Open Access Article
Open Access ArticleA rapid construction strategy of NaYF4:Yb,Er@CDs nanocomposites for dual-mode anti-counterfeiting†
Haopeng
Wei‡
ab,
Yihao
Zheng‡
*ab,
Xingcai
Zhang
 cd,
Ping
Liang
ab,
Xiaokai
Xu
ab,
Chaofan
Hu
cd,
Ping
Liang
ab,
Xiaokai
Xu
ab,
Chaofan
Hu
 ab,
Xuejie
Zhang
ab,
Xuejie
Zhang
 ab,
Bingfu
Lei
ab,
Bingfu
Lei
 ab,
Yingliang
Liu
ab,
Yingliang
Liu
 *ab and
Jianle
Zhuang
*ab and
Jianle
Zhuang
 *ab
*ab
aKey Laboratory for Biobased Materials and Energy of Ministry of Education/Guangdong Provincial Engineering Technology Research Center for Optical Agriculture, College of Materials and Energy, South China Agricultural University, Guangzhou 510642, China. E-mail: chemzhengyh@163.com; tliuyl@scau.edu.cn; zhuangjl@scau.edu.cn
bGuangdong Laboratory for Lingnan Modern Agriculture, Guangzhou 510642, China
cSchool of Engineering and Applied Sciences, Harvard University, Cambridge, MA 02138, USA
dSchool of Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA
First published on 4th May 2022
Abstract
A rapid construction strategy is for the first time developed to achieve the composite structure of NaYF4:Yb,Er and carbon dots (CDs) through electrostatic interactions in only half a minute. The resulting NaYF4:Yb,Er@CDs nanocomposites have superior dual-mode luminescence properties, showing promising application prospects in the field of advanced optical anti-counterfeiting.
Introduction
Optical anti-counterfeiting is a common and mature anti-counterfeiting method, which is widely used in many fields in our daily life such as banknotes, documents, invoices, medicine, tobacco, alcohol, electronic products, and so on.1–4 However, currently, counterfeit and shoddy products are increasing day by day, and traditional optical anti-counterfeiting technology is facing a huge challenge.5,6 As we all know, traditional optical anti-counterfeiting mainly uses single-mode fluorescent materials under ultraviolet (UV) excitation, which is very easy to be imitated and faked by some counterfeiters.7 Therefore, in order to meet the urgent demand for advanced anti-counterfeiting technology in many fields, it is of great significance to develop new optical anti-counterfeiting materials with multimode luminescence properties.8,9To the best of our knowledge, the currently reported luminescent materials for optical anti-counterfeiting have some problems. For traditional semiconductor quantum dots, the prominent toxicity problem restricts their development in the application of anti-counterfeiting.10,11 Perovskite nanocrystals exhibit lots of limitations in the anti-counterfeiting field due to their poor environmental stability, harsh synthesis process, and high cost.12,13 There are also many unfavorable factors for the application of organic luminescent materials in anti-counterfeiting, such as complicated molecular design, poor air stability, and non-negligible toxicity.14,15 Recently, carbon dots (CDs) have attracted intense attention and interest in security applications such as anti-counterfeiting, information encryption, and fingerprint identification, because of their excellent optical properties, easy preparation and structural regulation, good photostability, environmental friendliness, and low toxicity and cost.16–20 Nevertheless, the single downconversion (DC) luminescence mode and the well-known aggregation-caused quenching (ACQ) effect still make it difficult for pure CDs to directly achieve more complex anti-counterfeiting.21–23 To this end, we conceive combining CDs with upconversion (UC) materials to achieve dual-mode optical anti-counterfeiting, which can improve the safety factor and increase the difficulty of being forged. It is well known that lanthanide-doped upconversion nanoparticles (UCNPs) have excellent upconversion luminescence (UCL) properties and easily modified surface structures.24–27 However, it is difficult to combine UCNPs and CDs by constructing a single core–shell structure because of their lattice mismatch. In order to overcome this problem, many research groups have tried other methods. Wu et al. and Song et al. incorporated as-prepared UCNPs into the reaction system of CDs, in which the CDs were synthesized in situ and attached to the surface of the UCNPs.28,29 Unfortunately, this kind of construction is unstable, and the CDs could fall off relatively easily. Our group and others have tried to embed the CDs into the silica shell on the surface of UCNPs to obtain stable dual-mode luminescent nanocomposites, but a complex sol–gel method was required in the preparation process.30,31 Xing et al. achieved the covalent coupling of carboxyl groups on the surface of CDs and amine groups on the surface of UCNPs through the amidation reaction.32 This method is limited due to its long reaction time, low efficiency, high technical requirements and poor reproducibility. More importantly, the surface functional groups involved in the reaction can change the surface state of CDs, which will affect or even quench the luminescence of CDs. Therefore, it is highly desirable to construct a stable dual-mode luminescent nanocomposite by a facile and efficient method that does not change the surface groups of CDs.
In this work, we propose a novel and rapid strategy to construct composite structures of NaYF4:Yb,Er UCNPs and CDs through electrostatic interactions. The surface potentials of the as-prepared carboxylate radical-functionalized CDs and branched PEI-modified UCNPs are opposite, which provides an electrostatic driving force for rapid adsorption. After the addition of NaYF4:Yb,Er UCNPs to the CD solution, the CDs immediately begin to be adsorbed by NaYF4:Yb,Er, and the entire adsorption process can be completed in only half a minute. The branched PEI ligand chains on the surface of NaYF4:Yb,Er not only strongly adsorb CDs to form a stable composite structure, but also act as a matrix to disperse CDs and inhibit their aggregation. The resulting NaYF4:Yb,Er@CDs nanocomposites show excellent dual-mode luminescence properties and great application potential in the field of anti-counterfeiting.
Results and discussion
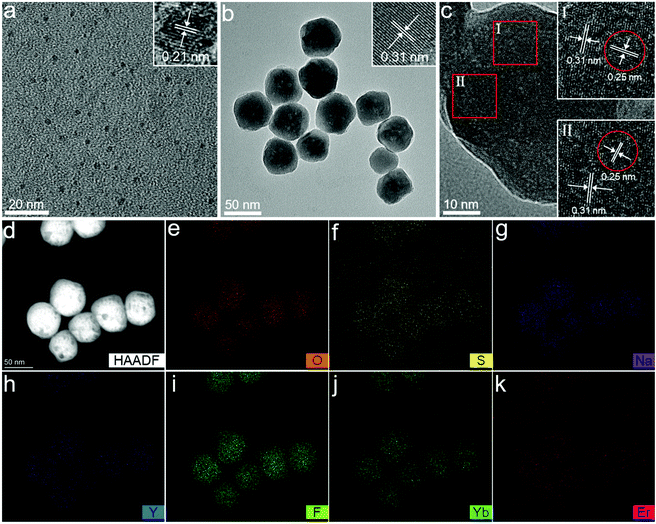
The CDs were synthesized by a solvothermal method using citric acid as the carbon source and thiourea as the nitrogen source. The surface of the as-prepared CDs contains abundant negative ionized carboxylate radicals. The NaYF4:Yb,Er UCNPs were synthesized by a solvothermal method with branched PEI as the surface ligand. Since there are lots of amine groups on the PEI polymer chains, the surface of NaYF4:Yb,Er is positively charged. After NaYF4:Yb,Er UCNPs were added to the CD solution, it could be observed that the luminescence of the CD solution decayed drastically under UV light as the CDs were rapidly adsorbed by NaYF4:Yb,Er. Importantly, the process was completed thoroughly only by manual shaking for half a minute. After standing, removing the supernatant, and freeze-drying, NaYF4:Yb,Er@CDs nanocomposites with dual-mode (UC and DC) luminescence properties were obtained (Scheme 1 and Fig. S1, ESI†).The morphology and structure were characterized by transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM), and energy dispersive spectroscopy (EDS) elemental mapping. As shown in Fig. 1a, the as-prepared CDs are uniform and monodisperse spherical particles with a diameter of about 2.1 nm (Fig. S2, ESI†). The HRTEM image of CDs in the inset of Fig. 1a exhibits clear lattice fringes with a 0.21 nm spacing, which is close to the (100) plane of graphite carbon.33–35 It can be observed from Fig. 1b that the as-prepared NaYF4:Yb,Er UCNPs show a typical sphere-like structure of about 50 nm size. The HRTEM images corresponding to the red squares in Fig. 1c demonstrate apparent lattice fringes with 0.31 nm and 0.25 nm spacing, which can be assigned to the (111) plane of cubic NaYF4 and the (020) plane of graphite carbon for the adsorbed CDs, respectively.33–35 The high-angle annular dark field (HAADF)-STEM image (Fig. 1d) and EDS elemental mapping (Fig. 1e–k) of NaYF4:Yb,Er@CDs composites further confirm the preferential distribution of the CDs containing oxygen and sulfur elements on the surface of NaYF4 particles and also prove the successful doping of Yb3+ and Er3+ ions.
The crystal phase and surface functional groups were investigated by X-ray diffraction (XRD), and Fourier transform infrared (FT-IR) spectroscopy. As shown in Fig. 2a, the diffraction peaks in the XRD pattern of NaYF4:Yb,Er are well matched with the cubic NaYF4 (JCPDS No. 77-2042). The weak and broad peak appearing in the XRD pattern of the NaYF4:Yb,Er@CDs nanocomposites can generally be attributed to the amorphous CDs.36,37 The surface functional groups of CDs, NaYF4:Yb,Er, and NaYF4:Yb,Er@CDs are shown in the FT-IR spectra of Fig. 2b. For the CDs, the broad absorption band at 3050–3600 cm−1 is assigned to the stretching vibrations of –OH/N–H. The characteristic peaks at 1640, 1580, and 1200 cm−1 indicate the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and C
C, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, respectively. Importantly, the absorption peak at 1350 cm−1 is ascribed to the vibration of carboxylate radicals (COO) in carboxylate groups, which are negatively charged groups. For the NaYF4:Yb,Er UCNPs, the absorption band at 2750–3000 cm−1 and the absorption peak at 1450 cm−1 are attributed to the stretching and bending vibrations of CH2, respectively. The broad absorption band at 3150–3650 cm−1 and the absorption peak at 1630 cm−1 are assigned to the vibrations of amine groups including NH2 and NH in PEI ligands, which belong to positively charged groups. It can be seen that the absorption processes of related groups at 3150–3650 and 1630 cm−1 in the NaYF4:Yb,Er@CDs were significantly enhanced, suggesting the adsorption of CDs. Furthermore, the zeta potentials of NaYF4:Yb,Er and CDs were determined to be +40.3 mV and −42.9 mV (Table S1, ESI†), respectively, which directly indicated that CDs were adsorbed on the surface of NaYF4:Yb,Er particles. Because part of the charges were offset, the zeta potential of the NaYF4:Yb,Er@CDs composites was +10.8 mV, which further confirmed the successful combination of CDs and NaYF4:Yb,Er owing to strong electrostatic interactions.
S, respectively. Importantly, the absorption peak at 1350 cm−1 is ascribed to the vibration of carboxylate radicals (COO) in carboxylate groups, which are negatively charged groups. For the NaYF4:Yb,Er UCNPs, the absorption band at 2750–3000 cm−1 and the absorption peak at 1450 cm−1 are attributed to the stretching and bending vibrations of CH2, respectively. The broad absorption band at 3150–3650 cm−1 and the absorption peak at 1630 cm−1 are assigned to the vibrations of amine groups including NH2 and NH in PEI ligands, which belong to positively charged groups. It can be seen that the absorption processes of related groups at 3150–3650 and 1630 cm−1 in the NaYF4:Yb,Er@CDs were significantly enhanced, suggesting the adsorption of CDs. Furthermore, the zeta potentials of NaYF4:Yb,Er and CDs were determined to be +40.3 mV and −42.9 mV (Table S1, ESI†), respectively, which directly indicated that CDs were adsorbed on the surface of NaYF4:Yb,Er particles. Because part of the charges were offset, the zeta potential of the NaYF4:Yb,Er@CDs composites was +10.8 mV, which further confirmed the successful combination of CDs and NaYF4:Yb,Er owing to strong electrostatic interactions.
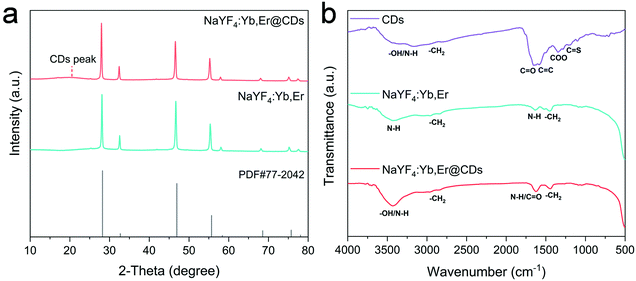 | ||
| Fig. 2 (a) XRD patterns of NaYF4:Yb,Er and NaYF4:Yb,Er@CDs, and the standard diffraction data of cubic NaYF4 (JCPDS No. 77-2042). (b) FT-IR spectra of CDs, NaYF4:Yb,Er, and NaYF4:Yb,Er@CDs. | ||
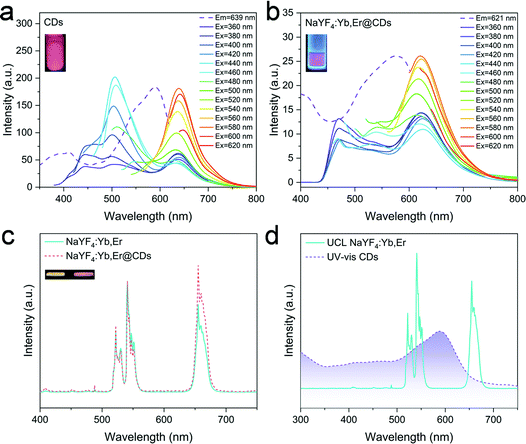
The optical properties of CDs, NaYF4:Yb,Er, and NaYF4:Yb,Er@CDs were investigated by fluorescence spectroscopy and absorption spectroscopy. As shown in Fig. 3a, the as-prepared CDs in DMSO solution have dual-emission peaks, including blue and red peaks. Under low-wavelength UV excitation, the CDs mainly emit blue light. As the excitation wavelength increases, the red emission gradually dominates over the PL spectra. In particular, the CDs exhibit pure red emission at about 640 nm under the excitation of green light. Such CDs with polychromatic optical properties at different excitation wavelengths are favorable for application in luminescence anti-counterfeiting. The inset in Fig. 3a shows the fluorescence image of the CD solution under UV lamp irradiation. In the NaYF4:Yb,Er@CDs nanocomposite powder, the CDs are uniformly adsorbed on the surface polymer ligand chains of NaYF4:Yb,Er particles, which can overcome the aggregation-caused quenching effect of solid CDs and consequently maintain the same fluorescence emission as in the solution. Therefore, we can observe the emission of CDs from the PL spectra of the NaYF4:Yb,Er@CDs nanocomposites in Fig. 3b. The inset shows the fluorescence image of the NaYF4:Yb,Er@CDs nanocomposites under UV light irradiation, which is similar to that of the CD solution in the inset of Fig. 3a. As shown in Fig. 3c, the normalized UCL spectra of NaYF4:Yb,Er and NaYF4:Yb,Er@CDs nanocomposites have the same emission peaks at 522 nm, 541 nm, and 654 nm. The images of NaYF4:Yb,Er and NaYF4:Yb,Er@CDs nanocomposites under 980 nm laser irradiation are shown in the insets of Fig. 3c. A notable phenomenon is that the red emission of NaYF4:Yb,Er@CDs nanocomposites is more stronger than that of pure NaYF4:Yb,Er (Fig. 3c and Fig. S3, ESI†), implying the existence of energy transfer between NaYF4:Yb,Er and CDs. Furthermore, there are spectral overlaps between the absorption spectrum of CDs and the UCL spectrum of NaYF4:Yb,Er (Fig. 3d), which provides a possibility for energy transfer from the green emission of Er3+ ions to CDs. In addition, the red emission wavelengths of CDs and NaYF4:Yb,Er are very close, which is beneficial for the red emission of CDs to be transmitted back to the red emissive energy level of Er3+, thus enhancing the red emission of NaYF4:Yb,Er. The luminescence decay curves and lifetime data are shown in Fig. S4 and Table S2 (ESI†). The almost unaltered UC green luminescence lifetimes (Fig. S4a, ESI†) of NaYF4:Yb,Er and NaYF4:Yb,Er@CDs suggest the radiation reabsorption of the green emission of Er3+ ions by CDs. The reduced red luminescence lifetime (Fig. S4b, ESI†) of NaYF4:Yb,Er@CDs compared to the pure CDs under 541 nm excitation as well as the enhanced UC red emission (Fig. 3c) of NaYF4:Yb,Er@CDs indicates the energy transfer from the excited state of CDs to the red emissive energy level of Er3+ ions through the resonance energy transfer process. Therefore, the energy transfer mechanism here is similar but not identical to our previous work.38 In addition, when such composites with internal energy transfer features are applied for anti-counterfeiting, it is difficult to imitate by using certain substitutes or simply mixing different luminescent materials. Taken together, the above results demonstrate that the NaYF4:Yb,Er@CDs nanocomposites possess dual-mode luminescence properties, which also further proves the successful combination of NaYF4:Yb,Er and CDs through electrostatic interactions. The structural stability of the NaYF4:Yb,Er@CDs nanocomposites was also investigated. On the one hand, stable composite structures were obtained when the CDs were adsorbed on the surface of NaYF4:Yb,Er. After 42 hours, the CDs were still not detached from the NaYF4:Yb,Er surface (Fig. S5a, ESI†). On the other hand, even if the as-prepared NaYF4:Yb,Er@CDs composites were immersed in common organic solvents and water with shaking, the CDs were still not separated from the composites (Fig. S5b, ESI†). Thus, these results proved the good stability of the NaYF4:Yb,Er@CDs composites.
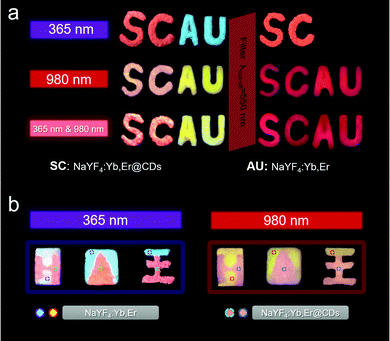
Given the excellent dual-mode luminescence properties of NaYF4:Yb,Er@CDs nanocomposites, they were used for dual-mode anti-counterfeiting applications. As shown in Fig. 4a, “SC” and “AU” patterns were made with NaYF4:Yb,Er@CDs and NaYF4:Yb,Er, respectively. These letter patterns display specific and multicolor images under the irradiation of 365 nm UV light, a 980 nm laser, and 365 nm UV light & a 980 nm laser, respectively, which reflects the multi-mode excitation characteristics. After using a 550 nm cut-off filter, the images with other colors can be read, which further improves the anti-counterfeiting level. In addition, some logos for graphic security made with NaYF4:Yb,Er@CDs and NaYF4:Yb,Er also show the role of dual-mode anti-counterfeiting in Fig. 4b. In short, it is meaningful for advanced anti-counterfeiting to gain particular information with different color characteristics by dual-mode excitation.
Conclusions
In summary, we have developed a simple and rapid construction strategy to prepare NaYF4:Yb,Er@CDs nanocomposites which are suitable for dual-mode anti-counterfeiting technology. The surface potentials of PEI-modified NaYF4:Yb,Er particles and carboxylate radical-functionalized CDs are exactly opposite. By simply mixing and oscillating NaYF4:Yb,Er and CDs in solution, the efficient adsorption of CDs on the surface polymer ligand chains of NaYF4:Yb,Er particles can be achieved in a very short time through electrostatic interactions, which also helps CDs to avoid the aggregation-caused quenching effect so that they can emit bright fluorescence in the solid state. Therefore, the resulting NaYF4:Yb,Er@CDs nanocomposites have superior dual-mode luminescence properties, including the UCL of NaYF4:Yb,Er and the DCL of CDs. As a proof of the prospective application, a preliminary demonstration of the dual-mode anti-counterfeiting scheme is carried out. Finally, more studies are expected to be performed such as the realization of tunable dual-mode luminescence by changing the kinds of rare earth ions and using different luminescent CDs.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (52172142 and 12174119), the Guangdong Basic and Applied Basic Research Foundation (2020A1515011210 and 2022A1515011958), the Science and Technology Planning Project of Guangdong Province (2021A0505060007), and the Science and Technology Planning Project of Guangzhou City (202102080288 and 202007020005).References
- X. Yu, H. Zhang and J. Yu, Aggregate, 2021, 2, 20–34 CrossRef.
- P. Kumar, S. Singh and B. K. Gupta, Nanoscale, 2016, 8, 14297–14340 RSC.
- B. Song, H. Wang, Y. Zhong, B. Chu, Y. Su and Y. He, Nanoscale, 2018, 10, 1617–1621 RSC.
- A. F. Smith and S. E. Skrabalak, J. Mater. Chem. C, 2017, 5, 3207–3215 RSC.
- W. Ren, G. Lin, C. Clarke, J. Zhou and D. Jin, Adv. Mater., 2019, 32, 1901430 CrossRef PubMed.
- J. Yuan, P. R. Christensen and M. O. Wolf, Chem. Sci., 2019, 10, 10113–10121 RSC.
- K. Du, M. Zhang, Y. Li, H. Li, K. Liu, C. Li, J. Feng and H. Zhang, Adv. Opt. Mater., 2021, 9, 2100814 CrossRef CAS.
- D. Gao, J. Gao, F. Gao, Q. Kuang, Y. Pan, Y. Chen and Z. Pan, J. Mater. Chem. C, 2021, 9, 16634–16644 RSC.
- D. Gao, D. Zhao, Y. Pan, R. Chai, Q. Pang a, X. Zhang and W. Chen, Ceram. Int., 2021, 47, 32000–32007 CrossRef CAS.
- J. C. Kays, A. M. Saeboe, R. Toufanian, D. E. Kurant and A. M. Dennis, Nano Lett., 2020, 20, 1980–1991 CrossRef CAS.
- F. P. García de Arquer, D. V. Talapin, V. I. Klimov, Y. Arakawa, M. Bayer and E. H. Sargent, Science, 2021, 373 Search PubMed.
- L. Xu, S. Yuan, H. Zeng and J. Song, Mater. Today Nano, 2019, 6, 100036 CrossRef.
- S. Sun, M. Lu, X. Gao, Z. Shi, X. Bai, W. W. Yu and Y. Zhang, Adv. Sci., 2021, 8, 2102689 CrossRef.
- M. Fang, J. Yang and Z. Li, Prog. Mater. Sci., 2022, 125, 100914 CrossRef CAS.
- Q. Li and Z. Li, Acc. Chem. Res., 2020, 53, 962–973 CrossRef CAS PubMed.
- B. Wang and S. Lu, Matter, 2022, 5, 110–149 CrossRef CAS.
- T. C. Wareing, P. Gentile and A. N. Phan, ACS Nano, 2021, 15, 15471–15501 CrossRef CAS PubMed.
- Z. Tian, X. Zhang, D. Li, D. Zhou, P. Jing, D. Shen, S. Qu, R. Zboril and A. L. Rogach, Adv. Opt. Mater., 2017, 5, 1700416 CrossRef.
- Y. Zheng, H. Wei, P. Liang, X. Xu, X. Zhang, H. Li, C. Zhang, C. Hu, X. Zhang, B. Lei, W.-Y. Wong, Y. Liu and J. Zhuang, Angew. Chem., Int. Ed., 2021, 60, 22253–22259 CrossRef CAS.
- J. Ge, Q. Jia, W. Liu, L. Guo, Q. Liu, M. Lan, H. Zhang, X. Meng and P. Wang, Adv. Mater., 2015, 27, 4169–4177 CrossRef CAS PubMed.
- L. Ai, Y. Yang, B. Wang, J. Chang, Z. Tang, B. Yang and S. Lu, Sci. Bull., 2021, 66, 839–856 CrossRef CAS.
- Y. Zhai, Y. Wang, D. Li, D. Zhou, P. Jing, D. Shen and S. Qu, J. Colloid Interface Sci., 2018, 528, 281–288 CrossRef CAS PubMed.
- A. Xu, G. Wang, Y. Li, H. Dong, S. Yang, P. He and G. Ding, Small, 2020, 16, 2004621 CrossRef CAS.
- X. Zhu, J. Zhang, J. Liu and Y. Zhang, Adv. Sci., 2019, 6, 1901358 CrossRef CAS.
- X. Zheng, R. K. Kankala, C.-G. Liu, S.-B. Wang, A.-Z. Chen and Y. Zhang, Coord. Chem. Rev., 2021, 438, 213870 CrossRef CAS.
- L. Sun, R. Wei, J. Feng and H. Zhang, Coord. Chem. Rev., 2018, 364, 10–32 CrossRef CAS.
- A. Sedlmeier and H. H. Gorris, Chem. Soc. Rev., 2015, 44, 1526–1560 RSC.
- M. Li, W. Yao, J. Liu, Q. Tian, L. Liu, J. Ding, Q. Xue, Q. Lu and W. Wu, J. Mater. Chem. C, 2017, 5, 6512–6520 RSC.
- A. Zhou, F. Song, W. Yao, Y. Han, F. Song, W. Wu, C. Ming, D. Ju and A. Khan, J. Alloys Compd., 2019, 775, 457–465 CrossRef CAS.
- X. Xu, W. Li, W. Zhou, G. Tan, Y. Zheng, C. Hu, B. Lei, X. Zhang, Y. Liu and J. Zhuang, J. Mater. Chem. C, 2018, 6, 10360–10366 RSC.
- H. Tan, G. Gong, S. Xie, Y. Song, C. Zhang, N. Li, D. Zhang, L. Xu, J. Xu and J. Zheng, Langmuir, 2019, 35, 11503–11511 CrossRef CAS.
- D. Zhang, L. Wen, R. Huang, H. Wang, X. Hu and D. Xing, Biomaterials, 2018, 153, 14–26 CrossRef CAS PubMed.
- X. Guo, C.-F. Wang, Z.-Y. Yu, L. Chen and S. Chen, Chem. Commun., 2012, 48, 2692 RSC.
- H. Nie, M. Li, Q. Li, S. Liang, Y. Tan, L. Sheng, W. Shi and S. X.-A. Zhang, Chem. Mater., 2014, 26, 3104–3112 CrossRef CAS.
- J. Liu, D. Li, K. Zhang, M. Yang, H. Sun and B. Yang, Small, 2018, 14, 1703919 CrossRef PubMed.
- X. Miao, D. Qu, D. Yang, B. Nie, Y. Zhao, H. Fan and Z. Sun, Adv. Mater., 2017, 30, 1704740 CrossRef PubMed.
- Y. Chen, M. Zheng, Y. Xiao, H. Dong, H. Zhang, J. Zhuang, H. Hu, B. Lei and Y. Liu, Adv. Mater., 2016, 28, 312–318 CrossRef CAS PubMed.
- X. Xu, X. Zhang, C. Hu, Y. Zheng, B. Lei, Y. Liu and J. Zhuang, J. Mater. Chem. C, 2019, 7, 6231–6235 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ma00418f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |