Open Access Article
Open Access ArticleMixed metal node effect in zeolitic imidazolate frameworks†
Rasmus S. K. Madsen‡
 a,
Malwina Stepniewska‡a,
Yongjian Yang‡b,
Ang Qiaoc,
Wessel M. W. Wintersa,
Chao Zhoua,
Jakob Königd,
John C. Mauro*b and
Yuanzheng Yue
a,
Malwina Stepniewska‡a,
Yongjian Yang‡b,
Ang Qiaoc,
Wessel M. W. Wintersa,
Chao Zhoua,
Jakob Königd,
John C. Mauro*b and
Yuanzheng Yue *a
*a
aDepartment of Chemistry and Bioscience, Aalborg University, Aalborg, DK9220, Denmark. E-mail: yy@bio.aau.dk
bDepartment of Materials Science and Engineering, The Pennsylvania State University, USA. E-mail: jcm426@psu.edu
cWuhan University of Technology, Wuhan, 430070, China
dAdvanced Materials Department, Jožef Stefan Institute, Ljubljana, 1000, Slovenia
First published on 7th April 2022
Abstract
We synthesized two series of bimetallic (zinc and cobalt) zeolitic imidazolate frameworks (ZIF-62) under different solvothermal conditions. It is found that the structure of the derived ZIF crystals is highly sensitive to synthesis conditions. One series possesses the standard ZIF-62 structure, whereas the other has a mixed structure composed of both the standard structure and an unknown one. The standard series exhibits a slight negative deviation from linearity of melting temperature (Tm) and glass transition temperature (Tg) with the substitution of Co for Zn. In contrast, the new series displays a stronger negative deviation. These negative deviations from linearity indicate the mixed metal node effect in bimetallic ZIF-62 due to the structural mismatch between Co2+ and Zn2+ and to the difference in their electronic configurations. The new series involves both cobalt-rich and zinc-rich phases, whereas the standard one shows one homogeneous phase. Density functional theory calculations predict that the substitution of Co for Zn increases the bulk modulus of the ZIF crystals. This work indicates that the structure, melting behaviour, and mechanical properties of ZIFs can be tuned by metal node substitution and by varying the synthetic conditions. Both series of ZIFs have higher glass forming abilities due to their higher Tg/Tm ratios (0.77–0.84) compared to most good glass formers.
Introduction
Metal–organic frameworks (MOFs) are a class of compounds consisting of metal nodes interconnected by organic ligands to form a 2D or 3D porous structure.1–4 MOFs have received intense interest from chemists and materials scientists since their tunable composition and structure enable potential applications such as catalysis, gas storage, gas separation, and sensors.5,6 Conventional synthesis approaches of MOFs enabled the formation of fine crystalline powders with the fabrication of large monolithic pieces that were difficult to be obtained.7 The fine powder of MOFs results in interface interactions and grain boundaries, which are two major engineering challenges in gas or liquid separation.8–10 It is known that some zeolitic imidazolate frameworks (ZIFs) (a subset of MOFs), which contain imidazole-based ligands that coordinate tetrahedrally to metal ions, forming zeolite crystal topologies, can be melted and then quenched to bulk glass.2,11–13 MOF glasses are a new family of melt-quenched glasses, which differ from other families such as inorganic, organic, and metallic glasses in terms of chemical bonds, chemical composition, and topological structure. MOF glasses have shown a range of superior properties, e.g., ZIF-62 (Zn Im2−x bImx, Im: imidazolate; bIm: benzimidazolate) possesses an ultrahigh glass forming ability (Tg/Tm > 0.84, where Tg is the glass transition temperature, and Tm is the melting point), and high resistance to crystallisation12. While other ZIF glasses like ZIF-76 (Zn Im2−x 5-CbImx, 5-CbIm: 5-chlorobenzimidazole) high porosity.13 Solid-state 67Zn nuclear magnetic resonance (NMR) spectroscopy revealed that the melting and glass formation of ZIFs led to a high degree of structural disorder.14 Wang et al.8 recently developed the first glassy MOF membrane, in which ZIF-62 glass was used as the active membrane material on an alumina support. Bulk ZIF-62 glass shows high light transmittance (90%) in the visible range,15 and possesses anomalous mechanical properties.16,17 Henke et al.18 revealed that substituting cobalt for zinc in ZIF-62 results in a meltable Co-ZIF-62 phase of the same space group as conventional ZIF-62, i.e., Pbca. Bimetallic ZIFs could be produced by introducing two types of metal nodes.19–21 Most research has focused on bimetallic ZIF-8/ZIF-67 [Zn/Co1 MeIm2], (MeIm = 2-methyl imidazole), within the realm of catalysis.19–21 A recent study by Bumstead et al.22 investigated the effect of structural disorder within a ZIF-62-type network. They found that the Tm decreased as they introduced cobalt into the zinc network. Zn/Co-ZIF-62 exhibits super-broadband mid-infrared (Mid-IR) luminescence,23 which is important for photonic applications. Recently, the computational modelling of ZIFs (and MOFs)24,25 through density functional theory (DFT)26–29 and molecular modelling30–36 has been performed to understand the effect of both nodes and linkers37 on the ZIF structure.In this work, we report a systematic study on the effect of metal substitution on thermodynamic, kinetic, and mechanical properties in Co/Zn-ZIF-62 series. Two series of Co/Zn ZIF crystals were synthesized via a solvothermal method. The first series was Co/Zn-ZIF-62 (Pbca space group) based on the work of Henke et al.18 The second series was synthesized by the experimental procedures described elsewhere,17 which is a biphasic Co/Zn-ZIF-new series, i.e., a series containing both a Zn-rich phase and a Co-rich phase. The two series of crystals were structurally characterized through powder X-ray diffraction (PXRD), scanning electron microscopy (SEM), as well as energy-dispersive X-ray spectroscopy (EDX), and chemically via 1H solution nuclear magnetic resonance (S-NMR). The Co/Zn-ZIF-62 series showed structural features similar to those known in the literature, whereas the Co/Zn-ZIF-new series featured both a different crystal structure and an unexpected molecular structure of the organic ligands. The Co/Zn-ZIF-new series was also analyzed via infrared spectroscopy (FT-IR) and inductively coupled plasma optical emission spectroscopy (ICP-OES) to investigate the nature of its structure. Both series were analyzed via differential scanning calorimetry (DSC) to investigate how Tm and Tg vary with the substitution of Co for Zn. In this work, we also conducted DFT and classical force field (FF) calculations on the crystalline phase of the bimetallic ZIF-62 systems, in order to predict the change in mechanical properties of the crystals, and how bond lengths and bond strength increase when substituting cobalt for zinc.
Experimental
Synthesis of ZIF crystals
| Sample | 0.14 M Zn(NO3)2·6H2O (mL) | 0.14 M Co(NO3)2·6H2O (mL) | 0.40 M Im (mL) | 0.06 M bIm (mL) |
|---|---|---|---|---|
| 0.0-ZIF-62 | 25 | — | 25 | 25 |
| 0.1-ZIF-62 | 22.5 | 2.5 | 25 | 25 |
| 0.2-ZIF-62 | 20 | 5 | 25 | 25 |
| 0.4-ZIF-62 | 15 | 10 | 25 | 25 |
| 0.6-ZIF-62 | 10 | 15 | 25 | 25 |
| 0.8-ZIF-62 | 5 | 20 | 25 | 25 |
| 0.9-ZIF-62 | 2.5 | 22.5 | 25 | 25 |
| 1.0-ZIF-62 | — | 25 | 25 | 25 |
| Sample | 0.6 M Zn(NO3)2·6H2O (mL) | 0.6 M Co(NO3)2·6H2O (mL) | 4 M Im (mL) | 0.5 M bIm (mL) | DMF (mL) |
|---|---|---|---|---|---|
| 0.0-ZIF-new | 17.45 | — | 33.82 | 21.82 | 1.91 |
| 0.1-ZIF-new | 15.71 | 1.75 | 33.82 | 21.82 | 1.91 |
| 0.2-ZIF-new | 13.96 | 3.49 | 33.82 | 21.82 | 1.91 |
| 0.4-ZIF-new | 10.47 | 6.98 | 33.82 | 21.82 | 1.91 |
| 0.6-ZIF-new | 6.98 | 10.47 | 33.82 | 21.82 | 1.91 |
| 0.8-ZIF-new | 3.49 | 13.96 | 33.82 | 21.82 | 1.91 |
| 1.0-ZIF-new | — | 17.45 | 33.82 | 21.82 | 1.91 |
Structural analysis
A PANalytical Empyrean equipped with a Cu X-ray source (λ = 1.54098 Å) was used to collect powder X-ray diffraction (PXRD) patterns. The crystalline powders were loaded onto an amorphous silicon sample holder (PANalytical) and measured using a Ni-filter, 0.04 rad soller, 1/8° divergence slit, and 1/4° anti-scatter slit on the incident side and a 7.5 mm anti-scatter slit and a 0.04 rad soller on the diffracted side. The diffraction spectra were collected with 2θ = [5.009583°, 79.995010°] using a step-size of 0.006565°. The samples were dried at 350 °C in an argon atmosphere before the measurements.Solution 1H-NMR (Bruker Avance III 600 MHz spectrometer) was used to determine the composition of organic linkers in each crystalline sample. All samples were digested using 200 μL of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 DCl (35% conc., 99% 2H, Aldrich)
5 DCl (35% conc., 99% 2H, Aldrich)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dimethyl sulfoxide (DMSO) (VWR, 99.80% 2H) solution. The pulse sequence used was a 1D experiment with composite pulses.38 A 5 seconds continuous-wave irradiation of γB1/2π = 50 Hz was used to suppress the water signal. The total recycle delay was 28 s. A Zeiss 1540 XB was used to perform both scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) to characterise the crystalline samples. EDX data acquisition and analysis were performed using the NSS3 X-ray microanalysis software (Thermo Fischer Scientific Inc.). The accelerating voltage of the electron beam is 10 kV. Fourier-transform infrared spectroscopy (FT-IR) measurements were performed on Co/Zn-ZIF-new samples in a wavenumber range 4000–400 cm−1 in absorbance mode using Bruker Tensor II equipped with platinum attenuated total reflectance (ATR).
dimethyl sulfoxide (DMSO) (VWR, 99.80% 2H) solution. The pulse sequence used was a 1D experiment with composite pulses.38 A 5 seconds continuous-wave irradiation of γB1/2π = 50 Hz was used to suppress the water signal. The total recycle delay was 28 s. A Zeiss 1540 XB was used to perform both scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) to characterise the crystalline samples. EDX data acquisition and analysis were performed using the NSS3 X-ray microanalysis software (Thermo Fischer Scientific Inc.). The accelerating voltage of the electron beam is 10 kV. Fourier-transform infrared spectroscopy (FT-IR) measurements were performed on Co/Zn-ZIF-new samples in a wavenumber range 4000–400 cm−1 in absorbance mode using Bruker Tensor II equipped with platinum attenuated total reflectance (ATR).
Thermal analysis
The melting, glass transition, and mass change of the samples were studied using a Netzsch STA 449 F1 instrument that combines differential scanning calorimetry (DSC) and thermal gravimetric analysis (TGA). The samples were placed in a platinum crucible situated on a sample holder of the STA at room temperature. The samples were held for 5 min at an initial temperature of 343 K, then heated at 10 K min−1 to 733 K, and then cooled back to 393 K at 10 K min−1, thus forming the standard glass. Subsequently, the second upscan was conducted from 393 K to 643 K at 10 K min−1. To determine the isobaric heat capacity of the samples, both the baseline (blank) and the reference sample (sapphire) were measured.In order to identify the decomposition gases, additional TGA were performed using a Jupiter 449 simultaneous thermal analysis (STA) instrument coupled with a 403 C Aëolos mass spectrometer (MS) (Netzch, Selb, Germany). The measurements were performed with a heating rate of 10 K min−1 in an argon atmosphere. A small amount of powder (11–14 mg) was inserted into an uncovered alumina crucible. The ionised species of the gases released from the heat-treated sample were detected by the MS and compared to the gas ionic spectra data from the NIST Standard Reference Database.58
Simulations of ZIF-62 based samples
The atomic configuration for crystalline Zn-ZIF-62 was obtained from the Cambridge Structural Database (CSD, CCDC number 671070). The original structure does not contain cobalt atoms. To create Zn(1−x)Cox-ZIF-62, an increasing number of zinc atoms were substituted by cobalt atoms. Because the distance between the nodes (zinc and cobalt atoms) in ZIF-62 is relatively large (>6 Å) with direct substitution of zinc atoms with cobalt atoms, the interaction between the cobalt sites can be ignored. Both the DFT calculation and the force field calculation were attempted. While the DFT calculation gives more accurate results, the force field calculation is much faster because it uses a classical force field and requires less computational resources.For the DFT calculation, the crystal Zn-ZIF-62 structure was relaxed using the projector augmented-wave PAW method as implemented in the Vienna ab initio simulation package (VASP). An energy cut-off of 650 eV was applied for the plane-wave basis set. Perdew–Burke–Ernzerhof (PBE) functional was used to evaluate the electronic exchange and correlation with a D3 van der Waals correction by Grimme.39 The Brillouin zone was sampled at the Γ-point, which is considered sufficient for the ZIF-62 unit cell dimension of 296 atoms. For the structural relaxation, we set the EDIFF to be 10−3–10−4 eV. The ionic relaxation stops when all forces are converged to less than 0.005 eV Å−1. In order to calculate the bulk modulus, the ZIF-62 structures of different Co concentrations had been relaxed with a series of volume changes from −4% to 2%. The Birch–Murnaghan equation of state40 was used to extract the bulk modulus from the energy curve at different volumes.
In the classical FF calculations, the bimetallic Zn(1−x)Cox-ZIF-62 structures from the above DFT calculations are first subjected to energy minimisation using the conjugate gradient algorithm as implemented in LAMMPS41 (ref. 41) with an energy tolerance the force tolerance of 4.3 × 10−17 eV and 7 × 10−16 nN, respectively, for the minimisation. For the interactions between atoms, the classical Universal Force Field for Metal–Organic Frameworks (UFF4MOF) force field42,43 is used. UFF4MOF is based on the universal force field44 and has been extended for MOF chemistries. UFF4MOF contains transition metals, including zinc and cobalt, which are commonly found in ZIFs. It has been shown that the UFF4MOF force field can accurately reproduce the structural characteristics and the bulk modulus for many different types of MOFs that have been verified experimentally.31,43,45 To calculate the bulk modulus, the ZIF-62 structure is relaxed with a series of volume changes from −3% to 3% around the equilibrium volume. The bulk modulus is obtained by fitting the equation of state using the Birch–Murnaghan equation.40
To calculate the Young's modulus, the ZIF-62 sample is stretched or compressed in one Cartesian direction up to ∼5%, while the external pressure in the two other Cartesian directions is kept around zero. The Young's modulus at 0 K is extracted from the stress–strain curve.
Results and discussion
Modelling and characterisation of standard Co/Zn-ZIF-62
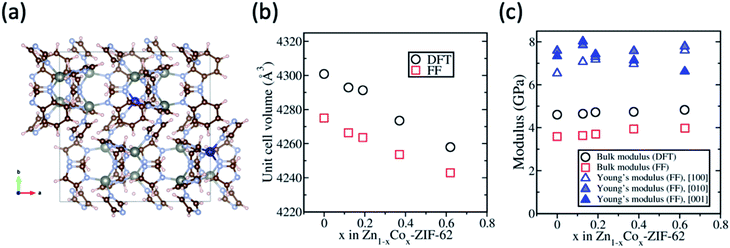
DFT and classical FF calculations were carried out using the Zn-ZIF-62 structure as an initial configuration. Fig. 1 shows the FF results for Zn-ZIF-62, viz., the unit cell volume, bulk modulus, and Young's modulus. Densities of crystalline Zn-ZIF-62 phase determined from DFT and FF methods are close to the published density data46 and the values obtained from the present experiment (Table S1†). As shown in Fig. 1b, the unit cell volumes from both DFT and FF calculations differ by ∼0.5%, and the volume of the cobalt substituted sample decreases up to 1% when the substitution of cobalt for zinc atoms reaches 62.5%. This decrease can be partially attributed to the shorter Co–N bond compared to Zn–N bond according to the DFT calculation by Krokidas et al.19,47 The decrease in bond length is hypothesised to stem from the different electronic configurations of the metal ions (3d10 for Zn2+ and 3d7 for Co2+). The unfilled 3d7 orbital is able to interact with electrons from the imidazolate linkers, which strengthens the interaction between cobalt and the linkers, resulting in the decrease in bond length. It has been experimentally observed that cobalt ZIFs have a smaller unit cell volume than their zinc counterparts.48For the powder samples (see Fig. S1†), the crystals are too small to measure the mechanical properties experimentally, and thus, only theoretical determination of bulk modulus and Young's modulus is reported here to show the effect of substitution of cobalt for zinc on the Zn-ZIF-62 structure. The bulk and uniaxial Young's moduli of the crystalline ZIF-62 are shown in Fig. 1c DFT and FF calculations yield an average bulk modulus of 3.8 ± 0.2 GPa and 4.7 ± 0.2 GPa for all samples. With increasing cobalt content, there is a moderate increase in the bulk modulus. The Young's modulus has a different trend with increasing substitution, depending on the crystal orientation, i.e., it increases in the [100] direction, decreases in the [001] direction, and remains roughly the same in the [010] direction. The finding that the unit cell volume decreases with increasing cobalt content agrees well with the finding of bulk modulus slightly increasing, as it has been found previously in the literature that the bond length is inversely proportional to the bulk modulus.49,50
The computational results predict that both zinc and cobalt ions are compatible with the ZIF-62 crystal structure. The random substitution of zinc ions with cobalt ions in the cag topology does not negatively impact the calculated mechanical strength of the crystal. Characterisation of the synthesized crystals was performed to confirm that there is no change in the space group, while the bond strength increases slightly, when substituting cobalt for zinc in ZIF-62.
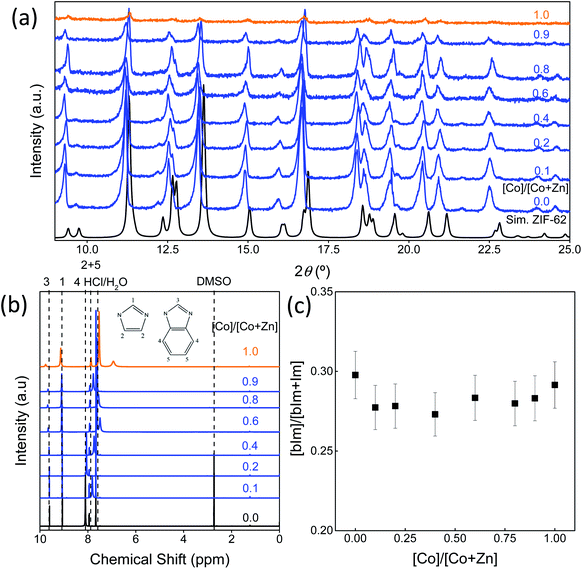
An image of the eight standard Co/Zn-ZIF-62 samples can be seen in Fig. S1,† revealing a color gradient as cobalt is substituted for zinc. The color gradient agrees with the relative cobalt content, as revealed by UV-Vis in Fig. S2.† Fig. 2a presents the XRD patterns of the bimetallic Co/Zn-ZIF-62 crystals synthesized by the standard approach.18 The patterns of all Co/Zn-ZIF-62 samples agree well with the reference crystallographic information files (CIF) (Cambridge Crystallographic Data Centre (CCDC)51 (ZIF-62: CCDC number 671070)) used to generate the XRD patterns. The decrease in the signal-to-noise ratio for the samples containing predominantly cobalt is due to the fluorescence caused by cobalt having its X-ray absorption edge close to the energy level of Cu-radiation.52 Fig. 2b and c confirm that the benzimidazole to imidazole ratio does not change noticeably when substituting cobalt for zinc in the Co/Zn-ZIF-62 samples. Thus, we can infer that the changes in both the mechanical properties (Fig. 1) and the thermal behaviours (shown below) are a consequence of metal substitution. Additionally, SEM and EDX reveal that cobalt is evenly distributed in the crystal of all the Co/Zn-ZIF-62 samples, as seen in Fig. S3–S10.† The shape of the crystals deviates from the octahedral morphology reported in the literature.8 This deviation can be explained by the removal of DMF from the pores during heat-treatment at 350 °C.
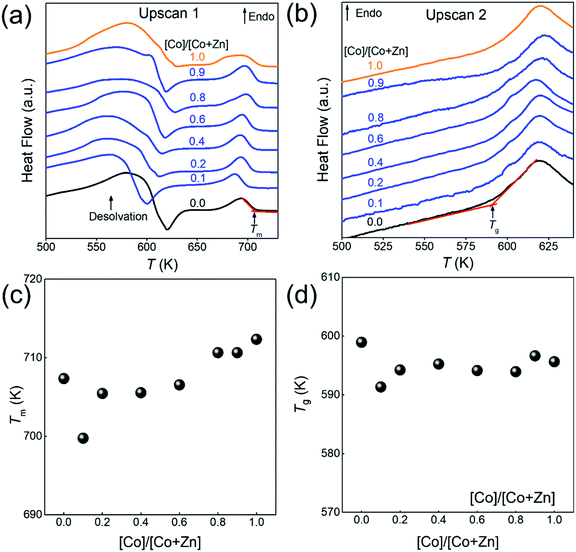
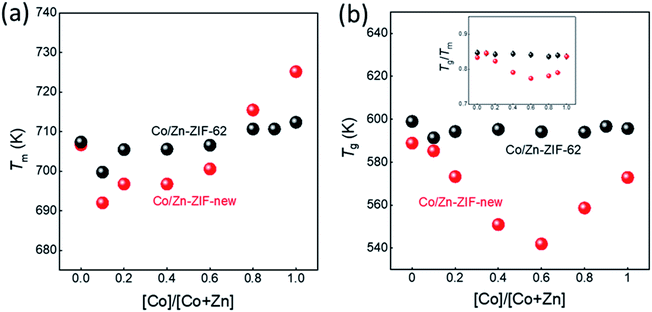
The structural characterisation of the standard series agrees well with the computational results, suggesting that zinc and cobalt ions can co-exist in the same bimetallic crystal structure without phase separation. Based on the computational results shown earlier, as well as previous experimental results for Co-ZIF-62,18 it is expected that Tm would linearly increase as cobalt is substituted for zinc in the ZIF-62 crystal structure due to stronger bonding between the cobalt nodes and the linkers. Fig. 3a and b show the thermal responses of the Co/Zn-ZIF-62 samples for the first and second upscans, respectively. The first upscan curve reveals two distinct endothermic responses. The first is attributed to the removal of DMF from the pores, while the second is ascribed to the melting process, with Tm defined as the offset of the melting peak. The second upscan curves in Fig. 3b for all the Co/Zn-ZIF-62 samples show clear glass transition peaks, strongly confirming the glassy nature of melt-quenched samples. Fig. 3c displays the Tm of the standard Co/Zn-ZIF-62 structures, which agrees well with that of the as-synthesized crystals in Henke group's work.18 Tm is seen to anomalously decrease as cobalt is introduced (0.1-ZIF-62), and then non-linearly increases with a gradual substitution of cobalt for zinc. This effect is rather unexpected, when looking at the simulation work of Fig. 1b and c. Fig. 3d demonstrates that Tg also has a similar drop, followed by an increase as the degree of cobalt substitution increases. Fig. 3c and d shows that both Tm and Tg follow a similar trend when substituting cobalt for zinc. Tm drops slightly from 707 K to 700 K and then non-linearly increases to a maximum of 712 K, slightly higher than reported previously. Similarly, Tg has a small drop from 599 K to 591 K and then increases to 597 K when all zinc nodes are substituted with cobalt. For the standard Co/Zn-ZIF-62 series, there is only one homogeneous crystalline phase, and the minima of both Tm and Tg are found at the composition of Co/(Co + Zn) = ∼0.1. The observed minimum in Tm could be a consequence of the mixed metal node effect that has the same fashion as the mixed modifier effect in oxide glasses.53,54 The mixed modifier effect in oxide glasses refers to a non-additive change of some transport properties when one type of modifier (e.g., sodium ion) is substituted by another (e.g., potassium), i.e., a positive or negative deviation from the linear trend of a property with the modifier substitution.53,54 In the studied standard Co/Zn-ZIF-62, the partial substitution of cobalt for zinc (and vice versa) causes an increase in structural instability, possibly due to structural mismatch effects caused by the difference in bond length and strength between Co–N and Zn–N bonds. This bond difference arises from the difference in electron configurations between the two types of metal ions (d7 for Co2+ and d10 for Zn2+). The structural instability enhances the atomic vibrations, and hence, the Lindemann criterion for melting would be more easily fulfilled.55 However, the pure Co-ZIF-62 exhibits higher Tm than its counterpart – pure Zn-ZIF-62. The higher Tm of pure Co-ZIF-62 can be attributed to the stronger interaction between the linkers and cobalt ions, as predicted from the DFT and FF calculations, and also from the experimental data reported by other authors.18
Impact of synthesis condition on Co/Zn-ZIF-62 structure
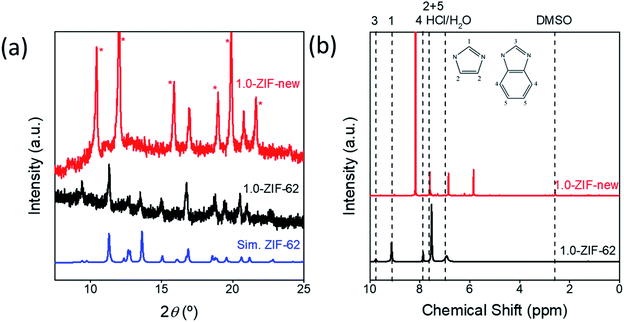
Fig. S11† shows the optical image of the seven ZIF samples. A clear relationship is seen between the intensity of the purple color of the sample and the amount of cobalt added during the synthesis. These samples were analyzed via X-ray diffraction to identify the crystal structure (Fig. S12†). When sufficient cobalt nitrate is used in the solvothermal synthesis, an additional phase is observed for the resulting crystals, with the peaks marked with asterisks (*). In Fig. 4a, the diffraction pattern of 1.0-ZIF-new is compared to that of 1.0-ZIF-62. A different XRD pattern is observed, confirming that 1.0-ZIF-new has a different structure from the standard 1.0-ZIF-62. This new type of ZIF crystal shows fewer peaks in the XRD pattern than the standard 1.0-ZIF-62. This suggests that the new structure is likely of higher symmetry than that of the standard sample, as fewer peaks (i.e., fewer unique d-spacings) are present. The absence of diffraction peaks at higher angles suggests that the structure is indeed a Co-based metal–organic framework. It is assumed that the formation of this structure is a result of the high reactant concentrations used during the solvothermal synthesis.S-NMR spectroscopy was employed to verify the presence of the organic linkers in the biphasic frameworks and the differences in linker composition between 1.0-ZIF-62 and 1.0-ZIF-new (Fig. 4b). Fig. S13† plots the NMR spectra, where the signals of 0.0-ZIF-new agree with those of the standard 0.0-ZIF-62 spectra. However, as cobalt nitrate is substituted for zinc nitrate during the synthesis, the imidazole and benzimidazole signals decrease in intensity for the resulting crystals. Two new peaks at ∼5.84 and 6.83 ppm (Fig. 4b) appear and increase in intensity with increasing the cobalt nitrate content. It was assumed that the high concentration of nitrate ions in the presence of cobalt would catalyze the nitration of imidazole to 4-nitroimidazole. The NMR spectrum of 4-nitroimidazole, dissolved in the same NMR solvent, can be found in the ESI (Fig. S14†). The spectrum shows no peaks in the 5–7 ppm range, thus excluding the possibility that the emerging signal arises from nitrated imidazole. To determine the distribution of zinc and cobalt in the two crystal phases, SEM and EDX analyses were performed on the 0.6-ZIF-new sample, as seen in Fig. 5. Two crystal particles with different morphologies are observed, i.e., octahedral and spherical shapes in column 1 of Fig. 5. The SEM image in Fig. S15a† shows the sample containing only Zn, i.e., 0.0-ZIF-new, which contains crushed ZIF-62 particles, where some of the flat surfaces of the standard ZIF-62 morphology remain. The SEM image in Fig. S15b† illustrates the sample containing only Co, i.e., 1.0-ZIF-new, where only spherical particles are present. The SEM images indicate that there is a difference in the crystal morphologies between the standard ZIF-62 structure (i.e., the standard phase) and the new structure (i.e., the new phase). The EDX elemental mapping in Fig. 5 (see columns 2 and 3) reveals that both morphologies contain zinc and cobalt, which confirms that both metal nodes are incorporated into the two crystal structures. EDX elemental analysis in Fig. S16† confirms that the octahedral ZIF-62 crystals contain zinc predominantly, while the new spherical crystal structure contains more cobalt. The metallic heterogeneity in the new series of ZIFs could be ascribed to the preferential incorporation of cobalt into the new phase.
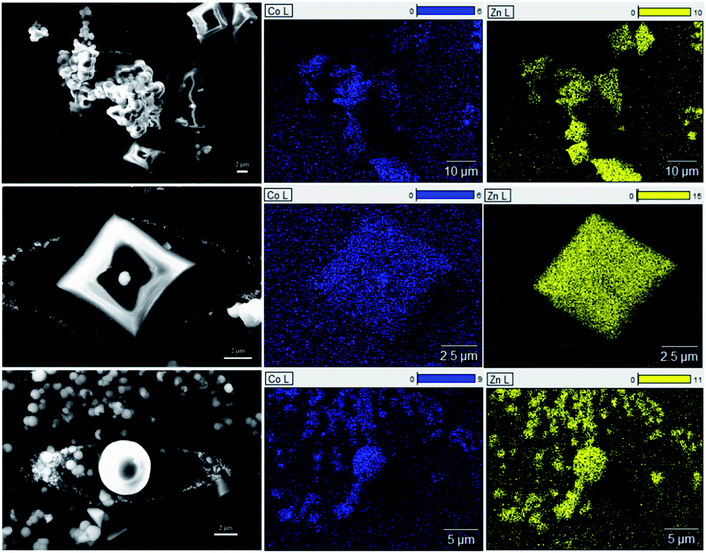 | ||
| Fig. 5 Left column is the SEM image of the area detected for EDX elemental mapping for cobalt (middle column) and zinc (right column). | ||
ATR FT-IR was employed to detect the changes of the chemical bonds in the Co/ZIF-new samples (Fig. S17†). Several changes in the signal can be observed. First, the peaks at 1677 and 1384 cm−1, which are assigned to the DMF,18,56,57 disappear with increasing the cobalt content, being attributed to the new phase with a denser structure. Second, some of the peaks around 1150–1300 cm−1 (assigned to C–N and C–C stretching) change in shape as cobalt is substituted for zinc. Simultaneously, the double peak at ∼1480 cm−1, which is assigned to aromatic ring stretching, varies in line shape with increasing the cobalt content. The FT-IR results (Fig. S17†) indicate that signals corresponding to imidazole and benzimidazole rings are still present. In combination with the S-NMR findings in Fig. S13,† it is evident that the imidazole and benzimidazole structures have changed, while the exact chemical structure of the linkers remains unknown. It is reasonable to infer that the formation of the unknown linker is caused by cobalt nitrate, and hence, cobalt preferentially stays in the new phase. The absolute Co/(Co + Zn) ratio of these samples was analysed via ICP-OES. Fig. S18† reveals that there is a non-linear relationship between the Co/(Co + Zn) ratio used during the synthesis and the ratio detected in the resulting ZIF samples. This is explained by the phase separation exhibited in Fig. S19,† where a portion of the new phase with higher cobalt content could have been lost during the washing and collecting process.
Fig. 6a and b shows the changes of both Tm and Tg values with the substitution of cobalt for zinc for two series of ZIFs. The data points are acquired from the first and second DSC scans shown in Fig. 3a, b and S20.† It is seen in Fig. 6a and b that both Tm and Tg show negative deviations from linearity with substitution of cobalt for zinc, which could be caused by two factors. The first is the mixed metal node effect as the two crystal phases both contain zinc-ions and cobalt-ions. The second is a eutectic effect caused by the presence of two discrete phases resulting in a drop in Tm. Given that 0.1-ZIF-new in Fig. S12† reveals no detectable quantity of the new phase, the mixed metal effect is likely to have a greater effect at lower Co/(Co + Zn) ratios on Tm and Tg. The deviations in both Tm and Tg are much larger for the new ZIF series than that of Co/Zn-ZIF-62. Due to the absence of the new phase at low cobalt content, it is reasonable to infer that the effect on Tm and Tg is initially driven by the mixed metal node effect, but at higher cobalt concentrations, multiple effects e.g., the effect of two discrete phases could cause the changing trend in Tm and Tg. These results imply that the atomic vibration in the new series of ZIF more easily meets Lindemann's criterion for melting. Moreover, it is seen in Fig. S21† that the Co/Zn-ZIF-new series undergoes a noticeable loss of mass during the melting process. The origin of the mass loss is investigated by a thermogravimetric analyzer coupled with mass spectrometry (TGA-MS) (Fig. S22†). The TGA-MS analysis shows that mainly NH3 and NO gases are released during melting, suggesting that the linkers in the new series are more unstable than those in the standard series. The 0.0-ZIF-new sample has a Tm at 706 K and a Tg at 588 K, which correspond to the values for standard Zn-ZIF-62. 1.0-ZIF-new has a higher Tm at 724 K and a lower Tg at 573 K. The higher Tm might be due to a denser structure, as indicated by the lack of DMF signals from the FT-IR results.
The inset of Fig. 6b shows the dependence of Tg/Tm ratios for the two studied ZIF series on the Co–Zn substitution. As is known, Tg/Tm is a measure of the glass forming ability (GFA) of a glass former, i.e., the higher the Tg/Tm ratio of a glass former is, the higher its GFA is. It is seen that both series show higher GFA since their Tg/Tm ratios (0.77–0.84) are significantly higher than that of most good glass formers (Tg/Tm = 0.67).12 Interestingly, there is no change in Tg/Tm with metal node substitution in the standard series, indicating that the GFA remains the same. However, in contrast to the standard series, the new series exhibits smaller Tg/Tm ratios, suggesting that the GFA of the latter is relatively lower. In addition, the Tg/Tm ratio shows a non-monotonic trend with substituting cobalt for zinc, i.e., there is a minimum Tg/Tm ratio at Co/(Co + Zn) = 0.6. This composition shows a pronounced phase separation, as shown in Fig. 5. This fact agrees with the general notion that a glass former with stronger phase separation tendency features a lower GFA.
Conclusions
We synthesized two series of bimetallic cobalt/zinc zeolitic imidazolate frameworks based on ZIF-62. It was found that the structure of the derived ZIF crystals strongly depended on synthesis conditions. One series of ZIF crystals were obtained by using the standard synthesis condition reported in the literature, which possessed the standard ZIF-62 structure. In contrast, the other series, which was produced at a lower temperature (120 °C) for a shorter duration (48 hours), had a mixed structure composed of both the standard structure and a new one. We predicted the mechanical properties of the standard series of Zn/Co-ZIF-62 through DFT and FF calculations.An interesting mixed metal node effect was observed in bimetallic ZIF-62 samples, i.e., negative deviations from linearity of Tm and Tg for both series of bimetallic ZIF-62 with substitution of Co for Zn. Notably, the new series displayed a stronger mixed metal node effect at lower Co substitution. This mixed metal node effect was attributed to the structural mismatch between Co2+ and Zn2+, and to the difference in their electronic configurations. The network became destabilised by introducing the dissimilar cobalt-ion into the ZIF crystal. Some of the samples in the new series contained both cobalt-rich and zinc-rich phases, whereas the standard one showed only one homogeneous phase. It was found that during melting, the Co/Zn-ZIF-new samples underwent a partial decomposition of an unknown species, suggesting that the new phase had a lower thermal stability than the standard phase. The above findings are instrumental to the understanding of the structure and thermodynamic properties of MOF glasses and to the design of novel MOF glass formers. Concerning the strong mixed metal node effect of the Co/Zn-ZIF-new series, the chemistry and structure of this series should be further investigated to reveal the melting mechanism of MOFs and to design new MOF glass formers.
Both series of ZIFs have higher GFA due to their higher Tg/Tm ratios (0.77–0.84) compared to most of the good glass formers. Tg/Tm remains the same with metal node substitution in the standard series, and hence there is no change in GFA. However, the new series exhibits smaller Tg/Tm ratios, i.e., lower GFA, than the standard series. In addition, the new series shows a minimum Tg/Tm ratio at Co/(Co + Zn) = 0.6.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors thank Villum Fonden (grant no. 13253) for the support. A. Q. acknowledges the National Natural Science Foundation of China (no. 22175135) and the Fundamental Research Funds for the Central Universities (WUT: 2021IVA099, 2021III018JC) for their support. Y. Y. thank the Independent Research Fund Denmark for the support (1026-00318B).References
- K. S. Park, Z. Ni, A. P. Cote, J. Y. Choi, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- T. D. Bennett and S. Horike, Nat. Rev. Mater., 2018, 3, 431–440 CrossRef.
- B. Chen, Z. Yang, Y. Zhu and Y. Xia, J. Mater. Chem. A, 2014, 2, 16811–16831 RSC.
- B. R. Pimentel, A. Parulkar, E. K. Zhou, N. A. Brunelli and R. P. Lively, ChemSusChem, 2014, 7, 3202–3240 CrossRef CAS PubMed.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- M. Safaei, M. M. Foroughi, N. Ebrahimpoor, S. Omidi, A. Jahani and M. Khatami, TrAC, Trends Anal. Chem., 2019, 118, 401–425 CrossRef CAS.
- M. I. Nandasiri, S. R. Jambovane, B. P. McGrail, H. T. Schaef and S. K. Nune, Coord. Chem. Rev., 2016, 311, 38–52 CrossRef CAS.
- Y. Wang, H. Jin, Q. Ma, K. Mo, H. Mao, A. Feldhoff, X. Cao, Y. Li, F. Pan and Z. Jiang, Angew. Chem., Int. Ed., 2020, 59, 4365–4369 CrossRef CAS PubMed.
- A. Kertik, L. H. Wee, K. Sentosun, J. A. R. Navarro, S. Bals, J. A. Martens and I. F. J. Vankelecom, ACS Appl. Mater. Interfaces, 2020, 12, 2952–2961 CrossRef CAS PubMed.
- H. T. Kwon and H. K. Jeong, J. Am. Chem. Soc., 2013, 135, 10763–10768 CrossRef CAS PubMed.
- T. D. Bennett, J. C. Tan, Y. Yue, E. Baxter, C. Ducati, N. J. Terrill, H. H. M. Yeung, Z. Zhou, W. Chen, S. Henke, A. K. Cheetham and G. N. Greaves, Nat. Commun., 2015, 6, 1–7 Search PubMed.
- A. Qiao, T. D. Bennett, H. Tao, A. Krajnc, G. Mali, C. M. Doherty, A. W. Thornton, J. C. Mauro, G. N. Greaves and Y. Yue, Sci. Adv., 2018, 4, eaao6827 CrossRef PubMed.
- C. Zhou, L. Longley, A. Krajnc, G. J. Smales, A. Qiao, I. Erucar, C. M. Doherty, A. W. Thornton, A. J. Hill, C. W. Ashling, O. T. Qazvini, S. J. Lee, P. A. Chater, N. J. Terrill, A. J. Smith, Y. Yue, G. Mali, D. A. Keen, S. G. Telfer and T. D. Bennett, Nat. Commun., 2018, 9, 1–9 CrossRef PubMed.
- R. S. K. Madsen, A. Qiao, J. Sen, I. Hung, K. Chen, Z. Gan, S. Sen and Y. Yue, Science, 2020, 367, 1473–1476 CrossRef CAS PubMed.
- A. Qiao, H. Tao, M. P. Carson, S. W. Aldrich, L. M. Thirion, T. D. Bennett, J. C. Mauro and Y. Yue, Opt. Lett., 2019, 44, 1623 CrossRef CAS PubMed.
- T. To, S. S. Sørensen, M. Stepniewska, A. Qiao, L. R. Jensen, M. Bauchy, Y. Yue and M. M. Smedskjaer, Nat. Commun., 2020, 11, 2593 CrossRef CAS PubMed.
- M. Stepniewska, K. Januchta, C. Zhou, A. Qiao, M. M. Smedskjaer and Y. Yue, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 10149–10154 CrossRef CAS PubMed.
- L. Frentzel-Beyme, M. Kloß, R. Pallach, S. Salamon, H. Moldenhauer, J. Landers, H. Wende, J. Debus and S. Henke, J. Mater. Chem. A, 2019, 7, 985–990 RSC.
- P. Krokidas, S. Moncho, E. N. Brothers, M. Castier and I. G. Economou, Phys. Chem. Chem. Phys., 2018, 20, 4879–4892 RSC.
- R. R. Kuruppathparambil, R. Babu, H. M. Jeong, G. Y. Hwang, G. S. Jeong, M. Kim, D. W. Kim and D. W. Park, Green Chem., 2016, 18, 6349–6356 RSC.
- G. Kaur, R. K. Rai, D. Tyagi, X. Yao, P. Z. Li, X. C. Yang, Y. Zhao, Q. Xu and S. K. Singh, J. Mater. Chem. A, 2016, 4, 14932–14938 RSC.
- A. M. Bumstead, M. F. Thorne and T. D. Bennett, Faraday Discuss., 2021, 225, 210–225 RSC.
- M. A. Ali, J. Ren, T. Zhao, X. Liu, Y. Hua and Y. Yue, ACS Omega, 2019, 4, 12081–12087 CrossRef CAS PubMed.
- F.-X. Coudert and A. H. Fuchs, Coord. Chem. Rev., 2016, 307, 211–236 CrossRef CAS.
- R. B. Getman, Y.-S. Bae, C. E. Wilmer and R. Snurr, Chem. Rev., 2011, 112, 703–723 CrossRef PubMed.
- J.-C. Tan, B. Civalleri, C.-C. Lin, L. Valenzano, R. Galvelis, P.-F. Chen, T. D. Bennett, C. Mellot-Draznieks, C. M. Zicovich-Wilson and A. K. Cheetham, Phys. Rev. Lett., 2012, 108, 095502 CrossRef PubMed.
- A. U. Ortiz, A. Boutin, A. H. Fuchs and F.-X. Coudert, Phys. Rev. Lett., 2012, 109, 195502 CrossRef PubMed.
- J. C. Tan, T. D. Bennett and A. K. Cheetham, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 9938–9943 CrossRef CAS PubMed.
- A. M. Walker, B. Civalleri, B. Slater, C. Mellot-Draznieks, F. Corà, C. M. Zicovich-Wilson, G. Román-Pérez, J. M. Soler and J. D. Gale, Angew. Chem., Int. Ed., 2010, 49, 7501–7503 CrossRef CAS PubMed.
- M. Gao, A. J. Misquitta, L. H. Rimmer and M. T. Dove, Dalton Trans., 2016, 45, 4289–4302 RSC.
- P. G. Boyd, S. M. Moosavi, M. Witman and B. Smit, J. Phys. Chem. Lett., 2017, 8, 357–363 CrossRef CAS PubMed.
- R. Gaillac, P. Pullumbi, K. A. Beyer, K. W. Chapman, D. A. Keen, T. D. Bennett and F.-X. Coudert, Nat. Mater., 2017, 16, 1149–1155 CrossRef CAS PubMed.
- L. Zhang, Z. Hu and J. Jiang, J. Am. Chem. Soc., 2013, 135, 3722–3728 CrossRef CAS PubMed.
- Z. Hu, Y. Chen and J. Jiang, J. Chem. Phys., 2011, 134, 134705 CrossRef PubMed.
- Y. Yang, Y. K. Shin, S. Li, T. D. Bennett, A. C. T. van Duin and J. C. Mauro, J. Phys. Chem. B, 2018, 122, 9616–9624 CrossRef CAS PubMed.
- Y. Yang, C. J. Wilkinson, K.-H. Lee, K. Doss, T. D. Bennett, Y. K. Shin, A. C. T. van Duin and J. C. Mauro, J. Phys. Chem. Lett., 2018, 9, 6985–6990 CrossRef CAS PubMed.
- D. Dubbeldam, K. S. Walton, D. E. Ellis and R. Q. Snurr, Angew. Chem., Int. Ed., 2007, 46, 4496–4499 CrossRef CAS PubMed.
- A. Bax, J. Magn. Reson., 1985, 65, 142–145 Search PubMed.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- F. Birch, Phys. Rev., 1947, 71, 809 CrossRef CAS.
- S. Plimpton, J. Comput. Phys., 1995, 117, 1–19 CrossRef CAS.
- M. A. Addicoat, N. Vankova, I. F. Akter and T. Heine, J. Chem. Theory Comput., 2014, 10, 880–891 CrossRef CAS PubMed.
- D. E. Coupry, M. A. Addicoat and T. Heine, J. Chem. Theory Comput., 2016, 12, 5215–5225 CrossRef CAS PubMed.
- A. K. Rappé, C. J. Casewit, K. S. Colwell, W. A. Goddard III and W. M. Skiff, J. Am. Chem. Soc., 1992, 114, 10024–10035 CrossRef.
- L. Vanduyfhuys, S. Vandenbrande, J. Wieme, M. Waroquier, T. Verstraelen and V. van Speybroeck, J. Comput. Chem., 2018, 39, 999–1011 CrossRef CAS PubMed.
- T. D. Bennett, Y. Yue, P. Li, A. Qiao, H. Tao, N. G. Greaves, T. Richards, G. I. Lampronti, S. A. T. Redfern, F. Blanc, O. K. Farha, J. T. Hupp, A. K. Cheetham and D. A. Keen, J. Am. Chem. Soc., 2016, 138, 3484–3492 CrossRef CAS PubMed.
- P. Krokidas, M. Castier, S. Moncho, E. Brothers and I. G. Economou, J. Phys. Chem. C, 2015, 119, 27028–27037 CrossRef CAS.
- S. Henke, M. T. Wharmby, G. Kieslich, I. Hante, A. Schneemann, Y. Wu, D. Daisenberger and A. K. Cheetham, Chem. Sci., 2018, 9, 1654–1660 RSC.
- K. Li, Z. Ding and D. Xue, Funct. Mater. Lett., 2010, 3, 241–244 CrossRef CAS.
- I. D. Brown, P. Klages and A. Skowron, Acta Crystallogr., Sect. B: Struct. Sci., 2003, 59, 439–448 CrossRef PubMed.
- P. Z. Moghadam, A. Li, S. B. Wiggin, A. Tao, A. G. P. Maloney, P. A. Wood, S. C. Ward and D. Fairen-Jimenez, Chem. Mater., 2017, 29, 2618–2625 CrossRef CAS.
- L. Chen, T. Mashimo, C. Iwamoto, H. Okudera, E. Omurzak, H. S. Ganapathy, H. Ihara, J. Zhang, Z. Abdullaeva, S. Takebe and A. Yoshiasa, Nanotechnology, 2013, 24, 045602 CrossRef CAS PubMed.
- J. Kjeldsen, M. M. Smedskjaer, J. C. Mauro and Y. Yue, J. Non-Cryst. Solids, 2014, 406, 22–26 CrossRef CAS.
- J. Kjeldsen, M. M. Smedskjaer, J. C. Mauro and Y. Yue, Appl. Phys. Lett., 2014, 104, 051913 CrossRef.
- F. A. Lindemann, Phys. Z., 1910, 11, 609–612 CAS.
- D. Radhakrishnan and C. Narayana, J. Chem. Phys., 2015, 143, 234703 CrossRef PubMed.
- G. Khandelwal, N. P. Maria Joseph Raj and S. J. Kim, J. Mater. Chem. A, 2020, 8, 17817–17825 RSC.
- NIST Chemistry WebBook, NIST Standard Reference Database Number 69, ed. P. J. Linstrom and W. G. Mallard, National Institute of Standards and Technology, Gaithersburg MD, 2005, p. 20899, http://webbook.nist.gov, accessed August 2021 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Optical images, SEM images, EDX elemental mapping, XRD patterns, 1H-NMR spectra, ATR FT-IR spectra, ICP-OES ratio, DSC scans, TGA data, and DTA-MS analysis. See DOI: 10.1039/d2ra00744d |
| ‡ These authors equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2022 |