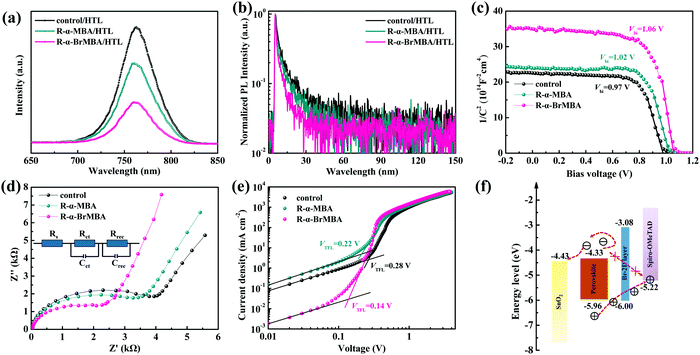
In order to elucidate the effect of MD phases on the charge dynamics at the perovskite/HTL interfaces, PL and TRPL spectra were measured on the perovskite films covered with Spiro-OMeTAD. Fig. 3a and Fig. S6a (ESI†) show that the PL quenching for the R-α-BrMBA-modified film is more obvious than that of the control, R-α-MBA-, R-α-FMBA- and R-α-ClMBA-modified film, indicating that the Br-2D perovskite promotes the charge transport and accelerates the carrier quenching. The TRPL decay spectra are shown in Fig. 3b and Fig. S6b (ESI†). The corresponding fitted parameters are listed in Table S2 (ESI†). The faster quenching lifetime of the R-α-BrMBA-modified film compared with the other films suggests improved hole transfer from the perovskite to the Spiro-OMeTAD, which is consistent with the PL results.
We then investigated Mott–Schottky (M–S) analyses of the perovskite films with different modification. As shown in Fig. 3c and Fig. S7, (ESI†) the built-in voltage (Vbi) increases from 0.97 V of the control perovskite film to 1.06 V of the R-α-BrMBA-modified perovskite device. The enhanced Vbi may well extract hole carriers and repel electron carriers, reducing charge transfer loss and preventing surface recombination.49 Therefore, the R-α-BrMBA-modified perovskite device with MD phases presents a more effective charge transfer, which is consistent with the PL and TRPL results.
Electrochemical impedance spectroscopy (EIS) was taken to further explore the charge transfer and recombination behavior for the devices. Nyquist plots of devices with different modification are shown in Fig. 3d and Fig. S8, (ESI†) and the inset in Fig. 3d displays the relevant equivalent circuit model. The left and right semicircles are corresponding to the charge transfer resistance (Rct) and the recombination resistance (Rrec), respectively. It is can be seen that the Rct decreases and the Rrec increases significantly after the devices are capped with 2D passivation layers (Table S3, ESI†). Moreover, the variation of the resistances for the R-α-BrMBA-modified PSC is more obvious than that of the other devices, indicating that MD phases can efficiently enhance the charge transfer and suppress the charge recombination at the interface.50
In addition, we prepared electron-only devices (FTO/SnO2/3D perovskite/2D passivation layer/PCBM/Ag, as depicted in Fig. S9, ESI†) to measure the space charge limited current (SCLC) curves under dark conditions, as shown in Fig. 3e and Fig. S10 (ESI†). The charge mobility (μ) of the devices can be extracted from the SCLC curves following the equation:μ = (8L3J)/(9ε0εrV2), where L, J, ε0, εr, and V are the bulk perovskite thickness, current density, vacuum dielectric constant, relative dielectric constant of perovskite, and applied voltage, respectively.57 The calculated μ increases from 1.38 × 10−2 cm2 V−1 s−1 (control) to 1.97 × 10−2 cm2 V−1 s−1 (R-α-BrMBA), as listed in Table S4 (ESI†). The highest μ for the R-α-BrMBA-modified device demonstrates that the interfacial MD phases well facilitate the charge transport at the perovskite/HTL interface, which is consistent with the above analyses. Furthermore, we quantitatively estimate the trap density of the devices via the equation: Nt = (2ε0εrVTFL)/(eL2), where VTFL is the trap-filled limit voltage.58 As shown in Table S4, (ESI†) the optimal device based on R-α-BrMBA displays the lowest trap density of 4.22 × 1015 cm−3, demonstrating that the passivation effect of R-α-BrMBA is much better than the others.
To further accurately explore the surface structure and chemical state of the perovskite film modified with R-α-BrMBA because of its preferable treatment effect, ultraviolet photoelectron spectroscopy (UPS) and X-ray photoelectron spectroscopy (XPS) were carried out. Fig. S11 (ESI†) suggests that the secondary electron cut-off edge and valence band edge for the 3D perovskite film are 16.56 eV and 1.30 eV, respectively, and those for the Br-2D/3D perovskite film are 16.54 eV and 1.32 eV, respectively. Based on the bandgaps (Eg) obtained from the ultraviolet-visible (UV-vis) absorption spectra and Tauc plots (Fig. S12, ESI†), Type I energy level alignment is depicted in Fig. 3f.26,59 The energy level diagram signifies that the Br-2D perovskite is able to inhibit the transportation of electrons into Spiro-OMeTAD and reduce the electron–hole recombination probability efficiently.53,60
The XPS spectra are displayed in Fig. S13 (ESI†). The C 1s peaks of the control film at 284.8 eV and 286.3 eV are assigned to the C–C and C–NH2 bonds, respectively, which are derived from the FA+ and MA+ of the perovskite. In addition, the C 1s peak at 288.1 eV is ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond caused by water/oxygen, as exhibited in Fig. S13a (ESI†). As for the Br-2D/3D perovskite film, the obviously increased C–NH2 peak intensity in Fig. S13b (ESI†) demonstrates the existence of R-α-BrMBA on the surface. More importantly, the intensity of the C
O bond caused by water/oxygen, as exhibited in Fig. S13a (ESI†). As for the Br-2D/3D perovskite film, the obviously increased C–NH2 peak intensity in Fig. S13b (ESI†) demonstrates the existence of R-α-BrMBA on the surface. More importantly, the intensity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak decreased dramatically, which indicates that the Br-2D perovskite capping layer is able to suppress the degradation of the absorber layer efficiently.15,61 Fig. S13c and d (ESI†) reveal that the intensity of the N 1s peak at 401.8 eV assigned to the C–NH2 bond increased obviously with the R-α-BrMBA modification, implying the existence of Br-2D perovskites.61 Furthermore, the introduction of R-α-BrMBA is demonstrated by Fig. S13e (ESI†). Compared with the control perovskite film, the peaks from Pb 4f are shifted to lower binding energies and the peaks from Pb0 are diminished in the perovskite film modified with R-α-BrMBA (Fig. S13f, ESI†), suggesting that the perovskite surface can be certainly passivated by R-α-BrMBA.62
O peak decreased dramatically, which indicates that the Br-2D perovskite capping layer is able to suppress the degradation of the absorber layer efficiently.15,61 Fig. S13c and d (ESI†) reveal that the intensity of the N 1s peak at 401.8 eV assigned to the C–NH2 bond increased obviously with the R-α-BrMBA modification, implying the existence of Br-2D perovskites.61 Furthermore, the introduction of R-α-BrMBA is demonstrated by Fig. S13e (ESI†). Compared with the control perovskite film, the peaks from Pb 4f are shifted to lower binding energies and the peaks from Pb0 are diminished in the perovskite film modified with R-α-BrMBA (Fig. S13f, ESI†), suggesting that the perovskite surface can be certainly passivated by R-α-BrMBA.62
Based on the above analyses, we can conclude that the R-α-BrMBA-modified device with MD phases possesses enhanced charge transport and suppressed non-radiative recombination at the perovskite/HTL interfaces compared with other devices. Next, we fabricated PSCs with a normal structure of FTO/SnO2/3D perovskite/2D perovskite/Spiro-OMeTAD/Ag. Fig. S14 (ESI†) shows the cross-sectional SEM images of these devices. The current density–voltage (J–V) curves for PSCs modified with different concentrations of R-α-BrMBA are depicted in Fig. S15 (ESI†) and the corresponding photovoltaic parameters are listed in Table S5 (ESI†). The control device delivers a PCE of 18.97% with an open-circuit voltage (VOC) of 1.12 V, a short-circuit current density (JSC) of 21.60 mA cm−2, and a fill factor (FF) of 78.41%. The PCEs of the PSCs first increased and then decreased with the addition of the R-α-BrMBA at different concentrations. This is because the covering of 2D phases is imperfect at low concentration, whereas the 2D layers are too thick to impair charge extraction at high concentrations, dramatically reducing the VOC and FF. The device achieves a champion PCE of 21.48% with a VOC of 1.20 V, a JSC of 21.71 mA cm−2, and a FF of 82.44% at a concentration of 4 mg mL−1 R-α-BrMBA. Besides, the PCE of 21.48% for PSCs modified with R-α-BrMBA is the highest compared with the PCEs of 20.32% and 20.87% for PSCs modified with R-α-FMBA and R-α-ClMBA, respectively, as shown in Fig. S16 and Table S6 (ESI†). The great improvement in PCEs of R-α-BrMBA-modified devices mainly originates from the increasing VOC and FF, which could be attributed to the suppressed non-radiative recombination and enhanced charge transport.
Based on previous discussions, the excellent performance of R-α-BrMBA-modified devices was further compared with the control and R-α-MBA-modified devices as below. As shown in the J–V characteristics of the devices in Fig. 4a, the hysteresis index (HI) of the control, R-α-MBA-modified, and R-α-BrMBA-modified PSCs was calculated according to the equation:63 HI = ((PCEreverse − PCEforward))/PCEreverse. The HI of R-α-BrMBA-modified PSCs is 3.07%, which is below the HI of 6.01% for the control PSCs and 4.69% for the R-α-MBA-modified PSCs (Table 1). The significantly reduced HI benefits from the more effective charge transport process and less trap density at the 3D/Br-2D/Spiro-OMeTAD interfaces as discussed previously. External quantum efficiency (EQE) spectra of the champion PSCs are depicted in Fig. 4b. The integrated JSC values of the control, R-α-MBA- and R-α-BrMBA-modified devices are 20.89, 21.02 and 21.12 mA cm−2, respectively, which match well with the values obtained from the measured J–V curves. The steady-state PCEs and current density were measured at the maximum power point of the PSCs, as shown in Fig. 4c and Fig. S17 (ESI†). A stabilized PCE of 20.47% and a stabilized JSC of 20.76 mA cm−2 were obtained for the PSCs modified with R-α-BrMBA, while those of the control/R-α-MBA-modified devices are 17.58%/18.69% and 19.85/20.45 mA cm−2, respectively. The PCEs of 50 devices of each type were statistically analyzed to study the reproducibility, as shown in Fig. 4d. It could be observed that the PSCs modified with R-α-BrMBA possess the optimum photovoltaic performance and narrowest distribution. The improvement in the PCEs was mainly due to the increase in the VOC and FF, derived from the optimized carrier dynamics and interfacial state.
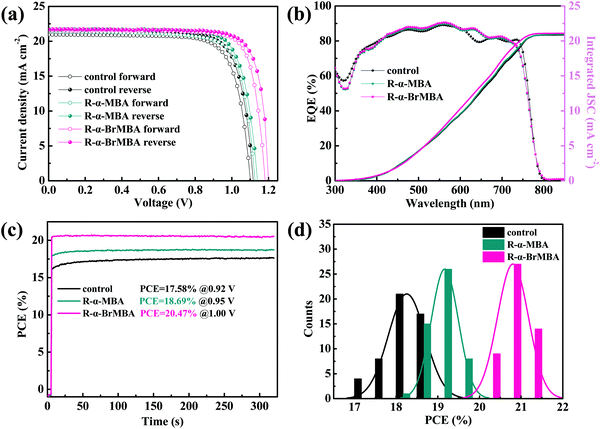 | ||
| Fig. 4 Perovskite devices with different modifications. (a) J–V curves under forward and reverse scan, (b) EQE spectra, (c) steady-state output efficiency and (d) statistical distribution of PCEs. | ||
| Samples | Directions | V OC (V) | J sc (mA cm−2) | FF (%) | PCE (%) | HI (%) |
|---|---|---|---|---|---|---|
| Control | Reverse | 1.12 | 21.60 | 78.41 | 18.97 | 6.01 |
| Forward | 1.10 | 20.87 | 77.68 | 17.83 | ||
| R-α-MBA | Reverse | 1.14 | 21.65 | 79.47 | 19.61 | 4.69 |
| Forward | 1.12 | 21.39 | 78.01 | 18.69 | ||
| R-α-BrMBA | Reverse | 1.20 | 21.71 | 82.44 | 21.48 | 3.07 |
| Forward | 1.18 | 21.59 | 81.73 | 20.82 |
Specifically, FF loss, which consists of non-radiative loss and charge transport loss, was further studied. The FF maximum (FFmax) without charge transport loss can be calculated according to the equation: FFmax = ((νOC − ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (νOC + 0.72)))/((νOC + 1)), where νOC = (qVOC)/nkT.64 The n is the ideal factor and is extracted from the dark J–V curves in Fig. S18 (ESI†).65 As shown in Fig. S19, (ESI†) compared with the control PSC, the non-radiative loss and charge transport loss are certainly suppressed for the PSC modified with R-α-MBA. These losses are further suppressed after modification with R-α-BrMBA, especially for the charge transport loss. The computation results again confirm that the Br-2D perovskite capping layer does not only reduce the surface trap states density and suppress non-radiative recombination, but also facilitates the charge carrier transport efficiently, resulting in boosted VOC, FF and PCE.
(νOC + 0.72)))/((νOC + 1)), where νOC = (qVOC)/nkT.64 The n is the ideal factor and is extracted from the dark J–V curves in Fig. S18 (ESI†).65 As shown in Fig. S19, (ESI†) compared with the control PSC, the non-radiative loss and charge transport loss are certainly suppressed for the PSC modified with R-α-MBA. These losses are further suppressed after modification with R-α-BrMBA, especially for the charge transport loss. The computation results again confirm that the Br-2D perovskite capping layer does not only reduce the surface trap states density and suppress non-radiative recombination, but also facilitates the charge carrier transport efficiently, resulting in boosted VOC, FF and PCE.
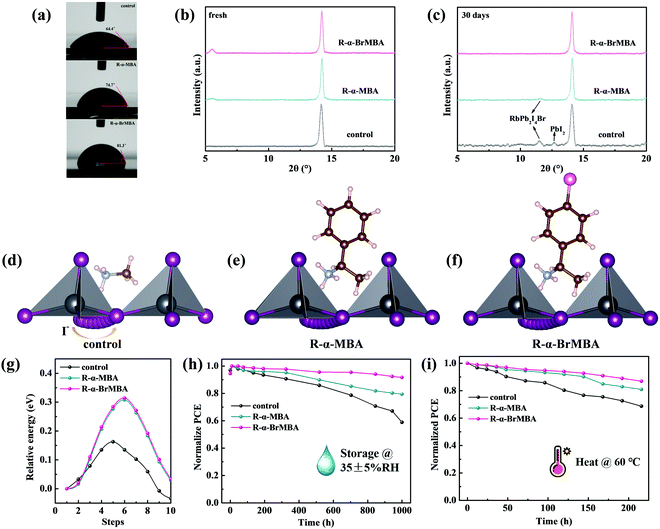
Device stability is another important factor for PSCs except for PCEs. The water contact angle of different films was determined in order to evaluate the water resistance of PSCs with and without modification, as shown in Fig. 5a. The water contact angle of the control film is only 64.4°. Moreover, the water contact angle for the R-α-MBA-modified film is 74.7° and increases to 81.3° for the R-α-BrMBA-modified film, which may be due to the more homogeneous morphology and bromine atom. XRD patterns were obtained on the fresh films and the films stored in the dry chamber (35 ± 5% RH and room temperature in dark) for 30 days. As shown in Fig. 5c, the control film has two additional peaks at 11.4° and 12.7°, which are assigned to RbPb2I4Br and PbI2, respectively, after being aged for 30 days (Fig. 5b).66 However, the degradation is certainly inhibited for the R-α-MBA- and R-α-BrMBA-modified films, which means that the I− migration is suppressed.
In order to theoretically study the mechanism of the distinct phenomena, we performed DFT simulations to model the ion migration pathway at interfaces (Fig. 5c–e) and calculated the I− migration energy between two I− vacancies (Fig. 5f). We can find that the migration distances are extended for both R-α-MBA- and R-α-BrMBA-incorporated cases.67 Furthermore, the calculated migration energy for R-α-MBA- and R-α-BrMBA-incorporated cases are around two-fold higher than that of the control.68 The substantially higher migration energy indicates that I− ion migration in the 2D/3D layers is suppressed significantly, which may be due to the steric impediment effect from larger spacer cations. Correspondingly, the unencapsulated PSCs modified with R-α-MBA and R-α-BrMBA maintained ∼80% and ∼90% of their initial PCEs after 1000 h of storage, respectively. Nevertheless, the control PSC only retained ∼58% of its initial PCE during the same period. In addition, the thermal stability of the MD heterojunction was investigated. The PL spectra of fresh and thermal aged perovskite films modified with R-α-BrMBA are shown in Fig. S20 (ESI†). The result implies that the dimensionality of the MD phases remains the same after annealing at 60 °C in N2 for 8 days. We also conducted thermal stability testing of the devices, in which the Spiro-OMeTAD was replaced by PTAA. The PCE of R-α-MBA- and R-α-BrMBA-modified PSCs without encapsulation maintained 81% and ∼87% of their original values after 216 h of annealing in N2, whereas the control PSC without encapsulation decreased to ∼68% of its initial PCEs under the same condition, respectively. The improved long-term storage and thermal stabilities of the R-α-BrMBA-modified PSC can be explained by the reduced trap density, suppressed ion migration and thermally robust MD heterojunction derived from the Br-2D passivation layers.
3. Conclusions
In summary, we employed R-α-MBA based halogen-substituted aromatic cations to prepare efficient and stable 2D/3D wide-bandgap PSCs. We found that the perovskite film modified with bromine-substituted aromatic ligand R-α-BrMBA presents the smoothest surface and the optimal passivation effect compared with the others, resulting in the reduction of the non-radiative loss. Besides, R-α-BrMBA induces MD in 2D phases on top of the bulk perovskite absorber layers, owing to its higher formation energy to develop the n = 1 phase for the 2D perovskite. Therefore, the charge transport behavior at the perovskite/HTL interface is optimized with Type I band alignment, leading to less energy loss. As a result, a champion PCE of 21.48% with an ultrahigh FF of 82.44% and an ultrahigh VOC of 1.20 V is achieved for 1.63 eV perovskite solar cells. Moreover, the increased surface hydrophobicity, inhibited ion migration and thermally robust MD heterojunctions obviously enhanced the stabilities of Br-2D/3D wide-bandgap PSCs. Our findings provide a promising method to construct more effective 2D/3D heterojunctions by regulating the dimensionality of covered 2D perovskites formed with aromatic ligands, and achieve highly efficient wide-bandgap PSCs with simultaneously improved stability for future applications.Author contributions
Jinkun Jiang: writing original draft, experiment and characterization, data analysis; Congcong Tian: synthesizing R-α-MBACl; Zhiang Zhang: data analysis, theoretical calculation, characterization; Xiao Liu: data analysis, characterization; Xin Wang: revising the draft; Yiting Zheng: data analysis, revising the draft; Zhanfei Zhang: revising the draft; Luyan Wang: experimental guidance; Xueyun Wu: characterization; Jianghu Liang: revising the draft; Chun-Chao Chen: supervision, revising the draft, project administration.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This study was funded by the Natural Science Foundation of Shanghai (22ZR1428200), the National Natural Science Foundation of China (Grant no. 51950410581), the Shanghai Government (20JC141500), and CATL–SJTU joint funding.References
- Y. Fu, H. Zhu, J. Chen, M. P. Hautzinger, X. Y. Zhu and S. Jin, Nat. Rev. Mater., 2019, 4, 169–188 CrossRef CAS.
- J. Huang, Y. Yuan, Y. Shao and Y. Yan, Nat. Rev. Mater., 2017, 2, 17042 CrossRef CAS.
- A. K. Jena, A. Kulkarni and T. Miyasaka, Chem. Rev., 2019, 119, 3036–3103 CrossRef CAS PubMed.
- Best-reserch-cell-efficiencies. https://www.nrel.gov/pv/cell-efficiency.html.
- M. De Bastiani, A. J. Mirabelli, Y. Hou, F. Gota, E. Aydin, T. G. Allen, J. Troughton, A. S. Subbiah, F. H. Isikgor, J. Liu, L. Xu, B. Chen, E. Van Kerschaver, D. Baran, B. Fraboni, M. F. Salvador, U. W. Paetzold, E. H. Sargent and S. De Wolf, Nat. Energy, 2021, 6, 167–175 CrossRef CAS.
- M. I. Hossain, A. M. Saleque, S. Ahmed, I. Saidjafarzoda, M. Shahiduzzaman, W. Qarony, D. Knipp, N. Biyikli and Y. H. Tsang, Nano Energy, 2021, 79, 105400 CrossRef CAS.
- A. Al-Ashouri, E. Köhnen, B. Li, A. Magomedov, H. Hempel, P. Caprioglio, J. A. Márquez, A. B.-M. Vilches, E. Kasparavicius, J. A. Smith, N. Phung, D. Menzel, M. Grischek, L. Kegelmann, D. Skroblin, C. Gollwitzer, T. Malinauskas, M. Jošt, G. Matič, B. Rech, R. Schlatmann, M. Topič, L. Korte, A. Abate, B. Stannowski, D. Neher, M. Stolterfoht, T. Unold, V. Getautis and S. Albrecht, Science, 2020, 370, 1300–1309 CrossRef CAS PubMed.
- K. Xiao, R. Lin, Q. Han, Y. Hou, Z. Qin, H. T. Nguyen, J. Wen, M. Wei, V. Yeddu, M. I. Saidaminov, Y. Gao, X. Luo, Y. Wang, H. Gao, C. Zhang, J. Xu, J. Zhu, E. H. Sargent and H. Tan, Nat. Energy, 2020, 5, 870–880 CrossRef CAS.
- B. Chen, Z. J. Yu, S. Manzoor, S. Wang, W. Weigand, Z. Yu, G. Yang, Z. Ni, X. Dai, Z. C. Holman and J. Huang, Joule, 2020, 4, 850–864 CrossRef CAS.
- J. Y. Kim, J. W. Lee, H. S. Jung, H. Shin and N. G. Park, Chem. Rev., 2020, 120, 7867–7918 CrossRef CAS PubMed.
- J.-P. Correa-Baena, W. Tress, K. Domanski, E. H. Anaraki, S.-H. Turren-Cruz, B. Roose, P. P. Boix, M. Grätzel, M. Saliba, A. Abate and A. Hagfeldt, Energy Environ. Sci., 2017, 10, 1207–1212 RSC.
- Y. Zhou, F. Wang, Y. Cao, J.-P. Wang, H.-H. Fang, M. A. Loi, N. Zhao and C.-P. Wong, Adv. Energy Mater., 2017, 7, 1701048 CrossRef.
- J. Zhuang, P. Mao, Y. Luan, X. Yi, Z. Tu, Y. Zhang, Y. Yi, Y. Wei, N. Chen, T. Lin, F. Wang, C. Li and J. Wang, ACS Energy Lett., 2019, 4, 2913–2921 CrossRef CAS.
- J. He, J. Liu, Y. Hou, Y. Wang, S. Yang and H. G. Yang, Nat. Commun., 2020, 11, 4237 CrossRef CAS PubMed.
- Q. Jiang, Y. Zhao, X. Zhang, X. Yang, Y. Chen, Z. Chu, Q. Ye, X. Li, Z. Yin and J. You, Nat. Photonics, 2019, 13, 460–466 CrossRef CAS.
- Q. Song, H. Gong, C. Ji, H. Zhang, F. Sun, F. You, Z. He, D. Li and C. Liang, Sol. RRL, 2021, 5, 2100472 CrossRef CAS.
- J. Suo, B. Yang, E. Mosconi, H. S. Choi, Y. Kim, S. M. Zakeeruddin, F. De Angelis, M. Grätzel, H. S. Kim and A. Hagfeldt, Adv. Funct. Mater., 2021, 31, 2102902 CrossRef CAS.
- E. Akman and S. Akin, Adv. Mater., 2021, 33, 2006087 CrossRef CAS PubMed.
- G. Grancini and M. K. Nazeeruddin, Nat. Rev. Mater., 2018, 4, 4–22 CrossRef.
- P. Chen, Y. Bai, S. Wang, M. Lyu, J.-H. Yun and L. Wang, Adv. Funct. Mater., 2018, 28, 1706923 CrossRef.
- R. Quintero-Bermudez, A. Gold-Parker, A. H. Proppe, R. Munir, Z. Yang, S. O. Kelley, A. Amassian, M. F. Toney and E. H. Sargent, Nat. Mater., 2018, 17, 900–907 CrossRef CAS PubMed.
- P. Gao, A. R. Bin Mohd Yusoff and M. K. Nazeeruddin, Nat. Commun., 2018, 9, 5028 CrossRef PubMed.
- T. Bu, J. Li, Q. Lin, D. P. McMeekin, J. Sun, M. Wang, W. Chen, X. Wen, W. Mao, C. R. McNeill, W. Huang, X.-L. Zhang, J. Zhong, Y.-B. Cheng, U. Bach and F. Huang, Nano Energy, 2020, 75, 104917 CrossRef CAS.
- Y. Lin, Y. Bai, Y. Fang, Z. Chen, S. Yang, X. Zheng, S. Tang, Y. Liu, J. Zhao and J. Huang, J. Phys. Chem. Lett., 2018, 9, 654–658 CrossRef CAS PubMed.
- H. Wang, C. Zhu, L. Liu, S. Ma, P. Liu, J. Wu, C. Shi, Q. Du, Y. Hao, S. Xiang, H. Chen, P. Chen, Y. Bai, H. Zhou, Y. Li and Q. Chen, Adv. Mater., 2019, 31, e1904408 CrossRef PubMed.
- M. He, J. Liang, Z. Zhang, Y. Qiu, Z. Deng, H. Xu, J. Wang, Y. Yang, Z. Chen and C.-C. Chen, J. Mater. Chem. A, 2020, 8, 25831–25841 RSC.
- G. Yang, Z. Ren, K. Liu, M. Qin, W. Deng, H. Zhang, H. Wang, J. Liang, F. Ye, Q. Liang, H. Yin, Y. Chen, Y. Zhuang, S. Li, B. Gao, J. Wang, T. Shi, X. Wang, X. Lu, H. Wu, J. Hou, D. Lei, S. K. So, Y. Yang, G. Fang and G. Li, Nat. Photonics, 2021, 15, 681–689 CrossRef CAS.
- R. Azmi, E. Ugur, A. Seitkhan, F. Aljamaan, A. S. Subbiah, J. Liu, G. T. Harrison, M. I. Nugraa, M. K. Eswaran, M. Babics, Y. Chen, F. Xu, T. G. Allen, A. U. Rehman, C.-L. Wang, T. D. Anthopoulos, U. Schwingenschlögl, M. D. Bastiani, E. Aydin and S. D. Wolf, Science, 2022, 376, 73–77 CrossRef CAS PubMed.
- J. Wang, S. Fu, X. Liu, H. Yuan, Z. Xu, C. Wang, J. Zhang, L. Huang, Z. Hu and Y. Zhu, J. Alloys Compd., 2021, 891, 161971 CrossRef.
- J. Ahn, E. Lee, J. Tan, W. Yang, B. Kim and J. Moon, Mater. Horiz., 2017, 4, 851–856 RSC.
- G. Long, C. Jiang, R. Sabatini, Z. Yang, M. Wei, L. N. Quan, Q. Liang, A. Rasmita, M. Askerka, G. Walters, X. Gong, J. Xing, X. Wen, R. Quintero-Bermudez, H. Yuan, G. Xing, X. R. Wang, D. Song, O. Voznyy, M. Zhang, S. Hoogland, W. Gao, Q. Xiong and E. H. Sargent, Nat. Photonics, 2018, 12, 528–533 CrossRef CAS.
- H. Lu, J. Wang, C. Xiao, X. Pan, X. Chen, R. Brunecky, J. J. Berry, K. Zhu, M. C. Beard and Z. V. Vardeny, Sci. Adv., 2019, 5, eaay0571 CrossRef CAS PubMed.
- J. Wang, H. Lu, X. Pan, J. Xu, H. Liu, X. Liu, D. R. Khanal, M. F. Toney, M. C. Beard and Z. V. Vardeny, ACS Nano, 2021, 15, 588–595 CrossRef CAS PubMed.
- Y.-H. Kim, Y. Zhai, H. Lu, X. Pan, C. Xiao, E. A. Gaulding, S. P. Harvey, J. J. Berry, Z. V. Vardeny, J. M. Luther and M. C. Beard, Science, 2021, 371, 1129–1133 CrossRef CAS PubMed.
- X. L. Xu, L. B. Xiao, J. Zhao, B. K. Pan, J. Li, W. Q. Liao, R. G. Xiong and G. F. Zou, Angew. Chem., Int. Ed., 2020, 59, 19974–19982 CrossRef CAS PubMed.
- X. Liu, Z. Zhang, J. Jiang, C. Tian, X. Wang, L. Wang, Z. Zhang, X. Wu, Y. Zheng, J. Liang and C.-C. Chen, Chem. Eng. J., 2022, 432, 134382 CrossRef CAS.
- Z. Zhang, J. Jiang, X. Xiao Liu, X. Wang, L. Wang, Y. Qiu, Z. Zhang, Y. Zheng, X. Wu, J. Liang, C. Tian and C. C. Chen, Small, 2022, 18, e2105184 CrossRef PubMed.
- S. Luo and W. A. Daoud, Materials, 2016, 9, 123 CrossRef PubMed.
- H. Pan, X. Zhao, X. Gong, Y. Shen and M. Wang, J. Phys. Chem. Lett., 2019, 10, 1813–1819 CrossRef CAS PubMed.
- B. Saparov and D. B. Mitzi, Chem. Rev., 2016, 116, 4558–4596 CrossRef CAS PubMed.
- J. Hu, I. W.-H. Ostwald, S. J. Stuard, M. M. Nahid, N. Zhou, O. F. Williams, Z. Guo, L. Yan, H. Hu, Z. Chen, X. Xiao, Y. Lin, Z. Yang, J. Huang, A. M. Moran, H. Ade, J. R. Neilson and W. You, Nat. Commun., 2019, 10, 1276 CrossRef PubMed.
- S. Zhao, J. Xie, G. Cheng, Y. Xiang, H. Zhu, W. Guo, H. Wang, M. Qin, X. Lu, J. Qu, J. Wang, J. Xu and K. Yan, Small, 2018, 14, e1803350 CrossRef PubMed.
- A. H. Proppe, A. Johnston, S. Teale, A. Mahata, R. Quintero-Bermudez, E. H. Jung, L. Grater, T. Cui, T. Filleter, C. Y. Kim, S. O. Kelley, F. De Angelis and E. H. Sargent, Nat. Commun., 2021, 12, 3472 CrossRef CAS PubMed.
- M. U. Rothmann, W. Li, J. Etheridge and Y. B. Cheng, Adv. Energy Mater., 2017, 7, 1700912 CrossRef.
- L. Wang, X. Wang, L. Zhu, S.-B. Leng, J. Liang, Y. Zheng, Z. Zhang, Z. Zhang, X. Liu, F. Liu and C.-C. Chen, Chem. Eng. J., 2022, 430, 132730 CrossRef CAS.
- S. Zhang, L. Zhang, Q. Tian, X. Gu, Y. Du, K. Zhao and S. Liu, Adv. Energy Mater., 2021, 12, 2103007 CrossRef.
- W. Li, X. Gu, C. Shan, X. Lai, X. W. Sun and A. K.-K. Kyaw, Nano Energy, 2022, 91, 106666 CrossRef CAS.
- J. Liang, Z. Zhang, Q. Xue, Y. Zheng, X. Wu, Y. Huang, X. Wang, C. Qin, Z. Chen and C.-C. Chen, Energy Environ. Sci., 2022, 15, 296–310 RSC.
- C. C. Zhang, Z. K. Wang, S. Yuan, R. Wang, M. Li, M. F. Jimoh, L. S. Liao and Y. Yang, Adv. Mater., 2019, 31, e1902222 CrossRef PubMed.
- F. Wang, M. Yang, Y. Zhang, J. Du, S. Yang, L. Yang, L. Fan, Y. Sui, Y. Sun and J. Yang, Nano Res., 2021, 14, 2783–2789 CrossRef CAS.
- W. Zhao, J. Xu, K. He, Y. Cai, Y. Han, S. Yang, S. Zhan, D. Wang, Z. Liu and S. Liu, Nanomicro Lett., 2021, 13, 169 CAS.
- M. E.-F. Bouduban, V. I.-E. Queloz, V. M. Caselli, K. T. Cho, A. R. Kirmani, S. Paek, C. Roldan-Carmona, L. J. Richter, J. E. Moser, T. J. Savenije, M. K. Nazeeruddin and G. Grancini, J. Phys. Chem. Lett., 2019, 10, 5713–5720 CrossRef CAS PubMed.
- Z. Liu, K. Meng, X. Wang, Z. Qiao, Q. Xu, S. Li, L. Cheng, Z. Li and G. Chen, Nano Lett., 2020, 20, 1296–1304 CrossRef CAS PubMed.
- J. Xing, Y. Zhao, M. Askerka, L. N. Quan, X. Gong, W. Zhao, J. Zhao, H. Tan, G. Long, L. Gao, Z. Yang, O. Voznyy, J. Tang, Z. H. Lu, Q. Xiong and E. H. Sargent, Nat. Commun., 2018, 9, 3541 CrossRef PubMed.
- T. Wu, Y. Wang, X. Li, Y. Wu, X. Meng, D. Cui, X. Yang and L. Han, Adv. Energy Mater., 2019, 9, 1803766 CrossRef.
- D. Xin, S. Tie, X. Zheng, J. Zhu and W.-H. Zhang, J. Energy Chem., 2020, 46, 173–177 CrossRef.
- M. Nikolka, K. Broch, J. Armitage, D. Hanifi, P. J. Nowack, D. Venkateshvaran, A. Sadhanala, J. Saska, M. Mascal, S. H. Jung, J. K. Lee, I. McCulloch, A. Salleo and H. Sirringhaus, Nat. Commun., 2019, 10, 2122 CrossRef PubMed.
- X. Wang, Y. Qiu, L. Wang, T. Zhang, L. Zhu, T. Shan, Y. Wang, J. Jiang, L. Kong, H. Zhong, H. Yu, F. Liu, F. Gao, F. Wang and C.-C. Chen, Nano Energy, 2021, 89, 106445 CrossRef CAS.
- S. Paek, C. Roldan-Carmona, K. T. Cho, M. Franckevicius, H. Kim, H. Kanda, N. Drigo, K. H. Lin, M. Pei, R. Gegevicius, H. J. Yun, H. Yang, P. A. Schouwink, C. Corminboeuf, A. M. Asiri and M. K. Nazeeruddin, Adv. Sci., 2020, 7, 2001014 CrossRef CAS PubMed.
- Y.-W. Jang, S. Lee, K. M. Yeom, K. Jeong, K. Choi, M. Choi and J. H. Noh, Nat. Energy, 2021, 6, 63–71 CrossRef CAS.
- G. Liu, H. Zheng, H. Xu, L. Zhang, X. Xu, S. Xu and X. Pan, Nano Energy, 2020, 73, 104753 CrossRef CAS.
- Y. Du, J. Wu, X. Zhang, Q. Zhu, M. Zhang, X. Liu, Y. Zou, S. Wang and W. Sun, J. Energy Chem., 2021, 52, 84–91 CrossRef.
- G. Kim, H. Min, K. S. Lee, D. Y. Lee, S. M. Yoon and S. I. Seok, Science, 2020, 370, 108–112 CrossRef CAS PubMed.
- M. A. Green, Sol. Cells, 1982, 7, 337–340 CrossRef CAS.
- T. He, S. Li, Y. Jiang, C. Qin, M. Cui, L. Qiao, H. Xu, J. Yang, R. Long, H. Wang and M. Yuan, Nat. Commun., 2020, 11, 1672 CrossRef CAS PubMed.
- Y. Hu, M. F. Aygüler, M. L. Petrus, T. Bein and P. Docampo, ACS Energy Lett., 2017, 2, 2212–2218 CrossRef CAS.
- J. Yang, W. Sheng, R. Li, L. Gong, Y. Li, L. Tan, Q. Lin and Y. Chen, Adv. Energy Mater., 2022, 2103652 CrossRef CAS.
- S. Tan, I. Yavuz, N. De Marco, T. Huang, S. J. Lee, C. S. Choi, M. Wang, S. Nuryyeva, R. Wang, Y. Zhao, H. C. Wang, T. H. Han, B. Dunn, Y. Huang, J. W. Lee and Y. Yang, Adv. Mater., 2020, 32, e1906995 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ma00391k |
| This journal is © The Royal Society of Chemistry 2022 |