Open Access Article
Open Access ArticleHydrogen bonds-triggered differential extraction efficiencies for bifenthrin by three polymeric ionic liquids with varying anions based on FT-IR spectroscopy
Xiaofan Zhang†
a,
Ming Gao†a,
Tingting Liub,
Huili Wanga and
Xuedong Wang *a
*a
aSchool of Environmental Science and Engineering, Suzhou University of Science and Technology, Suzhou 215009, China. E-mail: zjuwxd@163.com
bJiangsu Provincial Key Laboratory of Environmental Science and Engineering, Suzhou University of Science and Technology, Suzhou 215009, China
First published on 5th May 2022
Abstract
Herein, we fabricated three imidazolium-based polymeric ionic liquids (PILs) with different anions (P[VEIM]BF4, P[VEIM]PF6 and P[VEIM]Br), and analyzed their differential extraction efficiencies for bifenthrin through H-bonding induced effects. Three PILs all presented an irregular block structure with rough surface and lower specific-surface area (SSA, 11.2–18.7 m2 g−1) than carbon-based nanomaterials. They formed hydrogen bonds with free-water molecules in the lattice of PILs, including C2,4,5–H⋯O–H, Br⋯H–O–H⋯Br, O–H⋯Br, C2,4,5–H⋯F–P, P–F⋯H–O–H⋯F–P, C2,4,5–H⋯F–B and B–F⋯H–O–H⋯F–B. After extraction, the O–H stretching-vibration peak was prominently intensified, whereas the C–H bond varied slightly concomitant with reduced B–F and P–F vibration. Theoretically, the C–H vibration should become more intense in the C4,5–H⋯H2O and C2–H⋯H2O bonds after extraction in contrast to before extraction. These contrary spectral changes demonstrated that the hydrogen bonds between cations in the PILs and free-water molecules were broken after extraction, yielding the H-bonding occurrence between bifenthrin and H–O–H in the lattice. As a time indicator for the free-water binding and releasing process, the highest slope for the plot of It/I0 against time implied that the shortest time was required for P[VEIM]PF6 to reach an adsorption equilibrium. Overall, the strong hydrophobicity, small SSA and electrostatic-repulsion force for P[VEIM]PF6 are all not conducive to its efficient adsorption. Beyond our anticipation, P[VEIM]PF6 provided the highest extraction recovery for bifenthrin up to 92.4% among three PILs. Therefore, these data lead us to posit that the above high efficiency results from the strongest H-bonding effect between P[VEIM]PF6 and bifenthrin. These findings promote our deep understanding of PILs-triggered differential efficiency through a H-bonding induced effect.
1. Introduction
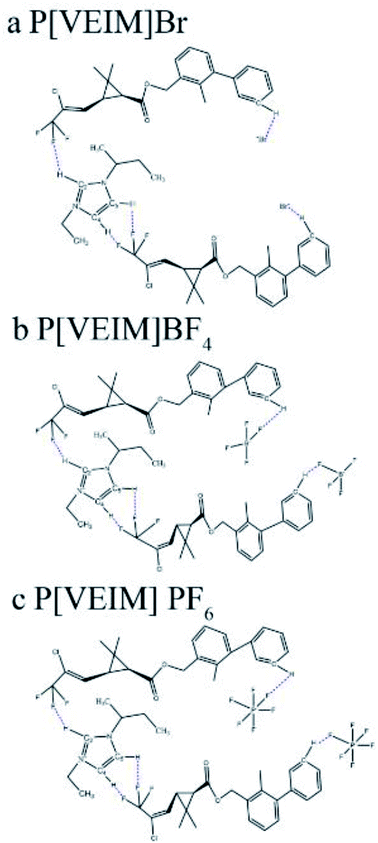
Polymeric ionic liquids (PILs) possess the advantages of both ionic liquids (ILs) and polymers, and thus have good ionic and conductive properties,1,2 which expand their applications as fit-for-purpose and functional ILs. Currently, two kinds of PILs have been widely reported: the first is that the polymerized groups are introduced into ILs to prepare functional PILs through polymerization; the second is that the composite metals or metal oxides with catalytic activity are integrated with PILs for the sake of fabricating supported PILs.3–5 Lin and coworkers (2021) fabricated polymeric self-solidifying ionic liquids as catalysts for producing biodiesel under mild conditions with good recyclability.3 Safa's group (2016) synthesized a series of PILs-based gel electrolytes, which were employed in lithium batteries and achieved higher catalytic efficiency than using ILs alone.4 The aliphatic hydroxyl-functionalized imidazole PILs were used to catalyze CO2-cycloaddition reaction of epoxides with high yields, and could be recycled many times with no significant decrease in their catalytic performance.5 Peng et al. (2020) prepared many kinds of PIL-based microspheres as the stationary phase in HPLC columns and achieved satisfactory separation efficiency for organic pollutants.6Also, PILs are extensively used in gas adsorption and separation, especially in CO2 adsorption, which solve the bottleneck problem of ILs in high viscosity and low-adsorption selectivity.7–9 Under the same experimental conditions, the PILs, based on 1-(4-vinylphenyl)-3-butyl-imidazole ILs, provided the adsorption capacity for CO2 3–5-fold greater than that of ordinary imidazole ILs.7,8 Xiong's group (2012) synthesized a type of PILs by introducing the IL groups into the main chain of a polymer, and observed that their adsorption capacity for CO2 reached up to 10% with shorter adsorption–desorption equilibrium time.9,10 Besides, PILs have been widely used in various nanocomposites. In common cases, nanomaterials easily lose the practical application value due to high specific surface area, which is dynamically unstable and shows strong agglomeration phenomenon. Therefore, stabilizers, such as surfactants and soluble polymers, are often added to nanomaterials to prevent the agglomeration of nanoparticles. Due to possessing polar ionic bonds and long spatial chain, PILs can stabilize nanoparticles by virtue of electrostatic and steric effects. Matandabuzo and Alibade synthesized vinyl-pyridinium PILs to further prepare the nanocomposites of PILs and multiwalled carbon nanotubes (PIL/MWCNT), which showed maximum adsorption capacity of 37% for Cr(VI) within 12 h agitation.10 Based on 1-vinyl-3-methylimidazole chloride and n-vinyl-2-carbonylpyrrolidine, the as-synthesized copolymers were mixed with [BMIM]BF4 and added to rhodium nanoparticles to improve the catalytic activity and lifetime.11 Although several reports on the utilization of PILs have been reported as catalysts and adsorbents, there is a paucity of information regarding their applications as extractant in analytical pretreatment procedures. Enlightened by the aforementioned analyses, our group synthesized three kinds of imidazolium-based PILs with different anions (BF4−, PF6− and Br−). In the lattice of PILs, many kinds of hydrogen bonds were found to be formed with free water molecules (Fig. 1). After adsorption/extraction of bifenthrin, the underlying mechanisms regarding differential extraction efficiencies were explored in detail by virtue of changes in the formation, reintegration and vibration of hydrogen bonds in PILs before and after extraction.
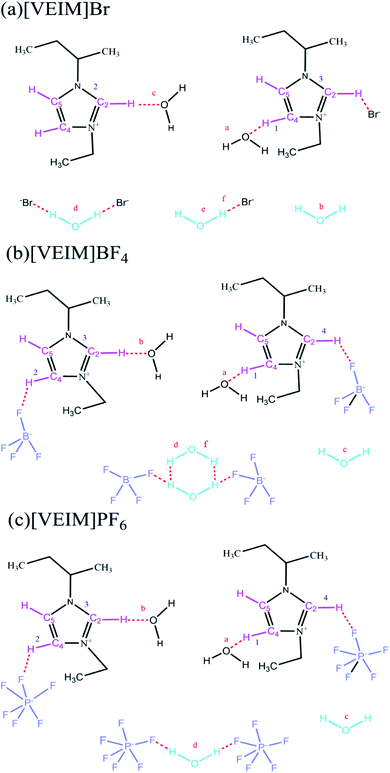 | ||
| Fig. 1 Types of hydrogen bonds in the lattice of PILs. (a) P[VEIM]Br; (b) P[VEIM]BF4, (c) P[VEIM]PF6. | ||
Among common pesticides, pyrethroids compounds are widely used to control agricultural/forestry insects all over the world due to high efficiency, rapidity, broad spectrum, and strong photostability. With the abuse increase of pyrethroids, they not only cause serious impact on the environment, but also accumulate excessive residues in fruits and vegetables, which affect central nervous system and lead to disturbance of consciousness.12,13 The maximum residue limits (MRLs) of pyrethroids in foods have been set at 0.02 to 0.5 mg kg−1 in China (GB2763-2019), and 0.02 to 0.3 mg kg−1 in European Union (Part A of Annex to Reg. 396/2005), respectively.14 Common analytical approaches for pyrethroids detection include GC-ECD, GC-MS, HPLC-DAD, and so on.15 At present, the sample pretreatment methods for pyrethroids in complex matrices include liquid–liquid extraction, solid-phase extraction, magnetic solid-phase extraction, and so on.16 However, owing to the presence of trace-level pyrethroids and various interference chemicals and ions, the rapid/sensitive assay of pyrethroids is still a challenging issue in environmental and food matrices.
Hydrogen bonds play crucial roles in determining the extraction efficiency of ILs for target analytes. Previous literature reported the effects of polymethylated imidazole cations, imidazole nitrogen cations and NTF2− anions on the properties of ILs (melting point and viscosity) by hydrogen bonds,17 and also investigated the H-bonding role in crystal structure.18 In typical imidazolium-based ILs, hydrogen bonds between cations and anions are considered to be the key factor to determine the microstructure and macroscopic properties. Hu and coworkers employed density functional theory (DFT) to illustrate the relationship between viscosity and hydrogen bonds in ILs.19 Due to the H-bonding importance in determining IL's property and structure, there have been many reports on the dependence of ILs on H-bonding strength.19–21 The optimized geometry of H-bonding ion pairs was predicted for imidazolium-based ILs with varying anions by DFT. Phadagi et al. and Liu et al. calculated the H-bonding strength changes of different cations and anions in PILs by FT-IR spectroscopy and DFT.20,21
As far as our information goes, no reports are related to the application of PILs for bifenthrin extraction as a model chemical in pyrethroids, the underlying H-bonding formation, and reintegration mechanism in the extraction process. Motivated by the above issue, we prepared three kinds of imidazolium-based PILs with different anions, explored their interactions with bifenthrin, and confirmed the crucial roles of anions in the H-bonding occurrence. Prior to adsorption, the anions formed hydrogen bonds with free water molecules in the lattice of PILs. Three kinds of H-bonding forms existed in the carbon atoms of imidazolium ring: C2–H, C4–H and C5–H.22,23 Free water molecules interacted with anions of PILs by hydrogen bonds as H2O⋯anion and anion⋯H2O⋯anion. Consequently, the formation and reintegration of O–H, C–H, P–F and B–F vibration peaks were investigated in detail by virtue of changes in FT-IR spectra before and after extraction. This work sheds new light on mechanistic insight into the differential extraction efficiencies for target analytes by PILs, resulting from the changes in H-bonding interactions between PILs and analytes.
2. Materials and methods
2.1. Reagents and chemicals
Bifenthrin with purity of 99.5% was purchased from the China Chemical Standard Corporation (Beijing, China), and its molecular structure is shown in Fig. 2a. N-Vinylimidazole (98.0%), and the analytical-grade bromoethane, sodium tetrafluoroborate, potassium hexafluorophosphate, and azobisisobutyronitrile (AIBN) were all obtained from Tansoole (Shanghai, China). Chromatographic-grade acetone, methanol, acetonitrile and ethyl acetate were sourced from Aladdin (Shanghai, China).2.2. Instrumentation
The structures and morphologies of three PILs were characterized by a scanning electron microscopy (Zeiss Sigma 300, Germany; accelerating voltage 20.0 kV, working distance 9.8–10.2 mm, ETD detector). The gold coating of the SEM used an Agar Auto Sputter Coater mod. 108 (Cressington, UK), with purity > 99.99%, current 40 mA, and 30 s coating time. The FT-IR spectra of PILs were measured with a scanning range of 400–4000 cm−1 by the KBr method in a Bruker Tensor II infrared spectrometer (Brook, Germany). The spectral intensity ratios (It/I0) reflect the situations on H-bonding breakage or reintegration, where It and I0 denote the intensities of O–H or C–H bonds after adsorption in different time intervals and prior to adsorption, respectively. X-ray photoelectron spectroscopy (XPS) measurements were performed using a PHI Quantera spectrometer with Al Kα X-ray (hν = 1486.6 eV) radiation. UV-Vis spectra were recorded on a UV-5500PC spectrophotometer (Metash, Malvern, UK). A SDT Q600 thermal gravimetric analyzer (PerkinElmer, MA, USA) was employed to analyze the thermal stability of PILs, and it was operated at a heating rate of 10 °C min−1 across the temperature range of 30–600 °C under a N2 flow. The decomposed temperature was analyzed based on the weight-loss curves. Also, a Setsys Ev 24 model thermal analyzer (Setaram, Paris, France) was used to detect the glass transition temperature (Tg) according to differential scanning calorimetric curves over the temperature range of 150–300 °C. The Brunauer–Emmett–Teller (BET) surface area was measured by N2 adsorption–desorption at 77 K using an ASAP 2020 System (Quantachrome, USA). Zeta potential was detected by a zeta potential analyzer (Malvern, UK). The measurement of solution pH was carried out using a Leici PHB-4 pH meter (Inesa Scientific Corporation, Shanghai, China). All stock and work solutions were prepared using ultrapure water (18.25 MΩ) from Hangzhou Jiejing Purification System (Hangzhou, China). The water-phase filter membrane (50 mm × 0.45 μm) was acquired from Tianjin Jinteng Experimental Equipment Corporation (Tianjin, China).2.3. Bifenthrin detection
The concentration of bifenthrin was determined by a Shimadzu LC-20AT liquid chromatography equipped with a diode-array detector (HPLC-DAD). Chromatographic separation was conducted on a Shim-Pack GIST C18 column (250 mm × 4 mm, 5 μm) under the following operational conditions: mobile phase, acetonitrile–water at 80%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20% by v/v; flow rate, 1 mL min−1; and column temperature, 30 °C. The detection wavelength was set at 210 nm, and the injection volume was 20 μL under a 10 μL sample loop. The differential extraction efficiencies for bifenthrin were assessed in the following optimized conditions: ultrapure water, 10 mL; the fortified bifenthrin level, 500 μg L−1; solution pH, 7.0; and elution solvent, 1.2 mL methanol. Briefly, after 10 min ultrasonic adsorption, the PILs containing analytes were centrifuged at 5000 rpm for 5 min, and the supernatant was discarded. The settled phase was eluted by methanol and filtered by a 0.22 μL membrane filter, and the resultant eluent was subjected to HPLC-DAD analysis.
20% by v/v; flow rate, 1 mL min−1; and column temperature, 30 °C. The detection wavelength was set at 210 nm, and the injection volume was 20 μL under a 10 μL sample loop. The differential extraction efficiencies for bifenthrin were assessed in the following optimized conditions: ultrapure water, 10 mL; the fortified bifenthrin level, 500 μg L−1; solution pH, 7.0; and elution solvent, 1.2 mL methanol. Briefly, after 10 min ultrasonic adsorption, the PILs containing analytes were centrifuged at 5000 rpm for 5 min, and the supernatant was discarded. The settled phase was eluted by methanol and filtered by a 0.22 μL membrane filter, and the resultant eluent was subjected to HPLC-DAD analysis.
2.4. Fabrication of PILs
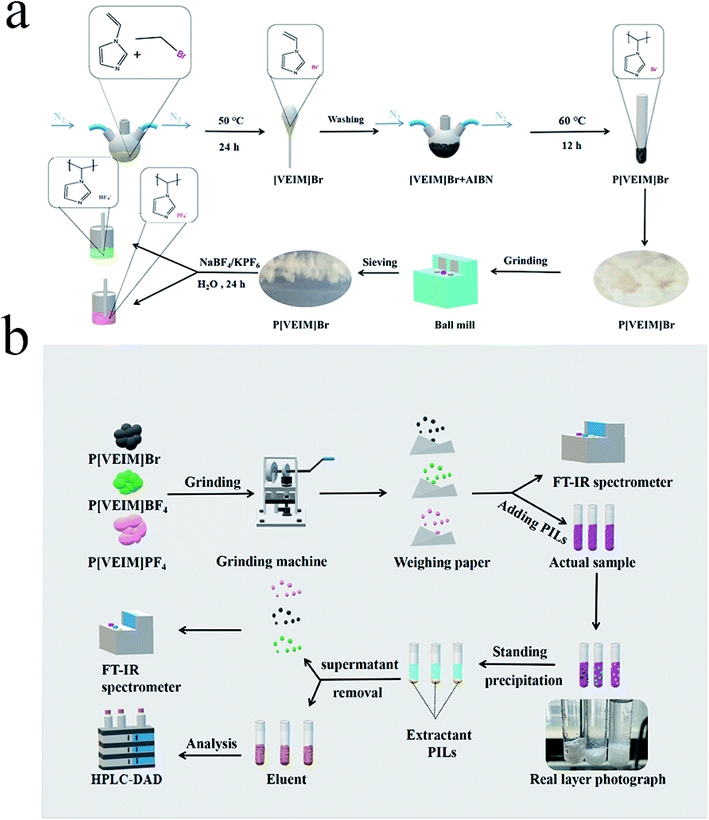
As vividly elaborated in Fig. 3, 0.05 mol of N-vinylimidazole was accurately weighed and added to a 100 mL round-bottom flask under N2 protection, and then 0.10 mol of bromoethane was added dropwise to the above flask and refluxed for 24 h at 50 °C. With increasing reaction time, the solution viscosity continued to rise to form a yellow-white solid product, which was further washed three times with ethyl acetate to remove an excessive reaction precursor and dried in a vacuum drying oven for 24 h. Finally, ∼8.4 g of the product was obtained with a yield of 82.2%. Upon addition of an initiator AIBN, ILs monomer [VEIM]Br with double bond was polymerized to form P[VEIM]Br. To be more precise, 6.14 g of [VEIM]Br, 0.08 g of AIBN, and 35 mL of chloroform were added to a 100 mL round-bottom flask, protected by N2 flow and reacted under reflux conditions at 50 °C. When the reaction solution was relatively stable, it was kept at 60 °C for 12 h reaction. Clearly, a yellow-white solid were gradually formed in the solution, and as the reaction continued, the resultant precipitate gradually became bulk solid. After the reaction, the precipitate was washed three times with chloroform, and the product obtained was vacuum-dried at 40 °C. Finally, 5.75 g of the yellow-white solid was achieved with a yield as high as 93.7%. Subsequently, other two kinds of PILs (P[VEIm]BF4 and P[VEIm]PF6) were prepared by virtue of anion exchanging reaction. Briefly, 5.30 g of P[VEIM]Br, and 5.50 g of NaBF4 for P[VEIM]BF4 (or 5.00 g of KPF6 for P[VEIM]PF6) were added to a 100 mL single-neck flask and stirred for 12 h at ambient conditions. After the reaction, the resultant precipitate was filtered and vacuum-dried at 60 °C for 6 h. The final bulk solid was ground into powder, washed with ultrapure water for three times, and further vacuum-dried for 6 h. The as-achieved yellow-white solid for P[VEIM]BF4 or P[VEIM]PF6 was employed in the following trials.2.5. FT-IR spectra of PILs before and after extraction
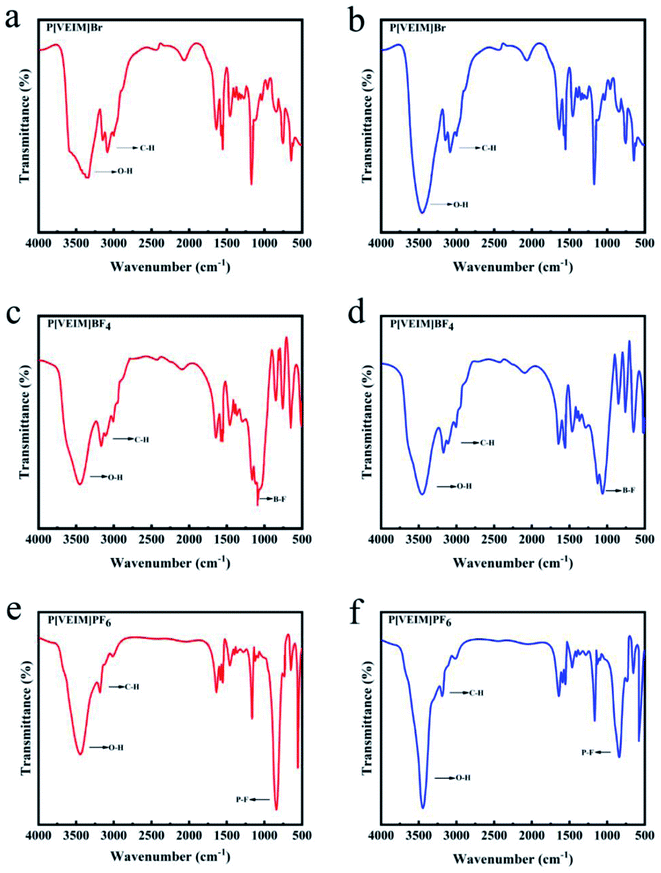
After the bulk solids of three PILs were ground into powder, aliquots of them were subjected to FT-IR analyses, which were referred to as the sample before extraction. After an appropriate time of adsorption, the PILs containing bifenthrin were eluted with methanol as described in Section 2.3, vacuum-dried for 6 h and subjected to FT-IR detection as the sample after extraction (Fig. 3b). For peak deconvolution, the FT-IR spectra were fitted with the Voigt function using the Origin 8.0 software. In such cases, the chi-squared value for each fitting curve was more than 0.99 in the deconvolution. As for the FT-IR spectra of PILs with varying anions, we focused on observation on the O–H stretching band at 3100–3700 cm−1, C–H stretching band at 2700–3200 cm−1, and B–F stretching band at 950–1152 cm−1, respectively.24–26 Based on changes in the above stretching bands, the H-bonding occurrence and reintegration information in PILs were deducted before and after extraction.3. Results and discussion
3.1. Characterization of three PILs with varying anions
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnification. Obviously, the imidazolium-based PILs with varying anions displayed the similar morphology. They presented irregular and asymmetric block structure with rough surface and no fixed crystal shape. From the overall appearance, PILs were close to the shape of jade and pile up with different sizes of particles, demonstrating the formation of amorphous polymers.27
000× magnification. Obviously, the imidazolium-based PILs with varying anions displayed the similar morphology. They presented irregular and asymmetric block structure with rough surface and no fixed crystal shape. From the overall appearance, PILs were close to the shape of jade and pile up with different sizes of particles, demonstrating the formation of amorphous polymers.27
3.2. Differential extraction efficiency for bifenthrin by PILs
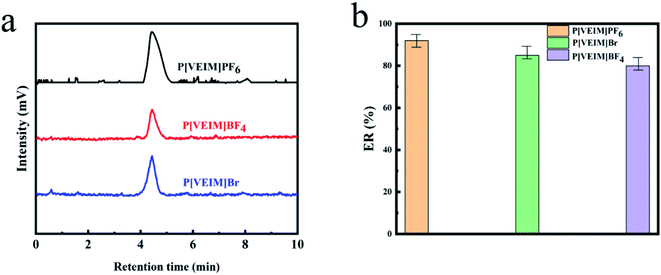
Under optimized experimental conditions (10 mL of ultrapure water, 500 μg L−1 of bifenthrin, solution pH, 7.0, and 1.2 mL methanol as elution solvent), we compared the extraction efficiencies of three PILs for bifenthrin. Fig. 6a shows the typical HPLC-DAD chromatograms of bifenthrin after extraction by PILs. The retention time of bifenthrin was observed at ∼4.5 min. As displayed in Fig. 6, the average extraction recoveries (ERs) for bifenthrin were 92.4% by P[VEIM]PF6, 85.3% by P[VEIM]Br, and 79.4% by P[VEIM]BF4, respectively. Obviously, P[VEIM]PF6 provided the highest ER for bifenthrin among three PILs, which were ∼13.0% higher than that by P[VEIM]BF4. The extraction efficiency for analytes by PILs is dependent on several factors, including the physico-chemical properties of PILs (SSA, zeta potential, pore size and so on), as well as the molecular interactions between PILs and analytes (hydrogen bond, π–π interaction and van der Waals force, and so on).29 In the following experiments, we focused on unveiling the H-bonding changes before and after extraction and its relationship with PILs bearing with different anions.3.3. H-bonding changes before and after extraction based on FT-IR spectra
Three PILs with imidazolium frameworks and varying anions were employed to analyze the FT-IR changes in hydrogen bonds before and after extraction (Fig. 7). P[VEIM]Br formed hydrogen bonds with free water molecules in the lattice of PILs, and the main types of hydrogen bonds were C2,4,5–H⋯O–H, Br−⋯H–O–H⋯Br−, and O–H⋯Br−.30,31 As for P[VEIm]PF6, the H-bonding forms were C2,4,5–H⋯O–H, C2,4,5–H⋯F–P, and P–F⋯H–O–H⋯F–P. With respect to P[VEIm]BF4, the main hydrogen bonds concerned were C2,4,5–H⋯O–H, C2,4,5–H⋯F–B, and B–F⋯H–O–H⋯F–B.The FT-IR spectra before and after extraction of bifenthrin are elaborated in Fig. 7a and b for P[VEIm]Br, Fig. 7c and d for P[VEIM]BF4, and Fig. 7e and f for P[VEIM]PF6, respectively. The vibration intensity of O–H reflected the absorption value of this bond in the FT-IR spectrum. For P[VEIM]Br, the O–H stretching-vibration peak at 3450 cm−1 was prominently intensified, whereas the C–H vibration at 3175 cm−1 varied slightly. As for P[VEIM]BF4, two peaks were observed to change significantly before and after extraction, which showed the intensified O–H vibration at 3495 cm−1 and decreased B–F vibration at 1190 cm−1, respectively. With regard to P[VEIM]PF6, the O–H vibration peak at 3490 cm−1 was prominently increased; in sharp contrast, the P–F vibration at 895 cm−1 was substantially decreased. In contrast to the FT-IR spectra before and after extraction, no remarkable variations were observed for the C–H vibration peaks at 3200 cm−1 for P[VEIM]BF4 and at 3270 cm−1 for P[VEIM]PF6, respectively.
Prior to extraction, the hydrogen bonds formed between the anions and free water in the grids of PILs limited the vibrations of O–H, C–H, P–F, and B–F. However, the stretching vibrations of the O–H absorption band were significantly enhanced after extraction, providing compelling evidence for the breakage of hydrogen bonds between anions and free water molecules in the PILs. As well known, many kinds of molecular forces exist between adsorbents and target analytes. From the theoretical point of view, C–H, P–F, and B–F should vibrate more strongly after adsorption, while the opposite consequence was observed. Besides, the corresponding shrinking of three characteristic peaks in the FT-IR spectra further proved that the anions in the PILs and bifenthrin formed stronger hydrogen bonds to limit or bind the vibrations of C–H, P–F, and B–F.
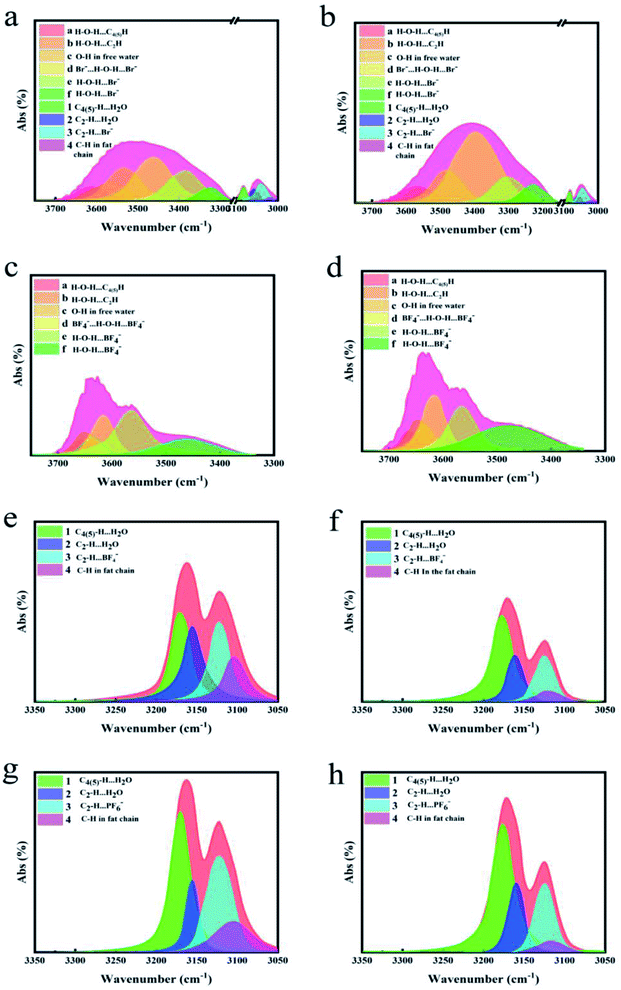
As described above, hydrogen bonds formed by virtue of PF6−, BF4−, Br− in PILs as receptors and hydrogen atoms in water molecules and on imidazolium ring as donors, respectively. Owing to the overlapping phenomenon of different O–H/C–H vibrations in total band, we deconvoluted the O–H/C–H peaks (3000–3800 cm−1) of three PILs for the sake of illustrating the detailed FT-IR spectral changes before and after extraction.
3.4. Strong H-bonding production between PILs and bifenthrin by XPS analysis
As summarized in Table 1, the stretching vibrations could be deconvoluted into four peaks for C–H, and six peaks for O–H,32–35 respectively. For P[VEIM]Br, five types of hydrogen bonds could form, which included H2O⋯Br−, Br−⋯H2O⋯Br−, H2O⋯C4(5)–H, H2O⋯C2–H, and C2–H⋯Br−.36,37 As similar as P[VEIM]Br, P[VEIM]BF4 could yield five H-bonding categories: H2O⋯BF4−, BF4−⋯H2O⋯BF4−, H2O⋯C4(5)–H, H2O⋯C2–H, and C2–H⋯BF4−.38 Likely, the following hydrogen bonds could occur in the homogeneous solution of P[VEIM]PF6, which were composed of H2O⋯PF6−, PF6−⋯H2O⋯PF6−, H2O⋯C4(5)–H, H2O⋯C2–H, and C2–H⋯PF6−. After extraction, the O–H stretching-vibration band was remarkably intensified in the P[VEIM]Br, which possibly resulted from fracture of the H2O⋯Br−, Br−⋯H2O⋯Br−, H2O⋯C4(5)–H, H2O⋯C2–H bonds in the lattice of PILs. By XPS analyses of P[VEIM]Br, the O–H stretching vibrations for H2O⋯C4(5)–H, H2O⋯C2–H and H–O–H became intense to a large extent; in stark contrast, the C–H vibrations for H2O⋯C4(5)–H and H2O⋯C2–H were evidently decreased, which implied the H-bonding breakage of C4(5)–H⋯H2O and C2–H⋯H2O after extraction (Fig. 8a and b). From a theoretical point of view, the C–H vibration should become more intense in the C4(5)–H⋯H2O and C2–H⋯H2O bonds after extraction when compared to before extraction. These contrary spectral changes provide compelling evidence that after extraction, the hydrogen bonds between cations in the PILs and free water molecules were broken, further resulting in the H-bonding occurrence between bifenthrin and H–O–H in the lattice of PILs.After extraction, more intense O–H vibration and weaken B–F bond were observed in the lattice of P[VEIM]BF4 as compared to before extraction (Fig. 8c and d). By XPS analysis, the following hydrogen bonds were confirmed to be fractured: H2O⋯BF4−, BF4−⋯H2O⋯BF4−, H2O⋯C4(5)–H, H2O⋯C2–H, C2–H⋯BF4− (Fig. 8).39 Theoretically, more strong vibration of C2–H⋯H2O, C4(5)–H⋯H2O and B–F bonds should occur after extraction of bifenthrin; with regret, the as-anticipated cases did not occur, demonstrating the stronger H-bonding occurrence between P[VEIM]BF4 and bifenthrin after extraction. Similarly, in the FT-IR spectra of P[VEIM]PF6, the O–H vibration at 3490 cm−1 became more intense, but the contrary changing trend was observed for P–F vibration at 895 cm−1 after extraction. Based on XPS analysis, a series of H-bonding strength was observed to be prominently weakened, including C4,5–H⋯H2O, C2–H⋯H2O, C2–H⋯PF6− and C–H in the imidazolium ring (Fig. 8g and h), which displayed the contrary trends as those for the O–H vibration in Fig. 8e and f. These phenomena provide compelling evidence that the distinct H-bonding changes occur, and more intense hydrogen bonds form between PILs and bifenthrin in the extraction process.
3.5. Quantitative changes in the O–H vibration intensity of PILs before and after extraction
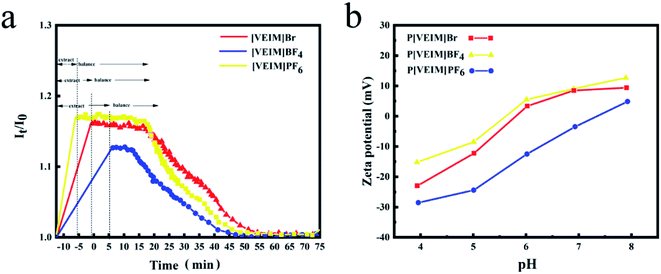
The H-bonding emergence mainly occurred at the positions of C2, C4, and anions (Br1−, PF61−, BF41−) on the imidazolium ring of PILs. As illustrated in Fig. 9a, I0 is the O–H vibration intensity prior to extraction, while It indicates that after extraction at varying extraction time. The ratio of It to I0 (It/I0) represents the binding and releasing processes of free water molecules by virtue of the breakage and reintegration of hydrogen bonds in the lattice of PILs.40 The gradual increases in the It/I0 values result from the breakage of hydrogen bonds between PILs and free water molecules. When rising up to a certain value, the It/I0 ratios do not change because the extraction process reaches an equilibrium, and then it gradually decreases and returns to an original level.40 The above changing trend for the It/I0 values may be explained by the recyclability of PILs, which also proves the breakage and reintegration of hydrogen bonds on the PILs during the extraction process. For the plot of It/I0 vs. time, the linear slopes of P[VEIM]BF4, P[VEIM]PF6 and P[VEIM]Br are 0.018, 0.013 and 0.12, respectively, suggesting that the shortest time is required for P[VEIM]PF6 to reach an adsorption equilibrium.3.6. Zeta potential changes of PILs before and after extraction
The zeta potentials of three PILs at varying solution pH are shown in Fig. 9b. When the solution pH spanned the range of 4.0–5.0, three PILs were all negatively charged from −28.7 mV to −10.2 mV. However, with further increases in solution pH from 6.0 to 8.0, the zeta potentials became positive for P[VEIM]Br and P[VEIM]BF4 with exception of P[VEIM]PF6. Under the optimized extraction conditions (pH = 6.0), P[VEIM]PF6 was negatively charged (−12.7 mV), while P[VEIM]BF4 and P[VEIM]Br were positively charged (9.6 and 10.7 mV). Similarly, the bifenthrin was negatively charged (−3.9 mV) under the solution pH of 6.0. As a consequence, there was electrostatic attraction between P[VEIM]Br and P[VEIM]BF4 and bifenthrin besides the hydrogen-bonding effect, whereas a repulsive force occurred between P[VEIM]PF6 and bifenthrin. As mentioned above, the P[VEIM]PF6 gave the highest extraction efficiency (92.4%) for bifenthrin among three PILs. Building upon the above data analysis, we posit that the highest extraction efficiency of P[VEIM]PF6 for bifenthrin mainly resulted from its stronger hydrogen-bonding effect in spite of the occurrence of a repulsive action.3.7. Mechanistic understanding of the strongest H-bonding form between P[VEIM]PF6 and bifenthrin
In common cases, the hydrophobicity of PILs may affect the H-bonding production speed and strength.41,42 For three imidazolium-based PILs, the hydrophobic orders of their anions are PF6− > BF4− > Br−. For P[VEIM]PF6, it forms hydrogen bonds with bifenthrin mainly through cations because its smaller SSAs and mesoporous structure are unfavorable for highly efficient adsorption of bifenthrin. In terms of electrostatic attraction, when the solution pH is 7.0, both P[VEIM]BF4 and P[VEIM]Br are negatively charged, which are opposite to the charge of bifenthrin (Fig. 9b). Thus, we posit that electrostatic attraction existed between P[VEIM]BF4 or P[VEIM]Br and bifenthrin.In sharp contrast, P[VEIM]PF6 has strong hydro P[VEIM]BF4 or P[VEIM]Br and bifenthrin. The above analyses demonstrate that in the process of adsorption, P[VEIM]BF4 and P[VEIM]Br not only form strong hydrogen bonds with target analytes, but also strong electrostatic attraction force exists between them. Due to strong hydrophobicity and small pore size in the P[VEIM]PF6, both of the characteristics are not conducive to its dispersion in aqueous phase and efficient adsorption for target adsorbates. When the solution pH is 7.0, both P[VEIM]PF6 and bifenthrin are negatively charged. Consequently, when P[VEIM]PF6 adsorbs bifenthrin, the H-bonding form between them needs to overcome the electrostatic repulsion force in order to acquire a high extraction efficiency. Among three PILs, P[VEIM]PF6 offers the highest extraction efficiency for bifenthrin up to 92.4%. Building upon the above analyses, we conclude that P[VEIM]PF6 forms the strongest hydrogen bond with bifenthrin during the extraction.
4. Conclusion
In this investigation, we synthesized three imidazolium-based PILs with different anions (BF4−, PF6−, and Br−) by employing N-vinyl group as a cross-linker and AIBN as an initiator. The morphologies and physical–chemical properties of three PILs were characterized/analyzed in detail by virtue of spectral techniques and several metrics, including SEM, FT-IR, TGA, N2 adsorption–desorption and glass transition temperature (Tg). After extraction, the O–H stretching-vibration peak (3450–3500 cm−1) was significantly intensified, whereas the C–H peak (∼3100 cm−1) remained nearly unchanged in concomitant with prominently decreased B–F (1190 cm−1) and P–F (895 cm−1) bonds. These spectral changes provide compelling evidence that after extraction, the hydrogen bonds between cations in the PILs and free water molecules were broken, further resulting in the H-bonding occurrence between bifenthrin and H–O–H in the lattice of PILs. For the plot of It/I0 vs. time, the linear slopes of P[VEIM]BF4, P[VEIM]PF6 and P[VEIM]Br were 0.018, 0.013 and 0.12, respectively, suggesting that the shortest time was required for P[VEIM]PF6 to reach an adsorption equilibrium. Because the following properties of P[VEIM]PF6 (strong hydrophobicity, small SSA and electrostatic repulsion force) were all not beneficial to its efficient adsorption, it gave the highest extraction efficiency for bifenthrin up to 92.4% among three PILs. As such, it can be concluded that P[VEIM]PF6 forms the strongest hydrogen bond with bifenthrin to achieve its high efficiency in the extraction process.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was jointly supported by the National Science Foundation of China (22076134 and 21876125), Jiangsu Provincial Natural Science Foundation (BK20211338), Key Science & Technology Project of Suzhou City (SS202028), and Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX20_2781).References
- G. Durga, P. Kalra, V. K. Verma, K. Wangdi and A. Mishra, Ionic liquids: from a solvent for polymeric reactions to the monomers for poly(ionic liquids), J. Mol. Liq., 2021, 335, 116540 CrossRef CAS.
- X. Lin, Y. Huang, L. Li, C. Ye, J. Chen and T. Qiu, Polymeric ionic liquids (PILs) with high acid density: tunable catalytic performance for biodiesel production, Chin. J. Chem. Eng., 2021, 38, 266–275 CrossRef.
- X. Lin, M. Li, Z. Chen, M. Li, Y. Huang and T. Qiu, One-step fabrication of polymeric self-solidifying ionic liquids as the efficient catalysts for biodiesel production, J. Cleaner Prod., 2021, 292, 125967 CrossRef CAS.
- M. Safa, A. Chamaani, N. Chawla and B. El-Zahab, Polymeric ionic liquid gel electrolyte for room temperature lithium battery applications, Electrochim. Acta, 2016, 213, 587–593 CrossRef CAS.
- S. Muhammad, M. N. Javed, F. I. Ali, A. Bari and I. A. Hashmi, Supramolecular polymeric aggregation behavior and its impact on catalytic properties of imidazolium based hydrophilic ionic liquids, J. Mol. Liq., 2020, 300, 112372 CrossRef CAS.
- Q. Peng, Y. Wu, H. Cong, Y. Shen, K. Mahmood and B. Yu, Preparation of monodisperse porous polymeric ionic liquid microspheres and their application as stationary phases for HPLC, Talanta, 2020, 208, 120462 CrossRef CAS PubMed.
- A. Eftekhari and T. Saito, Synthesis and properties of polymerized ionic liquids, Eur. Polym. J., 2017, 90, 245–272 CrossRef CAS.
- S. Y. Zhang, Q. Zhuang, M. Zhang, H. Wang, Z. Gao, J.-K. Sun and J. Yuan, Poly(ionic liquid) composites, Chem. Soc. Rev., 2020, 49, 1726–1755 RSC.
- G. Mittal, V. Dhand, K. Y. Rhee, S. J. Park and W. R. Lee, A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites, J. Ind. Eng. Chem., 2015, 21, 11–25 CrossRef CAS.
- M. Matandabuzo and P. A. Alibade, Vinyl pyridinium polymeric ionic liquid functionalized carbon nanotube composites as adsorbent for chromium(VI) in aqueous solution, J. Mol. Liq., 2019, 196, 111778 CrossRef.
- X. L. Meng, Y. Nie, J. Sun, W. G. Cheng, J. Q. Wang, H. Y. He and S. J. Zhang, Functionalized dicyandiamide-formaldehyde polymers as efficient heterogeneous catalysts for conversion of CO2 into organic carbonates, Green Chem., 2014, 16, 2771–2778 RSC.
- S. Chen, S. Gu, Y. Wang, Y. Yao, G. Wang, Y. Jin and Y. Wu, Exposure to pyrethroid pesticides and the risk of childhood brain tumors in East China, Environ. Pollut., 2016, 218, 1128–1134 CrossRef CAS PubMed.
- R. D. O. Silva, M. G. G. De Menezes, R. C. De Castro, C. D. A. Nobre, M. A. L. Milhome and R. F. Do Nascimento, Efficiency of ESI and APCI ionization sources in LC-MS/MS systems for analysis of 22 pesticide residues in food matrix, Food Chem., 2019, 297, 124934 CrossRef PubMed.
- M. Murcia-Morales, V. Cutillas and A. R. Fernández-Alba, Supercritical fluid chromatography and gas chromatography coupled to tandem mass spectrometry for the analysis of pyrethroids in vegetable matrices: a comparative study, J. Agric. Food Chem., 2019, 67, 12626–12632 CrossRef CAS PubMed.
- J. Yao, Z. Wang, L. Guo, X. Xu, L. Liu, L. Xu, S. Song, C. Xu and H. Kuang, Advances in immunoassays for organophosphorus and pyrethroid pesticides, TrAC, Trends Anal. Chem., 2020, 116022 CrossRef CAS.
- M. Moloney, S. Tuck, A. Ramkumar, A. Furey and M. Danaher, Determination of pyrethrin and pyrethroid residues in animal fat using liquid chromatography coupled to tandem mass spectrometry, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2018, 1077, 60–70 CrossRef PubMed.
- L. Chen, J. Fu, Q. Lu, L. Shi, M. Li, L. Dong, Y. Xu and R. Jia, Cross-linked polymeric ionic liquids ion gel electrolytes by in situ radical polymerization, Chem. Eng. J., 2019, 378, 122245 CrossRef CAS.
- C. Roth, T. Peppel, K. Fumino, M. Köckerling and R. Ludwig, The importance of hydrogen bonds for the structure of ionic liquids: single-crystal X-ray diffraction and transmission and attenuated total reflection spectroscopy in the terahertz region, Angew. Chem., Int. Ed. Engl., 2010, 49, 10221–10224 CrossRef CAS PubMed.
- K. H. Hu, H. W. Zhang, M. K. Kong, M. Y. Qin, M. Ouyang, G. W. Wang and L. H. Zhuang, Effect of alkyl chain length of imidazolium cations on foam properties of anionic surface active ionic liquids: experimental and DFT studies, J. Mol. Liq., 2021, 340, 117197 CrossRef CAS.
- M. Shukla, Spectroscopic and DFT Studies of cation-anion interaction in imidazolium and piperidinium based ionic liquids, J. Sci. Technol. Res., 2018, 8, 5–11 Search PubMed.
- R. Phadagi, S. Singh, H. Hashemi, S. Kaya, P. Venkatesu, D. Ramjugernath, E. E. Ebenso and I. Bahadur, Understanding the role of dimethylformamide as co-solvents in the dissolution of cellulose in ionic liquids: experimental and theoretical approach, J. Mol. Liq., 2021, 328, 115392 CrossRef CAS.
- Z. Liu, S. Z. E. Abedin and F. Endres, Raman and FTIR spectroscopic studies of 1-ethyl-3-methylimidazolium trifluoromethylsulfonate, its mixtures with water and the solvation of zinc ions, ChemPhysChem, 2015, 16, 970–977 CrossRef CAS PubMed.
- Y. Z. Zheng, N. N. Wang, J. J. Luo, Y. Zhou and Z. W. Yu, Hydrogen-bonding interactions between [BMIM]BF4 and acetonitrile, Phys. Chem. Chem. Phys., 2013, 15, 18055–18064 RSC.
- Q. B. Zhang, C. Yang, Y. X. Hua, Y. Li and P. Dong, Electrochemical preparation of nanostructured lanthanum using lanthanum chloride as a precursor in 1-butyl-3-methylimidazolium dicyanamide ionic liquid, Phys. Chem. Chem. Phys., 2015, 17, 4701–4707 RSC.
- M. S. Gruzdev, U. V. Chervonova, A. A. Ksenofontov, M. A. Krestianinov, A. I. Alexandrov and T. V. Pashkova, Schiff base complexes with different metals incorporating derivatives of 3,6-di-tert-butylcarbazole, Appl. Organomet. Chem., 2021, 35, e6145 CrossRef CAS.
- S. E. Lyubimov, E. A. Rastorguev and V. A. Davankov, The use of a new ionic phosphite ligand in the hydroformylation catalyzed by rhodium complexes: Effect of reaction media, Russ. J. Phys. Chem. B, 2014, 8, 953–957 CrossRef CAS.
- X. Yang, M. Wang, J. Zhao, C. Cui, S. Wang and J. Liu, Multichromic polymers containing alternating bithiophenes derivatives and 4-cyanotriphenylamine unit and their application for electrochromic devices, J. Electroanal. Chem., 2014, 714, 1–10 Search PubMed.
- X. M. Ye, C. M. Shao, Q. H. Fan, L. R. Shang and F. F. Ye, Porous carbon nanotube microspheres with tailorable surface wettability areas for oil adsorption, J. Colloid Interface Sci., 2021, 15, 737–745 CrossRef PubMed.
- S. Sadeghi and S. Oliaei, Microextraction of sulfathiazole from milk and honey samples using a polymeric ionic liquid membrane followed by fluorometric determination, J. Food Compos. Anal., 2021, 97, 103774 CrossRef CAS.
- Y. Zhang, H. Ding, Y. Wu, C. Zhang, B. Bai, H. Wang and M. Li, Ultrasound-induced controllable morphology and growth dimension in a dihydrazide-based self-assembly system, Soft Matter, 2014, 10, 8838–8845 RSC.
- D. Allen, Preparation and characterization of 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquids, J. Chem. Eng., 2013, 3, 187–197 Search PubMed.
- K. Goossens, K. Lava, C. W. Bielawski and K. Binnemans, Ionic liquid crystals: versatile materials, Chem. Rev., 2016, 116, 4643–4807 CrossRef CAS PubMed.
- M. Galib and G. Hanna, The role of hydrogen bonding in the decomposition of H2CO3 in water: mechanistic insights from ab initio metadynamics studies of aqueous clusters, J. Phys. Chem. B, 2014, 118, 5983–5993 CrossRef CAS PubMed.
- J. A. Abia and R. Ozer, Development of polyoxometalate-ionic liquid compounds for processing cellulosic biomass, Bioresources, 2013, 8, 2924–2933 CrossRef.
- S. Zhang, J. Wang, X. Lu and Q. Zhou, Structures and interactions of ionic liquids, Springer, Berlin, 2013 Search PubMed.
- A. A. Tietze, H. Pascal, S. Annegret and I. Diana, Ionic liquid applications in peptide chemistry: synthesis, purification and analytical characterization processes, Molecules, 2018, 17, 4158–4185 CrossRef PubMed.
- Z. D. Ding, Z. Chi, W. X. Gu, S. M. Gu, J. H. Liu and H. J. Wang, Theoretical and experimental investigation on dissolution and regeneration of cellulose in ionic liquid, Carbohydr. Polym., 2012, 89, 7–16 CrossRef CAS PubMed.
- N. J. Brooks, F. Castiglione, C. M. Doherty, A. Dolan, A. J. Hill, P. A. Hunt, R. P. Matthews, M. Mauri, A. Mele and R. Simonutti, Linking the structures, free volumes, and properties of ionic liquid mixtures, Chem. Sci., 2017, 8, 6359–6374 RSC.
- A. Mehrdad and M. Taleb-Abbasi, Viscometric behavior of hydroxyethyl cellulose in aqueous solutions of some imidazolium ionic liquids, Cellulose, 2019, 26(13), 7685–7693 CrossRef CAS.
- S. Yesudass, L. O. Olasunkanmi, I. Bahadur, M. M. Kabanda, I. B. Obot and E. E. Ebenso, Experimental and theoretical studies on some selected ionic liquids with different cations/anions as corrosion inhibitors for mild steel in acidic medium, J. Taiwan Inst. Chem. Eng., 2016, 64, 252–268 CrossRef CAS.
- L. A. Kartsova, E. A. Bessonova and E. A. Kolobova, Ionic liquids as modifiers of chromatographic and electrophoretic systems, J. Anal. Chem., 2016, 71, 141–152 CrossRef CAS.
- H. W. Cheng, J. N. Dienemann, P. Stock, C. Merola, Y. J. Chen and M. Valtiner, The effect of water and confinement on self-assembly of imidazolium based ionic liquids at mica interfaces, Sci. Rep., 2016, 6, 1–9 CrossRef PubMed.
Footnote |
| † Xiaofan Zhang and Ming Gao are the co-first authors. |
| This journal is © The Royal Society of Chemistry 2022 |