Open Access Article
Open Access ArticleModification of C.I. Pigment Red 146 with surfactants and graphene oxide
Dongjun Lv *,
Zilong Zhang,
Jiahui Zhang*,
Xiaolei Zhang,
Leifang Liu,
Yue Gong,
Jianghong Zhao and
Yi Li
*,
Zilong Zhang,
Jiahui Zhang*,
Xiaolei Zhang,
Leifang Liu,
Yue Gong,
Jianghong Zhao and
Yi Li
Department of Chemical Engineering, School of Chemistry and Chemical Engineering, De Zhou University, Dezhou 253023, China. E-mail: lvdongjun@dzu.edu.cn; zjhchem_dzu@163.com
First published on 8th August 2022
Abstract
Organic pigments are important in a range of fields, from printing ink to industrial coatings. Azo pigments are some of the most common pigments in use today, but they typically have poor solvent solubility and tend to agglomerate. Consequently, the size and crystal structure of the pigment particles has a crucial effect on their optical and physical properties, such as color strength and solvent resistance, respectively. Several technologies, such as microreactors, have been developed to control pigment particle size, but an in-depth study of the effects of modification conditions on pigment properties (color, flowability, and solvent resistance) has not been reported to date. Therefore, in this paper, we report the surface modification of C.I. Pigment Red 146 particles using anionic (Igepon T) and non-ionic surfactants (Peregal O-25) and additives (DB-60 as the second diazo component and graphene oxide) on the pigment properties. In addition, we examined the effect of hydrothermal treatment at different temperatures on the same properties. The various modifications resulted in an increase in the solvent resistance, a reduction in the particle size (from 30.581 to 12.252 μm), a narrowing of the particle size distribution, and an increase in hydrophilicity. In addition, the color brightness and brilliance were significantly improved, and the maximum color strength reached 112.6%. These findings have applications for the development of pigments having enhanced color properties, solvent resistance, and processability.
Introduction
Among the organic colored pigments, the azo pigments are the most well-known, accounting for more than 60% of total organic pigments produced.1,2 Color Index Pigment Red 146 (C.I. Pigment Red 146, denoted P.R. 146 hereafter) is a bluish-red azo pigment having good solvent resistance, heat stability, and light fastness3 and is mainly used in printing pastes and water-based coatings and inks.4,5 However, azo pigments are basically insoluble in water and most organic solvents.6,7 Thus, they are typically applied in solid form, for example, as colloidal dispersions and dry powders. Because the pigment particle properties are not altered during processing, the particle size, grain pattern, crystal structure, and surface properties produced during pigment synthesis have a significant impact on the optical and physical properties, especially color tone, glossiness, tinting strength, light fastness, and dispersibility.8Currently, P.R. 146 is prepared by reactive crystallization, but there has been relatively little research on the effects of synthetic conditions on the properties of this pigment, although the production operating parameters have been optimized. Currently, there are three main techniques to improve the properties of pigments such as P.R. 146: Method 1, modification with surfactants; Method 2, the use of microreactor technology; Method 3, the use of mini-emulsion technology. For Method 1, Lv et al.9 added an aqueous acidic solution of a diazo compound 3-amino-4-methoxy-N-phenylbenzamide, also known as Fast Red KD Base, to an alkaline aqueous solution of N-(4-chloro-2,5-dimethoxyphenyl)-3-hydroxynaphthalene-2-carboxamide, also known as Naphthol AS-LC, and then, added different surfactants (including red oil, sodium oleate, and a polymer dispersant) to improve the color strength, blue hue, and dispersibility. For Method 2, microreactor technology is a new method of reactive crystallization. Crucially, in the reactor, the mixing efficiency and mass and heat transport are enhanced significantly, which can improve the efficiency and yield of the reactions.10–12 Importantly, when using microreactor technology for the reactive crystallization of P.R. 146, the mean particle size can be significantly reduced.13 In addition, the lightness and green and blue hues can be enhanced compared to those of standard P.R. 146.14 For Method 3, Hao et al.15 proposed a method for preparing nanoscale P.R. 146 by using microemulsion technology. In this technique, the emulsion droplets are used as a soft template for reactive crystallization. However, although some of the properties of P.R. 146 can be improved by using these new technologies, further improvements are required.
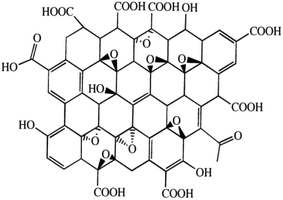
As mentioned above, in addition to the use of different synthetic technologies, additives can be used to modify pigment properties. Graphene oxide (GO, Fig. 1) is a popular additive used in many fields, particularly as it can be produced in large quantities cheaply and is easy to process.16 In addition, GO is rich in active oxygen-containing functional groups,17 making it more soluble than the parent graphene18 and enabling surface functionalization. For example, Lv et al. reported the modification of blue pigments based on copper phthalocyanine with single-layer GO; this modification improved the color strength significantly.19
In this paper, we used different amounts of surfactants (Igepon T and Peregal O-25) individually and in combination to increase the dissolution of the dye precursors, as well as GO as an additive to modify the P.R. 146 particles. In addition, we used a second diazotization component (3-amino-4-methylbenzamide, also known as DB-60) during synthesis to form a solid solution of pigment molecules. Finally, we also investigated the effect of hydrothermal treatment at different temperatures on the modified P.R. 146. As the concentrations of these components varied, and the effects of adding each single alone and combined treatment on the physical and optical properties of the resulting pigment particles were investigated. Overall, a multi-technology synergistic modification technology on the surface of Pigment Red 146 is developed, which integrates the compound surfactant and the mixed-coupling solid solution technology, the graphene modified organic pigment and the hydrothermally treated technology. We found that the modifications resulted in the prepared P.R. 146 particles having a brighter and stronger color, good solvent resistance, smaller particle size, and higher flowability.
Experimental section
Materials
3-Amino-p-anisanilide (Red Base KD, 98%) was provided by Wujiang Meiyan Sanyou Dyestuff Chemical Co., Ltd.N-(4-Chloro-2,5-dimethoxyphenyl)-3-hydroxynaphthalene-2-carboxamide (Naphthol AS-LC, 98%) was obtained from Jiangsu Tianbo Chemical Co., Ltd. 3-Amino-4-methylbenzamide (DB 60, 99%) was purchased from Shanghai Haohong Biomedical Technology Co., Ltd. Single-layer GO was provided by Tianjin Plam Nanotechnology Co., Ltd. Concentrated (36%) hydrochloric acid, sodium nitrite, glacial acetic acid, sodium hydroxide, and 1-octyl-2-pyrrolidone were supplied by Tianjin Kemiou Chemical Reagent Co., Ltd. All other chemicals were all analytical grade and were used as received without further purification.
Methods
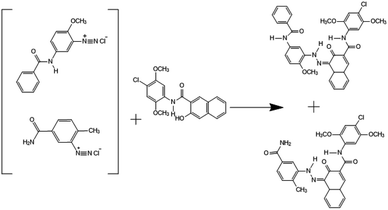
Solution 1 (diazonium salt). 3-Amino-4-methoxy-N-phenylbenzamide (Red Base KD, 2.3 g, 0.0095 mol) and different amounts of 3-amino-4-methylbenzamide (Red Base DB-60; 2%, 4%, and 6% of KD) were dissolved in deionized water (50 mL). HCl (36%, 3.9 mL) was added, and the mixture was stirred for 30 min. Next, crushed ice was added to adjust the temperature to 5–10 °C. The mixture was then stirred for a further 20 min, and a white slurry formed. This slurry was cooled in an ice-water bath (0–5 °C). Then, 30% sodium nitrite (0.775 g) was added dropwise over 5–10 min until the white slurry was completely dissolved. Next, deionized water was added to make the reaction solution 110 mL, and the reaction was stirred for 1 h. The diazo precursor was determined to have been formed when potassium iodide test paper turned blue. During the reaction, sulfamic acid was introduced to react with the excess sodium nitrite to avoid the generation of nitrous acid because this affects the coupling reaction.
Solution 2 (P.R. 146 coupling component). Naphthol AS-LC (3.44 g, 0.0096 mol) was dissolved in deionized water (50 mL) in a 500 mL beaker. Then, the surfactant Igepon T (2%, 4%, or 6% of the pigment) and sodium hydroxide (1.00 g) were added to the beaker, and the solution was stirred. Next, the solution was heated to 85 °C until the Naphthol AS-LC had dissolved completely. Then, crushed ice was added to reduce the temperature to 40 °C.
Solution 3 (synthesis and post-processing). Sodium hydroxide (0.115 g), HCl (1.95 mL), glacial acetic acid (0.715 mL), and crushed ice (50 g) were dissolved in deionized water (10 mL) in a 1 L beaker. Next, the surfactant Peregal O-25 (2%, 4%, or 6% of the pigment) was dissolved in deionized water and added to the 1 L beaker. Then, solution 2 was added dropwise to solution 3 with a peristaltic pump over 30 min. During the reaction, the temperature and pH were maintained below 20 °C and 4.5–5.2, respectively. Next, deionized water was added to make the reaction solution 200 mL, and the reaction was stirred for 10 min. Subsequently, the pH of the reaction solution was adjusted to 5.6. Finally, solution 1 was added dropwise to the coupling solution with a peristaltic pump over 120 min. During this reaction, the temperature was maintained between 38 and 40 °C, and the pH was maintained between 5.0 and 5.2 by the addition of 6% sodium hydroxide solution since the pH of the solution decreased as the reaction proceeded.
For GO modification, at the end of the coupling reaction, the temperature was increased to 46 °C, and GO (0.1 g) was added; this mixture was stirred for 15–30 min. Finally, the solution was heated at 80 °C for 30 min. After the reaction, a solid filter cake of modified P.R. 146 was isolated by suction filtration, washed with deionized water.
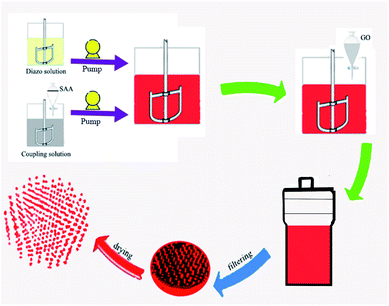
Solution 4 (hydrothermal treatment). The solid sample obtained in the previous step (2.0 g) was combined with 1-octyl-2-pyrrolidone (0.3 g) in deionized water (100 mL) in a 200 mL beaker. Next, a magnetic stir bar was added, and the suspension was stirred for 20–30 min until well mixed. Then, deionized water (20 mL) was added, and the solution was poured into an autoclave. The autoclave was placed in an oven and heated at 80, 105, 110, or 115 °C for 1 h. Finally, after cooling, the autoclave was taken out and unscrewed, and the treated pigment solution was filtered by suction, washed with deionized water to neutrality, and dried at 80 °C. The preparation process and synthesis routes of the modified pigments are illustrated in Fig. 2 and Scheme 1.
Characterization
The colors of the pigments were assessed using the CIE 1976 L*a*b* system. Color measurements were achieved using an X-rite 8400 automatic colorimeter (X-RITE, USA). The color strength and flowability were determined according to the Chinese National Standard GB/T 1708-79 and the National Industry Standard of People's Republic of China HG/T 3854-2006, respectively.The particle size distributions were determined using a Mastersizer 2000 (Malvern, UK) particle size analyzer after sonication of an aqueous suspension of the pigment for 15 min. The morphologies of the pigment samples were determined by scanning electron microscopy (SEM, Merlin Compact, Zeiss Corporation, Oberkochen, Germany). The pigment sample was sputtered with gold before scans. The UV-vis spectra were obtained with a UV-2700 spectrophotometer (Shimadzu, Japan). The optical absorption properties of the pigments were investigated in ethanol solutions with concentrations of 0.05 mg mL−1. The crystalline phases of the pigment were determined by X-ray diffractometry (XRD, D8A, Bruker, Germany). Cu Ka X-rays were generated at 40 kV and 40 mA. Powder X-ray diffraction data of the pigment was recorded over the 2θ range of 5–40°, and measurements were carried out at room temperature. The wettability of the pigments was determined by measuring the water contact angles of pelleted samples (EasyDrop, KRUSS GmbH, Germany).
Results and discussion
Color properties and flowability
The color performance of the modified pigments was compared with that of the unmodified pigment (sample 1-1 in the following tables), and the results are listed in Table 1. The P.R. 146 samples modified with Igepon T were brighter than the unmodified pigment, as shown by the higher L values. In addition, the modified samples showed high color saturation, as shown by the large and positive value of c. When 2% Igepon T was used, the resulting pigment had a strong yellow hue (H = 27.60) and high color strength (102.4%). Further, as the amount of added surfactant increased, the flowability of the modified pigment also increased.| No. | Name and amount of surfactant | L | a | b | c | H | Tinctorial strength (%) | Flowability (mm) |
|---|---|---|---|---|---|---|---|---|
| 1-1 | — | 42.81 | 50.80 | 23.78 | 56.09 | 25.08 | 100.0 | 23.0 |
| 1-2 | Igepon T 2% | 42.95 | 50.68 | 26.58 | 57.32 | 27.60 | 102.4 | 23.5 |
| 1-3 | Igepon T 4% | 43.86 | 52.65 | 24.77 | 58.19 | 24.98 | 101.4 | 24.0 |
| 1-4 | Igepon T 6% | 44.03 | 51.88 | 23.70 | 57.03 | 24.54 | 101.2 | 24.0 |
| 1-5 | O-25 2% | 43.37 | 53.02 | 24.23 | 58.27 | 24.47 | 101.9 | 23.0 |
| 1-6 | O-25 4% | 43.87 | 52.43 | 25.10 | 58.15 | 25.50 | 101.2 | 24.5 |
| 1-7 | O-25 6% | 43.68 | 52.33 | 25.56 | 56.27 | 25.95 | 99.3 | 25.0 |
| 1-8 | Igepon T 2%, O-25 4% | 43.19 | 51.54 | 24.52 | 58.97 | 29.18 | 101.6 | 25.0 |
When O-25 was used as the surfactant, the modified pigment samples (Table 1, entries 1-5–1-7) showed higher lightness and higher color saturation values than the unmodified pigment, as shown by the higher L and c values. When 4% and 6% O-25 were used, the modified pigments had strong yellow hues, delivering H values of 25.50 and 25.95, respectively. When 2% O-25 was used, the modified pigment color strength was slightly higher than that of the unmodified pigment, but the color strength decreased with increasing amounts of O-25 added. As also observed for Igepon T, an increase in the amount of O-25 added resulted in an increase in the flowability of the modified pigment.
The combined addition of 2% Igepon T and 4% O-25 resulted in modified pigments having lighter colors and greater brightness, as well as an obvious yellow hue. In addition, the color strength and flowability were improved to maximum values 101.6% and 25 mm, respectively.
Next, we investigated the effect of the addition of the second diazo component (DB-60) on the properties of the pigment (Table 2). The use of DB-60 resulted in the formation of a solid solution of pigment components, when 4% DB-60 was added, the color strength was the highest (105.1%) and the modified pigment showed greater lightness (L = 43.82), the pigment had a bright color and high color strength. Further, the color saturation was improved (higher C), but the flowability was reduced. DB-60 can also affect crystal growth in the modified pigment and can prevent pigment particles from aggregating. In aqueous media, there is an electrical double layer around the surface of the modified pigment particle. The repulsion between two particles increases as they approach each other. Since aggregation decreases the overall size of a pigment particle, flowability also decreases.20
| No. | DB-60 | L | a | b | c | H | Tinctorial strength (%) | Flowability (mm) |
|---|---|---|---|---|---|---|---|---|
| 1-8 | — | 43.19 | 51.54 | 24.52 | 58.97 | 29.18 | 101.6 | 25.0 |
| 2-1 | 2% | 43.14 | 51.27 | 29.26 | 58.90 | 29.81 | 102.7 | 24.0 |
| 2-2 | 4% | 43.82 | 52.04 | 28.08 | 59.23 | 28.26 | 105.1 | 23.5 |
| 2-3 | 6% | 42.97 | 50.43 | 29.55 | 58.40 | 30.38 | 103.9 | 22.5 |
The properties of the pigments modified with GO are listed in Table 3. For the sample modified with surfactant, DB-60, and GO, the pigment color strength was enhanced, yielding a color strength of 109.0% and enhanced flowability, ranging from 23.5 to 25.0 mm.
| No. | GO | L | a | b | c | H | Tinctorial strength (%) | Flowability (mm) |
|---|---|---|---|---|---|---|---|---|
| 2-2 | — | 43.82 | 52.04 | 28.08 | 59.23 | 28.26 | 105.1 | 23.5 |
| 3-1 | 2% | 41.91 | 50.38 | 25.62 | 56.52 | 26.92 | 109.0 | 23.5 |
| 3-2 | 4% | 41.07 | 46.89 | 24.75 | 53.05 | 27.72 | 106.6 | 24.5 |
| 3-3 | 6% | 40.41 | 45.52 | 24.60 | 51.78 | 27.24 | 103.7 | 25.0 |
The color values of the hydrothermally treated sample are shown in Table 4, showing that these samples have a stronger yellow hue (H = 28.87) than the unmodified P.R. 146 (H = 25.08), as well as increased flowability. After treatment at 105 °C, a high color strength (112.6%) was achieved, but, after treatment at 115 °C, the color strength was reduced (106.4%). Therefore, the use of excessively high temperatures reduces the pigment color strength.
| No. | Temperature (°C) | L | a | b | c | H | Tinctorial strength (%) | Flowability (mm) |
|---|---|---|---|---|---|---|---|---|
| 3-2 | 80 | 41.91 | 50.38 | 25.62 | 56.52 | 26.92 | 109.0 | 23 |
| 4-1 | 105 | 42.31 | 48.94 | 26.82 | 55.88 | 28.72 | 112.6 | 23 |
| 4-2 | 110 | 42.12 | 48.46 | 26.96 | 55.45 | 28.87 | 109.6 | 24 |
| 4-3 | 115 | 43.15 | 50.16 | 24.90 | 56.01 | 26.44 | 106.4 | 24 |
Ethanol resistance
The absorption of ethanol by the pigments was next tested using UV-vis absorption spectroscopy measurements (Table 5). The modification treatments increased the ethanol resistance, as shown by the reduction in the absorbance values. When 2% Igepon T or 4% O-25 were added, the absorption values were 0.0247 and 0.0183, respectively. In contrast, when both 2% Igepon T and 4% O-25 were used, the absorbance was 0.0168, indicating further increased ethanol resistance. Further, when DB-60 was used as an additional coupling agent, even better solvent resistance was obtained (absorbance of 0.012). Finally, after the addition of surfactants, DB-60, and GO, the lowest absorbance (0.0074) and, thus, best ethanol resistance was obtained. Thus, the addition of GO can enhance the solvent resistance of the pigments further.| No. | Surfactant | DB-60 | GO | Maximum absorption wavelength | Absorbance |
|---|---|---|---|---|---|
| 1-1 | — | — | — | 583.50 | 0.0474 |
| 1-2 | Igepon T | — | — | 583.50 | 0.0247 |
| 1-6 | O-25 | — | — | 583.50 | 0.0183 |
| 1-8 | Igepon T, O-25 | — | — | 583.50 | 0.0168 |
| 2-3 | Igepon T, O-25 | 4% | — | 583.50 | 0.0102 |
| 3-1 | Igepon T, O-25 | 4% | 2% | 583.50 | 0.0074 |
Hydrophilicity of modified pigments
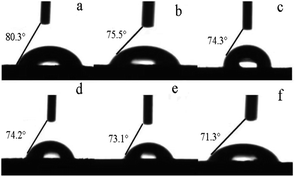
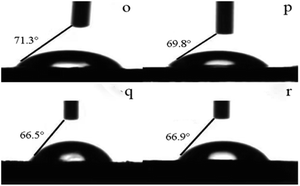
Pigment modification can affect the polarity and wettability of the pigment particles and, thus, their compatibility with water. Therefore, we measured the contact angles of the modified pigments with water (Table 6 and Fig. 3). The treatment with surfactants and GO resulted in smaller contact angles than that of the unmodified pigment, suggesting enhanced hydrophilicity. When 2% Igepon T or 4% O-25 were used, the contact angles of the pigments were 75.5° and 74.3°, respectively. The polyethylene oxide (PEO) groups, the hydrophilic part of O-25, provide steric stabilization by extending into aqueous media and make the surface hydrophilic.21 However, when 2% Igepon T and 4% O-25 were used together, the contact angle of the pigment was 74.2°. When DB-60 was added in addition to both surfactants, the modified pigment contact angle was further reduced.Next, we measured the contact angles of the modified pigments treated hydrothermally to evaluate their hydrophilicity (Table 7 and Fig. 4). After hydrothermal treatment at increasing temperatures, the contact angle reduced; the lowest contact angle (66.5°), which indicates the greatest hydrophilicity, was obtained after treatment at 110 °C.
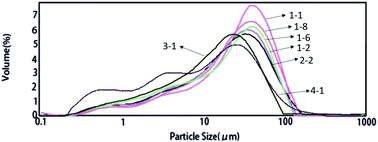
Particle size and distribution
The particle size and distribution affect pigment properties (e.g., shade, flow behavior, and color strength). Fig. 5 and Table 8 show the particle size and distribution data for the original and modified pigments.| No. | Surfactant | DB-60 | GO | Temperature (°C) | D (0.1)/μm | D (0.5)/μm | D (0.9)/μm |
|---|---|---|---|---|---|---|---|
| 1-1 | — | — | — | 80 | 3.756 | 30.581 | 76.359 |
| 1-2 | Igepon T | — | — | 80 | 3.881 | 28.329 | 79.768 |
| 1-6 | O-25 | — | — | 80 | 2.541 | 22.455 | 68.235 |
| 1-8 | Igepon T O-25 | — | — | 80 | 2.247 | 22.436 | 64.441 |
| 2-3 | Igepon T O-25 | 4% | — | 80 | 2.383 | 20.241 | 61.831 |
| 3-2 | Igepon T O-25 | 4% | 2% | 80 | 1.932 | 15.793 | 48.664 |
| 4-1 | Igepon T O-25 | 4% | 2% | 105 | 0.889 | 12.252 | 56.093 |
As shown by Fig. 5 and Table 8, the average particle size of the modified pigment is smaller than that of the unmodified pigment. In addition, the addition of DB-60 and GO had a significant effect on these parameters. When the second diazo component DB-60 was included, a solid solution was formed. It increased the surface charge of the pigment and the electrostatic repulsion between the modified pigment particles, which could hinder the aggregation of the pigment particles and result in a smaller particle size and narrower distribution.22 In the combined modified sample with surfactant, DB-60, and GO, further reduction in particle size and narrowing of the size distribution was observed. The results illustrate that GO has a large surface area, which allowed for the effective adsorption of the organic pigments on the GO surfaces. The interaction between GO and the organic pigment could reduce the surface tension and the interface energy of the organic pigment, restricting the aggregation among the pigment particles, which the reduced the particle size and size distribution of the modified pigment. After treatment at 105 °C, the small particle diameter (D (0.1)) and median particle diameter (D (0.5)) of hydrothermal treatment pigment were all smaller.
XRD analysis
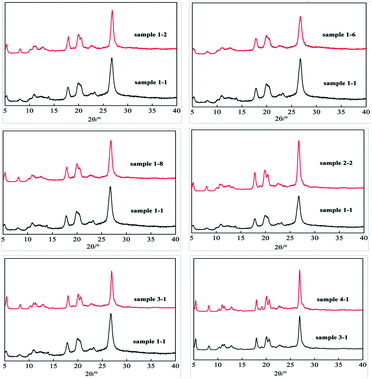
The crystal structure affects the color of pigments, so X-ray diffraction (XRD) analysis was performed (Table 9 and Fig. 6). The XRD pattern of unmodified P.R. 146 has characteristic reflections at 2θ = 5.3°, 8.0°, 10.7°, 17.8°, 19.9°, 20.5°, and 26.8°.| No. | I5.3°/% | I8.0°/% | I10.7°/% | I11.2°/% | I17.8°/% | I19.9°/% | I20.5°/% | I26.8°/% |
|---|---|---|---|---|---|---|---|---|
| 1-1 | 9.9 | 6.0 | 11.6 | 0 | 31.7 | 34.4 | 26.4 | 100 |
| 1-2 | 22.7 | 11.9 | 12.1 | 11.1 | 35.9 | 37.4 | 27.0 | 100 |
| 1-6 | 13.2 | 8.1 | 10.6 | 8.0 | 38.1 | 38.8 | 29.3 | 100 |
| 1-8 | 25.4 | 13.1 | 11.0 | 8.1 | 33.8 | 38.4 | 24.8 | 100 |
| 2-2 | 19.9 | 12.2 | 10.8 | 7.1 | 33.9 | 36.7 | 24.9 | 100 |
| 3-1 | 36.5 | 13.3 | 13.3 | 10.1 | 35.2 | 35.7 | 27.3 | 100 |
| 4-1 | 28.4 | 15.9 | 17.1 | 12.5 | 27.5 | 33.5 | 24.3 | 100 |
After modification, the XRD patterns had more intense peaks, suggesting greater crystallinity. For the sample modified with Igepon T, the reflections at 5.3°, 8.0°, 17.8°, and 19.9° are more intense than those of the unmodified sample, and a new peak at 11.2° was observed. When Igepon T and O-25 were used, the reflections at 5.3°, 8.0°, and 19.9° were enhanced. For the samples modified with surfactant, DB-60, and GO, the reflections at 5.3° and 17.8° were obviously enhanced. However, the XRD pattern of the hydrothermally treated sample was similar to that of the untreated sample.
SEM analysis
In addition, the morphologies of the unmodified, combined treatment, GO-treated, and hydrothermally treated samples were analyzed by scanning electron microscopy (SEM), and the results are shown in Fig. 7. The morphologies of the four analyzed products were all flaky or rod-like. Compared with the original products, the modified pigments are smaller, and their size distributions are more uniform. Additionally, the hydrothermally treated pigment, which had also been treated with surfactants and DB-60, were smallest and most uniform in size.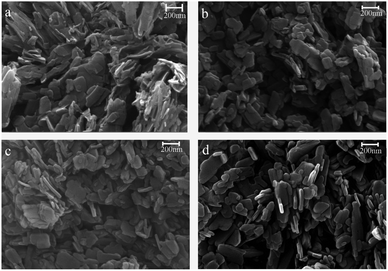 | ||
| Fig. 7 SEM analysis of the modified pigment: (a) no surfactant, (b) Igepon T, O-25, and DB-60, (c) GO, surfactant and DB-60 (d) hydrothermal treatment. | ||
Conclusion
C.I. Pigment Red 146 was modified with anionic and nonionic surfactants. In addition, DB-60 was used as a co-coupling agent during preparation to yield a solid solution of dye molecules, and graphene oxide was added to modify the pigment further. The modifications resulted in significantly improved color brightness and brilliance, and the color strength of the modified pigments reached 112.6%. After modification and hydrothermal treatment, the pigment particle size decreased from 30.581 to the lowest value of 12.252 μm and achieved the narrowest particle size distribution. In addition, the ethanol resistance was significantly improved compared to that of the original pigment. Moreover, the hydrophilicity of the modified pigments was also improved significantly. Therefore, these simple modification steps provide a way to increase the color properties and processability of C.I. Pigment Red 146 and have applications in the treatment of other azo pigments.Author contributions
Dongjun Lv: conceptualization, methodology, supervision, project administration. Zilong Zhang: methodology, investigation, formal analysis, writing – original draft. Jiahui Zhang: conceptualization, writing – review & editing. LeiFang Liu: conceptualization, formal analysis. Xiaolei Zhang: methodology, formal analysis. Yue Gong: investigation, formal analysis. Jianghong Zhao: formal analysis. Yi Li: formal analysis.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province (ZR2020MB134 and ZR2020QB082), and Foundation of Dezhou University (2020xjrc106, 2019xjrc306 and 2021xjrc305).Notes and references
- W. Li, Z. Li, L. J. Yu and L. T. Yang, CHN Pat., 106833005A, 2017.
- D. M. Yan, J. H. Xu, C. Zhou, F. J. Wang, D. Liu, J. P. Huang, L. T. Yang, W. C. Xu, D. S. Gong, X. L. Zhang, B. Liu and S. J. Yan, CHN Pat., 111378296, 2020.
- J. J. Yuan, S. X. Zhou, L. M. Wu and B. You, J. Phys. Chem. C, 2006, 110(1), 388–394 CrossRef CAS PubMed.
- H. Pennemann, S. Forster, J. Kinkel, V. Hessel, H. Löwe and L. Wu, Org. Process Res. Dev., 2005, 9, 188 CrossRef CAS.
- C. Wille, H. P. Gabski, T. Haller, H. Kim, L. Unverdorben and R. Winter, Chem. Eng. J., 2004, 101, 179 CrossRef CAS.
- R. R. Mather, Dyes Pigm., 1999, 42, 103–106 CrossRef CAS.
- W. T. Cheng, C. W. Hsu and Y. W. Chih, J. Colloid Interface Sci., 2004, 270, 106–112 CrossRef CAS PubMed.
- C. L. Zhou, Fine Spec. Chem., 2007, 15(7), 4–6 Search PubMed.
- D. J. Lv and J. C. Zhang, Dyest. Color., 2007, 44(6), 6–8 Search PubMed.
- H. Kim, K. Saitmacher, L. Unverdorben and C. Wille, Macromol. Symp., 2002, 187, 631–640 CrossRef CAS.
- J. S. Zhang, K. Wang, Y. C. Lu and G. S. Luo, Chem. Eng. Process., 2010, 49, 740 CrossRef CAS.
- K. Jähnisch, V. Hessel, H. Löwe and M. Baerns, Angew. Chem., Int. Ed., 2004, 43, 406 CrossRef PubMed.
- F. J. Wang, J. P. Huang and J. H. Xu, Org. Process Res. Dev., 2019, 23(12), 2637–2646 CrossRef CAS.
- F. J. Wang, Y. C. Ding and J. H. Xu, Ind. Eng. Chem. Res., 2019, 58, 16338–16347 CrossRef CAS.
- X. Z. Meng, Y. L. Wang, X. Li, X. Chen, D. J. Lv, C. Xie, Q. X. Yin, X. L. Zhang and H. X. Hao, Nanomaterials, 2019, 9, 379 CrossRef PubMed.
- Y. Wang, S. S. Li, H. Y. Yang and J. Luo, RSC Adv., 2020, 10(26), 15328–15345 RSC.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45(7), 1558–1565 CrossRef CAS.
- T. Kuilla, S. Bhadra, D. Yao, N. H. Kim, S. Bose and J. H. Lee, Prog. Polym. Sci., 2010, 35(11), 1350–1375 CrossRef CAS.
- D. J. Lv, H. S. Sung, X. J. Li, X. Zhang, Z. Li and D. F. Chen, Dyes Pigm., 2020, 180, 108449 CrossRef CAS.
- X. N. Fei, T. Y. Zhang and C. L. Zhou, Dyes Pigm., 2000, 44(2), 75–80 CrossRef CAS.
- H. Sis and M. Birinci, Colloids Surf., A, 2014, 455, 58–66 CrossRef CAS.
- F. X. Ren, X. N. Fei, L. F. Cui, L. Y. Cao, D. J. Lv, S. Zhu, X. Han and L. J. Liu, Dyes Pigm., 2020, 183, 108699 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |