The catalytic decarboxylative allylation of enol carbonates: the synthesis of enantioenriched 3-allyl-3′-aryl 2-oxindoles and the core structure of azonazine†
K. Naresh
Babu
a,
Souvik
Pal
a,
Arindam
Khatua
a,
Avishek
Roy
a and
Alakesh
Bisai
 *ab
*ab
aDepartment of Chemistry, Indian Institute of Science Education and Research Bhopal, Bhauri, Bhopal – 462 066, Madhya Pradesh, India. E-mail: alakesh@iiserkol.ac.in; alakeshb@gmail.com
bDepartment of Chemical Sciences, Indian Institute of Science Education and Research Kolkata, Mohanpur, Nadia – 741246, West Bengal, India
First published on 30th November 2021
Abstract
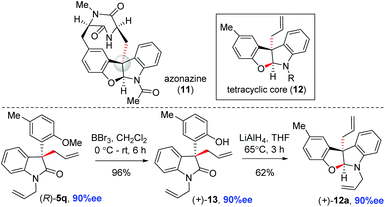
The catalytic asymmetric synthesis of 3-allyl-3′-aryl 2-oxindoles has been shown via the Pd(0)-catalyzed decarboxylative allylation of allylenol carbonates. This methodology provides access to a variety of 2-oxindole substrates (5a–v) with all-carbon quaternary stereocenters (up to 94% ee) at the pseudobenzylic position under additive-free and mild conditions. The synthetic potential of this method was shown by the asymmetric synthesis of the tetracyclic core of the diketopiparazine-based alkaloid azonazine (11).
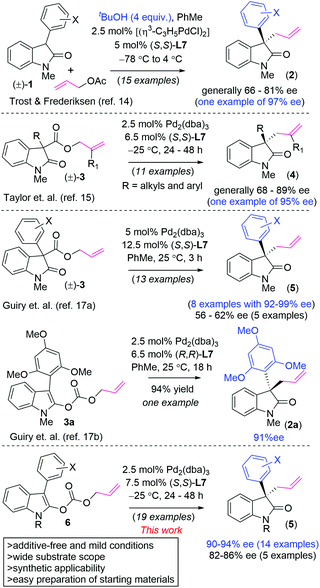
2-Oxindoles1 with quaternary stereogenic centers are common structural scaffolds2 found in a number of naturally occurring alkaloids3 and several pharmaceutically important compounds.4 In this regard, the generation of all-carbon quaternary stereocenters represents a fundamental problem in synthetic organic chemistry. Therefore, a number of elegant asymmetric methods describing the synthesis of 2-oxindoles with a quaternary center at the pseudobenzylic position have been reported. The most significant examples include catalytic asymmetric amide α-arylation by Hartwig,5a the asymmetric intramolecular Heck reaction by Overman,5b asymmetric acyl transfer strategy independently developed by Fu,6a Vedejs,6b and Guo,6c catalytic asymmetric Meerwein–Eschenmoser Claisen rearrangement by Kozlowski,7a,b asymmetric malonate addition onto 3-halo 2-oxindoles by Stoltz8a and others,8b–d malonate addition onto 3-hydroxy/3-sulfonyl 2-oxindoles by our group,8e,f and various thio-urea catalyzed deracemizations of 3-substituted 2-oxindoles.9 Towards this, palladium(0)-catalyzed asymmetric allylic alkylation10 contributes significantly to access molecules with quaternary stereogenic centers, particularly pioneering work led by the research groups of Stoltz,11 Trost,12 and Tunge.13 In 2005, Trost et al.14 (eqn (1), Scheme 1) showed the potential of this approach for the asymmetric construction of 3-allyl-3′-aryl 2-oxindoles via deracemization of (±)-1via allylation using allylacetate (Scheme 1). In this method 4 equiv. of tBuOH were used as an additive14 and the starting materials for this method, 3-aryl oxindoles, were prepared by Hartwig's Pd(0)-catalyzed amide α-arylation.15 Later, in 2011, Taylor et al.16 (eqn (2), Scheme 1) reported the decarboxylative allylation of allyl 2-oxindole 3-carboxylates to access both 3-allyl-3′-alkyl 2-oxindoles (up to 89% ee) and 3-allyl-3′-aryl 2-oxindoles (78–87% ee and one example with 95% ee). The starting materials for this method were synthesized (3 steps) via an intramolecular oxidative coupling.16
In 2017, Guiry et al.17a (eqn (3), Scheme 1) reported decarboxylative allylation at room temperature to access sterically congested 3-allyl-3′-aryl 2-oxindoles in a more general way with better enantioselectivity (see eqn (3)). Later, in 2020, the same group reported one example of the decarboxylative allylation of an allyl carbonate 3a with a bulky aryl group (see the 2,4,6-trimethoxyphenyl group in 3a) to access enantioenriched 2a in 91% ee (eqn (4), Scheme 1).17b However, the use of a higher loading of Pd(0)-catalyst (5 mol%)17a in addition to the use of toxic aryl lead triacetate18 in the preparation of the starting material limits the broader applicability of this approach. These reports clearly suggest that there remains an urgent need for a method for the asymmetric synthesis of 3-allyl-3′-aryl 2-oxindoles from readily available starting materials with better substrate scopes. We envisioned that structurally complex 3-aryl-3′-allyl 2-oxindoles with a quaternary stereocenter at the pseudobenzylic position could be obtained from allylenolcarbonates (eqn (5), Scheme 1),12b,19 which in turn could be accessed from simple and readily available starting materials. Herein, we report an efficient decarboxylative allylation of allyl enol carbonates as an alternative strategy for efficient entries to these targets (Scheme 2).
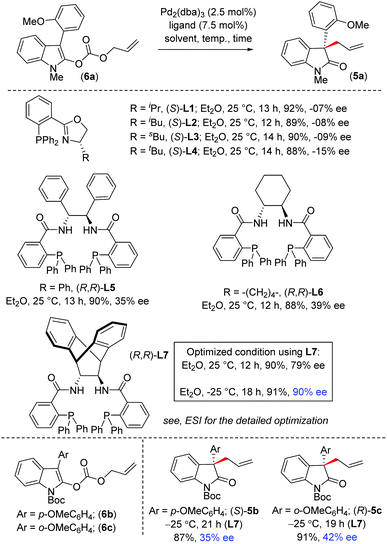
We carried out optimization (Scheme 1) of this reaction through the decarboxylative allylation of allyl carbonate 6a in the presence of [Pd2(dba)3] (2.5 mol%) in combination with ligands L1–L720 (7.5 mol%) in diethyl ether at room temperature (see ESI, Table S1,† entries 1–7). Interestingly, in almost all cases, decarboxylative allylation afforded the expected product 5a in excellent yield. Among various ligands that were screened, PHOX ligands (S)-L1–L4 were found to be unsuitable because they afforded products only up to 15% ee (see ESI, Table 1,† entries 1–4), whereas 2-phosphino-carboxamide ligands L5–L6 yielded the product 5a in up to 39% ee (see ESI, Table 1,† entries 5 and 6). By using the sterically hindered anthracenyl ligand L7,21 which is rigidly held in a specific conformation, we were pleased to find that product (R)-5a was obtained in 79% ee (see ESI, Table 1,† entry 7). Furthermore, solvent screening showed that diethyl ether was the best choice over other organic solvents (see ESI, Table 1,† entries 7–14).
Following exhaustive optimization, it was decided to proceed to investigation into the substrate scope of this reaction by using 2.5 mol% of [Pd2(dba)3] in combination with 7.5 mol% of (R,R)-L7 in Et2O at −25 °C (see ESI, Table 1,† entry 19). We then looked for the effect of different protecting groups, such as electron-withdrawing N-Boc on 2-oxindoles, such as 6b–c (see ESI, Table S1†). However, unfortunately, we only obtained product 5b–c in 35–42% ee, indicating that the nature of the N-protecting group plays a crucial role in achieving selectivity. Therefore, it was decided to use an electron-donating group, such as N-alkyl 2-oxindoles, for further substrate scope. With the optimized procedure in hand, we sought to explore the scope of our asymmetric reaction. Therefore, a number of electronically different aryl oxindoles were prepared and subjected to the standard conditions.
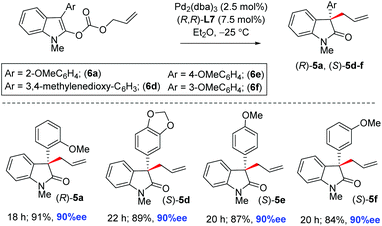
A variety of N-methyl enol carbonates 6d–f were tested to afford 2-oxindoles with an all-carbon quaternary stereogenic center at the pseudobenzylic position (5d–f) in 84–91% isolated yields with up to 90% ee (Scheme 3). Importantly, aromatic rings containing electron-rich methoxy groups at o-, m-, and p- are well tolerated under the standard conditions.
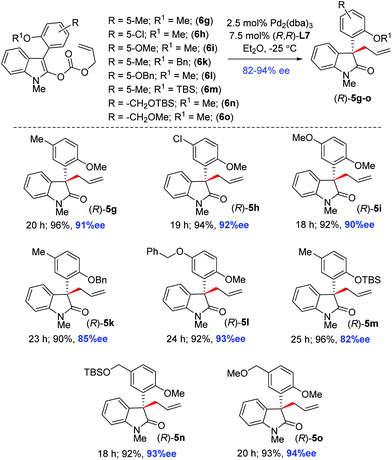
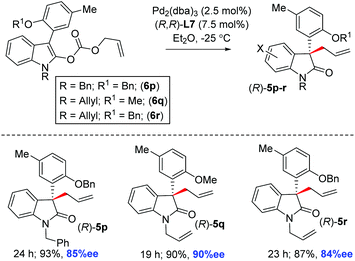
Therefore, we utilized various enol carbonates 6g–o sharing different functionalities on the aromatic ring in addition to an o-methoxy group, under the optimized conditions (Scheme 4). To our delight, a number of 2-oxindoles 5g–o were obtained in up to 94% ee with synthetically useful yields (90–96% isolated yields). Further scope was tested with an easily removable N-protecting group at 2-oxindoles, such as N-benzyl and N-allyl enol carbonates 6p–r. The decarboxylative allylation of enol carbonates 6p–r afforded 2-oxindoles with all-carbon quaternary stereogenic centers 5p–r in 84–90% ee (Scheme 5). In fact, one of these products, 5q, was later explored for the synthesis of the tetracyclic core of azonazine 11 (i.e., 12a) via a reductive cyclization strategy (see, Scheme 10).
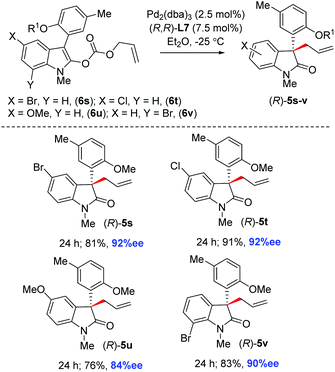
Furthermore, N-allyl enol carbonates 6s–v with the functionalized aromatic ring of 2-oxindole were tested under the optimized conditions. Delightfully, a number of 3-aryl 2-oxindoles 5s–v with a quaternary stereogenic center at the pseudobenzylic position were obtained with 84–92% ee and synthetically useful yields (Scheme 6).
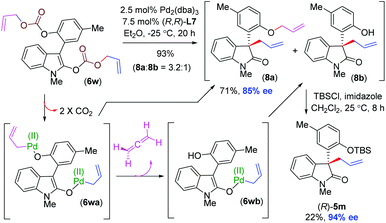
It is noteworthy that substrate 6w with two different allyl enol carbonates (arising from phenol and the enolization of 2-oxindole) afforded products 8a and 8b in 3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in favour of 85% ee and 95% ee, respectively (Scheme 7). This result is quite interesting in the sense that products 8a and 8b were obtained in different enantiomeric purities This probably indicates that the intermediate 6wa (formed after double decarboxylation) generates some amount of intermediate 6wbvia the elimination of allene (Scheme 7). Therefore, two distinct rates of allylation of intermediates 6wa and 6wb account for the unequally enantioenriched products 8a and 8b.
1 ratio in favour of 85% ee and 95% ee, respectively (Scheme 7). This result is quite interesting in the sense that products 8a and 8b were obtained in different enantiomeric purities This probably indicates that the intermediate 6wa (formed after double decarboxylation) generates some amount of intermediate 6wbvia the elimination of allene (Scheme 7). Therefore, two distinct rates of allylation of intermediates 6wa and 6wb account for the unequally enantioenriched products 8a and 8b.
Later, we envisioned a Pd-catalyzed dynamic kinetic asymmetric transformation (DYKAT),22 with rac-allylester (±)-9 and allyl enol carbonates 6, separately or from a mixture of (±)-9 and 6 in any ratio. However, since (±)-9 and 6 have two different reaction rates of Pd-catalyzed deallylation (Scheme 8), the DYKAT of such a complicated mixture would be challenging and worth testing. Interestingly, this effort led to the synthesis of 2-oxindoles 5d–e with similar efficiencies (Schemes 3vs.8).
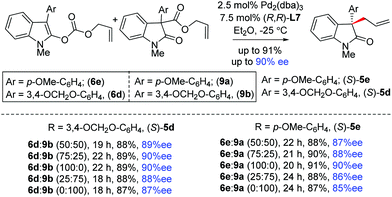 | ||
| Scheme 8 The catalytic decarboxylative allylation of a mixture of the enol carbonate 6 and allyl ester 9. | ||
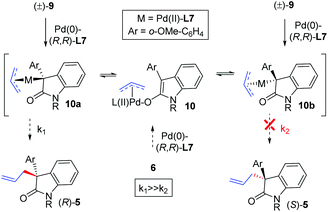
The rationale for the sense of asymmetric induction is shown in Scheme 9. In our strategy, the DYKAT of (±)-9 can be accomplished through a rapid interconversion of intermediates (diastereomeric Pd(II)-intermediates 10a and 10b) (Scheme 9).
Considering that enantioselectivity results from a kinetic reaction of one intermediate, this DYKAT requires a Curtin–Hammett condition to be established, wherein the enantioselectivity is not dependent, in principle, upon the thermodynamic ratio of the intermediates. The asymmetric induction results from a selective reaction with one intermediate (10a in the case of [(R,R)-L-Pd(0)]) through a selective differentiation of the enantiotopic faces of the nucleophile. In the reaction mixture, Pd(II)-intermediate 10 would equilibrate between two diastereomeric Pd(II)-intermediates 10a–b with two different energies of activation (Scheme 7). In the presence of an enantioenriched catalyst [(R,R)-L-Pd(0)], the allylation takes place from Pd(II)-intermediate 10a (when k1 ≫ k2) to afford a product with (R)-stereochemistry [(R)-5]. Whereas, in the case of [(R,R)-L-Pd(0)], allylation takes place from Pd(II)-intermediate 10b (if k2 ≫ k1) to afford a product with (S)-stereochemistry [(S)-5].
To demonstrate the utility of this methodology, we have started to employ these allylations as the key enantioselective reaction in the syntheses of the core structure of a indole alkaloid, azonazine (11).23 Towards this end, we have deprotected the O-methyl ether of the aromatic ring of enantioenriched 5q using BBr3 at 0 °C to afford 13 in 96% yield (Scheme 10). The latter was then reacted with LiAlH4 to afford tetracyclic core 12a in 62% yield via reductive cyclization.
In summary, we have disclosed the catalytic asymmetric synthesis of 3-aryl-3′-allyl oxindoles via the additive-free Pd(0)-catalyzed decarboxylative allylation of allyl enol carbonates. Our methodology affords products with good to excellent enantioselectivities, with the generation of sterically congested quaternary stereocenters under additive-free mild conditions with a broad substrate scope. The further application of this process has been shown in the enantioselective access to the tetracyclic core (12a) of azonazine (11) from readily available inexpensive starting materials. Further application of this strategy for the synthesis of complex alkaloids is underway in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
A. B. [CRG/2019/000113] sincerely thanks the SERB for financial support. A. K. and S. P. thank the INSPIRE and IISER Bhopal for SRFs, respectively. A. B. is a SERB-STAR fellow and sincerely thanks the SERB for generous support [STR/2020/000061].Notes and references
- (a) C. J. Douglas and L. E. Overman, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5363 CrossRef CAS PubMed; (b) A. W. G. Burgett, Q. Li, Q. Wei and P. G. Harran, Angew. Chem., Int. Ed., 2003, 42, 4961 CrossRef CAS PubMed; (c) B. M. Trost and J. Quancard, J. Am. Chem. Soc., 2006, 128, 6314 CrossRef CAS PubMed.
- (a) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748 CrossRef CAS PubMed; (b) Review: L. Chen, X.-P. Yin, C.-H. Wang and J. Zhou, Org. Biomol. Chem., 2014, 12, 6017 RSC.
- (a) Q.-X. Wu, M. S. Crew, M. Draskovic, J. Sohn, T. A. Johnson, K. Tenney, F. A. Valeriote, X.-J. Yao, L. F. Bjeldanes and P. Crews, Org. Lett., 2010, 12, 4458 CrossRef CAS PubMed; (b) N. Lindquist, W. Fenical, G. D. Van Duyne and J. Clardy, J. Am. Chem. Soc., 1991, 113, 2303 CrossRef CAS; (c) R. Fernandez, M. J. Martin, R. Rodriguez-Acebes, F. Reyes, A. Francesch and C. Cuevas, Tetrahedron Lett., 2008, 49, 2282 CrossRef.
- (a) R. R. Goehring, Y. P. Sachdeva, J. S. Pisipati, M. C. Sleevi and J. F. Wolfe, J. Am. Chem. Soc., 1985, 107, 435 CrossRef CAS; (b) A. W. Schammel, G. Chiou and N. K. Garg, J. Org. Chem., 2012, 77, 725 CrossRef CAS PubMed.
- (a) S. Lee and J. F. Hartwig, J. Org. Chem., 2001, 66, 3402 CrossRef CAS PubMed; (b) A. B. Dounay, K. Hatanaka, J. J. Kodanko, M. Oestreich, L. E. Overman, L. A. Pfeifer and M. M. Weiss, J. Am. Chem. Soc., 2003, 125, 6261 CrossRef CAS PubMed.
- (a) I. D. Hills and G. C. Fu, Angew. Chem., Int. Ed., 2003, 42, 3921 CrossRef CAS PubMed; (b) S. A. Shaw, P. Aleman and E. Vedejs, J. Am. Chem. Soc., 2003, 125, 13368 CrossRef CAS PubMed; (c) M. Shan, T. Liang, Y.-F. Zhang, M.-S. Xie, G.-R. Qu and H. M. Guo, Org. Chem. Front., 2019, 6, 3874 RSC.
- (a) E. C. Linton and M. C. Kozlowski, J. Am. Chem. Soc., 2008, 130, 16162 CrossRef CAS PubMed; (b) T. Cao, E. C. Linton, J. Deitch, S. Berritt and M. C. Kozlowski, J. Org. Chem., 2012, 77, 11034 CrossRef CAS PubMed.
- (a) S. Ma, X. Han, S. Krishnan, S. C. Virgil and B. M. Stoltz, Angew. Chem., Int. Ed., 2009, 48, 8037 CrossRef CAS PubMed; (b) Y.-H. Liao, Z.-J. Wu, W.-Y. Han, X.-M. Zhang and W.-C. Yuan, Chem. – Eur. J., 2012, 18, 8916 CrossRef CAS PubMed; (c) J. Zuo, Y.-H. Liao, X. Zhang and W.-C. Yuan, J. Org. Chem., 2012, 77, 11325 CrossRef CAS PubMed; (d) H. Zhang, L. Hong, H. Kang and R. Wang, J. Am. Chem. Soc., 2013, 135, 14098 CrossRef CAS PubMed; (e) K. N. Babu, A. Roy, M. Singh and A. Bisai, Org. Lett., 2018, 20, 6327 CrossRef CAS PubMed; (f) K. N. Babu, L. K. Kinthada, P. P. Das and A. Bisai, Chem. Commun., 2018, 54, 7963 RSC.
- (a) T. Bui, S. Syed and C. F. Barbas III, J. Am. Chem. Soc., 2009, 131, 8758 CrossRef CAS PubMed; (b) B. Tan, G. Hernández-Torres and C. F. Barbas III, J. Am. Chem. Soc., 2011, 133, 12354 CrossRef CAS PubMed; (c) S. De, M. K. Das, S. Bhunia and A. Bisai, Org. Lett., 2015, 17, 5922 CrossRef CAS PubMed; (d) S. De, M. K. Das, A. Roy and A. Bisai, J. Org. Chem., 2016, 81, 12258 CrossRef CAS PubMed.
- Reviews: (a) J. D. Weaver, A. Recio III, A. J. Grenning and J. A. Tunge, Chem. Rev., 2011, 111, 1846 CrossRef CAS PubMed; (b) J. R. James, M. Jackson and P. J. Guiry, Adv. Synth. Catal., 2019, 361, 3016 CrossRef CAS; (c) O. Pàmies, J. Margalef, S. Canellas, J. James, E. Judge, P. J. Guiry, C. Moberg, J.-E. Bäckvall, A. Pfaltz, M. A. Pericâs and M. Diéguez, Chem. Rev., 2021, 121, 4373 CrossRef PubMed.
- (a) J. T. Mohr, D. C. Behenna, A. W. Harned and B. M. Stoltz, Angew. Chem., Int. Ed., 2005, 44, 6924 CrossRef CAS PubMed; (b) J. A. Enquist Jr. and B. M. Stoltz, Nature, 2008, 453, 1228 CrossRef PubMed.
- (a) B. M. Trost, S. Malhotra and W. H. Chan, J. Am. Chem. Soc., 2011, 133, 7328 CrossRef CAS PubMed; (b) B. M. Trost and M. Osipov, Angew. Chem., Int. Ed., 2013, 52, 9176 CrossRef CAS PubMed; (c) B. M. Trost and M. K. Brennan, Org. Lett., 2006, 8, 2027 CrossRef CAS PubMed.
- (a) E. C. Burger and J. A. Tunge, J. Am. Chem. Soc., 2006, 128, 10002 CrossRef CAS PubMed; (b) A. Recio III and J. A. Tunge, Org. Lett., 2009, 11, 5630 CrossRef PubMed; (c) A. J. Grenning and J. A. Tunge, Org. Lett., 2010, 12, 740 CrossRef CAS PubMed.
- (a) B. M. Trost and M. U. Frederiksen, Angew. Chem., Int. Ed., 2005, 44, 308 CrossRef CAS PubMed; (b) B. M. Trost and Y. J. Zhang, J. Am. Chem. Soc., 2007, 129, 14548 CrossRef CAS PubMed.
- K. H. Shaughnessy, B. C. Hammann and J. F. Hartwig, J. Org. Chem., 1998, 63, 6546 CrossRef CAS.
- V. Frančkevicius, J. D. Cuthbertson, M. Pickworth, D. S. Pugh and R. J. K. Taylor, Org. Lett., 2011, 13, 4264 CrossRef PubMed.
- (a) M. Jackson, C. Q. O'Broin, H. Müller-Bunz and P. J. Guiry, Org. Biomol. Chem., 2017, 15, 8166 RSC; (b) B. V. Rokade and P. J. Guiry, J. Org. Chem., 2020, 85, 6172 CrossRef CAS PubMed.
- Use of aryllead triacetates and subsequent asymmetric strategies, see: (a) R. Akula and P. J. Guiry, Org. Lett., 2016, 18, 5472 CrossRef CAS PubMed; (b) J. James and P. J. Guiry, ACS Catal., 2017, 7, 1397 CrossRef CAS.
- For use of allyl enol carbonates in total syntheses of dimeric cyclotryptamine alkaloids, see: (a) See ref. 12b. ; (b) S. Ghosh, S. Bhunia, S. De, B. N. Kakde and A. Bisai, Chem. Commun., 2014, 50, 2434 RSC; (c) S. Ghosh, S. Chaudhuri and A. Bisai, Chem. – Eur. J., 2015, 21, 17479 CrossRef CAS PubMed; (d) Total syntheses of amaryllidaceae alkaloids, see: M. K. Das, N. Kumar and A. Bisai, Org. Lett., 2018, 20, 4421 CrossRef CAS PubMed.
- PHOX ligands L1–L4: (a) G. Helmchen and A. Pfaltz, Acc. Chem. Res., 2000, 33, 336 CrossRef CAS PubMed; (b) M. R. Krout, J. T. Mohr and B. M. Stoltz, Org. Synth., 2009, 86, 181 CrossRef CAS PubMed.
- Ligands L5–L7: (a) B. M. Trost and D. L. Van Vranken, Angew. Chem., Int. Ed. Engl., 1992, 31, 228 CrossRef; (b) B. M. Trost, D. A. Thaisrivongs and J. Hartwig, J. Am. Chem. Soc., 2011, 133, 12439 CrossRef CAS PubMed.
- Review on DYKAT: B. M. Trost and D. R. Fandrick, Aldrichimica Acta, 2007, 40, 59 CAS.
- Studies directed towards azonazine synthesis, see: (a) S. Ghosh, L. K. Kinthada, S. Bhunia and A. Bisai, Chem. Commun., 2012, 48, 10132 RSC; (b) R. Beaud, R. Guillot, C. Kouklovsky and G. Vincent, Angew. Chem., Int. Ed., 2012, 51, 12546 CrossRef CAS PubMed; (c) J.-C. Zhao, S.-M. Yu, Y. Liu and Z.-J. Yao, Org. Lett., 2013, 15, 4300 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ob02048j |
| This journal is © The Royal Society of Chemistry 2022 |