Open Access Article
Open Access ArticleSingle-molecule detection with enhanced Raman scattering of tungsten oxide nanostructure†
Yoshitaka
Shingaya
 *a,
Hirokazu
Takaki
*a,
Hirokazu
Takaki
 b,
Nobuhiko
Kobayashi
b,
Nobuhiko
Kobayashi
 b,
Masakazu
Aono
b,
Masakazu
Aono
 a and
Tomonobu
Nakayama
a and
Tomonobu
Nakayama
 *ab
*ab
aInternational Center for Materials Nanoarchitectonics (WPI-MANA), National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba 305-0044, Japan. E-mail: SHINGAYA.Yoshitaka@nims.go.jp; NAKAYAMA.Tomonobu@nims.go.jp
bFaculty of Pure and Applied Sciences, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8573, Japan
First published on 23rd September 2022
Abstract
We have found that tungsten oxide nanorods have a very large enhancement effect on Raman scattering. The nanorods with adsorbed 12CO and 13CO at the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were dispersed on a Si substrate and Raman mapping was performed. The Raman images of 12CO and 13CO were completely different, indicating that a very small number of molecules at the single-molecule level were observed. We also confirmed the characteristic blinking phenomenon when single-molecule detection was performed. The very large enhancement effect of Raman scattering can be attributed to the {001}CS structure of the tungsten oxide nanorods. It was confirmed from the DFT calculation results that the {001}CS structure exhibits two-dimensional electrical conduction properties.
1 were dispersed on a Si substrate and Raman mapping was performed. The Raman images of 12CO and 13CO were completely different, indicating that a very small number of molecules at the single-molecule level were observed. We also confirmed the characteristic blinking phenomenon when single-molecule detection was performed. The very large enhancement effect of Raman scattering can be attributed to the {001}CS structure of the tungsten oxide nanorods. It was confirmed from the DFT calculation results that the {001}CS structure exhibits two-dimensional electrical conduction properties.
1. Introduction
Raman scattering spectroscopy is a powerful method of identifying molecular species or obtaining information of the environment of molecules.1 However, an extremely small cross section2 of Raman scattering makes it difficult to realize single-molecule-level detection sensitivity, which is required for certain types of application such as medical diagnostics,3 water or environmental monitoring4 and food safety monitoring.5 Surface enhanced Raman scattering (SERS) can greatly amplify Raman signals as already demonstrated using roughened surfaces of Ag, Au or Cu6–8 or their nanoparticles. SERS with extremely high sensitivity has been realized with Ag nanoparticle aggregates, eventually demonstrating single-molecule-level detection sensitivity.9–11 The extremely large enhancement effect observed is mainly due to the surface plasmon resonance in a nanogap region between two adjacent Ag nanoparticles.12,13 Considerable efforts have been devoted so far to optimizing inter-nanoparticle distances14–16 or shapes of noble-metal islands on insulating substrates17 to maximize the enhancement effect. To date, metals for obtaining a substantial SERS effect have been limited to noble metals such as Ag and Au.In recent years, plasmonics based on semiconducting materials such as metal oxides, metal chalcogenide and metal nitrides have attracted considerable attention.18–20 Titanium nitride nanoparticles for photothermal conversion,21 tunable localized surface plasmon resonance using indium tin oxide nanoparticles22 or tungsten oxide nanocrystals,23 epsilon-near-zero materials using tungsten bronze24,25 and photo catalytic reaction using molybdenum oxide or tungsten oxide26–28 have been reported. SERS effect using semiconducting nanocrystals has also been reported,29–33 however, the enhancement factor is not very large and single-molecule detection is not achievable.
In this paper, we report that a metal oxide nanorod, an intermediate oxide of tungsten, WOx (x < 3),34–36 shows an extremely large enhancement effect of Raman scattering, and we also show that single-molecule detection becomes possible by using a certain activation procedure. Carbon monoxide (CO) adsorbed on WOx is clearly detected through the C–O stretching peak in Raman spectra; however it is very difficult to observe Raman scattering from such an adsorbed molecule. We further show that WOx enables us to detect even single-molecule through Raman spectroscopy. This result indicates that an extremely large enhancement effect of Raman scattering is indeed available with a metal oxide if it is appropriately engineered. WOx (x = 2.75) is an atomically well-defined single crystal with two-dimensional conducting layers as revealed by our theoretical calculations.
2. Results and discussion
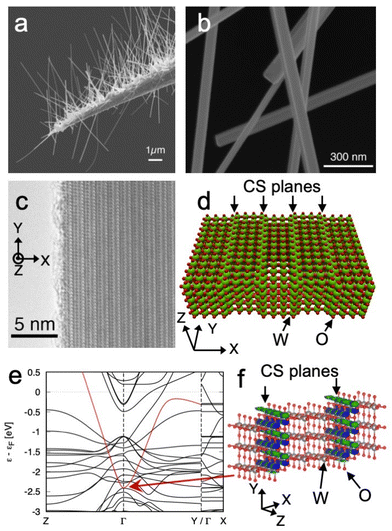
The large enhancement effect of Raman scattering that enables the observation of adsorbed molecule originates from WOx nanorod. Fig. 1a shows a scanning electron microscope (SEM) image of WOx nanorods grown on an electrochemically etched tungsten tip. The length of the nanorods is typically 1–10 μm. The thickness of the WOx nanorods is 20–150 nm, as can be determined from the SEM image shown in Fig. 1b. Fig. 1c shows a transmission electron microscope image of a WOx nanorod. The nanorod has planar defects of oxygen inside. The periodic stacking of the planar defects forms the {001}-type crystallographic shear (CS) structure described as WnO3n−1. The observed structure corresponds to W4O11, which is one of the {001}CS structures.34 A structural model of W4O11-CS is shown in Fig. 1d. Various intermediate oxides of tungsten show metallic conductivity.37–39 However, the electrical conductivity of the {001}CS structure remains unclear. Therefore, we carried out the first-principles electronic structure calculation for the W4O11-CS structure on the basis of density functional theory. We obtained the electronic band structure by the localized atomic orbital basis set method implemented in SAKE.40 We confirmed that the W4O11-CS structure exhibits a metallic band structure, as shown in Fig. 1e. A highly dispersive band that crosses the Fermi energy exists. The band has dispersion in the yz directions, but not in the x direction. This indicates two-dimensional conducting layers forming in the {001}CS structure. Our theoretical calculations showed that the wave function of that band localized in the CS plane as shown in Fig. 1f. It is expected that the plasmon resonance due to free electrons in the two-dimensional conducting layers may contribute to the enhancement of Raman scattering.We found that Raman spectra obtained from WOx nanorods contain Raman scattering attributable to adsorbed molecules, as will be shown later. Therefore, we expected that WOx nanorods would have the enhancement effect of Raman scattering. Since the peak frequency of an adsorbed molecule largely changes from position to position, we expect that the number of observed molecules will be very small and WOx nanorods will have an extremely large enhancement effect of Raman scattering. To prove this hypothesis, Raman spectroscopy was carried out for the WOx nanorods exposed to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of two isotopes. We used adsorbed CO molecules to estimate the enhancement effect. The CO molecules on WOx nanorods were formed by decomposition of 1
1 mixture of two isotopes. We used adsorbed CO molecules to estimate the enhancement effect. The CO molecules on WOx nanorods were formed by decomposition of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of methanol-12C and methanol-13C in an ultra high vacuum (UHV) chamber.41–43
1 mixture of methanol-12C and methanol-13C in an ultra high vacuum (UHV) chamber.41–43
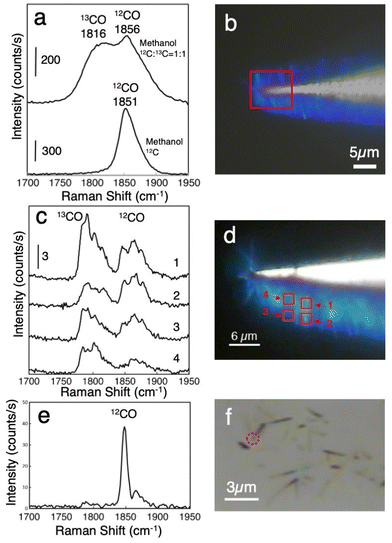
Fig. 2a shows accumulated Raman spectra of the adsorbed CO on WOx nanorods formed by the decomposition of methanol. The spectra were obtained at 225 different points in a 9 μm × 8 μm area on densely grown WOx nanorods, as indicated by the square in Fig. 2b. In this area, more than one hundred nanorods were included. Fig. 2b shows an optical microscope image of densely grown WOx nanorods on the tungsten tip. The lower spectrum in Fig. 2a was obtained after exposing the nanorods to methanol-12C at 600 °C and 5 × 10−5 torr for 15 min. Only the peak corresponding to 12CO molecules was observed. The upper spectrum of Fig. 2a was obtained after exposing the nanorods to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of methanol-12C and methanol-13C at 640 °C and 5 × 10−5 torr for 10 min. Two peaks correspond to the adsorbed 13CO and 12CO. Since we observed CO molecules on a large number of WOx nanorods in this case, the two peaks appear at a 1
1 mixture of methanol-12C and methanol-13C at 640 °C and 5 × 10−5 torr for 10 min. Two peaks correspond to the adsorbed 13CO and 12CO. Since we observed CO molecules on a large number of WOx nanorods in this case, the two peaks appear at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The difference in vibrational frequency between the two peaks is reasonably attributed to the isotope substitution of the carbon atom of the CO molecules. Normally, it is very difficult to observe Raman scattering from such adsorbed molecules. We expected that the WOx nanorods would have some enhancement effect of Raman scattering. Here, we note that as-grown nanorods did not show the Raman peak from CO molecules. After laser irradiation of 1 mW μm−2, CO peaks appeared. Once the peaks corresponding to CO molecules appear in the Raman spectrum, it is possible to observe them even when the laser intensity is reduced. These results indicate that the activation by laser irradiation is required for detecting adsorbed molecules.
1 ratio. The difference in vibrational frequency between the two peaks is reasonably attributed to the isotope substitution of the carbon atom of the CO molecules. Normally, it is very difficult to observe Raman scattering from such adsorbed molecules. We expected that the WOx nanorods would have some enhancement effect of Raman scattering. Here, we note that as-grown nanorods did not show the Raman peak from CO molecules. After laser irradiation of 1 mW μm−2, CO peaks appeared. Once the peaks corresponding to CO molecules appear in the Raman spectrum, it is possible to observe them even when the laser intensity is reduced. These results indicate that the activation by laser irradiation is required for detecting adsorbed molecules.
It is necessary to mention here the vibrational frequency of the adsorbed CO molecules observed in this study. The vibrational frequencies of observed CO molecules are much lower than that of adsorbed CO molecules shown in the previous SERS measurements.44 This is due to the difference in substrate materials. What is observed in the literature are CO molecules adsorbed on silver. CO molecules are weakly adsorbed on silver, therefore the intramolecular bonding of CO molecules is not very different from that of CO molecules in the gas phase. In contrast, CO molecules are strongly adsorbed on transition metals. As a result, the intramolecular bonding of CO become weaker and the intramolecular vibrations shift to lower frequencies. It is known that CO molecules adsorbed on the bridge site have a particularly large low frequency shift. The 1850 cm−1 obtained in the present study corresponds well to the peak position of CO molecules adsorbed on the bridge site of the transition metal surface.45 As can be seen from the structural model in Fig. 1d, tungsten oxide nanorods with CS planes have bridge sites on the CS planes. It is considered that the CO molecules adsorbed on the bridge sites on the CS planes are observed in this study.
Fig. 2c shows Raman spectra from an intermediate number of WOx nanorods. In this case, WOx nanorods were exposed to 1 × 10−6 torr methanol for 100 seconds at room temperature after growth. To reduce the number of observed molecule, the number of accumulated pixels was reduced to 9 in a 2 μm × 2 μm area. In this area, 20–50 nanorods were included. Four spectra were obtained at different positions on densely grown WOx nanorods, as shown in Fig. 2d with the correspondingly numbered squares. Although the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of methanol-12C and methanol-13C was used in this case, the intensity ratio of 13CO to 12CO is not anymore 1
1 mixture of methanol-12C and methanol-13C was used in this case, the intensity ratio of 13CO to 12CO is not anymore 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This indicates that the number of observed CO molecules was already very small. The appearance of multiple components in the CO stretching mode is due to a subtle difference between the adsorption states of CO on WOx nanorods46 that are averaged out when we observe many adsorbed molecules. Several components that originate from different adsorption sites appeared for a small number of adsorbed CO molecules. The intensity ratio differed from position to position.
1. This indicates that the number of observed CO molecules was already very small. The appearance of multiple components in the CO stretching mode is due to a subtle difference between the adsorption states of CO on WOx nanorods46 that are averaged out when we observe many adsorbed molecules. Several components that originate from different adsorption sites appeared for a small number of adsorbed CO molecules. The intensity ratio differed from position to position.
To further reduce their number, WOx nanorods were dispersed on a Si substrate, and Raman spectra were observed from 1–3 WOx nanorods. Fig. 2e shows the Raman spectra obtained at the single point indicated by the red circle in Fig. 2f. In this case, the intensity ratio of 12CO to 13CO increases further. Almost no 13CO peak appears except for a small peak at 1783 cm−1, whereas a very sharp and intense peak at 1848 cm−1 and a smaller peak at 1862 cm−1 appear for 12CO. Since the ratio of 12CO to 13CO should be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 if the Raman spectra correspond to many molecules, this result strongly suggests that single-molecule-level observation becomes possible for adsorbed CO.
1 if the Raman spectra correspond to many molecules, this result strongly suggests that single-molecule-level observation becomes possible for adsorbed CO.
To prove the single-molecule Raman scattering observation with an extremely large enhancement effect of WOx nanorods more clearly, Raman mapping was conducted for WOx nanorods with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
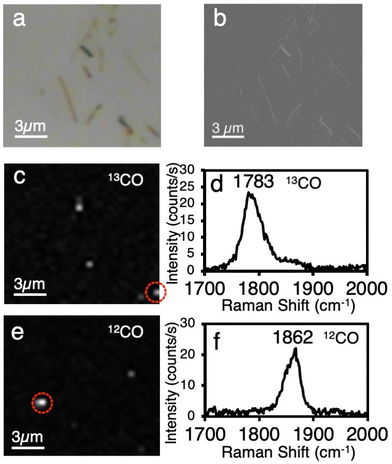
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 isotope mixture of CO. Fig. 3a and b show optical microscope and SEM images of WOx nanorods dispersed on a Si substrate. Fig. 3c and e respectively show Raman mappings of the 13CO and 12CO peaks obtained at the same area as in Fig. 3a and b. The result of mapping for the 13CO peak is totally different from that for the 12CO peak. Fig. 3d and f show the spectra obtained at the positions indicated by circles in Fig. 3c and e. We observed that only one isotope appears in each spectrum. This indicates that the observed peaks originate from a very small number of CO molecules, that is, the single-molecule-level.
1 isotope mixture of CO. Fig. 3a and b show optical microscope and SEM images of WOx nanorods dispersed on a Si substrate. Fig. 3c and e respectively show Raman mappings of the 13CO and 12CO peaks obtained at the same area as in Fig. 3a and b. The result of mapping for the 13CO peak is totally different from that for the 12CO peak. Fig. 3d and f show the spectra obtained at the positions indicated by circles in Fig. 3c and e. We observed that only one isotope appears in each spectrum. This indicates that the observed peaks originate from a very small number of CO molecules, that is, the single-molecule-level.
As mentioned above, as-grown nanorods did not show a large enhancement effect of Raman scattering to enable single-molecule detection. After laser irradiation as intense as 0.1–1.5 mW μm−2, single-molecule detection became possible. The threshold intensities of activation were dependent on the diameter and position of WOx nanorods. Nanorods with larger diameters tend to be activated at a lower intensity of an irradiated laser. In addition, the laser irradiation near the end of a nanorod can activate the nanorod of a lower laser power than the irradiation in the center of the nanorod. This activation procedure corresponds to cutting the CS planes by the local oxidation of WOx and localizing the plasmon at the active site formed by the oxidation.
It should be mentioned here that the enhancement factor (EF) become very large, over 1010. The calculation of the EF is described in ESI.† CO molecules do not have absorption in the visible light region. Therefore, the resonance Raman effect is not expected. We consider that this very large enhancement factor is mainly caused by the electromagnetic mechanism due to plasmon resonance. The results of additional experiments supporting this explanation are shown in Fig. S1 and S2 in ESI.† The light scattering spectrum obtained from a single WOx nanorod is shown in Fig. S1.† The light scattering with a peak around 450 nm is attributed to the plasmon resonance of the WOx nanorod. When the light within the peak wavelength region of the light scattering spectrum is used as the excitation light for Raman scattering, a large enhancement effect is obtained. Fig. S2† shows the results of electrical measurements of a single tungsten oxide nanorod using the prober in the SEM. A completely linear IV curves were obtained, indicating that the WOx nanorods have metallic electrical properties. The electrical resistivity determined from the diameter and the length of the nanorod was quite low, 2.9 × 10−4 Ω cm. These results support electromagnetic mechanism due to plasmon resonance. There is possibility that charge transfer mechanism also contribute to the large enhancement effect. The details of the enhancement mechanism are not yet clear, and are considered to be a target for future work.
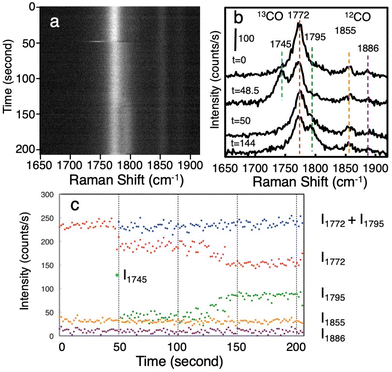
The enhanced Raman scattering of adsorbed CO on WOx nanorods shows time-dependent spectral changes, that is, the so-called blinking phenomenon. As the blinking is characteristic of single-molecule observation,9,47–49 the following result is additional evidence that the observed CO is at the single-molecule level. Fig. 4a shows time series Raman spectra of adsorbed CO on WOx nanorods dispersed on the Si substrate acquired at 1.5 s intervals. In this case also, 13CO and 12CO exist at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio on WOx nanorods. Fig. 4b indicates the Raman spectra obtained at various times. A peak at 1772 cm−1 corresponds to 13CO molecule and peaks at 1855 and 1886 cm−1 correspond to 12CO molecules. The large difference in peak intensity between 13CO and 12CO indicates that the number of observed CO molecules is very small (single-molecule level). The peak at 1772 cm−1 comes from single 13CO molecule, and it shows time-dependent changes in its intensity and position. These time-dependent changes are due to change of adsorption site of CO. At t = 48.5 s an intense peak appears at 1745 cm−1. The peak rapidly disappears because of the instability of the adsorption state that causes a rather low vibrational frequency of CO. After this event, a shoulder peak appears at 1795 cm−1. Fig. 4c shows plots of peak intensities as a function of time. The peak intensities of each peak were obtained by peak fitting. Although the peak intensities of 12CO at 1855 and 1886 cm−1 were constant throughout the measurements, the peak intensity of 13CO at 1772 cm−1 drastically changed. The splitting of the peak starting at t = 48.5 s corresponds to the rapid hopping of CO molecule between two adsorption states. The peak intensity indicates the occupation time of each adsorption state. The peak intensity at 1772 cm−1 gradually decreases from t = 124 s, whereas the peak intensity at 1795 cm−1 increases. The total intensity of peaks at 1772 cm−1 and 1795 cm−1 was almost constant after the start of splitting at t = 48.5 s. This result clearly indicates the negative correlation between two peaks and can be explained by the variation of the occupation time between two different adsorption states. When we observe thousands of molecules using a conventional spectroscopic technique, such detailed information of adsorption states is averaged out. Single-molecule detection with a large enhancement effect of Raman scattering enables us to examine in detail the dynamic behavior of the bonding state and environmental conditions of the target molecule.
1 ratio on WOx nanorods. Fig. 4b indicates the Raman spectra obtained at various times. A peak at 1772 cm−1 corresponds to 13CO molecule and peaks at 1855 and 1886 cm−1 correspond to 12CO molecules. The large difference in peak intensity between 13CO and 12CO indicates that the number of observed CO molecules is very small (single-molecule level). The peak at 1772 cm−1 comes from single 13CO molecule, and it shows time-dependent changes in its intensity and position. These time-dependent changes are due to change of adsorption site of CO. At t = 48.5 s an intense peak appears at 1745 cm−1. The peak rapidly disappears because of the instability of the adsorption state that causes a rather low vibrational frequency of CO. After this event, a shoulder peak appears at 1795 cm−1. Fig. 4c shows plots of peak intensities as a function of time. The peak intensities of each peak were obtained by peak fitting. Although the peak intensities of 12CO at 1855 and 1886 cm−1 were constant throughout the measurements, the peak intensity of 13CO at 1772 cm−1 drastically changed. The splitting of the peak starting at t = 48.5 s corresponds to the rapid hopping of CO molecule between two adsorption states. The peak intensity indicates the occupation time of each adsorption state. The peak intensity at 1772 cm−1 gradually decreases from t = 124 s, whereas the peak intensity at 1795 cm−1 increases. The total intensity of peaks at 1772 cm−1 and 1795 cm−1 was almost constant after the start of splitting at t = 48.5 s. This result clearly indicates the negative correlation between two peaks and can be explained by the variation of the occupation time between two different adsorption states. When we observe thousands of molecules using a conventional spectroscopic technique, such detailed information of adsorption states is averaged out. Single-molecule detection with a large enhancement effect of Raman scattering enables us to examine in detail the dynamic behavior of the bonding state and environmental conditions of the target molecule.
3. Conclusions
We have found that WOx nanorods show an extremely large enhancement effect of Raman scattering, and the vibrational spectra of a single adsorbed CO molecule become observable. Although as-grown nanorods did not show a large enhancement effect of Raman scattering, after laser irradiation at an intensity of 0.1–1.5 mW μm−2, single-molecule detection became possible. This activation procedure corresponds to cutting the CS planes by the local oxidation of WOx, and the locally oxidized part works as the active site for Raman enhancement. The Raman mapping of the isotope mixture proved the detection of a single molecule. The Raman mapping was performed for nanorods with adsorbed 12CO and 13CO at the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The Raman images of 12CO and 13CO were completely different, indicating that a very small number of molecules at the single-molecule level were observed. DFT calculations revealed the existence of a 2D conducting layer at the CS plane. Therefore, we considered that plasmon resonance using free electrons in 2D conducting layers in WOx nanorods is the main factor for the large enhancement effect. Conventional single-molecule SERS is always realized in a nearly closed system such as a nanogap or nanocavity. In contrast, the enhancement of Raman scattering by WOx is expected to be realized at the cleavage site of conducting planes at the surfaces of nanorods. It is considered that the active sites of Raman enhancement can be brought into close proximity to macromolecules such as proteins. Therefore, this technique has the potential to greatly expand the application of single-molecule SERS. WOx nanorods can be attached to the apex of probe for a scanning probe microscope.50 Therefore, chemical analysis with extremely high spatial resolution is expected to realize.
1. The Raman images of 12CO and 13CO were completely different, indicating that a very small number of molecules at the single-molecule level were observed. DFT calculations revealed the existence of a 2D conducting layer at the CS plane. Therefore, we considered that plasmon resonance using free electrons in 2D conducting layers in WOx nanorods is the main factor for the large enhancement effect. Conventional single-molecule SERS is always realized in a nearly closed system such as a nanogap or nanocavity. In contrast, the enhancement of Raman scattering by WOx is expected to be realized at the cleavage site of conducting planes at the surfaces of nanorods. It is considered that the active sites of Raman enhancement can be brought into close proximity to macromolecules such as proteins. Therefore, this technique has the potential to greatly expand the application of single-molecule SERS. WOx nanorods can be attached to the apex of probe for a scanning probe microscope.50 Therefore, chemical analysis with extremely high spatial resolution is expected to realize.
4. Experimental section
We have already developed an epitaxial growth technique for WOx nanorods on tungsten in a UHV chamber.50–52 The WOx nanorods were grown on electrochemically etched tungsten substrates. The substrates were annealed at 1100 °C to remove the residual oxide layer on the surface. Then the substrate was kept at 700 °C to 850 °C. Oxygen gas at 5 × 10−6 torr was introduced into the chamber, and the tungsten foil heated at 1100 °C was placed in front of the substrate in order to deposit tungsten oxide on the substrate. The growth time was 5 to 10 h. The obtained tungsten oxide showed a nanorod shape.Raman scattering spectral measurements were performed in air with a micro-Raman system (HORIBA Jobin-Yvon, HR-800) using an Ar-ion laser of 514.5 nm wavelength for excitation focused in a 1 μm diameter spot with a 100× objective lens (Olympus, 0.90 numerical aperture). The light that scattered back from the sample was collected with the same objective lens and detected through a polychromator with a liquid-nitrogen-cooled CCD camera. Detailed structures of WOx nanorods were observed by field emission-SEM (JEOL JSM-6500F) and TEM (JEOL JEM-2000EX). Highly pure oxygen gas (99.99995%) was used for WOx nanorod growth. Deuterated methanol-12C,13C (Cambridge Isotope Laboratories) was used for the formation of adsorbed molecules.
Author contributions
The manuscript was written by Y. S. and T. N. through the support of all the co-authors. Y. S. performed growth and characterization of WOx nanorods. Y. S. performed micro-Raman measurements. H. T. and N. K. performed theoretical calculations. N. K. and M. A. discussed band structure and possible enhancement mechanism. All the authors analyzed the data, discussed the results and commented on the manuscript.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
We would like to thank Dr K. Kurashima for TEM observation. This study was supported by the World Premier International Center (WPI) for Materials Nanoarchitectonics (MANA) of the National Institute for Materials Science (NIMS), Tsukuba, Japan, and JSPS Kakenhi grant number 20K05280.References
- S. Schlucker, Angew. Chem., Int. Ed., 2014, 53, 4756–4795 CrossRef PubMed.
- E. C. Le Ru and P. G. Etchegoin, Annu. Rev. Phys. Chem., 2012, 63, 65–87 CrossRef CAS PubMed.
- M. Mascini and S. Tombelli, Biomarkers, 2008, 13, 637–657 Search PubMed.
- C. I. L. Justino, A. C. Duarte and T. A. P. Rocha-Santos, Sensors, 2017, 17, 2918–2943 CrossRef PubMed.
- Z. Lin and L. He, Curr. Opin. Food Sci., 2019, 28, 82–87 CrossRef.
- M. Fleischmann, P. J. Hendra and A. J. McQuillan, Chem. Phys. Lett., 1974, 26, 163–166 CrossRef CAS.
- D. L. Jeanmaire and R. P. Van Duyne, J. Electroanal. Chem., 1977, 84, 1–20 CrossRef CAS.
- M. G. Albrecht and J. A. Creighton, J. Am. Chem. Soc., 1977, 99, 5215–5217 CrossRef CAS.
- S. Nie and S. R. Emory, Science, 1997, 275, 1102–1106 CrossRef CAS PubMed.
- K. Kneipp, Y. Wang, H. Kneipp, L. T. Perelman, I. Itzkan, R. R. Dasari and M. S. Feld, Phys. Rev. Lett., 1997, 78, 1667–1670 CrossRef CAS.
- H. Xu, E. J. Bjerneld, M. Käll and L. Börjesson, Phys. Rev. Lett., 1999, 83, 4358–4360 Search PubMed.
- M. Moskovits, Rev. Mod. Phys., 1985, 57, 783–826 CrossRef CAS.
- T. Itoh, Y. S. Yamamoto and Y. Ozaki, Chem. Soc. Rev., 2017, 46, 3904–3921 RSC.
- G. J. Kovacs, R. O. Loutfy, P. S. Vincett, C. Jennings and R. Aroca, Langmuir, 1986, 2, 689–694 CrossRef CAS.
- E. W. A. Visser, M. Horacek and P. Zijlstra, Nano Lett., 2018, 18, 7927–7934 CrossRef CAS PubMed.
- J. F. Li, Y. F. Huang, Y. Ding, Z. L. Yang, S. B. Li, X. S. Zhou, F. R. Fan, W. Zhang, Z. Y. Zhou, D. Y. Wu, B. Ren, Z. L. Wang and Z. Q. Tian, Nature, 2010, 464, 392–395 CrossRef CAS PubMed.
- C. L. Haynes and R. P. Van Duyne, J. Phys. Chem. B, 2001, 105, 5599–5611 CrossRef CAS.
- A. Comin and L. Manna, Chem. Soc. Rev., 2014, 43, 3957–3975 RSC.
- G. V. Naik, V. M. Shalaev and A. Boltasseva, Adv. Mater., 2013, 25, 3264–3294 CrossRef CAS PubMed.
- I. Kriegel, F. Scotognella and L. Manna, Phys. Rep., 2017, 674, 1–52 CrossRef CAS.
- S. Ishii, R. P. Sugavaneshwar and T. Nagao, J. Phys. Chem. C, 2016, 120, 2343–2348 CrossRef CAS.
- M. Kanehara, H. Koike, T. Yoshinaga and T. Teranishi, J. Am. Chem. Soc., 2009, 131, 17736–17737 CrossRef CAS PubMed.
- K. Manthiram and A. P. Alivisatos, J. Am. Chem. Soc., 2012, 134, 3995–3998 CrossRef CAS PubMed.
- L. Tegg, D. Cuskelly and V. J. Keast, Plasmonics, 2017, 13, 437–444 CrossRef.
- Z. Fusco, M. Taheri, R. Bo, T. Tran-Phu, H. Chen, X. Guo, Y. Zhu, T. Tsuzuki, T. P. White and A. Tricoli, Nano Lett., 2020, 20, 3970–3977 CrossRef CAS.
- H. Cheng, M. Wen, X. Ma, Y. Kuwahara, K. Mori, Y. Dai, B. Huang and H. Yamashita, J. Am. Chem. Soc., 2016, 138, 9316–9324 CrossRef CAS.
- X. Zhang, X. Wang, X. Yi, L. Liu, J. Ye and D. Wang, ACS Appl. Energy Mater., 2020, 3, 3569–3576 CrossRef CAS.
- G. Xi, S. Ouyang, P. Li, J. Ye, Q. Ma, N. Su, H. Bai and C. Wang, Angew. Chem., Int. Ed., 2012, 51, 2395–2399 CrossRef CAS PubMed.
- W. Li, R. Zamani, P. Rivera Gil, B. Pelaz, M. Ibanez, D. Cadavid, A. Shavel, R. A. Alvarez-Puebla, W. J. Parak, J. Arbiol and A. Cabot, J. Am. Chem. Soc., 2013, 135, 7098–7101 CrossRef CAS.
- L. Jiang, T. You, P. Yin, Y. Shang, D. Zhang, L. Guo and S. Yang, Nanoscale, 2013, 5, 2784–2789 RSC.
- A. Musumeci, D. Gosztola, T. Schiller, N. M. Dimitrijevic, V. Mujica, D. Martin and T. Rajh, J. Am. Chem. Soc., 2009, 131, 6040–6041 CrossRef CAS PubMed.
- D. Qi, L. Lu, L. Wang and J. Zhang, J. Am. Chem. Soc., 2014, 136, 9886–9889 CrossRef CAS PubMed.
- S. Cong, Y. Yuan, Z. Chen, J. Hou, M. Yang, Y. Su, Y. Zhang, L. Li, Q. Li, F. Geng and Z. Zhao, Nat. Commun., 2015, 6, 7800 CrossRef CAS.
- G. L. Frey, A. Rothschild, J. Sloan, R. Rosentsveig, R. Popovitz-Biro and R. Tenne, J. Solid State Chem., 2001, 162, 300–314 CrossRef CAS.
- Y. Q. Zhu, H. Weibing, W. K. Hsu, M. Terrones, N. Grobert, P. H. Jonathan, H. W. Kroto, D. R. M. Walton and H. Terrones, Chem. Phys. Lett., 1999, 309, 327–334 CrossRef CAS.
- J. Sloan, J. L. Hutchison, R. Tenne, Y. Feldman, T. Tsirlina and M. Homyonfer, J. Solid State Chem., 1999, 144, 18 CrossRef.
- W. Sahle and M. Nygren, J. Solid State Chem., 1983, 48, 154–160 CrossRef CAS.
- M. Remškar, J. Kovac, M. Viršek, M. Mrak, A. Jesih and A. Seabaugh, Adv. Funct. Mater., 2007, 17, 1974–1978 CrossRef.
- K. Viswanathan, K. Brandt and E. Salje, J. Solid State Chem., 1981, 36, 45–51 CrossRef CAS.
- H. Takaki, N. Kobayashi and K. Hirose, J. Phys.: Condens. Matter, 2020, 32, 325901 CrossRef CAS PubMed.
- J. Hrbek, R. A. DePaola and F. M. Hoffmann, J. Chem. Phys., 1984, 81, 2818–2827 CrossRef CAS.
- K. Franaszczuk, E. Herrero, P. Zelenay, A. Wieckowski, J. Wang and R. I. Masel, J. Phys. Chem., 1992, 96, 8509–8516 CrossRef CAS.
- R. Brito de Barros, A. R. Garcia and L. M. Ilharco, Surf. Sci., 2003, 532–535, 185–190 CrossRef CAS.
- I. Mrozek, C. Pettenkofer and A. Otto, Surf. Sci., 1990, 238, 192–198 CrossRef CAS.
- H. Hopster and H. Ibach, Surf. Sci., 1978, 77, 109–117 CrossRef CAS.
- A. Kudelski and B. Pettinger, Chem. Phys. Lett., 2004, 383, 76–79 CrossRef CAS.
- S. M. Stranahan and K. A. Willets, Nano Lett., 2010, 10, 3777–3784 CrossRef CAS.
- J. A. Dieringer, R. B. Lettan II, K. A. Scheidt and R. P. Van Duyne, J. Am. Chem. Soc., 2007, 129, 16249–16256 CrossRef CAS PubMed.
- Y. Sharaabi, T. Shegai and G. Haran, Chem. Phys., 2005, 318, 44–49 CrossRef CAS.
- O. Kubo, Y. Shingaya, M. Nakaya, M. Aono and T. Nakayama, Appl. Phys. Lett., 2006, 88, 254101–254103 CrossRef.
- Y. Shingaya, T. Nakayama and M. Aono, Sci. Technol. Adv. Mater., 2004, 5, 647–649 CrossRef CAS.
- T. Nakayama, O. Kubo, Y. Shingaya, S. Higuchi, T. Hasegawa, C.-S. Jiang, T. Okuda, Y. Kuwahara, K. Takami and M. Aono, Adv. Mater., 2012, 24, 1675–1692 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2nr03596k |
| This journal is © The Royal Society of Chemistry 2022 |