Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Simple Zn(II) complexes for the production and degradation of polyesters†
Jack Stewarta,
Martin Fuchsb,
Jack Paynea,
Oliver Driscolla,
Gabrielle Kociok-Köhn a,
Benjamin D. Ward
a,
Benjamin D. Ward c,
Sonja Herres-Pawlis
c,
Sonja Herres-Pawlis b and
Matthew D. Jones
b and
Matthew D. Jones *a
*a
aDepartment of Chemistry, University of Bath, Claverton Down, Bath BA27AY, UK. E-mail: mj205@bath.ac.uk
bLehrstuhl für Bioanorganische Chemie, Institut für Anorganische Chemie, RWTH Aachen University, Landoltweg 1, 52074 Aachen, Germany
cDepartment of Chemistry, Cardiff University, Park Place, Cardiff, CF10 3AT, UK
First published on 7th January 2022
Abstract
Nine new complexes based on thioether appended iminophenolate (ONS) ligands have been prepared and fully characterized in solution by NMR spectroscopy. Solid-state structures were also obtained for seven complexes. In solution, all complexes were monomeric. The complexes were highly active for the polymerization of purified rac-lactide ([M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Zn]
[Zn]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BnOH] = 10
[BnOH] = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 at 180 °C) reaching TOF values up to 250
30 at 180 °C) reaching TOF values up to 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. The kinetics of the polymerization have been probed by in situ Raman spectroscopy. The rate of reaction was dramatically reduced using technical grade rac-lactide with increased initiator loading. To move towards a circular economy, it is vital that catalysts are developed to facilitate chemical recycling of commodity and emerging polymeric materials. In this vein, the complexes have been assessed for their ability to break down poly(lactic acid) and poly(ethylene terephthalate). The results from both the polymerization and degradation reactions are discussed in terms of ligand functionality.
000 h−1. The kinetics of the polymerization have been probed by in situ Raman spectroscopy. The rate of reaction was dramatically reduced using technical grade rac-lactide with increased initiator loading. To move towards a circular economy, it is vital that catalysts are developed to facilitate chemical recycling of commodity and emerging polymeric materials. In this vein, the complexes have been assessed for their ability to break down poly(lactic acid) and poly(ethylene terephthalate). The results from both the polymerization and degradation reactions are discussed in terms of ligand functionality.
Introduction
The widespread use of polymers has revolutionised almost every aspect of modern life. However, the proliferation of these materials has come with serious ecological implications.1–3 Reducing our reliance on the fossil fuel feedstocks which are necessary for producing nearly all commercial plastics is imperative due to the catastrophic environmental impact of extracting and processing these finite resources.4 The use of renewable feedstocks to create new polymers or to replace hydrocarbon components of existing products is therefore one of the most important scientific challenges of the 21st century.5–8Poly(lactic acid) (PLA) is an important bio-renewable polymer that is sourced from starch-rich materials and is amenable to enzymatic degradation or chemical recycling.9,10 It is established as a packaging material and has also found use in the agricultural industry and the bio-medical industry where biocompatible materials are required.9,11–14 PLA is typically produced through the ring-opening polymerization (ROP) of lactide initiated by a metal complex. Industrially, Sn(Oct)2 is used but there are toxicity issues associated with tin residues and so the current focus is to achieve industrially relevant activity with environmentally benign metal initiators. Poly(L-lactide) dominates the PLA market due to the ease of L-lactic acid biosynthesis. However, the stereoselective ROP of rac-lactide can improve the material properties of the polymer and thus it is often studied in academic research. A diverse range of metals has been applied to lactide ROP including Mg(II),15,16 group IV,17–24 Fe(II/III),25–32 Al(III),33–49 and In(III).50–52
Initiators based on zinc(II) have consistently shown high activity under solvent-free conditions and, in some cases, stereocontrol is observed. Coates and co-workers published a β-diiminate zinc complex that produced heterotactic PLA (Pr = 0.94) at ambient conditions.53 The most isoselective zinc initiator was reported by Ma and co-workers (Pr = 0.08) using an aminophenolate complex in toluene at −20 °C.54 However, the low temperature required for high selectivity reduced the activity (TOF = 117 h−1) and high stereoselectivity was not maintained under solvent-free conditions (Pr = 0.19–0.20). A dizinc bis(imino)diphenylamido initiator reported by Williams and co-workers remains the most active solution-based zinc initiator for lactide ROP (TOF = 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1). Using high temperature (130–180 °C), solvent-free, industrial conditions, Jones et al. reported simple ethylenediamine monophenolate complexes that polymerised L-lactide with TOF values in excess of 100
000 h−1). Using high temperature (130–180 °C), solvent-free, industrial conditions, Jones et al. reported simple ethylenediamine monophenolate complexes that polymerised L-lactide with TOF values in excess of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1.55 Further work by the same group demonstrated the introduction of a propyl linker which increased the activity at 180 °C with L-lactide giving high conversion after just 1 minute at low initiator loading (10
000 h−1.55 Further work by the same group demonstrated the introduction of a propyl linker which increased the activity at 180 °C with L-lactide giving high conversion after just 1 minute at low initiator loading (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33).56 The most active zinc initiator to date for lactide ROP was reported by Herres-Pawlis and co-workers using a bisguanidine complex which significantly outperformed Sn(Oct)2 and gave highly crystalline PLLA whilst also being active for technical grade rac-lactide.57 Further work using similar complexes from Pellecchia and co-workers further demonstrated their effectiveness at industrial conditions whilst also achieving lactide copolymerisation with ε-caprolactone.58
33).56 The most active zinc initiator to date for lactide ROP was reported by Herres-Pawlis and co-workers using a bisguanidine complex which significantly outperformed Sn(Oct)2 and gave highly crystalline PLLA whilst also being active for technical grade rac-lactide.57 Further work using similar complexes from Pellecchia and co-workers further demonstrated their effectiveness at industrial conditions whilst also achieving lactide copolymerisation with ε-caprolactone.58
Most oil-based commodity plastics do not readily decompose in the environment and end up in landfill or accumulate in the oceans where plastic microparticles are devastating to marine ecosystems.59 Mechanical recycling can be effective in some cases but leads to material downcycling over time and this limits the number of potential cycles.60 Products must ultimately be repurposed and so this is a short-term solution to retaining value in the polymer economy. Conversely, chemical recycling to monomer or value-added products offers a much more long-term solution to value retention either through depolymerisation to virgin monomer or degradation to value-added products.61 Both offer an intrinsic economic incentive to industry either through a potentially infinite number of monomer to polymer cycles or through the upgrading of waste polymer to value-added and industrially relevant chemicals in the case of degradation.62 This approach has huge potential to offer a more sustainable approach to plastic usage and is crucial to attaining a circular economy for polymers.
Simple metal salts have been reported for PLA methanolysis and can give good methyl lactate yields (YMe–LA = 87% for FeCl3) but require high temperatures (130–180 °C).63–65 The first example of a zinc complex for PLA methanolysis was reported by Avilés et al. with a dizinc NHC complex capable of producing methyl lactate over 24 hours at ambient temperature.66 More recently, Jones and co-workers have reported several homoleptic zinc monophenolate complexes that are active for the degradation of PLA into alkyl lactates at mild conditions.56,67–71 An imine-monophenolate complex with a propylene diamine linker was able to achieve a high yield of methyl lactate (YMe–La = 89%) after just 30 minutes at 50 °C.56 This far exceeds the activity shown by the ethylenediamine-based analogues which required several hours to reach high conversion at comparable temperatures.70 The most effective propylenediamine catalyst was tested for the degradation of post-consumer PLA products into ethyl lactate.67 Although activity was lower than with the clear PLA cup used for the methanolysis experiments, good yields of ethyl lactate were observed and the variation in activity was attributed to additives and the ease of dissolution of each product. More recent work has demonstrated an ethylene diamine-based ligand with a catam (N–H) moiety that far exceeds the activity of the direct imine-based counterpart, achieving 85% Me–LA yields after 30 minutes at 50 °C.70,72 The aforementioned ligand is a building block of the tetradentate catalen system, zinc complexes of which were also active for PLA degradation to methyl lactate at moderate yields after 8 hours at 80 °C.71 Recent examples have also achieved PLA alcoholysis at ambient conditions and with no solvent, in some cases demonstrating full PLA conversion after 1 hour.73,74 Organocatalysts, such as DMAP,75 TBD,76 TMC77 and ionic liquids,78–80 have also been reported but typically require high catalyst loading and can be limited by issues relating to corrosivity, toxicity and high cost.
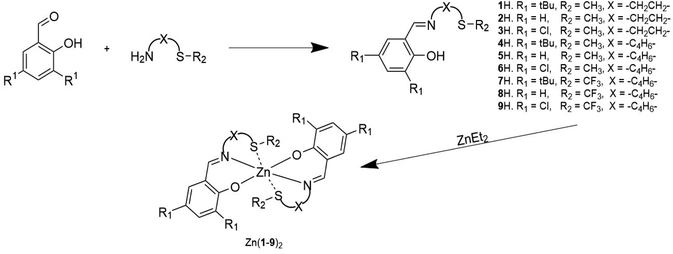
Herein, we report a series of nine Schiff base ligands each bearing a thioether motif where the phenolate substituents, thioether substituent and linking group have been varied systematically (Scheme 1). The homoleptic zinc(II) complexes were applied to lactide ROP under solvent-free conditions as well as the methanolysis of commercial PLLA and the glycolysis of PET.
Results and discussion
Synthesis and characterisation of complexes
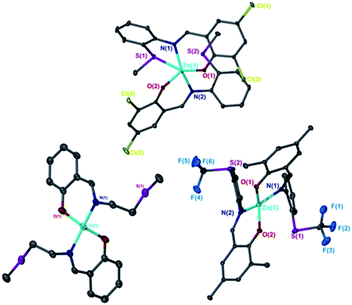
Ligands 1–9H were prepared via an imine condensation reaction between the relevant amine and salicylaldehyde derivative, with purity and structure confirmed through 1H NMR, 13C{1H} NMR and ESI-MS (Scheme 1 and Fig. SI1–SI18†). Complexation was performed under air-sensitive conditions in anhydrous toluene and the products were crystallised from varying mixtures of hexane and toluene. In all cases, the 1H NMR spectra were consistent with homoleptic complexes showing no evidence of ethyl groups remaining at the zinc centre (Fig. SI19–SI36†). This was supported by CHN analysis for all complexes.Solid state structures were obtained for seven complexes of which five, Zn(1, 2, 4, 5, 7)2, were shown to be four-coordinate with no Zn–S bonding (Table 1). Of all complexes, Zn(1)2 (R1 = tBu, R2 = CH3, X = –C2H4–) was closest to tetrahedral geometry 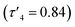 with coordination angles relatively close to the ideal tetrahedral angle of 109.5° {95.98(10)°–121.50(10)°}. The other ethylene-bridged structure, Zn(2)2 (R1 = tBu, R2 = CH3, X = –C2H4–) (Fig. 1), deviates further from the tetrahedral ideal
with coordination angles relatively close to the ideal tetrahedral angle of 109.5° {95.98(10)°–121.50(10)°}. The other ethylene-bridged structure, Zn(2)2 (R1 = tBu, R2 = CH3, X = –C2H4–) (Fig. 1), deviates further from the tetrahedral ideal 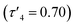 with a large coordination angle between the two nitrogen donors and the zinc centre {N(1)–Zn–N(2) = 136.49(13)}. For both Zn(1)2 and Zn(2)2, the average interatomic Zn–S distance is large: 4.4 Å and 4.5 Å respectively. These are greater than the sum of the van der Waals radii (Zn: 2.39 Å, S: 1.89)81 and thus it is reasonable to assume that there is no bonding interaction. The geometry of Zn(4)2 deviates further from tetrahedral
with a large coordination angle between the two nitrogen donors and the zinc centre {N(1)–Zn–N(2) = 136.49(13)}. For both Zn(1)2 and Zn(2)2, the average interatomic Zn–S distance is large: 4.4 Å and 4.5 Å respectively. These are greater than the sum of the van der Waals radii (Zn: 2.39 Å, S: 1.89)81 and thus it is reasonable to assume that there is no bonding interaction. The geometry of Zn(4)2 deviates further from tetrahedral 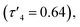 probably due to the rigid phenylene linker constraining chelation. This is also true for Zn(5)2 where two crystallographic structures were present in the sample, both with a relatively low geometry index {Zn(5)2A,
probably due to the rigid phenylene linker constraining chelation. This is also true for Zn(5)2 where two crystallographic structures were present in the sample, both with a relatively low geometry index {Zn(5)2A, 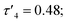 Zn(5)2B,
Zn(5)2B, 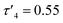 }. The average distance between zinc and sulphur atoms is considerably shorter than with the ethylene-bridged analogues, with values below the sum of van der Waals radii {Zn(4)2, 3.1 Å; Zn(5)2A, 3.1 Å; Zn(5)2B, 3.2 Å} suggesting that there could be a degree of bonding interaction. Again, this is likely a consequence of the more rigid structure forcing the sulphur to approach the metal centre more closely. Modification of the thioether substituent to CF3 {Zn(7)2: R1 = tBu, R2 = CF3, X = –C6H4–} (Fig. 1) caused the geometry to become more tetrahedral
}. The average distance between zinc and sulphur atoms is considerably shorter than with the ethylene-bridged analogues, with values below the sum of van der Waals radii {Zn(4)2, 3.1 Å; Zn(5)2A, 3.1 Å; Zn(5)2B, 3.2 Å} suggesting that there could be a degree of bonding interaction. Again, this is likely a consequence of the more rigid structure forcing the sulphur to approach the metal centre more closely. Modification of the thioether substituent to CF3 {Zn(7)2: R1 = tBu, R2 = CF3, X = –C6H4–} (Fig. 1) caused the geometry to become more tetrahedral 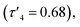 and caused the average Zn–S distance to increase to 3.4 Å. This reflects a combination of the modification in electron density at the potential sulphur donor atom and the steric difference between the substituents. The solid-state structure of Zn(6)2 indicates a five-coordinate geometry with one sulphur atom formally bonded to zinc (Fig. 1). The geometry index for a five-coordinate complex, τ5, was calculated as 0.02, indicating an almost perfectly square pyramidal geometry. Considering the similarity of the Zn–S bond lengths {Zn(1)–S(1) = 2.8032(18) Å, Zn(1)–S(2) = 2.9 Å}, an octahedral geometry is a more feasible description of the structure. This is supported by Bader's quantum theory of atoms in molecules (QTAIM) analysis, in which a bond critical point (BCP) was located between Zn(1) and S(2) with ρ = 0.145 e Å−3 (cf. BCP from Zn(1) to S(1) has ρ = 0.185 e Å−3). Assuming an octahedral geometry, the ligands wrap in a mer–mer orientation with the thioether and phenoxy donors of the respective ligands adopting a pseudo axial position {O(1)–Zn–S(1) = 157.45(3)°, O(2)–Zn–S(2) = 153.02°}. The complex based on ligand 9H gave a dimeric structure of the form Zn2(9)4 with two five coordinate zinc centres (Fig. 2). Zn(1) tended slightly towards trigonal bipyramidal (τ5 = 0.54) and Zn(2) was much closer to square pyramidal (τ5 = 0.33). There are a range of Zn–S interatomic distances with the closest (Zn–S = 3.4 Å) situated trans to the apical Zn(2)–N(4) bond. As with Zn(6)2, some degree of Zn–S interaction could be influencing the geometry at Zn(2). DOSY NMR was used to assess whether the dimeric structure is maintained in solution through comparison with Zn(4)2 and Zn(7)2. One species was observed for each complex. Comparison between the diffusion constant of the complex and the solvent (Table SI2†) was used to calculate the hydrodynamic radii of the complexes. All were sufficiently similar to indicate a consistent structure {rZn(4)2 = 7.25 Å, rZn(7)2 = 6.30 Å, rZn(9)2 = 7.13 Å}. Based on an approximate measurement of the complex diameter from the solid-state structures, it is likely that the monomeric homoleptic complex is present exclusively in solution.
and caused the average Zn–S distance to increase to 3.4 Å. This reflects a combination of the modification in electron density at the potential sulphur donor atom and the steric difference between the substituents. The solid-state structure of Zn(6)2 indicates a five-coordinate geometry with one sulphur atom formally bonded to zinc (Fig. 1). The geometry index for a five-coordinate complex, τ5, was calculated as 0.02, indicating an almost perfectly square pyramidal geometry. Considering the similarity of the Zn–S bond lengths {Zn(1)–S(1) = 2.8032(18) Å, Zn(1)–S(2) = 2.9 Å}, an octahedral geometry is a more feasible description of the structure. This is supported by Bader's quantum theory of atoms in molecules (QTAIM) analysis, in which a bond critical point (BCP) was located between Zn(1) and S(2) with ρ = 0.145 e Å−3 (cf. BCP from Zn(1) to S(1) has ρ = 0.185 e Å−3). Assuming an octahedral geometry, the ligands wrap in a mer–mer orientation with the thioether and phenoxy donors of the respective ligands adopting a pseudo axial position {O(1)–Zn–S(1) = 157.45(3)°, O(2)–Zn–S(2) = 153.02°}. The complex based on ligand 9H gave a dimeric structure of the form Zn2(9)4 with two five coordinate zinc centres (Fig. 2). Zn(1) tended slightly towards trigonal bipyramidal (τ5 = 0.54) and Zn(2) was much closer to square pyramidal (τ5 = 0.33). There are a range of Zn–S interatomic distances with the closest (Zn–S = 3.4 Å) situated trans to the apical Zn(2)–N(4) bond. As with Zn(6)2, some degree of Zn–S interaction could be influencing the geometry at Zn(2). DOSY NMR was used to assess whether the dimeric structure is maintained in solution through comparison with Zn(4)2 and Zn(7)2. One species was observed for each complex. Comparison between the diffusion constant of the complex and the solvent (Table SI2†) was used to calculate the hydrodynamic radii of the complexes. All were sufficiently similar to indicate a consistent structure {rZn(4)2 = 7.25 Å, rZn(7)2 = 6.30 Å, rZn(9)2 = 7.13 Å}. Based on an approximate measurement of the complex diameter from the solid-state structures, it is likely that the monomeric homoleptic complex is present exclusively in solution.
| Zn(1)2 | Zn(2)2 | Zn(4)2 | Zn(5)2A | Zn(5)2B | Zn(7)2 | |
|---|---|---|---|---|---|---|
| a Calculated from the two largest coordination angles.b Average of Zn–S interatomic distance. | ||||||
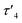 a a |
0.84 | 0.70 | 0.64 | 0.48 | 0.55 | 0.68 |
| Zn–Sb | 4.4 | 4.5 | 3.1 | 3.1 | 3.2 | 3.4 |
| Zn–O(1) | 1.896(2) | 1.944(2) | 1.9304(11) | 1.9555(15) | 1.9251(14) | 1.9188(17) |
| Zn–O(2) | 1.921(2) | 1.944(2) | 1.9304(11) | 1.9478(15) | 1.9251(14) | 1.9199(17) |
| Zn–N(1) | 1.989(3) | 1.993(2) | 2.0539(14) | 2.0755(17) | 2.0454(16) | 2.019(2) |
| Zn–N(2) | 2.003(3) | 1.933(2) | 2.0539(14) | 2.0701(17) | 2.0454(16) | 2.028(2) |
| O(1)–Zn–O(2) | 121.50(10) | 115.34(13) | 118.05(7) | 97.62(6) | 97.03(9) | 105.10(7) |
| O(1)–Zn–N(1) | 97.49(10) | 95.89(9) | 93.10(5) | 91.34(7) | 94.70(6) | 94.46(8) |
| O(1)–Zn–N(2) | 115.15(10) | 107.08(9) | 106.63(5) | 149.11(7) | 140.88(7) | 133.98(8) |
| O(2)–Zn–N(1) | 119.01(10) | 107.08(9) | 106.63(5) | 139.28(7) | 140.88(7) | 127.79(8) |
| O(2)–Zn–N(2) | 95.98(10) | 95.89(9) | 93.10(5) | 91.84(7) | 94.70(6) | 94.13(8) |
| N(1)–Zn–N(2) | 108.15(10) | 136.49(13) | 141.39(8) | 100.47(7) | 99.30(9) | 106.11(9) |
 | ||
| Fig. 1 Solid-state structures of Zn(2)2 (left), Zn(7)2 (right) and Zn(6)2 (top). Ellipsoids shown at the 30% probability level. H atoms and tBu groups have been removed for clarity. | ||
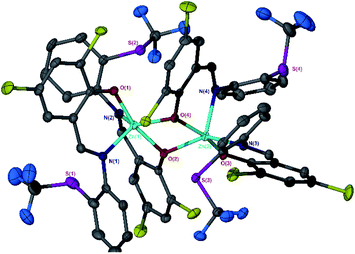 | ||
| Fig. 2 Solid-state structure of Zn(9)2 {Zn2(9)4}. Ellipsoids are shown at the 50% probability level. H atoms have been removed for clarity. | ||
Lactide polymerisation
Complexes Zn(1–9)2 were all active for solvent-free, rac-lactide polymerisation at relatively high initiator loading ([LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I]
[I]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BnOH] = 300
[BnOH] = 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 2). The complexes with ethylene linkers, Zn(1–3)2, were less active than their more rigid, phenylene bridged counterparts at this loading. The introduction of an electron withdrawing CF3 group, Zn(7–9)2, had minimal effect on the activity giving broadly similar results to the CH3 substituted complexes, Zn(4–6)2. Interestingly, the complexes with chloro-phenolate substitution were the least active in each series, despite the electron withdrawing properties which might be expected to increase the Lewis acidity at the zinc centre and hence the activity. Dispersities ranged from 1.21–1.88 with the unsubstituted complexes, Zn(2, 5, 8)2, giving the highest dispersities in their respective series, as may be expected from the lack of steric hindrance. The PLA produced by Zn(1–9)2 was essentially atactic (Pr = 0.51–0.60) and this is in keeping with other zinc monophenolate complexes under these conditions.56,69,71 When the lactide to initiator ratio was increased, ([LA]
1) (Table 2). The complexes with ethylene linkers, Zn(1–3)2, were less active than their more rigid, phenylene bridged counterparts at this loading. The introduction of an electron withdrawing CF3 group, Zn(7–9)2, had minimal effect on the activity giving broadly similar results to the CH3 substituted complexes, Zn(4–6)2. Interestingly, the complexes with chloro-phenolate substitution were the least active in each series, despite the electron withdrawing properties which might be expected to increase the Lewis acidity at the zinc centre and hence the activity. Dispersities ranged from 1.21–1.88 with the unsubstituted complexes, Zn(2, 5, 8)2, giving the highest dispersities in their respective series, as may be expected from the lack of steric hindrance. The PLA produced by Zn(1–9)2 was essentially atactic (Pr = 0.51–0.60) and this is in keeping with other zinc monophenolate complexes under these conditions.56,69,71 When the lactide to initiator ratio was increased, ([LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I]
[I]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BnOH] = 3000
[BnOH] = 3000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) the activity differences between the complexes became more apparent (Table 3). Ethylene bridge complexes, Zn(1–3)2, took much longer to reach high conversion (t = 30–60 min) than the phenylene bridge equivalents. The chlorinated analogues were the least active in each series whilst also showing a slight increase in heterotacticity compared to the other initiators. This reflects an alteration of the coordination sphere, facilitated by the chloride substituents, that increases steric crowding of the zinc centre. The octahedral structure of Zn(6)2 is evidence of this effect, which could also apply to Zn(3)2. There is no significant difference in activity between the –CH3 and –CF3 substituted complexes, except in the case of Zn(9)2, where the CF3 group appears to partially negate the drop in activity that is associated with the chloride substituents. Phenylene bridged complexes, where R1 = H or tBu [Zn(4, 5, 7, 8)], were clearly the most active initiators converting sufficient lactide after less than 5 minutes to stop stirring. Reasonable molecular weight control was maintained throughout and there was a narrowing of dispersity, (Đ = 1.08–1.48) that is often associated with increasing the monomer to initiator ratio. The differences in activity between the complexes show some correlation with the measured Zn–S interatomic distance (Table 1) in a volcano-style relationship (Fig. SI47†). Ethylene bridge complexes, Zn(1, 2)2, where the Zn–S distance is large (4.4–4.5 Å), and there is presumably no interaction, are comparatively slow. Conversely, the direct phenylene-bridged analogues {Zn(4, 5, 7)2} have a smaller average Zn–S distance (3.1–3.4 Å) and are the most active for lactide polymerisation. Zn(9)2 has an average Zn–S distance of 3.9 Å, intermediate to the two aforementioned groups of complexes, and this is reflected in the activity. The zinc centre in Zn(6)2 is considerably closer to the two sulphur atoms (Zn–S = 2.8–2.9 Å). The activity is similar to the ethylene-bridged complexes. This trend can be rationalised by considering the coordination of lactide to the saturated zinc centre. When the Zn–S distance is small, the complexes tend to distort further from tetrahedral geometry (e.g. Zn(1)2:
10) the activity differences between the complexes became more apparent (Table 3). Ethylene bridge complexes, Zn(1–3)2, took much longer to reach high conversion (t = 30–60 min) than the phenylene bridge equivalents. The chlorinated analogues were the least active in each series whilst also showing a slight increase in heterotacticity compared to the other initiators. This reflects an alteration of the coordination sphere, facilitated by the chloride substituents, that increases steric crowding of the zinc centre. The octahedral structure of Zn(6)2 is evidence of this effect, which could also apply to Zn(3)2. There is no significant difference in activity between the –CH3 and –CF3 substituted complexes, except in the case of Zn(9)2, where the CF3 group appears to partially negate the drop in activity that is associated with the chloride substituents. Phenylene bridged complexes, where R1 = H or tBu [Zn(4, 5, 7, 8)], were clearly the most active initiators converting sufficient lactide after less than 5 minutes to stop stirring. Reasonable molecular weight control was maintained throughout and there was a narrowing of dispersity, (Đ = 1.08–1.48) that is often associated with increasing the monomer to initiator ratio. The differences in activity between the complexes show some correlation with the measured Zn–S interatomic distance (Table 1) in a volcano-style relationship (Fig. SI47†). Ethylene bridge complexes, Zn(1, 2)2, where the Zn–S distance is large (4.4–4.5 Å), and there is presumably no interaction, are comparatively slow. Conversely, the direct phenylene-bridged analogues {Zn(4, 5, 7)2} have a smaller average Zn–S distance (3.1–3.4 Å) and are the most active for lactide polymerisation. Zn(9)2 has an average Zn–S distance of 3.9 Å, intermediate to the two aforementioned groups of complexes, and this is reflected in the activity. The zinc centre in Zn(6)2 is considerably closer to the two sulphur atoms (Zn–S = 2.8–2.9 Å). The activity is similar to the ethylene-bridged complexes. This trend can be rationalised by considering the coordination of lactide to the saturated zinc centre. When the Zn–S distance is small, the complexes tend to distort further from tetrahedral geometry (e.g. Zn(1)2: 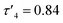 , TOF = 2080 h−1; Zn(1)2:
, TOF = 2080 h−1; Zn(1)2: 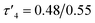 , TOF = 124
, TOF = 124![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 h−1). The displacement of weakly bound sulphur by lactide is easier than coordination to an undistorted tetrahedral centre. This explains the increase in activity up to a Zn–S distance of around 3 Å, after which the sulphur becomes more difficult to displace and the activity drops sharply.
200 h−1). The displacement of weakly bound sulphur by lactide is easier than coordination to an undistorted tetrahedral centre. This explains the increase in activity up to a Zn–S distance of around 3 Å, after which the sulphur becomes more difficult to displace and the activity drops sharply.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a
1a
| Init. | Time/min | Conv.b% | Prc | Mne | Mn calc.d | Đe | TOF/h−1f |
|---|---|---|---|---|---|---|---|
a Conditions: rac-LA (1 g), [LA]/[Zn]/[BnOH] = 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, solvent free.b Determined by 1H NMR spectroscopy.c Probability of racemic enchainment, determined by 1H{1H} NMR spectroscopy.d Theoretical molecular weight calculated from conversion (rounded to the nearest 50): {(conversion × 3 × Mn [LA]) + Mn [BnOH]}.e Determined from GPC (in tetrahydrofuran) referenced against polystyrene standards × 0.58.f TOF = [LA]t/([Zn] × t). 1, solvent free.b Determined by 1H NMR spectroscopy.c Probability of racemic enchainment, determined by 1H{1H} NMR spectroscopy.d Theoretical molecular weight calculated from conversion (rounded to the nearest 50): {(conversion × 3 × Mn [LA]) + Mn [BnOH]}.e Determined from GPC (in tetrahydrofuran) referenced against polystyrene standards × 0.58.f TOF = [LA]t/([Zn] × t). |
|||||||
| Zn(1)2 | 3 | 59 | 0.55 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.32 | 3550 |
| Zn(2)2 | 7 | 73 | 0.55 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 450 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
1.34 | 1900 |
| Zn(3)2 | 25 | 60 | 0.60 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 050 |
1.26 | 450 |
| Zn(4)2 | 2 | 81 | 0.57 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.59 | 7300 |
| Zn(5)2 | <1 | 56 | 0.57 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 050 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.88 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| Zn(6)2 | 3 | 61 | 0.57 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.21 | 3650 |
| Zn(7)2 | 1 | 72 | 0.59 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 950 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250 |
1.52 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 950 |
| Zn(8)2 | 2 | 72 | 0.51 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250 |
1.66 | 6500 |
| Zn(9)2 | 10 | 46 | 0.58 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 950 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.27 | 850 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10a
10a
| Init. | Time/min | Conv.b% | Pmc | Mne | Mn calc.d | Đe | TOF/h−1f |
|---|---|---|---|---|---|---|---|
a Conditions: rac-LA (1.5 g), [LA]/[Zn]/[BnOH] = 3000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, solvent free.b Determined by 1H NMR spectroscopy.c Probability of racemic enchainment, determined by 1H{1H} NMR spectroscopy.d Theoretical molecular weight calculated from conversion (rounded to the nearest 50): {(conversion × 3 × Mn [LA]) + Mn [BnOH]}.e Determined from GPC (in tetrahydrofuran) referenced against polystyrene standards × 0.58.f TOF = [LA]t/([Zn] × t). 10, solvent free.b Determined by 1H NMR spectroscopy.c Probability of racemic enchainment, determined by 1H{1H} NMR spectroscopy.d Theoretical molecular weight calculated from conversion (rounded to the nearest 50): {(conversion × 3 × Mn [LA]) + Mn [BnOH]}.e Determined from GPC (in tetrahydrofuran) referenced against polystyrene standards × 0.58.f TOF = [LA]t/([Zn] × t). |
|||||||
| Zn(1)2 | 45 | 52 | 0.56 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.17 | 2080 |
| Zn(2)2 | 30 | 73 | 0.58 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 150 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
1.14 | 4380 |
| Zn(3)2 | 60 | 40 | 0.62 | 4750 | 5850 | 1.08 | 1200 |
| Zn(4)2 | 4 | 71 | 0.60 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.23 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 950 |
| Zn(5)2 | 1 | 69 | 0.57 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 150 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 950 |
1.28 | 124![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
| Zn(6)2 | 40 | 53 | 0.61 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 050 |
1.16 | 2385 |
| Zn(7)2 | 2 | 73 | 0.57 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 950 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
1.48 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
| Zn(8)2 | 4 | 70 | 0.56 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.38 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| Zn(9)2 | 12 | 64 | 0.60 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.36 | 9600 |
The polymer produced by Zn(3)2 at this loading was suitable for MALDI-ToF analysis (Fig. SI43†). The repeat unit of the main series is 144 g mol−1, and the spectrum is centred around 4457.9 g mol−1; similar to that measured by GPC and to the theoretical molecular weight (Mn GPC = 4750 g mol−1, Mn calc. = 5850 g mol−1). At low molecular weight there is evidence of ionisation by incidental potassium and a small amount of transesterified PLA.
The most active initiators, Zn(4, 5, 7, 8, 9)2, were tested for rac-lactide polymerisation at very low catalyst loading ([LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I]
[I]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BnOH] = 10
[BnOH] = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30); similar to the concentrations employed in an industrial setting with Sn(Oct)2 (Table 4).82 Under these conditions, Zn(5)2 (R1 = H, R2 = CH3, X = C6H4) and Zn(7)2 (R1 = tBu, R2 = CF3, X = C6H4) were clearly the most active initiators achieving 68% and 51% conversion in 3 and 9 minutes respectively. These were selected for further kinetic analysis. With the exception of Zn(5)2, conversion was limited to around 50%, suggesting some initiator decomposition is taking place. The molecular weight control was maintained at this loading and the dispersities remained consistent (Đ = 1.20–1.44). To further probe the effectiveness of these complexes at industrial conditions, the most active complexes, Zn(4, 5, 7, 8, 9)2, were tested at 180 °C (Table 5). All the initiators tested were sufficiently robust to show activity at this temperature and were consistently more active than at 130 °C (t = 2–12 min, conversion = 56–83%), due both to the increase in temperature and the decreased viscosity allowing for better mixing at higher conversions. As might be expected at elevated temperatures, the dispersities were broader (Đ = 1.45–1.68) although reasonable molecular weight control was maintained. Zn(5)2 and Zn(7)2 were also tested for the polymerisation of L-lactide, the most commonly used industrial monomer. There was a small reduction in activity with L-lactide for both initiators, although the activity was still high. The reaction was very well controlled with comparatively narrow dispersities (Zn(5)2, Đ = 1.16; Zn(7)2, Đ = 1.15) and excellent molecular weight control (Zn(5)2, Mn = 32
30); similar to the concentrations employed in an industrial setting with Sn(Oct)2 (Table 4).82 Under these conditions, Zn(5)2 (R1 = H, R2 = CH3, X = C6H4) and Zn(7)2 (R1 = tBu, R2 = CF3, X = C6H4) were clearly the most active initiators achieving 68% and 51% conversion in 3 and 9 minutes respectively. These were selected for further kinetic analysis. With the exception of Zn(5)2, conversion was limited to around 50%, suggesting some initiator decomposition is taking place. The molecular weight control was maintained at this loading and the dispersities remained consistent (Đ = 1.20–1.44). To further probe the effectiveness of these complexes at industrial conditions, the most active complexes, Zn(4, 5, 7, 8, 9)2, were tested at 180 °C (Table 5). All the initiators tested were sufficiently robust to show activity at this temperature and were consistently more active than at 130 °C (t = 2–12 min, conversion = 56–83%), due both to the increase in temperature and the decreased viscosity allowing for better mixing at higher conversions. As might be expected at elevated temperatures, the dispersities were broader (Đ = 1.45–1.68) although reasonable molecular weight control was maintained. Zn(5)2 and Zn(7)2 were also tested for the polymerisation of L-lactide, the most commonly used industrial monomer. There was a small reduction in activity with L-lactide for both initiators, although the activity was still high. The reaction was very well controlled with comparatively narrow dispersities (Zn(5)2, Đ = 1.16; Zn(7)2, Đ = 1.15) and excellent molecular weight control (Zn(5)2, Mn = 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 g mol−1, Mn (theo.) = 35
050 g mol−1, Mn (theo.) = 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 g mol−1; Zn(7)2, Mn = 28
750 g mol−1; Zn(7)2, Mn = 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 g mol−1, Mn (theo.) = 27
050 g mol−1, Mn (theo.) = 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 g mol−1). DSC analysis showed that the melting point of PLLA produced by Zn(5)2 and Zn(7)2 was 162 °C and 167 °C respectively (Fig. SI45 and SI46†). This suggests that there is limited epimerisation and the presence of a single methine peak in the 1H{1H} NMR spectrum supports this conclusion. Despite the yellow colouring of the initiators, white PLA was produced at the highest ratios employed and this is desirable from a commercial standpoint (Fig. SI44†). The performance of Zn(5)2 and Zn(7)2 with L-lactide provides a direct comparison with analogous zinc ONN complexes. Ethylene-bridged complexes reported by Jones and co-workers gave comparable results at similar conditions (180 °C, [LA]
050 g mol−1). DSC analysis showed that the melting point of PLLA produced by Zn(5)2 and Zn(7)2 was 162 °C and 167 °C respectively (Fig. SI45 and SI46†). This suggests that there is limited epimerisation and the presence of a single methine peak in the 1H{1H} NMR spectrum supports this conclusion. Despite the yellow colouring of the initiators, white PLA was produced at the highest ratios employed and this is desirable from a commercial standpoint (Fig. SI44†). The performance of Zn(5)2 and Zn(7)2 with L-lactide provides a direct comparison with analogous zinc ONN complexes. Ethylene-bridged complexes reported by Jones and co-workers gave comparable results at similar conditions (180 °C, [LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I]
[I]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BnOH] = 10
[BnOH] = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33) taking 3 minutes to achieve a higher conversion of 94%.55
33) taking 3 minutes to achieve a higher conversion of 94%.55
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30a
30a
| Init. | Time/min | Conv.b% | Pmc | Mne | Mn calc.d | Đe | TOF/h−1f |
|---|---|---|---|---|---|---|---|
a Conditions: rac-LA (3 g), [LA]/[Zn]/[BnOH] = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, solvent free.b Determined by 1H NMR spectroscopy.c Probability of racemic enchainment, determined by 1H{1H} NMR spectroscopy.d Theoretical molecular weight calculated from conversion (rounded to the nearest 50): {(conversion × 3 × Mn [LA]) + Mn [BnOH]}.e Determined from GPC (in tetrahydrofuran) referenced against polystyrene standards × 0.58.f TOF = [LA]t/([Zn] × t). 30, solvent free.b Determined by 1H NMR spectroscopy.c Probability of racemic enchainment, determined by 1H{1H} NMR spectroscopy.d Theoretical molecular weight calculated from conversion (rounded to the nearest 50): {(conversion × 3 × Mn [LA]) + Mn [BnOH]}.e Determined from GPC (in tetrahydrofuran) referenced against polystyrene standards × 0.58.f TOF = [LA]t/([Zn] × t). |
|||||||
| Zn(4)2 | 30 | 51 | 0.58 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.23 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
| Zn(5)2 | 3 | 68 | 0.57 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850 |
1.20 | 136![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Zn(7)2 | 9 | 51 | 0.58 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 450 |
1.28 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Zn(8)2 | 45 | 54 | 0.58 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.44 | 7200 |
| Zn(9)2 | 30 | 55 | 0.61 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.31 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30a
30a
| Init. | Time/min | Conv.b% | Pmc | Mne | Mn calc.d | Đe | TOF/h−1h |
|---|---|---|---|---|---|---|---|
a Conditions: rac-LA (3 g), [LA]/[Zn]/[BnOH] = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, solvent free.b Determined by 1H NMR spectroscopy.c Probability of racemic enchainment, determined by 1H{1H} NMR spectroscopy.d Theoretical molecular weight calculated from conversion (rounded to the nearest 50): {(conversion × 3 × Mn [LA]) + Mn [BnOH]}.e Determined from GPC (in tetrahydrofuran) referenced against polystyrene standards × 0.58.f L-Lactide used.g One peak present in 1H{1H} NMR spectrum.h TOF = [LA]t/([Zn] × t). 30, solvent free.b Determined by 1H NMR spectroscopy.c Probability of racemic enchainment, determined by 1H{1H} NMR spectroscopy.d Theoretical molecular weight calculated from conversion (rounded to the nearest 50): {(conversion × 3 × Mn [LA]) + Mn [BnOH]}.e Determined from GPC (in tetrahydrofuran) referenced against polystyrene standards × 0.58.f L-Lactide used.g One peak present in 1H{1H} NMR spectrum.h TOF = [LA]t/([Zn] × t). |
|||||||
| Zn(4)2 | 3 | 74 | 0.57 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
1.62 | 148![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Zn(5)2 | 2 | 83 | 0.57 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 050 |
1.53 | 249![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Zn(5)2f | 4 | 74 | 0.00g | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 050 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
1.16 | 111![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Zn(7)2 | 6 | 67 | 0.58 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350 |
1.45 | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Zn(7)2f | 6 | 56 | 0.00g | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 050 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 050 |
1.15 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Zn(8)2 | 3 | 69 | 0.58 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 150 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.60 | 138![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Zn(9)2 | 12 | 65 | 0.62 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.68 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
In order to probe the polymerisation mechanism and the role of the co-initiator, a stoichiometric reaction was carried out. A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solution of rac-lactide and Zn(7)2 in CDCl3 was heated to 50 °C for 90 min and monitored by NMR spectroscopy (Fig. SI48†). Resonances similar to those of opened lactide increased significantly upon addition of BnOH to 60% in ten minutes at room temperature and 94% conversion after a further 90 min at 50 °C. This shows that a co-initiator is required for high activity but that a degree of activity might be observed in its absence. This could be explained through initiation by impurities in rac-lactide or through a coordination–insertion mechanism facilitated by a dissociated ligand as observed by McKeown et al. with zinc ONN complexes.55 Some evidence of ligand dissociation was observed in the methyl region of the 1H NMR spectrum where two minor resonances corresponding to t-butyl groups steadily increased throughout the reaction (Fig. SI49†). Furthermore, a new signal was present in the 19F NMR spectrum at the end of the reaction (Fig. SI50†). DOSY NMR analysis of the final reaction mixture showed that the ring-opened lactide had a distinctly different diffusion constant to the metal complex (Fig. SI51†). This suggests an activated monomer mechanism wherein the components of the reaction are never fully bonded to the metal and the co-initiator is exogenous. Therefore, the best explanation for the conversion observed prior to BnOH addition is through initiation by impurities in the monomer. The observation of additional resonances in the 1H and 19F NMR spectra is evidence of complex decomposition and could account for the limited conversion achieved by these systems.
1 solution of rac-lactide and Zn(7)2 in CDCl3 was heated to 50 °C for 90 min and monitored by NMR spectroscopy (Fig. SI48†). Resonances similar to those of opened lactide increased significantly upon addition of BnOH to 60% in ten minutes at room temperature and 94% conversion after a further 90 min at 50 °C. This shows that a co-initiator is required for high activity but that a degree of activity might be observed in its absence. This could be explained through initiation by impurities in rac-lactide or through a coordination–insertion mechanism facilitated by a dissociated ligand as observed by McKeown et al. with zinc ONN complexes.55 Some evidence of ligand dissociation was observed in the methyl region of the 1H NMR spectrum where two minor resonances corresponding to t-butyl groups steadily increased throughout the reaction (Fig. SI49†). Furthermore, a new signal was present in the 19F NMR spectrum at the end of the reaction (Fig. SI50†). DOSY NMR analysis of the final reaction mixture showed that the ring-opened lactide had a distinctly different diffusion constant to the metal complex (Fig. SI51†). This suggests an activated monomer mechanism wherein the components of the reaction are never fully bonded to the metal and the co-initiator is exogenous. Therefore, the best explanation for the conversion observed prior to BnOH addition is through initiation by impurities in the monomer. The observation of additional resonances in the 1H and 19F NMR spectra is evidence of complex decomposition and could account for the limited conversion achieved by these systems.
Raman kinetics
To further probe the effectiveness of these complexes at industrial conditions, the kinetics were investigated using in situ Raman spectroscopy. In these experiments Zn(5)2 and Zn(7)2 were taken forward, initially testing at 150 °C at a rac-LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn ratio of 2500
Zn ratio of 2500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using technical grade lactide, as a direct comparison to previous systems reported by Herres-Pawlis.57,83,84 Results were disappointing with low conversion (ca. 15–40%) being achieved after 3 h. This may indicate that the complexes herein are less stable to impurities in the monomer. More promising results were achieved using recrystallised L-LA and 4-methylbenzyl alcohol in a 3000
1 using technical grade lactide, as a direct comparison to previous systems reported by Herres-Pawlis.57,83,84 Results were disappointing with low conversion (ca. 15–40%) being achieved after 3 h. This may indicate that the complexes herein are less stable to impurities in the monomer. More promising results were achieved using recrystallised L-LA and 4-methylbenzyl alcohol in a 3000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ([LA]
10 ([LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Zn]
[Zn]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [4-MeBnOH]) ratio (Fig. SI53†). Zn(5)2 proved more active with a conversion of 48% (Mn = 17
[4-MeBnOH]) ratio (Fig. SI53†). Zn(5)2 proved more active with a conversion of 48% (Mn = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 g mol−1, Đ = 1.06 after 3 h), and a kapp = 1.3 × 10−3 s−1. The corresponding value for Zn(7)2 was kapp = 1.7 × 10−4 s−1. The process was modified to mimic that of the smaller scale testing with Zn(7)2 and benzyl alcohol as a co-initiator (Fig. SI55†). A marked increase in activity was observed (kapp = 2.2 × 10−3 s−1) and a conversion of 75% was achieved after 45 min (Mn = 31
900 g mol−1, Đ = 1.06 after 3 h), and a kapp = 1.3 × 10−3 s−1. The corresponding value for Zn(7)2 was kapp = 1.7 × 10−4 s−1. The process was modified to mimic that of the smaller scale testing with Zn(7)2 and benzyl alcohol as a co-initiator (Fig. SI55†). A marked increase in activity was observed (kapp = 2.2 × 10−3 s−1) and a conversion of 75% was achieved after 45 min (Mn = 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g mol−1, Đ = 1.22). Zn(7)2 was further tested at a ratio of 10
200 g mol−1, Đ = 1.22). Zn(7)2 was further tested at a ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 ([LA]
100 ([LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Zn]
[Zn]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH) achieving 34% after 6 hours (kapp = 9.95 × 10−5 s−1) (Fig. SI57†).
BnOH) achieving 34% after 6 hours (kapp = 9.95 × 10−5 s−1) (Fig. SI57†).
Polyester degradation
Zn(1–9)2 were investigated for PLA methanolysis at 80 °C in THF. The product, Me–LA, can be converted to lactide for the production of virgin PLA and is also an emerging green solvent.62 Furthermore, Me–LA can replace lactic acid in many transformations so has potential as a platform chemical.85 Waste polymer from a commercial source (0.25 g, PLLA cup, Mn = 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510 g mol−1) and catalyst (20 mg, 8 wt%) were added in a glovebox and subsequently dissolved in THF. When the polymer was fully solubilised, methanol was added to initiate the degradation reaction.
510 g mol−1) and catalyst (20 mg, 8 wt%) were added in a glovebox and subsequently dissolved in THF. When the polymer was fully solubilised, methanol was added to initiate the degradation reaction.
This reaction has been shown to proceed via a two-step mechanism with PLA initially broken down into oligomers before being converted to the product.70 Analysis of the methine region (ca. δ = 4.2–5.2 ppm) of the 1H NMR spectrum gives three key parameters: internal methine conversion (Xint), Me–LA yield (YMe–LA) and the selectivity to Me–LA (SMe–LA).
All complexes were active for the methanolysis of PLA (Table 6). Of the ethylene bridge complexes, Zn(1–3)2, the highest activity was recorded for Zn(2)2 (Xint = 87%) and this complex produced the most Me–LA of all the catalysts that were tested (SMe–LA = 72%, YMe–LA = 63%). This contrasts with the polymerisation studies where Zn(2)2 significantly underperformed most of the phenylene-bridged analogues. This can be rationalised by considering the equilibrium between oligomer and methyl lactate where the backward reaction (k−2) will be slower with a less effective polymerisation catalyst thus driving the reaction towards the product. The most active catalysts were Zn(4)2 (R1 = tBu, R2 = CH3, X = C6H4) and Zn(4)2 (R1 = H, R2 = CH3, X = C6H4) which gave internal methine conversions of 94% and 96% respectively. The latter also gave high selectivity and yield of Me–LA (YMe–LA = 59%, SMe–LA = 62%). As observed during polymerisation, Zn(6)2 was the slowest in this series (R1 = Cl, R2 = CH3, X = C6H4) presumably resulting from steric crowding from the pseudo-octahedral structure. Interestingly, the introduction of a CF3 group at the R2 position resulted in a sharp decline in activity, particularly for the non-halogenated complexes Zn(7–8)2, both of which converted 39% of internal methine after 8 hours with correspondingly low Me–LA yields. However, Zn(9)2 (R1 = Cl, R2 = CF3, X = C6H4) achieved good activity and selectivity (Xint = 81%, SMe–LA = 50%, YMe–LA = 41%) under the same conditions.
| Cat. | Time/h | Cat. loading/wt% | YMe–LA/% | SMe–LA/% | Xint/% |
|---|---|---|---|---|---|
a Reaction conditions: 0.25 g of PLLA cup (Mn = 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510 g mol−1), VTHF 510 g mol−1), VTHF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VMeOH = 4 VMeOH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, nMeOH 1, nMeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nester = 7 nester = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8 wt% cat. loading (1.3–2.1 mol% relative to ester linkages). YMe–LA, SMe–LA and Xint determined by 1H NMR upon solvent removal. 1, 8 wt% cat. loading (1.3–2.1 mol% relative to ester linkages). YMe–LA, SMe–LA and Xint determined by 1H NMR upon solvent removal. |
|||||
| Zn(1)2 | 8 | 8 | 24 | 33 | 72 |
| Zn(2)2 | 8 | 8 | 63 | 72 | 87 |
| Zn(3)2 | 8 | 8 | 13 | 20 | 65 |
| Zn(4)2 | 8 | 8 | 47 | 50 | 94 |
| Zn(5)2 | 8 | 8 | 59 | 62 | 96 |
| Zn(6)2 | 8 | 8 | 32 | 41 | 78 |
| Zn(7)2 | 8 | 8 | 6 | 16 | 39 |
| Zn(8)2 | 8 | 8 | 7 | 17 | 39 |
| Zn(9)2 | 8 | 8 | 41 | 50 | 81 |
The most active catalysts [Zn(2, 4, 5, 9)2 were tested at 50 °C for 18 hours (Table 7). There was a reduction in activity and selectivity compared with the 80 °C reactions and this is consistent with literature examples.69,86 Performance particularly decreased for Zn(4)2 (Xint = 57%, SMe–LA = 21%, YMe–LA = 12%) and Zn(5)2 (Xint = 56%, SMe–LA = 25%, YMe–LA = 14%). There was an indication that Zn(4–5)2 are sensitive to residual moisture in the THF and this could lead to deactivation over extended reaction times. Due to the lack of labile alkyl groups around the metal, it is likely that this hydrolytic sensitivity comes from the active species or a reaction intermediate. Zn(2)2 remained the most selective catalyst and gave reasonable conversion of internal methine (Xint = 77%).
| Cat. | Time/h | Cat. loading/wt% | YMe–LA/% | SMe–LA/% | Xint/% |
|---|---|---|---|---|---|
a Reaction conditions: 0.25 g of PLLA cup (Mn = 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510 g mol−1), VTHF 510 g mol−1), VTHF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VMeOH = 4 VMeOH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, nMeOH 1, nMeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nester = 7 nester = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8 wt% cat. loading (1.3–2.1 mol% relative to ester linkages). YMe–LA, SMe–LA and Xint determined by 1H NMR upon solvent removal. 1, 8 wt% cat. loading (1.3–2.1 mol% relative to ester linkages). YMe–LA, SMe–LA and Xint determined by 1H NMR upon solvent removal. |
|||||
| Zn(2)2 | 18 | 8 | 31 | 41 | 77 |
| Zn(4)2 | 18 | 8 | 12 | 21 | 57 |
| Zn(5)2 | 18 | 8 | 14 | 25 | 56 |
| Zn(9)2 | 18 | 8 | 16 | 26 | 62 |
Kinetic analysis of Zn(2, 4, 5, 9)2 was attempted through taking aliquots every hour for 4–6 hours with a final point measured after 8 hours. Based on previous literature, pseudo first-order kinetics of PLA consumption were assumed.70 Zn(4)2 and Zn(5)2 were not amenable to sampling and the reactivity was quenched.
To expand the scope of polyester degradation, the most active PLA degradation catalysts were applied to the glycolysis of PET to bis(2-hydroxyethyl) terephthalate (BHET); a useful product that can be polymerised to make virgin PET or can be used to make unsaturated polymer resins.87,88 PET is one of the most prevalent commodity plastics accounting for 9% of global plastic demand in 2015.89 Although widely mechanically recycled, it is still a major contributor to plastic pollution and is not degradable in the environment.90 Zn[2,4,5,9]2 were all active for the glycolysis of PET, with full dissolution observed after 1.5–4 hours for a standard carbonated drinks bottle (Table 8). Full degradation of PET was assumed at this point and BHET was isolated through recrystallisation from water. Isolated yields between 42% and 55% were observed, and this is consistent with previous literature examples using zinc catalysts.71,72 The most active catalyst {Zn(2)2: t = 1.5 h, YBHET = 0.16 g (48%) was tested with thin-film PET; a proxy for manufacturing waste. As expected, the reaction time was significantly reduced (t = 0.75 h) and a percentage yield of 51% was attained, again in keeping with previous results by Payne et al.71,72
| Cat. | Time/hours | T/°C | Cat. loading/wt% | YBHET (g/%) |
|---|---|---|---|---|
a Reaction conditions: 0.25 g of carbonated drinks bottle (Mn ∼ 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), 27.5 equivalents of EG (relative to ester linkages), 8 wt% cat. loading (0.02 g, 1.9–3.4 mol% relative to ester linkages).b 0.25 g PET thin film. 000 g mol−1), 27.5 equivalents of EG (relative to ester linkages), 8 wt% cat. loading (0.02 g, 1.9–3.4 mol% relative to ester linkages).b 0.25 g PET thin film. |
||||
| Zn(2)2 | 1.5 | 180 | 8 | 0.16 (48%) |
| Zn(2)2b | 0.75 | 180 | 8 | 0.17 (51%) |
| Zn(4)2 | 4 | 180 | 8 | 0.18 (55%) |
| Zn(5)2 | 4 | 180 | 8 | 0.14 (42%) |
| Zn(9)2 | 3.5 | 180 | 8 | 0.17 (51%) |
In the reaction with Zn(2)2, colourless BHET was obtained after recrystallisation and this is important when considering the quality of PET if it were repolymerized (Fig. SI62†).91
Conclusions
A series of nine ONS zinc complexes were prepared and characterized through NMR spectroscopy and single-crystal X-ray diffraction. The rapid polymerization of rac-lactide was demonstrated under industrially relevant, solvent-free conditions. Up to 83% conversion was achieved in 2 minutes at 180 °C with TOF values up to 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. Crystalline PLLA was also produced rapidly with minimal epimerization. Good control of molecular weight and dispersity (Đ = 1.08–1.88) was maintained throughout. Raman kinetic experiments showed that the activity is significantly reduced when using technical grade rac-lactide even at relatively high catalyst loading. For the optimised preparation with Zn(7)2, an apparent rate constant kapp = 2.23 × 10−3 s−1 was measured. All complexes were active for the degradation of PLA to methyl lactate under mild conditions. Two waste sources of PET were also degraded to colourless BHET with full PET consumption observed after 0.75–4 hours (Scheme 1).
000 h−1. Crystalline PLLA was also produced rapidly with minimal epimerization. Good control of molecular weight and dispersity (Đ = 1.08–1.88) was maintained throughout. Raman kinetic experiments showed that the activity is significantly reduced when using technical grade rac-lactide even at relatively high catalyst loading. For the optimised preparation with Zn(7)2, an apparent rate constant kapp = 2.23 × 10−3 s−1 was measured. All complexes were active for the degradation of PLA to methyl lactate under mild conditions. Two waste sources of PET were also degraded to colourless BHET with full PET consumption observed after 0.75–4 hours (Scheme 1).
Experimental section
All experimental details are provided in the ESI† with the original data.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank the EPSRC for funding a PhD studentship to JS (EP/L016443/1) and for funding for JP (EP/L016354/1). The University of Bath (OD) and MC2 are acknowledged for their use of facilities. We thank the EPSRC National Crystallographic Service center for collection of data for Zn(2)2. This study was partly funded under the Excellence Strategy of the Federal Government and the Länder (G: (DE-82)EXS-PF-KA007).References
- J. R. Jambeck, R. Geyer, C. Wilcox, T. R. Siegler, M. Perryman, A. Andrady, R. Narayan and K. L. Law, Science, 2015, 347, 768–771 CrossRef CAS PubMed.
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, e1700782 CrossRef PubMed.
- H. Namazi, BioImpacts, 2017, 7, 73–74 CrossRef CAS PubMed.
- R. C. Thompson, C. J. Moore, F. S. V. Saal and S. H. Swan, Philos. Trans. R. Soc., B, 2009, 364, 2153–2166 CrossRef CAS PubMed.
- I. Vroman and L. Tighzert, Materials, 2009, 2, 307–344 CrossRef CAS.
- Y. Zhu, C. Romain and C. K. Williams, Nature, 2016, 540, 354–362 CrossRef CAS PubMed.
- L. Yu, K. Dean and L. Li, Prog. Polym. Sci., 2006, 31, 576–602 CrossRef CAS.
- A. K. Mohanty, M. Misra and G. Hinrichsen, Macromol. Mater. Eng., 2000, 276–277, 1–24 CrossRef.
- A. K. Urbanek, A. M. Mirończuk, A. García-Martín, A. Saborido, I. de la Mata and M. Arroyo, Biochim. Biophys. Acta, Proteins Proteomics, 2020, 1868, 140315 CrossRef CAS PubMed.
- E. Feghali, L. Tauk, P. Ortiz, K. Vanbroekhoven and W. Eevers, Polym. Degrad. Stab., 2020, 179, 109241 CrossRef CAS.
- M. Gumienna and B. Górna, Molecules, 2021, 26, 3735 CrossRef CAS PubMed.
- R. Auras, B. Harte and S. Selke, Macromol. Biosci., 2004, 4, 835–864 CrossRef CAS PubMed.
- J. E. Oliveira, E. S. Medeiros, L. Cardozo, F. Voll, E. H. Madureira, L. H. C. Mattoso and O. B. G. Assis, Mater. Sci. Eng. C, 2013, 33, 844–849 CrossRef CAS PubMed.
- D. K. Gilding and A. M. Reed, Polymer, 1979, 20, 1459–1464 CrossRef CAS.
- M. J. Walton, S. J. Lancaster and C. Redshaw, ChemCatChem, 2014, 6, 1892–1898 CrossRef CAS.
- J. Hu, C. Kan, H. Wang and H. Ma, Macromolecules, 2018, 51, 5304–5312 CrossRef CAS.
- M. Hu, M. Wang, P. Zhang, K. Jin, Y. Chen and L. Sun, Polym. Bull., 2012, 68, 1789–1799 CrossRef CAS.
- A. Stopper, T. Rosen, V. Venditto, I. Goldberg and M. Kol, Chem.–Eur. J., 2017, 23, 11540–11548 CrossRef CAS PubMed.
- B. Rajashekhar, S. K. Roymuhury, D. Chakraborty and V. Ramkumar, Dalton Trans., 2015, 44, 16280–16293 RSC.
- P. Mckeown, M. G. Davidson, G. Kociok-Kohn and M. D. Jones, Chem. Commun., 2016, 3, 10431–10434 RSC.
- Y.-L. Duan, Z.-J. Hu, B.-Q. Yang, F.-F. Ding, W. Wang, Y. Huang and Y. Yang, Dalton Trans., 2017, 46, 11259–11270 RSC.
- M. Hu, F. Han, W. Zhang, W. Ma, Q. Deng, W. Song, H. Yan and G. Dong, Catal. Sci. Technol., 2017, 7, 1394–1403 RSC.
- M. D. Jones, L. Brady, P. McKeown, A. Buchard, P. M. Schäfer, L. H. Thomas, M. F. Mahon, T. J. Woodman and J. P. Lowe, Chem. Sci., 2015, 6, 5034–5039 RSC.
- M. Hu, W. Zhang, W. Ma, F. Han and W. Song, Polymer, 2018, 153, 445–452 CrossRef CAS.
- M. Qi, Q. Dong, D. Wang and J. A. Byers, J. Am. Chem. Soc., 2018, 140, 5686–5690 CrossRef CAS PubMed.
- P. McKeown, M. D. Jones, O. J. Driscoll, M. F. Mahon and C. K. C. Leung, Eur. J. Inorg. Chem., 2018, 2018, 5129–5135 CrossRef.
- O. J. Driscoll, C. H. Hafford-Tear, P. McKeown, J. A. Stewart, G. Kociok-Köhn, M. F. Mahon and M. D. Jones, Dalton Trans., 2019, 48, 15049–15058 RSC.
- R. Duan, C. Hu, X. Li, X. Pang, Z. Sun, X. Chen and X. Wang, Macromolecules, 2017, 50, 9188–9195 CrossRef CAS.
- M. Cozzolino, V. Leo, C. Tedesco, M. Mazzeo and M. Lamberti, Dalton Trans., 2018, 47, 13229–13238 RSC.
- P. Marin, M. J.-L. Tschan, F. Isnard, C. Robert, P. Haquette, X. Trivelli, L.-M. Chamoreau, V. Guérineau, I. del Rosal, L. Maron, V. Venditto and C. M. Thomas, Angew. Chem., Int. Ed., 2019, 58, 12585–12589 CrossRef CAS PubMed.
- Y. Liang, R. L. Duan, C. Y. Hu, L. L. Li, X. Pang, W. X. Zhang and X. S. Chen, Chin. J. Polym. Sci., 2018, 36, 185–189 CrossRef CAS.
- J. A. Stewart, P. Mckeown, O. J. Driscoll, M. F. Mahon, B. D. Ward and M. D. Jones, Macromolecules, 2019, 52, 5977–5984 CrossRef CAS.
- N. Nomura, R. Ishii, Y. Yamamoto and T. Kondo, Chem.–Eur. J., 2007, 13, 4433–4451 CrossRef CAS PubMed.
- E. D. Cross, L. E. N. Allan, A. Decken and M. P. Shaver, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 1137–1146 CrossRef CAS.
- M. Wisniewski, A. Le Borgne and N. Spassky, Macromol. Chem. Phys., 1997, 198, 1227–1238 CrossRef CAS.
- N. Spassky, M. Wisniewski, C. Pluta and A. L. Borgne, Macromol. Chem. Phys., 1996, 197, 2627–2637 CrossRef CAS.
- P. Hormnirun, E. L. Marshall, V. C. Gibson, A. J. P. White and D. J. Williams, J. Am. Chem. Soc., 2004, 126, 2688–2689 CrossRef CAS PubMed.
- H. Du, A. H. Velders, P. J. Dijkstra, J. Sun, Z. Zhong, X. Chen and J. Feijen, Chem.–Eur. J., 2009, 15, 9836–9845 CrossRef CAS PubMed.
- S. Gesslbauer, H. Cheek, A. J. P. White and C. Romain, Dalton Trans., 2018, 47, 10410–10414 RSC.
- S. Gesslbauer, R. Savela, Y. Chen, A. J. P. White and C. Romain, ACS Catal., 2019, 9, 7912–7920 CrossRef CAS.
- S. Gesslbauer, G. Hutchinson, A. J. P. White, J. Burés and C. Romain, ACS Catal., 2021, 11, 4084–4093 CrossRef CAS.
- B. Gao, D. Li, Y. Li, Q. Duan, R. Duan and X. Pang, New J. Chem., 2015, 39, 4670–4675 RSC.
- Z. Zhong, P. J. Dijkstra and J. Feijen, Angew. Chem., Int. Ed., 2002, 41, 4510–4513 CrossRef CAS PubMed.
- Y. Cui, D. Li, B. Gao, Y. Zhou, L. Chen, B. Qiu, Y. Li, Q. Duan and N. Hu, J. Coord. Chem., 2016, 69, 656–667 CrossRef CAS.
- J. Beament, M. F. Mahon, A. Buchard and M. D. Jones, Organometallics, 2018, 37, 1719–1724 CrossRef CAS.
- A. Pilone, N. De Maio, K. Press, V. Venditto, D. Pappalardo, M. Mazzeo, C. Pellecchia, M. Kol and M. Lamberti, Dalton Trans., 2015, 44, 2157–2165 RSC.
- A. Pilone, K. Press, I. Goldberg, M. Kol, M. Mazzeo and M. Lamberti, J. Am. Chem. Soc., 2014, 136, 2940–2943 CrossRef CAS PubMed.
- S. M. Kirk, G. Kociok-Köhn and M. D. Jones, Organometallics, 2016, 35, 3837–3843 CrossRef CAS.
- X. Pang, R. Duan, X. Li, C. Hu, X. Wang and X. Chen, Macromolecules, 2018, 51, 906–913 CrossRef CAS.
- J.-C. Buffet, J. Okuda and P. L. Arnold, Inorg. Chem., 2010, 49, 419–426 CrossRef CAS PubMed.
- M. Normand, E. Kirillov, T. Roisnel and J.-F. Carpentier, Organometallics, 2012, 31, 1448–1457 CrossRef CAS.
- S. Ghosh, R. R. Gowda, R. Jagan and D. Chakraborty, Dalton Trans., 2015, 44, 10410–10422 RSC.
- M. Cheng, A. B. Attygalle, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 1999, 121, 11583–11584 CrossRef CAS.
- C. Kan, J. Hu, Y. Huang, H. Wang and H. Ma, Macromolecules, 2017, 50, 7911–7919 CrossRef CAS.
- P. McKeown, S. N. McCormick, M. F. Mahon and M. D. Jones, Polym. Chem., 2018, 9, 5339–5347 RSC.
- P. McKeown, L. A. Román-Ramírez, S. Bates, J. Wood and M. D. Jones, ChemSusChem, 2019, 12, 5233–5238 CrossRef CAS PubMed.
- A. Hermann, S. Hill, A. Metz, J. Heck, A. Hoffmann, L. Hartmann and S. Herres-Pawlis, Angew. Chem., Int. Ed., 2020, 59, 21778–21784 CrossRef CAS PubMed.
- I. D'Auria, V. Ferrara, C. Tedesco, W. Kretschmer, R. Kempe and C. Pellecchia, ACS Appl. Polym. Mater., 2021, 3, 4035–4043 CrossRef.
- K. L. Law and R. C. Thompson, Science, 2014, 345, 144–145 CrossRef CAS PubMed.
- K. Ragaert, L. Delva and K. Van Geem, Waste Manage., 2017, 69, 24–58 CrossRef CAS PubMed.
- A. Rahimi and J. M. Garciá, Nat. Rev. Chem., 2017, 1, 0046 CrossRef.
- V. Piemonte, S. Sabatini and F. Gironi, J. Polym. Environ., 2013, 21, 640–647 CrossRef CAS.
- H. Liu, X. Song, F. Liu, S. Liu and S. Yu, J. Polym. Res., 2015, 22, 135 CrossRef.
- M. Hofmann, C. Alberti, F. Scheliga, R. R. R. Meißner and S. Enthaler, Polym. Chem., 2020, 11, 2625–2629 RSC.
- C. Alberti, N. Damps, R. R. R. Meißner, M. Hofmann, D. Rijono and S. Enthaler, Adv. Sustain. Syst., 2020, 4, 1900081 CrossRef CAS.
- C. Fliedel, D. Vila-Viçosa, M. J. Calhorda, S. Dagorne and T. Avilés, ChemCatChem, 2014, 6, 1357–1367 CrossRef CAS.
- L. A. Román-Ramírez, M. Powders, P. McKeown, M. D. Jones and J. Wood, J. Polym. Environ., 2020, 28, 2956–2964 CrossRef.
- L. A. Román-Ramírez, P. McKeown, C. Shah, J. Abraham, M. D. Jones and J. Wood, Ind. Eng. Chem. Res., 2020, 59, 11149–11156 CrossRef PubMed.
- J. Payne, P. McKeown, M. F. Mahon, E. A. C. Emanuelsson and M. D. Jones, Polym. Chem., 2020, 11, 2381–2389 RSC.
- L. A. Román-Ramírez, P. Mckeown, M. D. Jones and J. Wood, ACS Catal., 2019, 9, 409–416 CrossRef.
- J. Payne, P. McKeown, O. Driscoll, G. Kociok-Köhn, E. A. C. Emanuelsson and M. D. Jones, Polym. Chem., 2021, 12, 1086–1096 RSC.
- J. M. Payne, G. Kociok-Köhn, E. A. C. Emanuelsson and M. D. Jones, Macromolecules, 2021, 54, 8453–8469 CrossRef CAS.
- R. Yang, G. Xu, C. Lv, B. Dong, L. Zhou and Q. Wang, ACS Sustain. Chem. Eng., 2020, 8, 18347–18353 CrossRef CAS.
- F. Santulli, M. Lamberti and M. Mazzeo, ChemSusChem, 2021, 14, 5470–5475 CrossRef CAS PubMed.
- C. Alberti, N. Damps, R. R. R. Meißner and S. Enthaler, ChemistrySelect, 2019, 4, 6845–6848 CrossRef CAS.
- F. A. Leibfarth, N. Moreno, A. P. Hawker and J. D. Shand, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 4814–4822 CrossRef CAS.
- P. Mckeown, M. Kamran, M. G. Davidson, M. D. Jones, L. A. Román-Ramírez and J. Wood, Green Chem., 2020, 22, 3721–3726 RSC.
- X. Song, H. Wang, X. Zheng, F. Liu and S. Yu, J. Appl. Polym. Sci., 2014, 131, 40817–40823 CrossRef.
- H. Liu, R. Zhao, X. Song, F. Liu, S. Yu, S. Liu and X. Ge, Catal. Lett., 2017, 147, 2298–2305 CrossRef CAS.
- X. Song, Z. Bian, Y. Hui, H. Wang, F. Liu and S. Yu, Polym. Degrad. Stab., 2019, 168, 108937 CrossRef CAS.
- S. Alvarez, Dalton Trans., 2013, 42, 8617–8636 RSC.
- K. Masutani and Y. Kimura, in Poly(lactic acid) Science and Technology: Processing, Properties, Additives and Applications, The Royal Society of Chemistry, 2015, pp. 1–36 Search PubMed.
- R. D. Rittinghaus, P. M. Schäfer, P. Albrecht, C. Conrads, A. Hoffmann, A. N. Ksiazkiewicz, O. Bienemann, A. Pich and S. Herres-Pawlis, ChemSusChem, 2019, 12, 2161–2165 CrossRef CAS PubMed.
- P. M. Schäfer, M. Fuchs, A. Ohligschläger, R. Rittinghaus, P. McKeown, E. Akin, M. Schmidt, A. Hoffmann, M. A. Liauw, M. D. Jones and S. Herres-Pawlis, ChemSusChem, 2017, 10, 3547–3556 CrossRef PubMed.
- Y. Fan, C. Zhou and X. Zhu, Catal. Rev., 2009, 51, 293–324 CrossRef CAS.
- R. Petrus, D. Bykowski and P. Sobota, ACS Catal., 2016, 6, 5222–5235 CrossRef CAS.
- F. M. Lamberti, L. A. Román-Ramírez and J. Wood, J. Polym. Environ., 2020, 28, 2551–2571 CrossRef CAS.
- S. M. Al-Salem, P. Lettieri and J. Baeyens, Waste Manag., 2009, 29, 2625–2643 CrossRef CAS PubMed.
- I. Taniguchi, S. Yoshida, K. Hiraga, K. Miyamoto, Y. Kimura and K. Oda, ACS Catal., 2019, 9, 4089–4105 CrossRef CAS.
- H. Zhang and Z.-G. Wen, Waste Manag., 2014, 34, 987–998 CrossRef CAS PubMed.
- H. Yao, L. Liu, D. Yan, Q. Zhou, J. Xin, X. Lu and S. Zhang, Chem. Eng. Sci., 2022, 248, 117109 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2121321–2121327. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra09087a |
| This journal is © The Royal Society of Chemistry 2022 |