Open Access Article
Open Access ArticleStepwise synthesis of a Zr–C–Si main chain polymer precursor for ZrC/SiC/C composite ceramics†
Qiang Gao,
Cheng Han *,
Xiaozhou Wang and
Yingde Wang
*,
Xiaozhou Wang and
Yingde Wang *
*
Science and Technology on Advanced Ceramic Fibers and Composites Laboratory, National University of Defense Technology, Changsha, Hunan, China. E-mail: hancheng@nudt.edu.cn; wangyingde@nudt.edu.cn
First published on 14th January 2022
Abstract
Novel polymers containing a refractory metal in the main chain are highly desired as ultra-high temperature ceramic precursors. Herein, a low oxidation state active species Cp2Zr(II) with two semi-filled outer orbits was firstly obtained using Cp2ZrCl2 with Mg. This active species of Cp2Zr(II) was subsequently copolymerized with (CH3)2Si(CH2Cl)2 to form a PZCS precursor with a Zr–C–Si main chain. The pathways of constructing the Zr–C–Si main chain were proposed based on the active species Cp2Zr(II) polymerization combined with the auxiliary function of ·MgCl, quite different from the conventional understanding of using the Grignard reaction mechanism to explain the synthesis of metal-containing polymer precursors. The ceramic yield of the PZCS precursor at 900 °C is 43.9 wt%, and the ZrC/SiC/C composite preceramic powder was prepared by the pyrolysis of the PZCS precursor, which has important application prospects in ultra-high sonic aircraft and aero rocket engines. It is expected that this stepwise method of using refractory metal-containing active species to synthesize main-chain metallopolymers would provide significant guidance for preparing novel UHTCs precursors.
1 Introduction
Refractory metal (Zr, Hf, Ta, etc.) borides, nitrides and carbides commonly serve as ultra-high temperature ceramics (UHTCs) due to their high thermal stabilities and chemical inertness. They are heat- and wear-resistant candidate materials for solid rocket motors, hypersonic vehicles and nuclear protection.1,2 Furthermore, the incorporation of Si, B, N-containing components into the above refractory compounds enables an even greater protection against oxidation, further promoting their applications in extreme thermal and oxidizing environments.3–6 In contrast to the conventional manufacturing of preceramic powder at high temperatures, the polymer derived ceramics (PDCs) route offers a number of advantages in regulating the phase composition and microstructure of the ceramics.7At present, the synthesis of refractory metal-containing polymeric precursors can broadly be divided into three categories of (i) chemical modification of polymeric precursors with metal compounds,8–10 (ii) blending of the precursors with metal or metal oxide powders and (iii) direct synthesis of metallopolymers as single source precursors.11 Of note, the elemental composition, solubility, fusibility and viscosity of a single source precursor are adjustable by regulating its molecular structure, which provides possibilities for preparation of high-performance ceramics including UHTCs fibers.11,12 Generally, there are mainly two kind of metal bonds, including M–N and M–C in metallopolymers as UHTCs precursors.13,14 As one of the most popular reactions of building new chemical bonds, Grignard reaction has been applied to synthesize M-C polymeric precursors.7,15–17 The Grignard reaction-based one-pot copolymerization is facile and easy to control, thus has been widely used for preparing pre-ceramic polymers. However, it is worth noting that most of previous investigations were focused on the property characterization of polymer-derived ceramics, but provided very few insights into the reaction mechanism of synthesizing polymer precursors.
Considering that the complexity of one-pot copolymerization system would bring much difficulties in explicating the reaction mechanism, we creatively proposed a stepwise method to synthesize Zr–C–Si main chain polymer as UHTCs precursors. To be specific, the low oxidation state active species Cp2Zr(II) with two semi-filled outer orbits was firstly obtained by the reaction of bis(cyclopentadienyl) zirconium dichloride (Cp2ZrCl2) with magnesium (Mg). After separation, this active species of Cp2Zr(II) was subsequently copolymerized with bis(chloromethyl)-dimethylsilane ((CH3)2Si(CH2Cl)2) to form a Zr–C–Si main chain polymeric precursor with certain polymerization degree, labeled as polyzirconosilane (PZCS). Moreover, olefin polymerization occurred when the active species Cp2Zr(II) was used to react with bischloromethylvinylsilane (CH3Si(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)Cl2), further confirming the reactivity of Cp2Zr(II) as a reaction monomer. This result was different from previous reports that no olefin polymerization occurred when using Na, Cp2ZrCl2, and CH3Si(CH
CH2)Cl2), further confirming the reactivity of Cp2Zr(II) as a reaction monomer. This result was different from previous reports that no olefin polymerization occurred when using Na, Cp2ZrCl2, and CH3Si(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)Cl2 as raw chemicals in a one-pot system.18,19 The reasons might be related to the removal of residual Mg from Cp2Zr(II) but need further research and verification. To have a better understanding on the reaction mechanism of Cp2Zr(II), we also performed DFT calculations on the synthetic path of the PZCS precursor. The results showed that the Cl atom in (CH3)2Si(CH2Cl)2 was attracted by Cp2Zr(II) or ·MgCl, and the subsequent complex polymerization proceeded under the combined impacts of free radical reaction and Grignard reaction. Last but not in the least, we prepared a ZrC/SiC/C composite preceramic powder by pyrolyzing of PZCS precursor, which have important application prospects in ultra-high sonic aircraft and aero rocket engines. To the best of our knowledge, such a stepwise method of using refractory metal-containing active specie to synthesize main-chain metallopolymers as UHTCs precursor has rarely been reported.
CH2)Cl2 as raw chemicals in a one-pot system.18,19 The reasons might be related to the removal of residual Mg from Cp2Zr(II) but need further research and verification. To have a better understanding on the reaction mechanism of Cp2Zr(II), we also performed DFT calculations on the synthetic path of the PZCS precursor. The results showed that the Cl atom in (CH3)2Si(CH2Cl)2 was attracted by Cp2Zr(II) or ·MgCl, and the subsequent complex polymerization proceeded under the combined impacts of free radical reaction and Grignard reaction. Last but not in the least, we prepared a ZrC/SiC/C composite preceramic powder by pyrolyzing of PZCS precursor, which have important application prospects in ultra-high sonic aircraft and aero rocket engines. To the best of our knowledge, such a stepwise method of using refractory metal-containing active specie to synthesize main-chain metallopolymers as UHTCs precursor has rarely been reported.
2 Experimental procedures
2.1 Materials and characterization methods
(CH3)2Si(CH2Cl)2 was purchased from TCI (Tokyo, Japan). CH3Si(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)Cl2, vinyltrimethylsilane((CH3)3Si(CH
CH2)Cl2, vinyltrimethylsilane((CH3)3Si(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)) were purchased from Thermo Fisher Scientific (Massachusetts, USA). Cp2ZrCl2 and tetrahydrofuran (THF) were purchased from Innochem reagent Co., Ltd (Beijing, China). Magnesium (Mg) and toluene were purchased from Tianjin Chemical Factory (Tianjin, China). All reagents and solvents were analytically pure, and all solvents were refluxed with CaH2 to remove water before use.
CH2)) were purchased from Thermo Fisher Scientific (Massachusetts, USA). Cp2ZrCl2 and tetrahydrofuran (THF) were purchased from Innochem reagent Co., Ltd (Beijing, China). Magnesium (Mg) and toluene were purchased from Tianjin Chemical Factory (Tianjin, China). All reagents and solvents were analytically pure, and all solvents were refluxed with CaH2 to remove water before use.
All calculations were carried out with the Gaussian 09 software. The Gibbs free energy profile (kcal mol−1) for the reaction of ZrCp2(II)/·MgCl and R–Cl in toluene was calculated at PBE0-D3BJ/def2-TZVP/TZVP/SMD level. The PBE0 functional20 was adopted for all calculations in combination with the D3BJ dispersion correction.21 For geometry optimization, the Ahlrich's def2-TZVP basis set22 was used for Mg and Zr, and the TZVP basis set for the other atoms. Gibbs free energies were obtained by frequency calculations at the same level. The SMD implicit solvation model23 was used to account for the solvation effect of toluene for all calculations.
The structure of the PZCS precursor was analysed by 1H-nuclear magnetic resonance spectroscopy (1H NMR, Agilent NMR Systems 400 MHz, USA) and Fourier transform infrared spectroscopy (FT-IR/FIR Spectrum Frontier, USA) using KBr disks, detection was performed from 4000 to 400 cm−1. The molecular weight of the PZCS was measured by gel permeation chromatography (GPC, EcoSEC HLC-8320GPC, Japan) using tetrahydrofuran as eluent, and the acquisition time was 0–20 min with a sampling interval of 100 ms. A thermogravimetric analyser (TGA) machine (Pyris 1 TGA, USA) was used to test the ceramic yield of the precursor with heat from 50 °C to 910 °C at 10 °C min−1. X-ray diffraction (XRD) measurements were performed with a powder diffractometer (Bruker D8, Germany) using Cu-Kα radiation to determine the phase composition and crystallinity of the final preceramic powder. The elemental compositions and the bonding states in the precursors and preceramic powder were characterized by X-ray photoelectron spectroscopy analysis (XPS, Thermo Fisher Scientific K-Alpha, USA). The oxygen content in the preceramic powder was characterized by an oxygen and nitrogen analyzer (HORIBA, EMGA-820, Japan), and a carbon and sulfur analyzer (HORIBA, EMIA-320V2, Japan) was used to measure and analyse the C content with SiC powder as the standard sample at a test power of 6.0 kW. The content of silicon and zirconium was determined by inductively coupled plasma-optical emission spectrometer (ICP-OES, Thermo ICP 6300). Transmission electron microscope (TEM) (Tecnai G2 F20 S-TWIN, USA) characterization was performed to study the microstructure and crystallization behaviour of preceramic powder. Scanning electron microscope (SEM) (TESCAN MIRA4 LMH, Czech Republic) and elemental analysis (EDS) was adopted to observe the microstructure or to analyse the element distribution of preceramic powder surface.
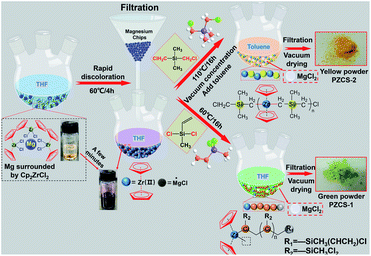
2.2 Synthesis of precursor
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) CH2)Cl2. A dropping funnel was used to slowly add 4.47 ml (34.20 mmol) of CH3Si(CH
CH2)Cl2. A dropping funnel was used to slowly add 4.47 ml (34.20 mmol) of CH3Si(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)Cl2 into Cp2Zr(II) solution. After stirring for 30 min, the solution was heated to 60 °C and reacted for 16 h. Then the solution was cooled down to an ambient temperature, filtered and concentrated under vacuum to yield green flaky solid, which was marked as PZCS-1.
CH2)Cl2 into Cp2Zr(II) solution. After stirring for 30 min, the solution was heated to 60 °C and reacted for 16 h. Then the solution was cooled down to an ambient temperature, filtered and concentrated under vacuum to yield green flaky solid, which was marked as PZCS-1.
2.3 Pyrolysis of PZCS precursor
PZCS-2 precursor was pyrolyzed in a quartz tube furnace in an ultra-high purity argon atmosphere. The furnace was heated from room temperature to 400, 600, 800 and 1000 °C, respectively, at a heating rate of 5 °C min−1, and this temperature was held for 1 h. To investigate the structure evolution behaviour of the as-prepared preceramic powder at high-temperature, the 1000 °C-pyrolyzed products was further heat-treated in graphite furnace at 1200, 1400, 1600, and 1800 °C for 1 h under argon atmosphere with heating rate of 5 °C min−1.3 Results and discussion
3.1 The synthesis of active species Cp2Zr(II) and its reactivity
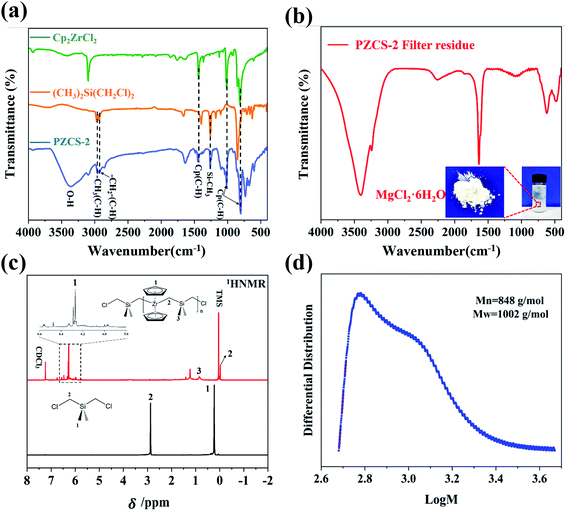
In our stepwise method of synthesizing main-chain metallopolymers, the first and crucial step lied in the generation of active species Cp2Zr(II) from Cp2ZrCl2 reacting with Mg, which subsequently acted as active species to trigger the polymerization of Zr–C–Si main chain. Since the existent state of the active species Cp2Zr(II) are difficult to directly measure, we use effective characterization combined with experimental phenomena to indirectly explain the structure and reactivity of active species. In order to explicit the active species, the content of metal elements in the product of Cp2ZrCl2 reacting with Mg was analyzed by ICP after removal of excessive Mg granules and evaporation of solvent. As shown in Table 1, the atomic ratio of Zr/Mg was measured to be 1/1.56, which indicated that the Zr–Cl bond in Cp2ZrCl2 did react with Mg to generate active species similar to the reaction with sodium.18,19 Moreover, there was no MgCl2 sediment being observed or tested in the filtered residue. Based on the redox theory of valence state change, as well as the Grignard reaction mechanism with free radical of ·MgX as intermediates, we thus inferred that a redox reaction occurred between Cp2ZrCl2 and Mg to produce low oxidation state species Cp2Zr(II) and ·MgCl in THF as active species. According to the reaction equation, the atomic ratio of Zr/Mg in active species solution should be 1/2, in good agreement with the above ICP result. It is worth noting that when toluene was used as the reaction solvent instead of THF, the system colour did not change and no reaction occurred. However, when DMF was used instead of THF, similar products were obtained. Although we did not perform a more detailed study on the coordination structure of Cp2Zr(II) with THF solvent, we inferred that the O atom in nonprotonic solvent THF/DMF indeed acted as an electronegative center to promote the reaction.| Element | Content | Atomic ratio |
|---|---|---|
| Mg | 6.83% | Zr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg = 1 Mg = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.56 1.56 |
| Zr | 16.43% | |
| Remark: 0.1% = 1000 mg kg−1 | ||
In order to prove the reactivity of Cp2Zr(II) and ·MgCl active specie, we firstly tried using the filtrate solution from Cp2ZrCl2 reacting with Mg to further react with CH3Si(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)Cl2. A green flaky solid was obtained and remarked as PZCS-1, which was further characterized by using FTIR spectroscopy and 1H NMR, as shown in ESI-1(Fig. S1a and b†). The result shows that the vinyl bond of CH3Si(CH
CH2)Cl2. A green flaky solid was obtained and remarked as PZCS-1, which was further characterized by using FTIR spectroscopy and 1H NMR, as shown in ESI-1(Fig. S1a and b†). The result shows that the vinyl bond of CH3Si(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)Cl2 was broken under the impact of Cp2Zr(II). Meanwhile, the Mn and Mw of PZCS-1 were measured to be 1013 and 1833 g mol−1 respectively, through GPC analysis, indicating the polymerization of vinyl group to construct a saturated main chain of single bonds.
CH2)Cl2 was broken under the impact of Cp2Zr(II). Meanwhile, the Mn and Mw of PZCS-1 were measured to be 1013 and 1833 g mol−1 respectively, through GPC analysis, indicating the polymerization of vinyl group to construct a saturated main chain of single bonds.
It was thus worthy to deeply analyze the polymerization mechanism, namely the role of Cp2Zr(II) and ·MgCl active species in constructing a polymeric chain. Since zirconocene compounds are commonly used as olefin polymerization catalysts,24–26 we believed the mechanism of coordination insertion polymerizations of 1-olefin by metallocenes18,19 would inspire our understanding on the role of Cp2Zr(II) in creating a polymeric structure in PZCS-1, and the synthesis path was shown in Fig. S1c.† By the way, it was found that the atom transfer between ·MgCl/Cp2Zr(II) and R–Cl played significant roles to initiate olefin insertion polymerization. When Si(CH3)2(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) was used as the reactant instead of SiCH3(CH
CH2) was used as the reactant instead of SiCH3(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)Cl2, the obtained sample only possessed Mn of 324 g mol−1 and Mw of 373 g mol−1 analysed from GPC, which meant no polymerization occurred (ESI-2†).27 Through the above experiments and analysis, we believed that the Cp2Zr(II) species in the Cp2Zr(II)/·MgCl/THF system would not spontaneously change from a low oxidation state to a high oxidation state but were active to initiate the polymerization of Zr–C–Si chain. This also provided a theoretical guideline for our subsequent synthesis of PZCS precursor with incorporated Zr in the main chain.
CH2)Cl2, the obtained sample only possessed Mn of 324 g mol−1 and Mw of 373 g mol−1 analysed from GPC, which meant no polymerization occurred (ESI-2†).27 Through the above experiments and analysis, we believed that the Cp2Zr(II) species in the Cp2Zr(II)/·MgCl/THF system would not spontaneously change from a low oxidation state to a high oxidation state but were active to initiate the polymerization of Zr–C–Si chain. This also provided a theoretical guideline for our subsequent synthesis of PZCS precursor with incorporated Zr in the main chain.
3.2 Synthesis mechanism and structure characterization of PZCS-2
In order to insert the Zr element into the polymer backbone structure, we used the Cp2Zr(II)/·MgCl/THF system to react with (CH3)2Si(CH2Cl)2, a chemical with two C–Cl bonds. The structure of the as-prepared PZCS-2 precursor was firstly analysed by using FTIR spectra, as shown in Fig. 2a. For the PZCS-2 spectrum, the characteristic peak at 2960 cm−1 could be assigned as the –C–H stretching vibration in –CH3, the peaks centred at 808, 1018 and 1446 cm−1 were contributed by the C–H bending vibration in Cp, the 2927 cm−1 peak corresponded well with the C–H stretching vibration of –CH2– structure, while the 1260 cm−1 peak was caused by the Si–CH3 bending vibration. The above results proved the presence of zirconium metallocene and silane structural units in PZCS-2, indicating a copolymerization or blend occurred between the two monomers of Cp2Zr(II) and (CH3)2Si(CH2Cl)2. It is noteworthy that a by-product of white precipitate was obtained and identified as MgCl2·6H2O (Fig. 2b). It indicated that the C–Cl bond in (CH3)2Si(CH2Cl)2 was broken by active species ·MgCl or Cp2Zr(II), which was similar with the above discussion of Fig. S1c.† Moreover, there is no characteristic peak of MgCl2·6H2O found in PZCS-2, indicating that the by-products have been cleanly removed.The 1H-NMR spectra were carried out to further investigate the bonding structures of PZCS-2. Seen from the PZCS-2 spectra in Fig. 2c, the peaks in the range of 6.25–6.35 ppm were associated with the Cp group, and the peak located at −0.02 ppm was ascribed to –Si–CH2– fragment. Meanwhile, the formation of Zr–C bond in PZCS-2 was confirmed by the Zr3d XPS spectrum, which would be detailed analysed in Fig. 6e. In contrast with the spectra of (CH3)2Si(CH2Cl)2, the 2.88 ppm peak corresponding to the –CH2Cl was not observed in PZCS-2, because the Si–CH2Cl structure has reacted with Cp2Zr(II)/·MgCl to generate a main chain structure of –(Zr–C–Si)n– and a by-product of MgCl2. In addition, Gel Permeation Chromatography (GPC) as a powerful method for measuring the molecular weight of organic matter was performed to characterize our prepared PZCS-2. The Mn and Mw of PZCS-2 were mainly measured to be 848 and 1002 g mol−1 respectively, through the GPC curve in Fig. 2d, indicating that the polymerization indeed occurred and the polymerization degree of –(Zr–C–Si)n– was about to be 2–3.
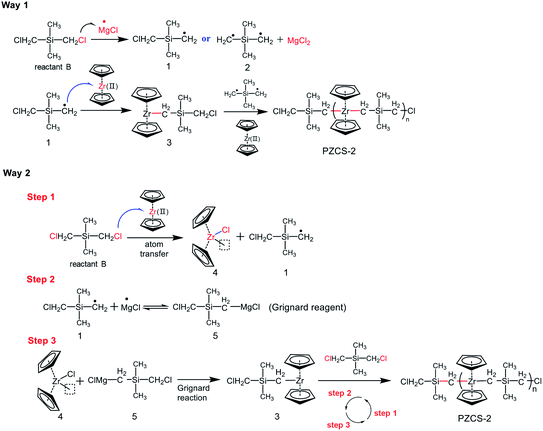
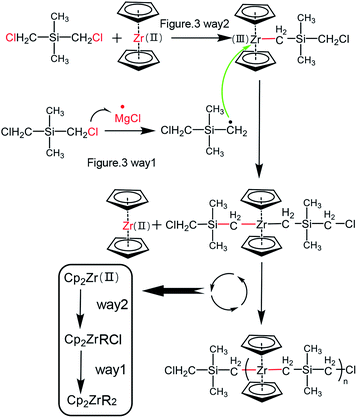
Through the above results and analysis, we have demonstrated the feasibility of using an active species Cp2Zr(II) to synthesize polymer precursor with Zr–C–Si main chain structure. Priority was thus given to the polymerization mechanism, namely how the Cp2Zr(II) was incorporated into the polymeric main chain. From the main chain structure of –(Zr–C–Si)n–, it seemed pretty clear that the C–Cl bond in (CH3)2Si(CH2Cl)2 monomer was broken by reacting with the active species of whether ·MgCl or Cp2Zr(II). This matter might offer a breakthrough to investigate the polymerization mechanism. On the one hand, the formation of by-product MgCl2 inspired the possibility of ·MgCl capturing Cl atom from R–Cl bond. On the other hand, since the outer electrons distribution state of Zr is [Kr]4d25s2, its stable valence state should be +4. Thus the transition from low oxidation state Zr(II) to high oxidation state Zr(IV) might provide the vacant site for Cl atom transfer. This process was very similar with the common atom transfer radical polymerization.28,29 Based on the above description of Cl atoms transfer to whether ·MgCl or Cp2Zr(II), we proposed two possible reaction mechanisms (Fig. 3). The first pathway is that MgCl· attracts Cl atoms in (CH3)2Si(CH2Cl)2 to generate R· radicals and MgCl2, and then R· radicals reacted with Cp2Zr(II) to form Zr–C bond18 (way 1 in Fig. 3). The second pathway mainly includes three steps of (i) Cp2Zr(II)attracted Cl atoms and generated R· radicals; (ii) R· radical and ·MgCl reversibly generated Grignard reagent of RMgCl at high temperature,30,31 and (iii) the Grignard reaction occurred between RMgCl and Cp2ZrCl. Similar with way 2, an idealized reaction process was proposed and discussed in ESI-3.†
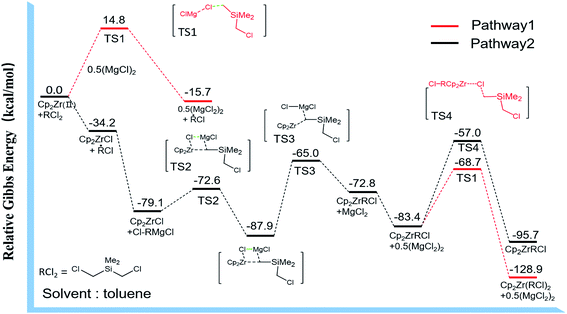
In order to further proved the proposed pathways, we performed DFT calculations of the two possible pathways, and the results were shown in Fig. 4. The first pathway was described as ·MgCl attracting Cl atoms from R–Cl2 to form the transition state TS1. Although the thermodynamic energy change (ΔG) of generating R· was about −15.7 kcal mol−1, the form of transition state TS1 needs to overcome a kinetic energy barrier of 14.8 kcal mol−1. While in the second pathway (black line in Fig. 4), the active species of Cp2Zr(II) would grab Cl atoms from one of the C–Cl bonds in (CH3)2Si(CH2Cl)2. The energy change of the initial step was −34.2 kcal mol−1, indicating it could proceed spontaneously and there was no kinetic energy barrier limitation. Under the comprehensive considerations of thermodynamics and kinetics limitation, the reaction process was more likely to proceed through pathway 2 and went on through Grignard reaction transition states of TS2, TS3 to generate Cp2Zr–RCl. Thereafter, the kinetic energy barrier of Cp2Zr–RCl reacting with (CH3)2Si(CH2Cl)2 to form TS4 was −57.0 kcal mol−1, which was higher than the kinetic energy barrier for TS1 and Cp2Zr–RCl (eqn (1)). Therefore, the possible reaction process was that Cp2Zr–RCl was generated through pathway 2, while Cp2Zr–R2 might be generated according to the pathway 1 as shown in Fig. 5. The main possible reaction processes as follows:
(1) Cp2Zr(II) attracted Cl atoms of Cl–R–Cl ((CH3)2Si(CH2Cl)2) to generated Cl–R· radicals and Cp2Zr–Cl.
(2) The Grignard reaction occurred between Cl–R–MgCl (Cl–R· and ·MgCl) and Cp2Zr–Cl to generated Cp2Zr(III)–R–Cl.
(3) ·MgCl attracted Cl atoms of Cl–R–Cl ((CH3)2Si(CH2Cl)2) to generated Cl–R· radicals and MgCl2
(4) The product Cl–R· of step (3) was reacted with Cp2Zr(III)–R–Cl of step (2) to generated Cp2Zr(R–Cl)2.
Repeat the above steps, the polymer precursor with –(Zr–C–Si)n– was obtained.
| Cp2Zr(II) + RCl2 + ·MgCl → Cp2ZrRCl + TS1 + 0.5(MgCl2)2 ΔG = −68.7 kcal mol−1 | (1) |
We believe our findings here provide fresh insights into the polymerization mechanism of incorporating refractory metal elements into the main chain of M–C–Si molecular structure. This mechanism was based on the active species Cp2Zr(II) radical polymerization combined with the auxiliary function of ·MgCl, quite different from the conventional understanding of using Grignard reaction mechanism (R–MgX directly react with Cp2MCl2) to explain the synthesis of metal-containing polymer precursors.
According to the above analysis, the PZCS-2 precursor with –(Zr–C–Si)n– structure was obtained through a complicated pathway. Our work effectively explaining the formation of Cp2Zr(II) and ·MgCl, which promoted the transfer of Cl atoms in R–Cl to generate R· radical. Because Mg reacts with X–R–X (X = Cl, Br) and Cp2MCl2 to form a complex Grignard reagent/radical system, we concluded that the Grignard reaction mechanism between XMg–R–MgX and Cp2MCl2 did not well match the synthesis of UHTCs precursor through the one-pot method of Mg, X–R–X (X = Cl, Br) and Cp2MCl2 (M = Hf, Zr, Ti). At the same time, the formation of R· radical as well as the possible reaction mechanism between free radicals and active species Cp2Zr(II) was well explained in our work. Such a stepwise method of using refractory metal-containing active specie to synthesize main-chain metallopolymers provides significant guidance for preparing UHTCs precursor with M–C main chain structure.
3.3 Characterization of PZCS-derived UHTCs
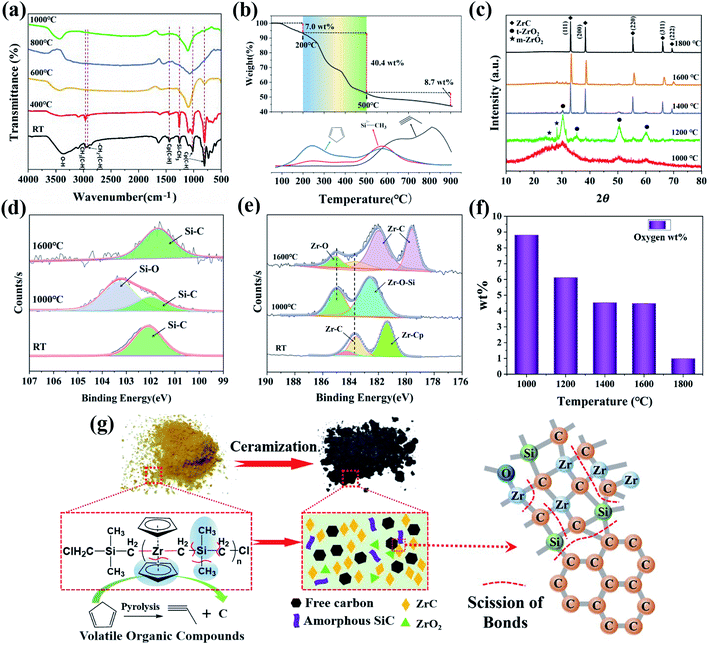
In order to investigate whether the synthesized PZCS-2 was suitable as a ceramic precursor, we further studied its ceramization features. Theoretically speaking, the Zr, Si, and C atom were well defined and covalently linked in the main chain structure of PZCS-2, thus would produce preceramic powder materials with highly homogenous distribution of elements and components. In addition, the PZCS-2 precursor was soluble in some solvents such as tetrahydrofuran, xylene and toluene, and meltable with a softening point of 140–185 °C. These features of PZCS-2 make it potential for the preparation of ceramic matrixes or dense monoliths, thin films, and coatings, etc.The pyrolysis of PZCS-2 precursor was performed at different temperature and the structure of products was firstly characterized using FTIR spectroscopy, as shown in Fig. 6a. After pyrolysis treatment at 400 °C, the C–H bending vibration peak of the Cp ring weakened, indicating that some of the Cp cracked and escaped. The peak positions of other groups did not change much as compared with the PZCS-2 precursor spectroscopy, indicating that the precursor was still in organic at this stage. When pyrolysis temperature raised to 600 °C., the Si–CH3 group in PZCS-2 almost disappeared. Meanwhile, the weaker C–H vibration peak of Cp ring still existed at 808 cm−1, which would be cracked and rearranged at temperature higher than 600 °C. To generate small propyne molecules and free carbon. The organic components of PZCS-2 were completely cracked and transformed into inorganic components when pyrolyzed at 1000 °C.
According to the TG-FTIR-GC-MS results in Fig. 6b and ESI-4,† the PZCS-2 experienced three stages of structural evolution as follows. The weight loss below 200 °C was about 7.0 wt%, mainly due to the volatilization of solvent and a small amount of small methyl silane molecules. When the temperature was raised to 200–500 °C, the rearrangements of chemical bonds occurred, which released a large amount of small molecules such as methylsilane, Cp and methane, caused a main weight loss of about 40.4 wt%.7,16 The completion of inorganization process occurred between 500 and 900 °C with a weight loss of 8.7 wt%. The ceramic yield of PZCS-2 was finally 43.9 wt% when heated to 900 °C in N2 atmosphere.
Whereas mainly amorphous components were obtained by pyrolyzing the PZCS precursor at 1000 °C, subsequent annealing at higher temperature would result in the crystallization of amorphous phase. According to the XRD results in Fig. 6c, a broad and weak diffraction peak of ZrO2 appeared after 1000 °C pyrolysis treatment, indicating that the precursor was oxidized during the preparation or transfer process, and gradually transformed into ZrO2 crystallites at 1000 °C. When annealed at 1200 °C, a faint diffraction peak of ZrC was observed, suggesting that the Zr–C bond structure in PZCS-2 began to crystallize. The diffraction peaks of ZrC (111) (2θ = 33.0°), (200) (2θ = 38.3°), (220) (2θ = 55.3°), (311) (2θ = 65.9°) and (222) (2θ = 69.2°) crystal planes became remarkable and sharp in the 1400 °C-annealing sample. At 1800 °C, the diffraction peaks of ZrO2 phase completely disappeared, mainly because CO gas was generated at temperature above 1600 °C,32,33 the oxygen content decreased gradually with the increase of pyrolysis temperature, as shown in Fig. 6f. The process of inorganization and ceramization was shown in Fig. 6g.
In order to further analyse the change of element bonding state during the thermal treatment of precursor, the XPS characterization was carried out. The XPS survey spectra in Fig. S5† indicate the presence of Zr, C, Si and O elements. As presented in the Si2p spectra of Fig. 6d, the fitted peak at 102 eV was ascribed to the Si–C bond in the sample of 1000 °C treating, which was also observed in the spectrum of pristine PZCS-2. Another peak at 103.3 eV was observed and could be assigned to Si–O bonds.34,35 Due to the carbothermal reduction reaction of SiO2, the Si–C bond was generated with amorphous SiC phase, supported by the Si–C peak in the Si2p spectrum of sample treated at 1600 °C. As for the Zr3d spectra of precursor pyrolyzed at 1000 °C (Fig. 6e), the peaks of 185.1 and 182.6 eV are corresponded to Zr–O bonds of ZrO2 phase and Zr–O–Si bonds of ZrSiO4 phase,36 respectively. As revealed by the above XRD results, the ZrC phase would not crystallize under 1000 °C annealing, and thus there was no ZrC peak observed in the spectrum. When the precursor was treated at 1600 °C, the main peaks of 182.0 eV, 183.6 eV and 179.6 eV are corresponded to Zr–C bonds.37–39 At this temperature, ZrC began to crystallize and grow up, and the ZrO2 phase underwent carbothermal reduction to further generate ZrC grain, which was in good agreement with the XRD analysis.
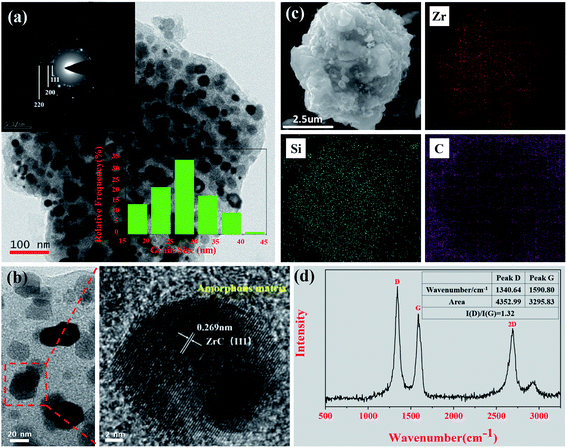
The elemental composition of the obtained preceramic powder heat-treated at 1600 °C was shown in Table 2. The relative content of Si in the preceramic powder was lower than that in PZCS-2 precursor, which might be caused by the released small molecules of Si–CH3 during the pyrolysis process. The microstructure of preceramic powder was studied by TEM and SEM subsequently. The selected area electron diffraction (SAED) pattern (inset in Fig. 7a) mainly corresponds to the (111), (200) and (220) crystal planes of the ZrC crystal, in accordance with the XRD results. From the statistical analysis of the TEM image, the average size of the ZrC grains is 28 nm, which is consistent with the grain size calculated by the Scherer formula (32 nm). The HRTEM (Fig. 7b) illustrates that the crystal has an interplanar spacing of 0.269 nm, which belongs to the (111) crystal plane of the ZrC grain.40 As shown in Fig. 7c, the elemental mapping results confirmed the uniform distribution of Zr, Si, and C elements in preceramic powder. Raman analysis was performed to analyse the elemental state of carbon atoms in the preceramic powder. Two strong peaks were detected at 1341.7 and 1596.7 cm−1, corresponding to the D peak of disordered graphite and the G peak of the graphite structure. In addition, the intensity ratio of D to G peak I(D)/I(G) is 1.32, showing that the preceramic powder mainly contains free carbon and some graphite structure.41 Combined with the SEM (Fig. 7c), it is speculated that the ZrC grains and SiC are embedded in the free carbon to form a uniform “coagulation-framework structure”. Thus, a ZrC/SiC/C preceramic https://www.baidu.com/link?url=T2NAtS2IMyaj8hclFobbfCCLfR-lpIEEBVemOmkWixfbqoGKQrtrZVqiXcB34DJ55wJUhV4q_JzXW6b025Xb9a%26wd=%26eqid=f51c90df00018ab50000000660c0341d powder was obtained by pyrolyzing of PZCS-2 precursor. When applied in high-temperature aerobic environment, the binary MC ceramics will be easily oxidized. Nevertheless, the addition of SiC to prepare MC/SiC composite ceramics can bring excellent oxidation resistance in a wide temperature range.42–44 Therefore, we believe the prepared ZrC/SiC/C composite preceramic powder in this work have important application prospects in ultra-high sonic aircraft and aero rocket engines.
| Element | C | Zr | Si | O | Composition |
|---|---|---|---|---|---|
| wt% | 44.34 | 51.38 | 2.65 | 4.48 | Zr0.56C3.69Si0.10O0.28 |
4 Conclusions
In summary, combining experimental results and theoretically calculations, we have demonstrated a novel synthesis method of preparing refractory metal containing polymers with metal in the main chain. The active species Cp2Zr(II) were formed by the reaction of Cp2ZrCl2 and reducing metal Mg in THF. First, metallocene-catalysed olefin polymerization was used to synthesize Cp2Zr–R end-capped polymer, and its synthesis mechanism was analysed. Secondly, the reaction between Cp2Zr(II) and (CH3)2Si(CH2Cl)2 generated a single-source polymer precursor containing main chain structure of –(Zr–C–Si)n–. Through DFT calculations, we found that the formation of Cp2ZrR2 was based on the active species Cp2Zr(II) radical polymerization combined with the auxiliary function of ·MgCl. The pyrolysis process of PZCS precursor was studied. The ceramic yield of PZCS-2 precursor at 900 °C was 43.9 wt%. Meanwhile, the ZrO2 crystals appeared after the PZCS-2 precursor was treated at 1000 °C, indicating that the precursor was oxidized during the preparation or transfer process. With the temperature raised, ZrC crystal grains appeared and gradually grew up to be a diameter size of 28 nm. This study mainly proposes a new method to synthesize UHTCs precursor with M–C–Si backbone structure, which is an important supplementary for the synthesis of polymer precursors by Grignard reaction. More importantly, this method can be applied to the reaction of Cp2Zr(II) and other dihaloalkanes, which provides a new idea for the synthesis of precursors of single-source backbone structure.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was sponsored by the National Natural Science Foundation of China (No. 51872329). The authors are also thankful for the Fund of Natural Science Foundation of Hunan Province (No. 2019JJ40336).References
- C. B. Guo and W. J. Li, Aerodynamic Missiles Journal, 2010, 04, 88–94 Search PubMed.
- N. P. Padture, Nat. Mater., 2016, 15, 804–809 CrossRef CAS PubMed.
- Q. B. Wen, X. G. Luan, L. Wang, X. Xu, E. Ionescu and R. J. Riedel, J. Eur. Ceram. Soc., 2019, 39, 2018–2027 CrossRef CAS.
- Y. Q. Wei, Y. Yang, M. Liu and Z. Huang, Ceram. Int., 2019, 46, 3927–3934 CrossRef.
- Y. Kubota, M. Yano, R. Inoue, Y. Kogo and K. O. Goto, J. Eur. Ceram. Soc., 2018, 38, 1095–1102 CrossRef CAS.
- S. H. Wang, Y. C. Zhang, Y. Sun, Y. Xu and M. Yang, J. Alloys Compd., 2016, 685, 828–835 CrossRef CAS.
- S. G. Chen, J. Wang and H. Wang, Mater. Des., 2016, 90, 84–90 CrossRef CAS.
- X. Long, C. W. Shao, H. Wang and J. Wang, Ceram. Int., 2016, 42, 19206–19211 CrossRef CAS.
- Z. J. Yu, Y. J. Yang, K. W. Mao, Y. Feng, Q. B. Wen and R. J. Riedel, Adv. Ceram., 2020, 9, 320–328 CrossRef CAS.
- Q. B. Wen, Z. J. Yu, R. Riedel and E. Ionescu, J. Eur. Ceram. Soc., 2021, 41, 3002–3012 CrossRef CAS.
- E. Ionescu, S. Bernard, P. Kroll, S. Ushakov and R. Riedel, Adv. Eng. Mater., 2019, 21, 1900269 CrossRef.
- E. Ionescu, H. J. Kleebe and R. Riedel, Chem. Informationsdienst, 2012, 43, 5032–5052 Search PubMed.
- J. Cheng, X. Z. Wang, J. Wang and H. Wang, J. Alloys Compd., 2018, 764, 387–396 CrossRef CAS.
- Y. H. Wu, F. H. Chen, W. J. Han and T. Zhao, Ceram. Int., 2020, 46, 22102–22107 CrossRef CAS.
- J. Cheng, X. Z. Wang, H. Wang and J. Wang, J. Am. Ceram. Soc., 2017, 100, 5044–5055 CrossRef CAS.
- Z. J. Yu, L. Yang, H. Min, P. Zhang, A. H. Liu and R. J. Riedel, J. Eur. Ceram. Soc., 2015, 35, 851–858 CrossRef.
- K. Inzenhofer, T. Schmalz, B. Wrackmeyer and G. Motz, Dalton Trans., 2011, 40, 4741–4745 RSC.
- Y. L. Tian, W. G. Zhang, M. Ge, S. Q. Yu, X. X. Lv and T. T. Zhang, RSC Adv., 2016, 6, 21048–21055 RSC.
- Y. L. Tian, M. Ge, W. G. Zhang, X. X. lV and S. Yu, Sci. Rep., 2015, 5, 16274 CrossRef CAS PubMed.
- C. Adamo and V. Barone, J. Chem. Phys., 1999, 110, 6158–6170 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2009, 113, 6378–6396 CrossRef CAS PubMed.
- I. Kim, H. K. Chang, J. S. Kim, G. W. Son and J. K. Lee, Macromol. Res., 2004, 12, 316–321 CrossRef CAS.
- K. Czaja, M. Bialek and A. Utrata, Polym. Chem., 2004, 42, 2512–2519 CrossRef CAS.
- L. G. Echevskaya, V. A. Zakharov, N. V. Semikolenova and T. B. Mikenas, Polimery, 2001, 46, 40–43 CrossRef CAS.
- Marek and Llan, Chem. Informationsdienst, 2002, 35, 1–49 Search PubMed.
- J. S. Wang and K. Matyjaszewski, J. Am. Chem. Soc., 1995, 117, 5614–5615 CrossRef CAS.
- P. Krys and K. Matyjaszewski, Eur. Polym. J., 2017, 89, 482–523 CrossRef CAS.
- Z. N. Chen, G. Fu and X. Xu, Org. Biomol. Chem., 2012, 10, 9491–9500 RSC.
- H. R. Rogers, C. L. Hill, Y. Fujiwara and R. J. Rogers, Chem. Informationsdienst, 1980, 11, 217–226 Search PubMed.
- F. P. Li, W. H. Wang, W. Dang and Y. Tang, Ceram. Int., 2019, 45, 24941–24945 CrossRef CAS.
- M. W. Chen, H. P. Qiu, W. G. Zhang, J. Jiao and R. G. Wang, Key Eng. Mater., 2014, 591, 20–25 CAS.
- Y. Lyu, H. Tang and G. D. Zhao, J. Eur. Ceram. Soc., 2020, 40, 324–332 CrossRef.
- S. Kaur, G. Mera, R. Riedel and E. Ionescu, J. Eur. Ceram. Soc., 2016, 36, 967–977 CrossRef CAS.
- Q. C. Zhang, Y. Z. Gou, J. Wang, H. Wang, K. Jian and Y. Wang, J. Eur. Ceram. Soc., 2017, 37, 1909–1916 CrossRef CAS.
- D. Briggs, Surf. Interface Anal., 1981, 3 DOI:10.1002/sia.740030412.
- G. Dorcioman, N. Stefan, E. Lambers and V. Craciun, Appl. Surf. Sci., 2011, 257, 5332–5336 CrossRef.
- A. Chu, M. L. Qin, L. Zhang and X. Qu, Int. J. Refract. Met. Hard Mater., 2013, 36, 204–210 CrossRef CAS.
- Z. J. Yu, X. Lv and S. Y. Lai, J. Adv. Ceram., 2019, 8, 112–120 CrossRef CAS.
- T. Cai, W. F. Qiu, D. Liu, W. J. Han, L. Ye and A. J. Zhao, Dalton Trans., 2013, 42, 4285–4290 RSC.
- Q. B. Wen, R. Riedel and E. Ionescu, Corros. Sci., 2018, 145, 191–198 CrossRef CAS.
- Y. T. Chen, W. Sun and X. Xiong, Ceram. Int., 2019, 45, 4685–4691 CrossRef CAS.
- G. H. Feng, H. J. Li, X. Y. Yao, M. Chen and Y. Xue, Ceram. Int., 2019, 45, 17936–17945 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra07783j |
| This journal is © The Royal Society of Chemistry 2022 |