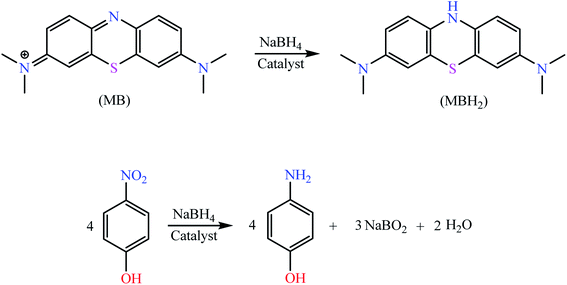
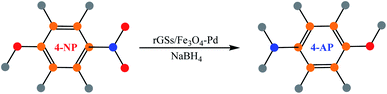Open Access Article
Open Access ArticleRecent developments in hydrogels containing copper and palladium for the catalytic reduction/degradation of organic pollutants
Jaber Dadashi a,
Mohammad Ali Ghasemzadeh
a,
Mohammad Ali Ghasemzadeh *b and
Masoud Salavati-Niasari
*b and
Masoud Salavati-Niasari c
c
aCatalysts and Organic Synthesis Research Laboratory, Department of Chemistry, Iran University of Science and Technology, Tehran, Iran
bDepartment of Chemistry, Qom Branch, Islamic Azad University, Qom, Iran. E-mail: ma.ghasemzadeh@iau.ac.ir; qasemzade.a@gmail.com
cInstitute of Nano Science and Nano Technology, University of Kashan, Kashan, Iran
First published on 18th August 2022
Abstract
The elimination of toxic and hazardous contaminants from different environmental media has become a global challenge, causing researchers to focus on the treatment of pollutants. Accordingly, the elimination of inorganic and organic pollutants using sustainable, effective, and low-cost heterogeneous catalysts is considered as one of the most essential routes for this aim. Thus, many efforts have been devoted to the synthesis of novel compounds and improving their catalytic performance. Recently, palladium- and copper-based hydrogels have been used as catalysts for reduction, degradation, and decomposition reactions because they have significant features such as high mechanical strength, thermal stability, and high surface area. Herein, we summarize the progress achieved in this field, including the various methods for the synthesis of copper- and palladium-based hydrogel catalysts and their applications for environmental remediation. Moreover, palladium- and copper-based hydrogel catalysts, which have certain advantages, including high catalytic ability, reusability, easy work-up, and simple synthesis, are proposed as a new group of effective catalysts.
1. Introduction
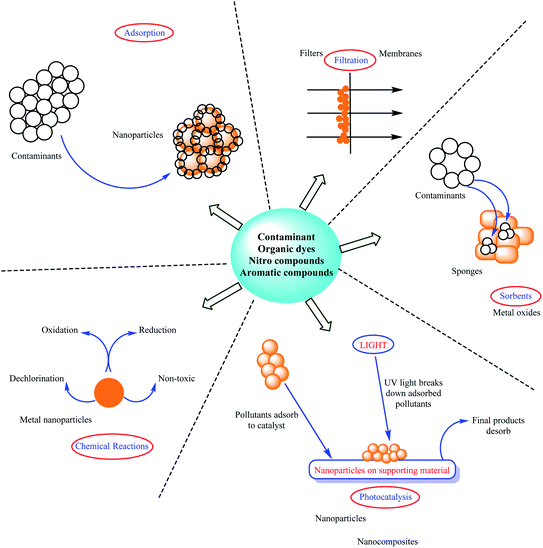
Environmental pollution induced by industrial development and increase in population affects the life of living organisms by increasing the production of dangerous and toxic compounds such as organic dyes and food waste. Thus, this global problem has received much attention from researchers.1Among the various pollutants, oil spills, heavy metals, herbicides, organic compounds, fertilizers, and sewage are considered the most important.2 Nitro compounds as one of the main pollutant groups are usually found in water. For instance, 4-nitrophenol, a key industrial pollutant, poses a threat to the ecosystem and human health when released into the environment owing to its high chemical stability and toxicity.3–5 Organic dyes are another group of compounds that pollute the environment. Synthetic organic dyes, which are poisonous, bio refractory, and carcinogenic pollutants, have become a significant source of water contamination. Because these dyes are chemically and physiologically persistent in the environment, the typical degradation processes are unsuitable for their separation.6–8 Furthermore, although there are various methods to remove pollutants from water and the environment, some of them are specific to certain uses. For example, coagulation, chemical reduction, reverse osmosis, and photodegradation procedures are used to remove nitro compounds and organic dyes.9–11 Scheme 1 provides the different approaches for the elimination of pollutants including absorption, chemical reactions, adsorption, photocatalysis, and filtration.12–23 Some of these methods are simple, inexpensive, with low energy consumption and high yield, and do not produce toxic products or require complex instruments; however, eco-friendly approaches are preferable.24 Chemical degradation is one of the most extensively utilized strategies for removing pollution from the environment, including ozone/UV, irradiation/H2O2 oxidation, photocatalytic degradation, supercritical water oxidation, and electrochemical methods.25
Recently, there have been many attempts to build efficient and novel catalytic systems for environmental applications.26 Also, the use of homogeneous catalysts in industry is a challenge, and thus heterogeneous catalysts are more suitable because of their high stability, recyclability, and simple separation from the reaction media.27 Metal complexes are widely employed as catalysts for eco-friendly applications.28–30 Furthermore, the catalytic yield of metal complexes can be improved by increasing their active surface area by decreasing their size to the nanoscale (1–100 nm), causing fascinating properties and differences from bulk materials to emerge.31,32 Metal nanoparticles (MNPs) such as gold, palladium, silver, platinum, and copper have recently been explored as catalysts for removing organic dyes and nitro chemicals.33–37
2. Hydrogel
Hydrogels with three-dimensional networks are insoluble polymeric materials comprised of numerous hydrophilic groups, which can load a vast amount of water. It has been reported that hydrogels are versatile carriers of active compounds including enzymes, drugs, and nanoparticles. Recently, smart hydrogels with the ability to respond to stimulation from the external environment, such as additional chemicals, pH, temperature, and light, have received increasing attention.38–43 Thus, due to these attractive properties, these smart hydrogels can be potential materials with promising applications in drug delivery, chemical sensors, catalysts, and optics.44–502.1. Classification of hydrogels
Hydrogels can be divided into numerous types based on their preparation procedure, ionic charge, and physical structures, including homopolymer hydrogels, co-polymer hydrogels, multi-polymer hydrogels, and interpenetrating network hydrogels. Homo-polymer hydrogels are cross-linked networks of hydrophilic monomer units of one type, whereas co-polymer hydrogels, are made by cross linking chains made up of two co-monomer units, one of which must be hydrophilic for them to swell in water. Three or more comonomers are reacted together to form multi-polymer hydrogels. Finally, interpenetrating network hydrogels can be made in two ways, i.e., in a premade network and solution. The typical strategy is the polymerization of one monomer within various cross-linked hydrogel networks. The monomer polymerizes to form a polymer or a second cross-linked network that is intermeshed with the first network.Hydrogels can also be categorized in a variety of ways. Ionic hydrogels with ionic charges on the backbone polymers are classed as follows: (A) uncharged, (B) anionic hydrogels (containing only negative charges), (C) cationic hydrogels (having only positive charges) and (D) ampholytic hydrogels (having both positive and negative charges). The net charge on these last gels can be harmful, positive, or neutral.
Hydrogels can be characterized as either (A) amorphous hydrogels (with covalent cross-links) or (B) semicrystalline hydrogels, depending on the physicochemical structural properties of their network (may or may not have covalent cross-links). The macromolecular chains in amorphous hydrogels are randomly organized. Alternatively, semicrystalline hydrogels (crystallites) consist of self-assembled regions of organized macromolecular chains.38–40
2.2. Preparation of hydrogels
Small multifunctional molecules such as monomers and oligomers are frequently combined, reacting to form a network structure, for the fabrication of covalently cross-linked hydrogels. Large polymer molecules can sometimes be cross-linked with small multifunctional molecules. The reaction of two chemical groups on two distinct molecules, which can be initiated by catalysts, photopolymerization or radiation cross-linking, can produce this type of cross-linking.Free radical reactions are used various ways to make cross-linked hydrogels. One approach is a copolymerization-cross-linking reaction involving one or more monomers and one multifunctional monomer in small amounts. Two water-soluble polymers can be cross-linked using a similar mechanism involving the generation of free radicals on both polymer molecules, which combine to create a cross-link. These reactions are known as free radical polymerization or cross-linking reactions. Moreover, they can be triggered by the decomposition of peroxides or azo compounds, or by ionizing radiation or UV light. Ionizing radiation involves the use of gamma rays, X-rays, or electron beams to excite a polymer, causing free radical reactions to generate a cross-linked structure. These free radical reactions, which normally occur in the absence of oxygen or air (note: some polymers are damaged by radiation, especially in air), result in the rapid development of a three-dimensional network.38
Chemical cross-linking is another method that involves the reaction of a linear or branched polymer directly with a difunctional or multifunctional low-molecular-weight cross-linking agent. This agent frequently connects two higher-molecular-weight chains through its di- or multifunctional groups. There are several well-known processes for joining hydrophilic polymers together or with cross-linkers to generate hydrogels, including some newer techniques that are gaining traction, such as the Michael addition of dithiol compounds with divinyl compounds. A related method involves the cross-linking of a tiny bifunctional molecule with linear polymeric chains that have pendant or terminal reactive groups such as –OH, –NH2, NCO, and –COOH. Enzymes can also be used to cross-link natural polymers such as proteins in a similar way.
2.3. Formation mechanism of hydrogels
The different types of gelation mechanisms are summarized in Table 1. Gelation can take place either by physical cross-linking (physical gelation) or by chemical cross-linking (chemical gelation) and radiation cross-linking. Physical gels can be sub-categorized as strong physical gels and weak gels. Strong physical gels have strong physical bonds between their polymer chains and effectively permanent under a given set of experimental conditions. Weak physical gels have reversible links formed from temporary associations between their chains. Alternatively, chemical gelation involves the formation of covalent bonds and always results in a strong gel.51| Physical cross-linking | Chemical cross-linking | Radiation cross-linking |
|---|---|---|
| Heating/cooling a polymer | Chemical cross-linkers | Aqueous-state radiation |
| Ionic interaction | Grafting (chemical grafting and radiation grafting) | Radiation in paste |
| Complex coacervation | Solid-state radiation (cross-linking in the solid state, natural polymers and synthetic/natural polymer blends) | |
| H-bonding | ||
| Maturation (heat-induced aggregation) | ||
| Freeze-thawing |
2.4. The techniques for the characterization of hydrogels
To design hydrogels with the desired performance and structure, the determination and characterization of their network parameters are significant. Table 2 presents the techniques for the characterization of hydrogels including physico-chemical, structural and thermal characterization, and the tools for their physicochemical characterization such as infrared spectroscopy, atomic force microscopy, X-ray diffraction analysis, electron microscopy, and many other techniques are described to characterize their structure.52| Physico-chemical characterization | Structural characterization | Thermal characterization |
|---|---|---|
| Solubility | Transmission electron microscopy | Differential scanning calorimetry |
| Swelling measurement | Scanning electron microscopy | Thermogravimetric analysis |
| Sol gel analysis | Atomic force microscopy | Dynamic mechanical thermal analysis |
| Rheology | Dynamic force microscopy | |
| UV-Vis absorption spectroscopy | Confocal microscopy | |
| Infrared spectroscopy | ||
| Mass spectroscopy | ||
| Nuclear magnetic resonance | ||
| Light scattering | ||
| X-ray diffraction analysis |
2.5. The reaction mechanism for the catalytic reduction/degradation of organic pollutants by hydrogels
Contaminants may be adsorbed on hydrogels through various methods including complexation, coordination, chelation, ion exchange, adsorption by physical forces, and ion trapping in their inter- and intra-fibrillar capillaries.53 These methods are always influenced by the properties of the hydrogels, the physical and chemical features of the specific type of pollutant being targeted, and the micro-environmental conditions (such as the contact solution pH and ionic strength).54 Hydrogels have the ability to remove pollutants through a variety of mechanisms or by combining the impacts of several different mechanisms, for example, the reduction of nitrophenols to aminophenols.The analysis of the reaction mechanism is beneficial for the development of more effective catalysts. An example of a heterogeneous catalytic reaction is the catalytic reduction of nitrophenols using hydrogel-supported metal nanoparticles. The Eley–Rideal and Langmuir–Hinshelwood models can both be used to predict the course of a heterogeneous catalytic reaction. In the case of the Eley–Rideal model, only one of the educts will adhere to the surface of the catalyst and interact with the other educt in the solution. According to the Langmuir–Hinshelwood model, both educts adsorb on the surface of the catalyst and undergo reactions there. Accordingly, by conducting two sets of tests, namely, examining the dependence of the rate constant on the concentration of PNP and sodium borohydride, the appropriate reaction mechanism for the reduction of nitrophenols can be proposed.
In the Eley–Rideal model, the rate increases with an increase in the concentration of PNP, which is not adsorbed on the surface. Alternatively, the rate constant decreases with an increase in the concentration of PNP according to the Langmuir–Hinshelwood model.55,56
In the present review, we describe the advantages and properties of copper and palladium-based hydrogels. In addition, the role of copper- and palladium-based hydrogels in the development of novel, safe, and efficient catalysts is explained.
3. Catalytic applications of synthesized palladium- and copper-based hydrogels
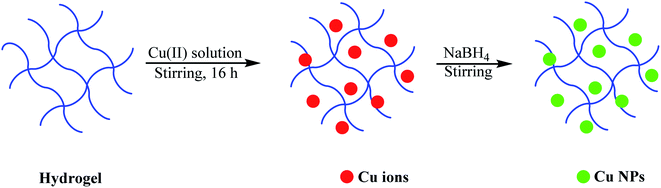
Some hydrogels have a large number of polar functional groups such as hydroxyl and carboxyl groups, which can strongly chelate with transition metals (Au,57 Ag,58 Cu,59 Pd,60 Ni,61 Co,62 etc.), leading to the effective loading of metal ions/nanoparticles and preventing their aggregation. Furthermore, they can increase the ion/nanoparticle loading and number of catalytic sites for catalytic reactions. Also, some hydrogels are biocompatible and biodegradable, and thus can be applied as green, low-cost, and suitable candidates for the removal of pollutants from the environment.63,64 Generally, nanocomposite hydrogels can be prepared by immersing a hydrogel in aqueous solutions of the respective metal salts. Subsequently, the metal ions are converted to metal nanoparticles via reduction by a typical reducing agent, such as NaBH4. Nitro compounds, phenols, and heavy metals are the most common pollutants, and thus an overwhelming number of works has been focused on their destruction.65–67 Au-,64 Pt-,68 and Ag-based69 hydrogels are commonly used for the catalytic reduction of 4-nitrophenol (4-NP) despite their high cost. Alternatively, copper (Cu) is a good candidate to replace these expensive metals because it is an abundant element in the Earth's crust.70–75 Also, palladium nanoparticles (Pd NPs) are one of the most promising candidates for pollutant removal due to their outstanding catalytic activity. Thus, among the different transition metals, Pd and Cu nanoparticles or Cu and Pd-based complexes have been significantly used in catalytic applications, especially considering their exclusive features and low cost. Besides, Cu NPs and Pd NPs play critical roles in degrading organic pollutants.76–79 Recently, Pt- and Cu-based hydrogels have been regarded as active metals with excellent performances for different chemical transformations. Different synthetic (such as polyacrylamide and polyacrylic acid) and natural-based polymers (such as chitosan and cellulose) are used as supports. The purpose of the extensive research in this area is to develop low-cost approaches for the scalable production of biocompatible catalysts. In this section, we present the catalytic uses of these compounds in the degradation of environmental pollutants.3.1. Reduction and degradation of environmental pollutants using Cu-based hydrogels
In 2012, Ozay et al. reported a simple route for the preparation of Cu(0) nanoparticles inside p(2-acrylamido-2-methyl-1-propansulfonic acid) (p(AMPS)) hydrogel networks.81 The p(2-acrylamido-2-methyl-1-propansulfonic acid)–Cu composites were utilized for the reduction of 4-NP as a model reaction in the presence of excess NaBH4, exhibiting superior catalytic activity (Scheme 3). Even in the presence of a significantly lower quantity of catalyst (1.1 mg Cu(0)), the degradation rate of 4-NP by the p(2-acrylamido-2-methyl-1-propansulfonic acid)–Cu composites at a temperature close to room temperature (30 °C) led to a much higher value (0.1032 min−1) compared to Ni(0) and Co(0) catalysts synthesized under very similar conditions. At various activation energies and reaction temperatures, the kinetic parameters defined for 4-NP and 4-AP were 39.2 and 28.2 kJmol−1, respectively.
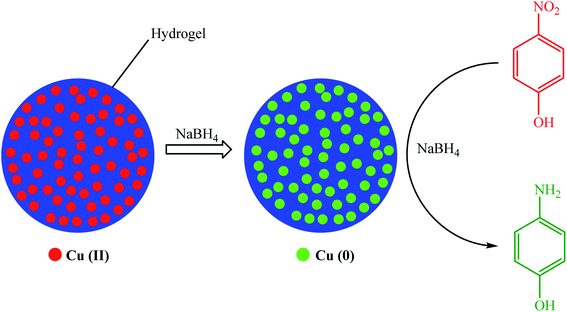 | ||
| Scheme 3 Schematic of the creation of Cu NPs inside p(AMPS) hydrogel network and their use for the reduction of 4-NP to 4-AP. | ||
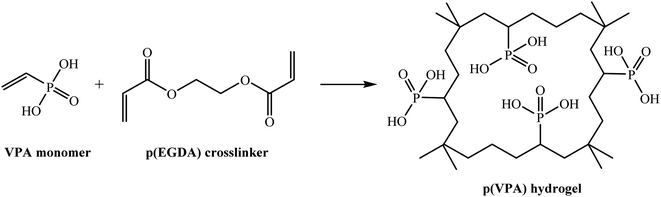
In 2013, Sahiner and Sagbas synthesized bulk poly(vinyl phosphonic acid) (p(VPA)) hydrogels through a photo-polymerization method utilizing polyethylene glycol diacrylate with various molecular weights as the cross-linker (Scheme 4).82 The prepared hydrogel was used as a template for the in situ synthesis of Ni, Co, and Cu nanoparticles. Initially, the equivalent ions from aqueous media were loaded in the poly(vinyl phosphonic acid) hydrogel complexes. Subsequently, the metal ions were reduced by sodium borohydride to the (p(VPA)) hydrogel matrices. They investigated the catalytic performance of p(VPA)–M (M: Co, Ni, Cu, etc.) for the reduction of 4-NP to 4-AP.
In 2017, Guin and co-workers synthesized a thermally stable mixed elastic poly(acrylic acid–sodium-sulfonate-styrene graphene oxide) (poly(AAc–SSS–GO)) superabsorbent hydrogel via addition polymerization induced by free gamma radiation after crosslinking (Fig. 1).83 The composite material adsorbed 98 mg g−1 copper(II) from an aqueous solution of copper(II) at room temperature at pH 5 with the chemi-adsorption of copper(II) ions by its various functional groups with high energy. Through the chemical reduction of the pre-adsorbed copper(II) ions, Cu NPs with a size of 12 ± 8 nm were prepared in situ. They confirmed the excellent catalytic activity of Cu NP–poly(AAc–SSS–GO) for the reduction of 4-NP to 4-AP.
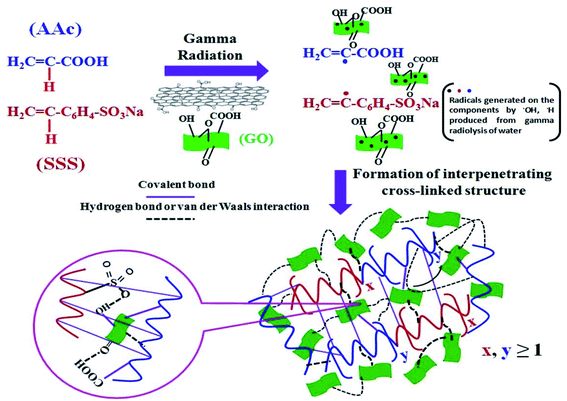 | ||
| Fig. 1 Preparation of the blended poly(AAc10–SSS15–GO0.01) composite hydrogel via a crosslinking process based on gamma radiation. This figure has been reproduced from ref. 83 with permission from John Wiley & Sons Inc. Copyright 5316550528241. | ||
In 2017, Naseer and co-workers synthesized poly(acrylic acid-co-acrylamide) (p(AAc-co-AAm)) hydrogels, which were subsequently used as templates for the synthesis of Cu nanoparticles (Scheme 5).84 The Cu NPs were prepared via the in situ reduction of Cu(II) ions. The synthesized p(AAc-co-AAm)–Cu composites were utilized as catalysts for the reduction of 4-NP and as an adsorbent in aqueous media to remove malachite. Moreover, the p(AAc-co-AAm)–Cu catalyst could be reutilized 4 times with no decline in its catalytic activity.
In 2019, Li and colleagues synthesized some Cu2O/Cu/reduced graphene oxide @carbon nanomaterial (Cu2O/Cu/rGO@CN) photocatalysts via a new sodium alginate hydrogel technique (Fig. 2).85 Cu2O NPs (∼50 nm) were synthesized via a calcination method under the protection of a nitrogen atmosphere. Inevitably, Cu nanoparticles (∼6 nm) were found over the surface of Cu2O. Thus, a Cu2O/Cu heterostructure was formed, which was identified as a Schottky junction. Synchronously, graphene oxide was reduced in situ by sodium alginate during the synthetic procedure, and eventually its 3-D structure contributed to the formation of the hydrogel skeleton. The photocatalytic activity and adsorption capacity of Cu2O/Cu/rGO@CN improved considerably when it was modified by 3-D rGO. Subsequently, the synthesized Cu2O/Cu/rGO@CN was employed as an effective catalyst for the degradation of p-nitrochlorobenzene. The advantages of this method included facile synthesis, no need for complex instruments, and use of nontoxic raw materials.
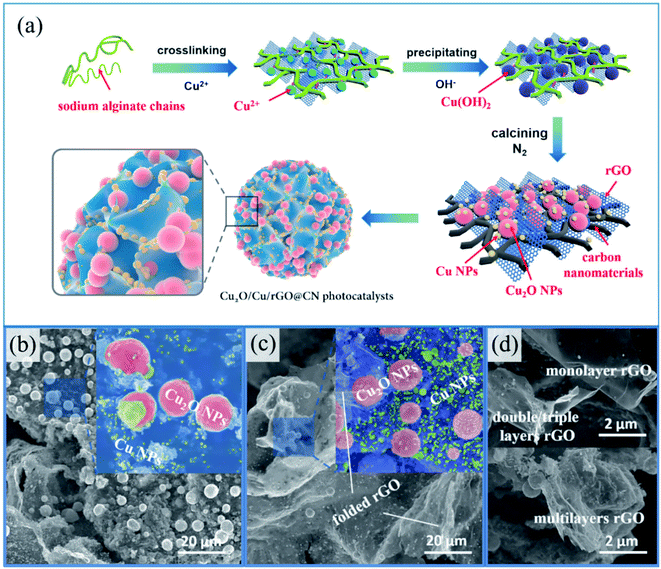 | ||
| Fig. 2 (a) Formation of Cu2O/Cu/rGO@CN photocatalysts using sodium alginate hydrogel as a template. SEM images of (b) Cu2O/Cu@CN, (c) Cu2O/Cu/rGO@CN-4 and (d) rGO. This figure has been reproduced from ref. 85 with permission from Elsevier. Copyright 5316550031989. | ||
Li et al. synthesized nanostructures of steady flexible electrospun hydrogel nanofibers comprised of poly(allylamine hydrochloride) and poly(acrylic acid).86 The metal NPs were formed by loading the hydrogel fibers with metal ions such as Ag and Cu and immersing them in solutions with metal ions after chemical reduction. Finally, the metal ions were absorbed from the medium by the hydrogel fibers. They presented a viscous environment for promoting the creation of alloy particles with a size of less than 25 nm. The reduction of methylene blue (MB) and 4-NP was carried out to examine and compare the catalytic activity of Ag–Cu bimetallic NPs and monometallic NPs (Scheme 6). It was found that the Ag–Cu bimetallic NPs presented desirable selectivity to reduce 4-NP and better catalytic activity for the degradation of MB.
In the same year, a simple procedure was reported for the synthesis of Cu NPs in a chitosan/fluorescein (CS/F) hydrogel and film matrix by immersing the particular matrix in an aqueous medium comprised of a metallic salt precursor and reducing agent under an N2 atmosphere at 75 °C for 3 h (Scheme 8).88 The high catalytic yield of the prepared nanocomposites was demonstrated by reducing methylene blue using N2H4 as a reducing agent. According to the experimental results, the order of the degradation reaction of the MB–N2H4 system and catalytic activity were strongly dependent on the type of nanocomposite used.
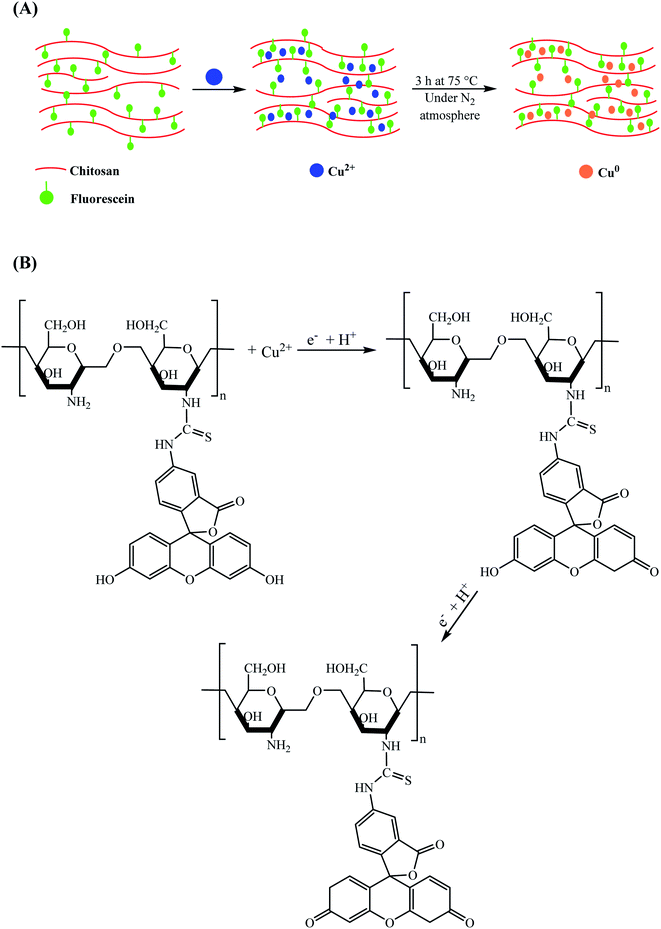 | ||
| Scheme 8 (A) Preparation of CS/F–Cu film and hydrogel nanocomposites and (B) proposed mechanism for the reduction of Cu2+ to Cu0. | ||
Sharma and colleagues synthesized a non-toxic biocompatible chitosan-based hydrogel nanocomposite with Cu NPs through in situ free radical polymerization utilizing acrylamide as the monomer. Chitosan was employed as the substrate, glutaraldehyde as the cross-linking agent, and Cu NPs and ammonium persulphate as the initiator (CH–PAmCu–NP catalyst).89 They employed the prepared hydrogel nanocomposite as a recyclable catalyst with high efficiency for the reduction of 4-NP to 4-AP (Scheme 9).
In 2018, Xiao and co-workers established an internal solidification procedure induced by a rigid template for the preparation of ultrafine MNPs immobilized on calcium alginate, polyethylene imine, and glutaraldehyde beads (Cu@GLA–PEI–CA). During the synthetic procedure, CaCO3 nanoparticles with bi-functional properties were applied as a hard skeleton for adjusting the structure of the catalyst and also as a subsidiary cross-linking agent to obtain hydrogel beads.90 Copper nanoparticles with a narrow size distribution of 2–3 nm were homogeneously supported on the surface of the GLA–PEI–CA beads with a well-developed hierarchical pore structure and high specific surface area. They observed that the Cu@GLA–PEI–CA beads demonstrated good catalytic activity for the reduction of 4-NP to 4-AP with sodium borohydride in aqueous solution (Scheme 10). Furthermore, this catalytic system could be simply separated and reused.
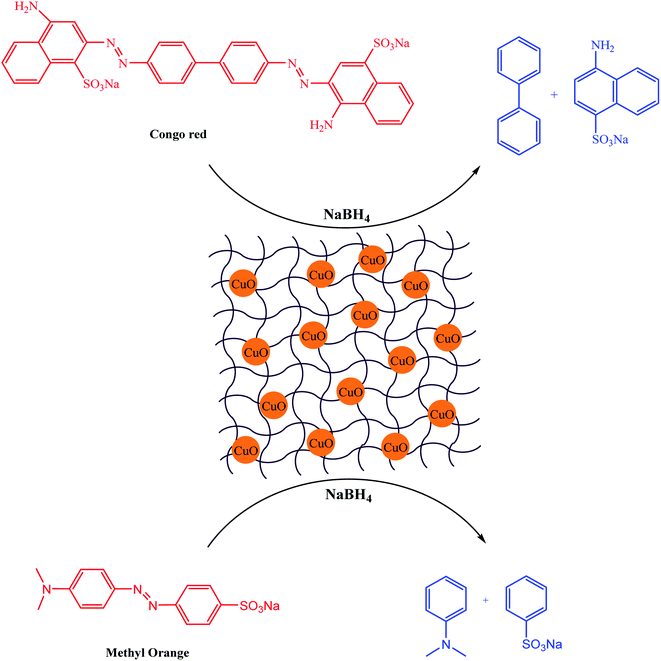
In 2020, a new bionanocomposite of gelatin as a polypeptide hydrogel comprised of CuO nanomaterial (GL–CuO) was synthesized by Ahmad and co-workers.91 After their dispersal, the synthesized CuO NPs were cross-linked with the addition of a CH2O solution in the GL aqueous solution. The synthesized GL–CuO with an average size of about 60–80 nm showed durable and extraordinary activity for the reduction of Congo red (CR) and methyl orange (MO) in an eco-friendly medium (Scheme 11). This method had the advantages of simple catalyst synthesis, high efficiency, and easy work up.
In 2020, a simple route was reported by Kamal et al. for the preparation of carbon nitride (g-C3N4)-incorporated sodium alginate (Alg) beads. The Alg–CNBs (alginate carbon nitride beads) were synthesized by adding an Alg aqueous solution containing dispersed g-C3N4 to an aqueous solution containing calcium(II) ions (Fig. 3).92 Then, Alg–CNBs were further treated with various transition metal salt solutions. Subsequently, employing sodium borohydride as a potent reducing agent, the transition metal ions covering the surface of Alg–CNBs were reduced to the zero-valent state (M0) and utilized for the catalytic degradation of various organic dyes. Finally, the synthesized M0/Alg–CNBs (where M0 = Ag0, Fe0, Cu0, and Ni0) catalysts were examined for the reduction of 4-NP to determine the best one. Among them, it was found that Cu0/Alg–CNBs reduced 4-nitrophenol to 4-aminophenol efficiently. Also, Cu0/Alg–CNBs was further employed for the reduction of CR, MO, 2-NP and 2,6-DNP.
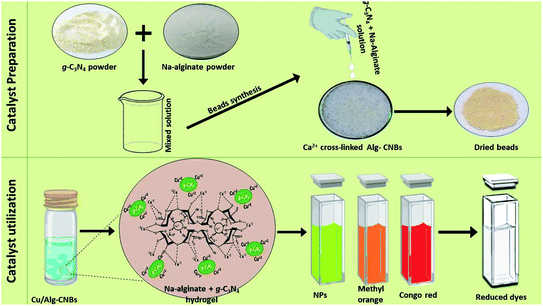 | ||
| Fig. 3 Preparation of Cu0/Alg–CNB catalytic beads and their catalytic utilization. This figure has been reproduced from ref. 92 with permission from Elsevier. Copyright 5316550823396. | ||
In 2021, Xiao et al. successfully prepared an N-doped sandwich-like carbon layer coating on copper NPs supported on N-doped porous carbon (N–C/Cu/N–C) by the deposition of a polydopamine (PDA) layer over alginate-based hydrogel beads after high-temperature pyrolysis in N2.93 The results demonstrated that copper nanoparticles with a narrow size distribution in the range of 10–30 nm were immobilized uniformly over the porous carbon bead framework by protecting the outer N–C layer. Moreover, the mechanical strength was enhanced effectively by the layer extracted from PDA on the porous beads, therefore preventing the loss of copper NPs during the catalytic reaction. Subsequently, the performance of the prepared catalyst (N–C/Cu/N–C) was assessed for the reduction of 4-NP to 4-AP (Scheme 12).
In 2022, Andou and co-workers designed a facile method for the in situ preparation of a new three-dimensional photocatalyst comprised of Cu2O/TiO2 supported on a cellulose nanofiber (CNF)/reduced graphene hydrogel (rGH) through a simple freeze-drying and hydrothermal treatment.94 The three-dimensional macrostructure acted as an appropriate template for anchoring TiO2 and Cu2O, providing an effective electron transport path to improve the photocatalytic activity. According to their findings, TiO2 and Cu2O were homogeneously incorporated in the aerogel template, resulting in structures with a larger surface area in the uncovered active sites. In comparison to the bare rGH, Cu2O/CNF/rGH, CNF/rGH, and TiO2/CNF/rGH, Cu2O/TiO2/CNF/rGH presented enhanced activity for the removal of methyl orange (Scheme 13).
The reports on the use of Cu-based hydrogels for environmental remediation are presented in Table 3.
| Cu-based hydrogels | Size of Cu nanoparticles | Application | Catalytic activity/benefits | Year | Ref. |
|---|---|---|---|---|---|
| a Discussed in the review. | |||||
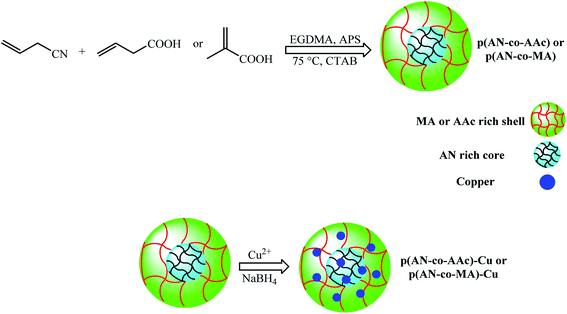
| Cu NPs inside p(AN-co-MA) and p(AN-co-AAc) particles | — | Reduction of 4-NP to 4-AP | Multipurpose | 2011 | 80a |
| p(AMPS)–Cu | 30–35 | High conductivity | 2012 | 81a | |
| p (VPA)–M | — | Potential applications in bone mineralization | 2013 | 82a | |
| Cu NW–Ag (CuAg-3) | 100 | Superior catalytic activity | 2014 | 96 | |
| DNA–Cu-hydrogel | 2–3 | Potential for green organic synthesis in aqueous media and for biomedical in vivo applications | 2016 | 100 | |
| Clean synthesis of the catalyst | |||||
| Cu NP–poly(AAc–SSS–GO) | 12 | Economically viable | 2017 | 83a | |
| CH/f-Cu hydrogel | — | Utilized 4 times with no loss in catalytic activity | 2017 | 88a | |
| p(AAc-co-AAm)–Cu | — | Separable with a magnetic field | 2017 | 84a | |
| CH–PAmCu–NPs | — | Good chelation with Cu NPs | |||
| Multi-functional | 2017 | 89a | |||
| Selective adsorption and recycling copper element for waste disposal industry | |||||
| Cu NPs-loaded SPI/PEI | 10–80 | Retained 70% activity after being stored for 30 days | 2017 | 101 | |
| WSC-g-PAA/PVA–Cu | 20–30 | Beyond 90% conversion in 10 cycles | 2017 | 102 | |
| Tunable structure and properties | |||||
| Cu@GLA–PEI–CA beads | 2–3 | Cost-effective and biocompatible | 2018 | 90a | |
| Cu NP-impregnated cellulose | 4–16 | Scalable reduction and reusable for 6 times | 2019 | 112 | |
| Chitosan–Cu | — | Enhanced mechanical strength and stability | 2020 | 110 | |
| Alg–CNB beads | — | 2020 | 92a | ||
| N–C/Cu/N–C | 10–30 | 2021 | 93a | ||
| Cu/CHCMM | 48 | 2-NP, 4-NP and cresyl blue | Economical and environmentally friendly | 2016 | 87a |
| Cu2O/Cu/rGO@CN photocatalysts | ∼6 | Degradation of p-nitrochlorobenzene | Suitable chemical stability during recycling | 2019 | 86a |
| GL–CuO | 83 | Reduction of Congo red and methyl orange | Good catalytic reduction properties toward different environmental pollutants and easy separation through filtration | 2020 | 91a |
| p(EP-g-AA)–Cu | 100 | Reduction of MB and 4-NP | Maintained 80–90% catalytic activity after 5 times use and 30 days storage | 2018 | 103 |
| poly(allylamine hydrochloride) and poly(acrylic acid) nanofibers | <20 | Simplicity, controllability, and versatility | 2021 | 86a | |
| Cu(II)–poly(EGDE–DA) | — | Reduction of MO | Oxidation of pharmaceutical product (epinephrine) | 2014 | 97 |
| Cu2O/TiO2/CNF/rGH | 4.3–6.3 | Enhanced photoactivity | 2021 | 94a | |
| PEI–Cu | — | Reduction of 4-NP and 2-NP | Very fast reduction times of 1.2 and 0.67 min to 4-AP and 2-AP, respectively | 2015 | 99 |
| Amid–p(Mac–Cu–AN)–M | 10–50 | Reduction of 4-NP, 2-NP, MO, MB, eosin Y | Individual and simultaneous degradation of cationic/anionic dyes double triple compounds | 2016 | 95 |
| Amid–p(AAm)–Cu | — | Reduction of 4-NP, 2-NP, MB, eosin Y | Fast response to external stimuli, good mechanical strength, and durability | 2015 | 98 |
| WSC-g-PAA/PVA–Cu | 20–50 | Degradation of chloramphenicol | Great potential for future applications for heavy metal recovery and reuse | 2018 | 104 |
| m-WSC/FP–Cu | 2–5 | Reduction of 2-aminobenzoic acid | Maintained 99.02% over 5 cycles utilization and 90.60% after 30 days storage | 2019 | 105 |
| AG–CuO | 15–92 | Reduction of 4-NP, 2-NP, 2,6-DNP | Easy recyclability and high catalytic activity | 2019 | 106 |
| BSA–Cu | — | Reduction of 4-NP, CR, MB | Robust hydrogel incorporating metal ions | 2020 | 107 |
| Fe3O4–CuO@C | — | Reduction of MB | Enhanced photocatalytic activity | 2020 | 108 |
| Cu/MC | 25 | Reduction of 4-NP and rhodamine B | Magnetic responsiveness to an external magnetic field | 2020 | 109 |
| CuS@CNs | 5 | 2,4-Dichlorphenol | Improved the separation of photogenerated charge carriers | 2021 | 111 |
3.2. Reduction and degradation of environmental pollutants using Pd-based hydrogels
 | ||
| Scheme 14 Reduction of 4-NP in the presence of 3D Pd–CNT–GH.113 | ||
In 2017, Shen and colleagues designed and synthesized new three-dimensional graphene oxide nanosheets reduced by molybdenum disulfide and supported by Pd (Pd/MoS2–rGO) through a convenient one pot self-assembly process (Fig. 4).114 The restacking of rGO nanosheets was prevented by the presence of MoS2 and their specific surface area increased. Moreover, a further transport platform was also afforded for palladium NPs to enhance their catalytic features. Excellent catalytic activity for the reduction of 4-NP to 4-AP was demonstrated by the synthesized Pd/MoS2–rGO NPs in water in the presence of borohydride.
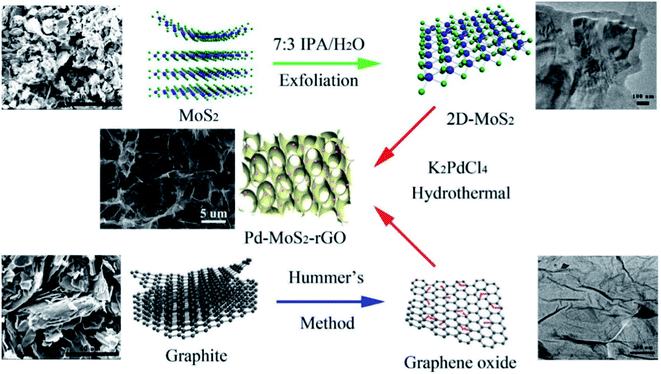 | ||
| Fig. 4 Procedure for the preparation of the 3D Pd/MoS2–rGO composite hydrogel. This figure has been reproduced from ref. 114 with permission from Elsevier. Copyright 5316560270510. | ||
In the same year, Wu and co-workers utilized a two-step technique to synthesize a reduced graphene oxide nanosheet/magnetite–palladium (rGSs/Fe3O4–Pd) aerogel with superior recyclability and catalytic performance.115 In the first step, graphene oxide nanosheet hydrogels were prepared via the self-assembly of GSs during the hydrothermal procedure. Meanwhile, the palladium nanoparticles (NPs) and hematite (α-Fe2O3) were synthesized and anchored on the hydrogel surface. Subsequently, upon heat-treatment, the graphene oxide nanosheets were reduced to rGSs, whereas the nonmagnetic α-Fe2O3 NPs changed to magnetite Fe3O4 nanoparticles. The as-achieved rGSs/Fe3O4–Pd aerogel possessed a 3D interconnected porous hierarchical architecture rich in macro-pores and mesopores. This structure was appropriate for catalysis because it not only enhanced the transport and mass diffusion but also simply exposed the catalytic palladium nanoparticles to the substrates. The potential catalytic performance of the synthesized Pd NPs was demonstrated for the reduction of 4-nitrophenol (Scheme 15).
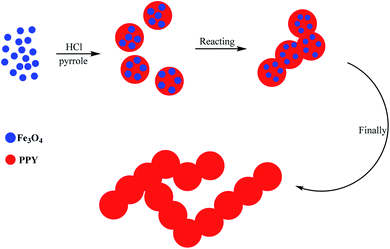
In 2018, Yao and co-workers synthesized a nanobead-based polypyrrole (PPy) hydrogel via a reactive-template technique in one step (Scheme 16).116 Fe3O4 NPs were chosen as the reactive template and used as the oxidant for initiating the polymerization of the pyrrole monomer and also guiding the growth of the polymer chains. Given that the PPy hydrogels were formed by the cross-linking of the individual nanobeads, they bridged the macro-dimension and nano-dimension; thus, a three-dimensional hierarchical porous structure was formed. The PPy hydrogels possessed a large surface area and several interconnected channels, and by taking advantage of their structural merits, they acted as superior candidates for adsorbing rhodamine B. The PPy hydrogels for loading Pd nanoclusters could serve as a support and used as adsorbents.
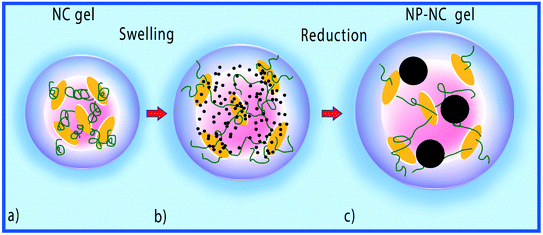
In the same year, Haraguchi and Varade described a novel approach to prepare discrete monometallic (silver, gold, and palladium) and bimetallic (Au–Pd, Pt–Pd) nanoparticles using a nanocomposite hydrogel comprised of a polymer–clay network (Fig. 5).117 Thermo-responsive nanocomposite hydrogels (NC gels) were prepared via the in situ free radical polymerization of N-isopropylacrylamide in the presence of clay (synthetic hectorite) nanosheets (CNSs). Given that the CNSs have robust affinities for metal ions, the concentration of the surrounding metal precursors was balanced and non-aggregated and well dispersed. Spherical bimetallic and monometallic NPs were obtained by reducing metal ions using ascorbic acid in NC gels, which were strongly immobilized in the polymer–clay network. The resultant hybrid NP–NC gels, which contained monometallic or bimetallic nanoparticles, exhibited high catalytic activities for the reduction of NP to AP.
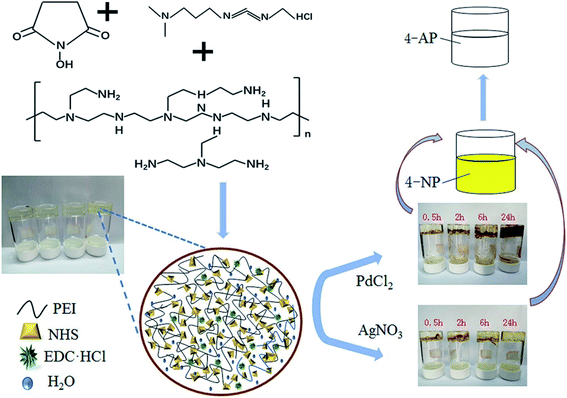
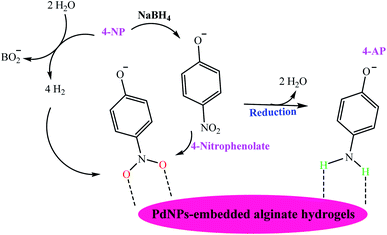
In 2020, PEI–Pd and PEI–Ag composite hydrogels were successfully synthesized by Jiao et al.118 Furthermore, the prepared PEI–Pd and PEI–Ag showed extraordinary and durable activity for the reduction of 4-nitrophenol to 4-aminophenol in an environmentally friendly medium (Fig. 6). This process has many advantages such as the high effectiveness of the composite hydrogels, simple process, and easy workup.
Lee and co-workers described a simple technique for immobilizing palladium NPs with high physicochemical stability in uniform hydrogel microparticles. Simultaneously, it resulted in the high physicochemical stability and dispersion of the immobilized palladium nanoparticles inside the hydrogel microparticles. The PVP (polyvinylpyrrolidone)-stabilized palladium nanoparticles were synthesized via a microwave-assisted method. The palladium nanoparticles were immobilized utilizing a micro-molding approach in the UV enhanced hydrogel precursor.119 The immobilized palladium nanoparticles were physically stabilized because they were trapped in the porous hydrogel framework. Meanwhile, they were chemically stabilized by the H-bonding induced among the PEGDA (polyethylene glycol diacrylate) hydrogel matrix, PVP, and PEG (Fig. 7). Also, the stability of the immobilized palladium nanoparticles against oxidation was confirmed by the formed hydrogen bonding. They investigated the catalytic performance of the synthesized Pd NPs for the reduction of 4-NP to 4-AP.
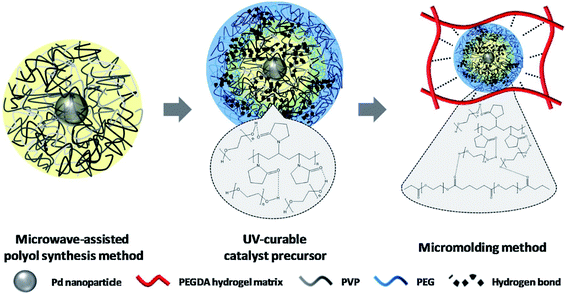 | ||
| Fig. 7 Postulated stabilization mechanism for Pd NPs immobilized on hydrogels. This figure has been reproduced from ref. 119 with permission from Elsevier. Copyright 5318331364158. | ||
In the same year, Wang and colleagues synthesized Pd-immobilized rGO and black phosphorus nanosheet-based (rGO–BP–Pd) hydrogels via a facile self-assembly method.120 Precisely, GO was reduced to the rGO hydrogel by addition of L-ascorbic acid. Furthermore, the rGO hydrogel was utilized as a skeleton for loading palladium nanoparticles and the catalytic features of the obtained template hydrogels were investigated (Fig. 8). They also investigated the effects of the hydrogels prepared by adding various quantities of black phosphorus nanosheets (BPNS) on the catalytic reduction of 4-nitrophenol. The outcomes demonstrated that increasing the quantity of BPNS could improve the catalytic activity owing to the interaction between Pd NPs and BPNS.
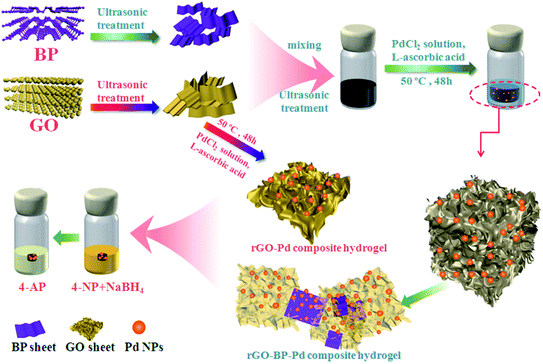 | ||
| Fig. 8 Catalytic performances and process for the synthesis of rGO-based composite hydrogels. This figure has been reproduced from ref. 120 with permission from Elsevier. Copyright 5318330689362. | ||
 | ||
| Scheme 17 Synthesis of cellulosic hydrogel anchoring 1,1,3,3-tetramethylguanidine poly-ionic liquid moiety. | ||
In the same year, Park and co-workers assessed the catalytic efficiency of alginate hydrogels as a support for catalysts.121 An alginate solution comprised of magnetite NPs and Pd was continuously dropped via a tapered glass capillary utilizing a syringe pump, and the created alginate droplets were gathered in an aqueous solution containing divalent cations. The created Pd NP/MNP-embedded alginate particles were considered as the catalyst support, catalyst, and magnetic stirrer for the reduction of 4-NP (Scheme 18). It was found that the catalytic reactions depended on the magnetic stirring of the alginate hydrogels.
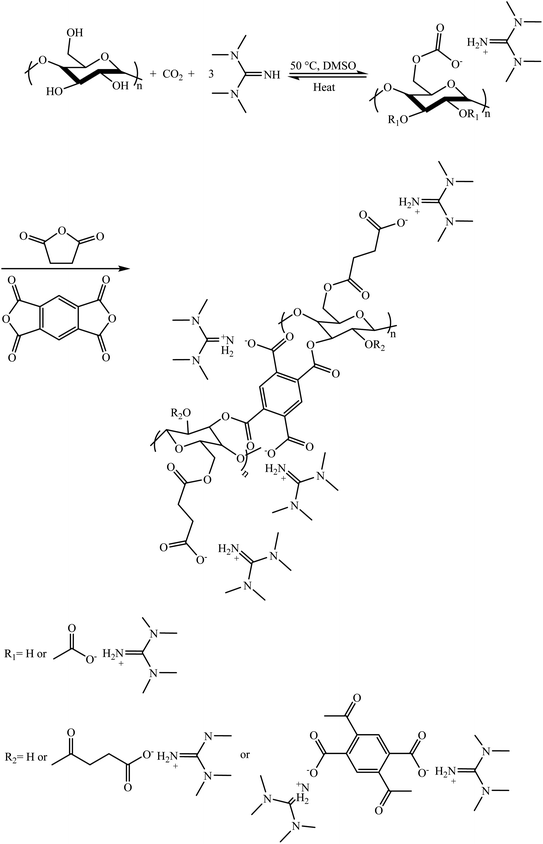
In 2020, Jiao and co-workers synthesized a series of palladium NP-incorporated chitosan (CS)-based composite hydrogels by adding carbon nanotubes, graphene oxide, and layered double hydrotalcites (LDHs) to a CS hydrogel with glutaraldehyde as the cross-linking agent and in situ growth of palladium nanoparticles.122 The catalytic reduction of 4-nitrophenol was carried out to evaluate the performance of the chitosan-based composite hydrogel (Scheme 19). The prepared chitosan-based composite hydrogel catalysts presented a higher catalytic performance in comparison with the chitosan hydrogel catalyst-doped palladium nanoparticles (CS-PdNPs) and recoverability for reducing 4-NP in aqueous media. Furthermore, the chitosan-based composite hydrogel catalysts comprised of CNTs possessed the highest catalytic activity among the three types of chitosan-based composite hydrogel catalysts because the particle size of the palladium nanoparticles was the smallest.
 | ||
| Scheme 19 Reduction of 4-NP to 4-AP applying Pd NPs. This figure has been reproduced from ref. 122 with permission from Elsevier. Copyright 5316560975161. | ||
In 2021, An and co-workers fabricated N-doped mesoporous carbon modified by highly dispersion nickel NPs (Ni–N@C) through the pyrolysis of Ni2+/histidine cross-linked alginate hydrogels.123 Next, the obtained Ni–N@C nano-catalyst was treated using a solution of palladium(II). Small amounts of the deposited palladium nanoparticles were found on the surface of nickel by the reduction of nickel to obtain a higher distribution of the valuable metal material (Fig. 9). The catalyst presented a good performance, where the pollutants were completely degraded in the presence of highly concentration 4-NP within a very short time. It was shown that the pre-embedded nickel nanoparticles could not only enhance the activity of palladium nanoparticles but also endow simple separation features to the catalyst. Also, this catalyst maintained favorable catalytic activity even after 5 reaction cycles.
 | ||
| Fig. 9 Synthesis of Pd Ni–N@C. This figure has been reproduced from ref. 123 with permission from Elsevier. Copyright 5357550496279. | ||
The reports on the use of Pd-based hydrogels for environmental remediation are presented in Table 4.
| Pd-based hydrogels | Size of Cu nanoparticles | Application | Catalytic activity/benefits | Year | Ref. |
|---|---|---|---|---|---|
| a Discussed in the review. | |||||
| Pd–CNT–GH | 2–3 | Reduction of 4-NP to 4-AP | Catalyzation over about 30 s at room temperature | 2014 | 113a |
| Pd/MoS2–rGO | 5 | Catalyzation within 30 s and 20 successive cycles without obvious deactivation | 2017 | 114a | |
| rGSs/Fe3O4–Pd | — | Magnetically separable | 2017 | 115a | |
| NP–NC gels | — | Environmentally friendly catalyst | 2018 | 117a | |
| Pd NPs@GC | 2–4 | Good immobilization capacity of Pd NPs | 2019 | 63a | |
| Pd NPs/MNP-embedded alginate particles | — | At least 10 consecutive catalytic reactions | 2019 | 121a | |
| PEI–Pd | — | Easily separated | 2020 | 118a | |
| Pd-immobilized on PEGDA-based hydrogel | 2–4 | Enhanced stability | 2020 | 119a | |
| CH–PdNPs | — | High stability and easy recyclability | 2020 | 122a | |
| rGO–BP–Pd | 5–10 | Enhanced catalytic performance | 2020 | 120a | |
| Ni–N@C | — | Favorable catalytic activity for 5 reaction cycles | 2021 | 123 | |
| PPy/Pd hydrogel | — | Adsorption of rhodamine B | Good TOF rate constant | 2017 | 116a |
| Pd_TMV_PEG | 2–3 | Reduction of Cr(VI) | Robust, and scalable synthesis | 2013 | 124 |
| Pd–TMV nano-biocomplexes | 1–2 | Retaining catalytic activity for at least 3 days | 2019 | 126 | |
| Pd–GHJ | 10 | Reduction of nitroarenes | Use of ammonia borane as a hydrogen storage material for the first time | 2017 | 125 |
| MarGO–Pd | 30 | Reduction of 4-NP, CR, MB | Suitable for several biochemical applications | 2020 | 127 |
| Pd NPs@CNCC–AHB | 9 | Reduction of MB | Suitable catalyst for C–C coupling along with MB reduction | 2020 | 128 |
4. Conclusion and future prospects
The industrial development and increasing population have resulted in an increase in the release of pollutants in the environment, threatening the health of all living organisms. These pollutants can be removed from the environment in different ways; however, it is vital to find an effective way to remove these pollutants. Catalysts are compounds that can degrade pollutants by different mechanisms, which are safe and effective for this aim. The high diversity of compounds possessing catalytic activities and possibilities of improving their catalytic yield by decreasing their size or increasing their surface area have resulted in significant progress in this field. However, although homogeneous catalysts have various advantages, including high selectivity and reactivity, they are not generally commercialized owing their difficult separation, leading to fouling and corrosion. To create heterogeneous catalysts, homogeneous catalysts can be loaded on different supports, among which hydrogels are suitable owing to their numerous advantages including high thermal stability, high mechanical strength, easy modification, and high surface area.By applying catalytic reduction or degradation reactions, different organic pollutants such as including nitro compounds, organic dyes, hexavalent chromium, and medicinal compounds can be removed. As stated above, copper and palladium-based hydrogels can be used as an effective catalyst to remove these contaminants. Copper and palladium with fascinating features such as antibacterial, antifungal, and antimicrobial activities can be used for environmental remediation. Herein, we summarized the recent progress on the use of copper- and palladium-based hydrogels for environmental remediation. Despite the many studies on copper- and palladium-based hydrogels, there are still some challenges for their future application. In the future, hydrogel-based carriers can be an excellent candidate for the successful administration of drugs at the desired rate and site in the body. With the creation of new hydrogels with various structural properties and hydrophobicity/hydrophilicity ratios, specific release rates and dissolution profiles may be achieved. Accordingly, these methods can be used to treat pathological illnesses such as diabetes and even cancer and enhance the delivery of more sensitive molecules. More advancements are anticipated, specifically, in the delivery of therapeutic proteins and peptides via hydrogels.
• Despite the progress in environmental remediation, some compounds have to be still eliminated from the environment.
• Use of different natural-based polymers such as lignin, chitin, chitosan, gum, cellulose, starch, and gelatin as supports.
• Use of low-cost approaches for the scalable production of catalysts.
• Manufacture of industrial-scale copper- and palladium-based hydrogels can be useful to eliminate environmental pollutants.
• Magnetic copper- and palladium-based hydrogels can be upgraded to exhibit high recyclability and catalytic activity to remove environmental pollutants.
Abbreviations
| MNPs | Metal nanoparticles |
| 4-NP | 4-Nitrophenol |
| PNP | para-Nitrophenol |
| Cu | Copper |
| Pd NPs | Palladium nanoparticles |
| AN | Acrylonitrile |
| AAc | Acrylic acid |
| MA | Methacrylic acid |
| CTAB | Hexadecyl trimethyl ammonium bromide |
| 4-AP | 4-Aminophenol |
| (P(AMPS)) | P(2-acrylamido-2-methyl-1-propansulfonic acid) |
| P(VPA) | P(vinyl phosphonic acid) |
| P(EGDA) | Polyethylene glycol diacrylate |
| (Poly(AAc–SSS–GO)) | Poly(acrylic acid–sodium-sulfonate-styrene graphene oxide) |
| (P(AAc-co-AAm)) | Poly(acrylic acid-co-acrylamide) |
| Cu2O/Cu/rGO@CN | Cu2O/Cu/reduced graphene oxide@carbon nanomaterial |
| MB | Methylene blue |
| CS | Chitosan |
| CMM | Cellulose microfiber mat |
| CB | Cresyl blue dye |
| CS/F | chitosan/fluorescein |
| CH–PAmCu–NPs | Cu NPs and ammonium persulphate |
| Cu@GLA–PEI–CA | Calcium alginate, polyethylene imine, and glutaraldehyde |
| GL | Gelatin |
| CR | Congo red |
| MO | Methyl orange |
| g-C3N4 | Carbon nitride |
| Alg | Sodium alginate |
| Alg–CNBs | Alginate carbon nitride beads |
| N–C/Cu/N–C | N-doped porous carbon |
| PDA | Polydopamine |
| CNF | Cellulose nanofiber |
| rGH | Reduced graphene hydrogel |
| CNT | Carbon nanotube |
| GH | Graphene hydrogel |
| GO | Graphene oxide |
| Pd/MoS2-rGO | Molybdenum disulfide and supported by Pd |
| rGSs/Fe3O4–Pd | Reduced graphene oxide nanosheets/magnetite-palladium |
| NPs | Nanoparticles |
| α-Fe2O3 | Hematite |
| PPy | Polypyrrole |
| NC gels | Nanocomposite hydrogels |
| CNSs | Clay (synthetic hectorite) nanosheets |
| PVP | Polyvinylpyrrolidone |
| PEGDA | Polyethylene glycol diacrylate |
| rGO–BP–Pd | Pd-immobilized rGO and black phosphorus nanosheets |
| BPNS | Black phosphorus nanosheets |
| GC | Guanidinium-cellulose |
| LDHs | Layered double hydrotalcites |
| CS–Pd NPs | Chitosan hydrogel catalyst doped palladium nanoparticles |
| Ni–N@C NPs | Carbon modified by highly dispersion nickel nanoparticles |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Here, the partial support of the Research Council of Islamic Azad University, Qom Branch are acknowledged.References
- F. Han, V. S. R. Kambala, M. Srinivasan, D. Rajarathnam and R. Naidu, Appl. Catal., A, 2009, 359, 25–40 CrossRef CAS.
- J. A. Tkaczyk, K. Mitrowska and A. Posyniak, Sci. Total Environ., 2020, 717, 137222 CrossRef PubMed.
- O. P. Zhao, X. Feng, D. Huang, G. Yang and D. Astruc, Coord. Chem. Rev., 2015, 287, 114–136 CrossRef.
- M. Zhang, X. Su, L. Ma, A. Khan, L. Wang, J. Wang, A. Maloletnev and C. Yang, J. Hazard. Mater., 2021, 403, 123870 CrossRef CAS PubMed.
- M. A. Zakaria, A. Menazea, A. M. Mostafa and E. A. Al-Ashkar, Surf. Interfaces, 2020, 19, 100438 CrossRef CAS.
- S. Xia, L. Zhang, G. Pan, P. Qian and Z. Ni, Phys. Chem. Chem. Phys., 2015, 17, 5345–5351 RSC.
- V. C. Lops, A. Ancona, K. Di Cesare, B. Dumontel, N. Garino, G. Canavese, S. Hérnandez and V. Cauda, Appl. Catal., B, 2019, 243, 629–640 CrossRef PubMed.
- V. A. Sakkas, M. A. Islam, C. Stalikas and T. A. Albanis, J. Hazard. Mater., 2010, 175, 33–44 CrossRef CAS PubMed.
- D. Ayodhya and G. Veerabhadram, Mater. Today Energy, 2018, 9, 83–113 CrossRef.
- S. Nataraj, K. Hosamani and T. Aminabhavi, Desalination, 2009, 249, 12–17 CrossRef CAS.
- B. Shi, G. Li, D. Wang, C. Feng and H. Tang, J. Hazard. Mater., 2007, 143, 567–574 CrossRef CAS PubMed.
- J. Ma, X. Tang, Y. He, Y. Fan and J. Chen, Desalination, 2020, 480, 114328 CrossRef CAS.
- I. C. da Costa Soares, Á. R. L. da Silva, E. C. M. de Moura Santos, E. V. dos Santos, D. R. da Silva and C. A. Martínez-Huitle, J. Solid State Electrochem., 2020, 24, 3245–3256 CrossRef CAS.
- K. B. Tan, M. Vakili, B. A. Horri, P. E. Poh, A. Z. Abdullah and B. Salamatinia, Sep. Purif. Technol., 2015, 150, 229–242 CrossRef CAS.
- G. Liu, M. R. Abukhadra, A. M. El-Sherbeeny, A. M. Mostafa and M. A. Elmeligy, J. Environ. Manage., 2020, 254, 109799 CrossRef CAS PubMed.
- M. Wawrzkiewicz, M. Wiśniewska, A. Wołowicz, V. M. Gun'ko and V. I. Zarko, Microporous Mesoporous Mater., 2017, 250, 128–147 CrossRef CAS.
- G. Liao, Y. Gong, L. Zhong, J. Fang, L. Zhang, Z. Xu, H. Gao and B. Fan, Nano Res., 2019, 12, 2407–2436 CrossRef CAS.
- G. Liao, J. Fang, Q. Li, S. Li, Z. Xu and B. Fang, Nanoscale, 2019, 11, 7062–7096 RSC.
- G. Liao, J. Chen, W. Zeng, C. Yu, C. Yi and Z. Xu, J. Phys. Chem. C, 2016, 45, 25935–25944 CrossRef.
- G. Liao, Q. Li, W. Zhao, Q. Pang, H. Gao and Z. Xu, Appl. Catal., A, 2018, 549, 102–111 CrossRef CAS.
- G. Liao, W. Zhao, Q. Li, Q. Pang and Z. Xu, Chem. Lett., 2017, 46, 1631–1634 CrossRef CAS.
- Q. Wu, J. Wang, Z. Wang, Y. Xu, Z. Xing, X. Zhang, Y. Guan, G. Liao and X. Li, J. Mater. Chem. A, 2020, 8, 13685–13693 RSC.
- H. Zhu, S. Cai, G. Liao, Z. F. Gao, X. Min, Y. Huang, S. Jin and X. Fan, ACS Catal., 2021, 11(24), 14751–14771 CrossRef CAS.
- R. Sankar, P. Manikandan, V. Malarvizhi, T. Fathima, K. S. Shivashangari and V. Ravikumar, Spectrochim. Acta, Part A, 2014, 121, 746–750 CrossRef CAS PubMed.
- M. M. Khin, A. S. Nair, V. J. Babu, R. Murugan and S. Ramakrishna, Energy Environ. Sci., 2012, 5, 8075–8109 RSC.
- W.-J. Liu, T.-T. Qian and H. Jiang, Chem. Eng. J., 2014, 236, 448–463 CrossRef CAS.
- M. D. Argyle and C. H. Bartholomew, Catalysts, 2015, 5, 145–269 CrossRef CAS.
- M. Nasrollahzadeh, M. Sajjadi, J. Dadashi and H. Ghafuri, Adv. Colloid Interface Sci., 2020, 276, 102103 CrossRef CAS PubMed.
- M. Chen, P. Liu, C. Wang, W. Ren and G. Diao, New J. Chem., 2014, 38, 4566–4573 RSC.
- K. M. Lee, C. W. Lai, K. S. Ngai and J. C. Juan, Water Res., 2016, 88, 428–448 CrossRef CAS PubMed.
- J. K. H. Wong, H. K. Tan, S. Y. Lau, P.-S. Yap and M. K. Danquah, J. Environ. Chem. Eng., 2019, 7, 103261 CrossRef CAS.
- R. K. Ibrahim, M. Hayyan, M. A. AlSaadi, A. Hayyan and S. Ibrahim, Environ. Sci. Pollut. Res., 2016, 23, 13754–13788 CrossRef CAS PubMed.
- N. Y. Nadaf and S. S. Kanase, Arabian J. Chem., 2019, 12, 4806–4814 CrossRef CAS.
- H. Dabhane, S. Chatur, G. Jadhav, P. Tambade and V. Medhane, Environ. Chem. Ecotoxicol., 2021, 3, 160–171 CrossRef CAS.
- A. J. Kora and L. Rastogi, Arabian J. Chem., 2018, 11, 1097–1106 CrossRef CAS.
- Z. Gong, T. Ma and F. Liang, J. Alloys Compd., 2021, 873, 159802 CrossRef CAS.
- S. Joseph and B. Mathew, J. Mol. Liq., 2015, 204, 184–191 CrossRef CAS.
- N. A. Peppas, J. Z. Hilt, A. Khademhosseini and R. Langer, Adv. Mater., 2006, 18, 1345–1360 CrossRef CAS.
- S. Wang, T. Vincent, C. Faur and E. Guibal, Appl. Sci., 2018, 8, 264–281 CrossRef.
- B. V. Slaughter, S. S. Khurshid, O. Z. Fisher, A. Khademhosseini and N. A. Peppas, Adv. Mater., 2009, 21, 3307–3329 CrossRef CAS PubMed.
- G.-J. Jiao, J. Ma, Y. Li, D. Jin, J. Zhou and R. Sun, J. Hazard. Mater., 2022, 421, 126722 CrossRef CAS PubMed.
- Q. Luo, H. Yuan, M. Zhang, P. Jiang, M. Liu, D. Xu, X. Guo and Y. Wu, J. Hazard. Mater., 2021, 401, 123432 CrossRef CAS PubMed.
- D. Dou, D. Wei, X. Guan, Z. Liang, L. Lan, X. Lan, P. Liu, H. Mo and P. Lan, J. Hazard. Mater., 2022, 423, 127137 CrossRef CAS PubMed.
- Y. Meng, J. Yin, T. Jiao, J. Bai, L. Zhang, J. Su, S. Liu, Z. Bai, M. Cao and Q. Peng, J. Mol. Liq., 2020, 298, 112010 CrossRef CAS.
- M. Liao, H. Qin, W. Guo, P. Gao and H. Xiao, Ceram. Int., 2021, 47, 33667–33677 CrossRef CAS.
- Y. Zhang, S. Liu, T. Li, L. Zhang, U. Azhar, J. Ma, C. Zhai, C. Zong and S. Zhang, Carbohydr. Polym., 2020, 236, 116021 CrossRef CAS PubMed.
- A. L. Mohamed, A. A. Soliman, E. A. Ali, N. Y. Abou-Zeid and A. A. Nada, Int. J. Biol. Macromol., 2020, 163, 888–897 CrossRef CAS PubMed.
- J. F. Souza, G. P. Costa, R. Luque, D. Alves and A. R. Fajardo, Catal. Sci. Technol., 2019, 9, 136–145 RSC.
- H. Ozay, P. Ilgin and O. Ozay, Int. J. Hydrogen Energy, 2020, 45, 17613–17624 CrossRef CAS.
- D. George, P. U. Maheswari and K. M. S. Begum, Int. J. Biol. Macromol., 2019, 132, 784–794 CrossRef CAS PubMed.
- S. K. H Gulrez, S. Al-Assaf and G. O Phillips, Prog. Mol. Environ. Bioeng.: Anal. Model. Technol. Appl., 2011, 117150, 117–150 Search PubMed.
- E. M. Ahmed, J. Adv. Res., 2015, 6, 105–121 CrossRef CAS PubMed.
- D. Zhou, L. Zhang and S. Guo, Water Res., 2005, 39, 3755–3762 CrossRef CAS PubMed.
- B. Benguella and H. Benaissa, Water Res., 2002, 36, 2463–2474 CrossRef CAS PubMed.
- S. Wunder, F. Polzer, Y. Lu, Y. Mei and M. Ballauff, J. Phys. Chem. C, 2010, 114, 8814–8820 CrossRef CAS.
- P. Herves, M. Perez-Lorenzo, L. M. Liz-Marzan, J. Dzubiella, Y. Lu and M. Ballauff, Chem. Soc. Rev., 2012, 41, 5577–5587 RSC.
- A. Zinchenko, Y. Miwa, L. I. Lopatina, V. G. Sergeyev and S. Murata, ACS Appl. Mater. Interfaces, 2014, 6, 3226–3232 CrossRef CAS PubMed.
- A. Lim Teik Zheng, T. Phromsatit, S. Boonyuen and Y. Andou, FlatChem, 2020, 23, 100174 CrossRef CAS.
- K. Akhtar, S. B. Khan, E. M. Bakhsh, T. Kamal, S. Ahmad, A. M. Asiri and Y. Anwar, Int. J. Biol. Macromol., 2022, 208, 56–69 CrossRef CAS PubMed.
- L. Ge, M. Zhang, R. Wang, N. Li, L. Zhang, S. Liu and T. Jiao, RSC Adv., 2020, 10, 15091–15097 RSC.
- T. Kamal, S. B. Khan and A. M. Asiri, Environ. Pollut., 2016, 218, 625–633 CrossRef CAS PubMed.
- A. Khalil, N. Ali, A. Khan, A. M. Asiri and T. Kamal, Int. J. Biol. Macromol., 2020, 164, 2922–2930 CrossRef CAS PubMed.
- X. Li, F. Dong, L. Zhang, Q. Xu, X. Zhu, S. Liang, L. Hu and H. Xie, Chem. Eng. J., 2019, 372, 516–525 CrossRef CAS.
- J. Li, C.-y. Liu and Y. Liu, J. Mater. Chem., 2012, 22, 8426–8430 RSC.
- N. Sahiner, H. Ozay, O. Ozay and N. Aktas, Appl. Catal., A, 2010, 385, 201–207 CrossRef CAS.
- N. Jabeen, Z. H. Farooqi, A. Shah, A. Ali, M. Khurram, K. Mahmood, N. Sahiner and M. Ajmal, J. Porous Mater., 2021, 28, 1563–1576 CrossRef CAS.
- J. Dhiman, S. O. Prasher, E. ElSayed, R. M. Patel, C. Nzediegwu and A. Mawof, J. Cleaner Prod., 2021, 311, 127644 CrossRef CAS.
- D. Berillo and A. Cundy, Carbohydr. Polym., 2018, 192, 166–175 CrossRef CAS PubMed.
- N. Alhokbany, T. Ahama, Ruksana, M. Naushad and S. M. Alshehri, Composites, Part B, 2019, 173, 106950 CrossRef CAS.
- Z. Khorsandi, A. R. Hajipour, M. R. Sarfjoo and R. S. Varma, Green Chem., 2021, 23, 5222–5229 RSC.
- H.-C. Li, W.-J. Liu, H.-X. Han and H.-Q. Yu, J. Mater. Chem. A, 2016, 4, 11680–11687 RSC.
- N. Ali, T. Kamal, M. Ul-Islam, A. Khan, S. J. Shah and A. Zada, Int. J. Biol. Macromol., 2018, 111, 832–838 CrossRef CAS PubMed.
- M. Mifsud, K. V. Parkhomenko, I. W. Arends and R. A. Sheldon, Tetrahedron, 2010, 66, 1040–1044 CrossRef CAS.
- J. Zhong, Q. Wang, J. Zhou, D. Chen and Z. Ji, Appl. Surf. Sci., 2016, 367, 342–346 CrossRef CAS.
- C. Zhou, J. Bai, Y. Zhang, J. Li, Z. Li, P. Jiang, F. Fang, M. Zhou, X. Mei and B. Zhou, J. Hazard. Mater., 2021, 401, 123232 CrossRef CAS PubMed.
- M. Noman, M. Shahid, T. Ahmed, M. B. K. Niazi, S. Hussain, F. Song and I. Manzoor, Environ. Pollut., 2020, 257, 113514 CrossRef CAS PubMed.
- M. I. Din, R. Khalid, Z. Hussain, T. Hussain, A. Mujahid, J. Najeeb and F. Izhar, Crit. Rev. Anal. Chem., 2020, 50, 322–338 CrossRef CAS PubMed.
- R. Kottappara, S. C. Pillai and B. K. Vijayan, Inorg. Chem. Commun., 2020, 121, 108181 CrossRef CAS.
- A. J. Kora and L. Rastogi, Ind. Crops Prod., 2016, 81, 1–10 CrossRef CAS.
- N. Sahiner, S. Butun and P. Ilgin, Colloids Surf., A, 2011, 386, 16–24 CrossRef CAS.
- N. Sahiner and O. Ozay, Curr. Nanosci., 2012, 8, 367–374 CrossRef CAS.
- N. Sahiner and S. Sagbas, Colloids Surf., A, 2013, 418, 76–83 CrossRef CAS.
- J. Paul Guin, Y. K. Bhardwaj and L. Varshney, J. Appl. Polym. Sci., 2018, 135, 46200–46212 CrossRef.
- F. Naseer, M. Ajmal, F. Bibi, Z. H. Farooqi and M. Siddiq, Polym. Compos., 2018, 39, 3187–3198 CrossRef CAS.
- R. Su, S. Ge, H. Li, Y. Su, Q. Li, W. Zhou, B. Gao and Q. Yue, Sci. Total Environ., 2019, 693, 133657 CrossRef CAS PubMed.
- Y. Y. Li Sip, D. W. Fox, L. R. Shultz, M. Davy, H.-S. Chung, D.-X. Antony, Y. Jung, T. Jurca and L. Zhai, ACS Appl. Nano Mater., 2021, 4, 6045–6056 CrossRef CAS.
- S. Haider, T. Kamal, S. B. Khan, M. Omer, A. Haider, F. U. Khan and A. M. Asiri, Appl. Surf. Sci., 2016, 387, 1154–1161 CrossRef CAS.
- C. Saldías, D. D. Díaz, S. Bonardd, C. Soto-Marfull, A. Cordoba, S. Saldías, C. Quezada, D. Radic and Á. Leiva, Carbohydr. Polym., 2018, 180, 200–208 CrossRef PubMed.
- S. Sharma, Deepak, A. Kumar, S. Afgan and R. Kumar, ChemistrySelect, 2017, 2, 11281–11287 CrossRef CAS.
- S. Wang, Z. Xiao, X. Ma, Z. Zhao, D. Guo, Y. Chen, S. Zhai, Q. An and D. Yang, Appl. Catal., A, 2018, 568, 105–113 CrossRef CAS.
- S. Ahmad, S. B. Khan, A. M. Asiri, H. M. Marwani and T. Kamal, J. Sol-Gel Sci. Technol., 2020, 96, 382–394 CrossRef CAS.
- S. B. Khan, S. Ahmad, T. Kamal, A. M. Asiri and E. M. Bakhsh, Int. J. Biol. Macromol., 2020, 164, 1087–1098 CrossRef CAS PubMed.
- Z. Zhao, Z. Xiao, C. Qin, H. Lv, L. Qin, W. Niu, S. Zhai and Q. An, Ind. Crops Prod., 2021, 164, 113413 CrossRef CAS.
- A. L. T. Zheng, S. Sabidi, T. Ohno, T. Maeda and Y. Andou, Chemosphere, 2022, 286, 131731 CrossRef CAS PubMed.
- M. Ajmal, S. Demirci, M. Siddiq, N. Aktas and N. Sahiner, New J. Chem., 2016, 40, 1485–1496 RSC.
- Y. Sun, F. Zhang, L. Xu, Z. Yin and X. Song, J. Mater. Chem. A, 2014, 2, 18583–18592 RSC.
- L. V. Lombardo Lupano, J. M. Lázaro Martínez, L. L. Piehl, E. Rubín de Celis, R. M. Torres Sánchez and V. Campo Dall’ Orto, Langmuir, 2014, 30, 2903–2913 CrossRef CAS PubMed.
- N. Sahiner, F. Seven and H. Al-lohedan, Water, Air, Soil Pollut., 2015, 226, 122–133 CrossRef.
- S. Demirci and N. Sahiner, Water, Air, Soil Pollut., 2015, 226, 64–76 CrossRef.
- A. Zinchenko, Y. Che, S. Taniguchi, L. I. Lopatina, V. G. Sergeyev and S. Murata, J. Nanopart. Res., 2016, 18, 179–187 CrossRef.
- J. Liu, D. Su, J. Yao, Y. Huang, Z. Shao and X. Chen, J. Mater. Chem. A, 2017, 5, 4163–4171 RSC.
- J. Ding, Q. Li, L. Zhao, X. Li, Q. Yue and B. Gao, RSC Adv., 2017, 7, 17599–17611 RSC.
- R. Su, Q. Li, R. Huang, L. Zhao, Q. Yue, B. Gao and Y. Chen, J. Taiwan Inst. Chem. Eng., 2018, 91, 235–242 CrossRef CAS.
- J. Ding, Q. Li, X. Xu, X. Zhang, Y. Su, Q. Yue and B. Gao, Carbohydr. Polym., 2018, 190, 12–22 CrossRef CAS PubMed.
- R. Su, F. Wang, J. Ding, Q. Li, W. Zhou, Y. Liu, B. Gao and Q. Yue, Carbohydr. Polym., 2019, 220, 202–210 CrossRef CAS PubMed.
- T. Kamal, Polym. Test., 2019, 77, 105896–105903 CrossRef CAS.
- A. Upadhyay, A. Narula and C. P. Rao, ACS Appl. Bio Mater., 2020, 3, 8619–8626 CrossRef CAS PubMed.
- L. Qin, R. Ru, J. Mao, Q. Meng, Z. Fan, X. Li and G. Zhang, Appl. Catal., B, 2020, 269, 118754 CrossRef CAS.
- P. Xu, C. Cen, M. Zheng, Y. Wang, Z. Wu and Z. Teng, Mater. Chem. Phys., 2020, 253, 123444 CrossRef CAS.
- A. Intanin, P. Inpota, T. Chutimasakul, J. Tantirungrotechai, P. Wilairat and R. Chantiwas, Molecules, 2020, 25, 1798 CrossRef CAS PubMed.
- Y. Chen, R. Su, F. Wang, W. Zhou, B. Gao, Q. Yue and Q. Li, Chemosphere, 2021, 270, 129295 CrossRef CAS PubMed.
- W. Peng, Y. Yan, D. Zhang, Y. Zhou, D. Na, C. Xiao, C. Yang, G. Wen and J. Zhang, Colloids Surf., A, 2021, 624, 126809 CrossRef CAS.
- Z. Zhang, T. Sun, C. Chen, F. Xiao, Z. Gong and S. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 21035–21040 CrossRef CAS PubMed.
- J. Ji, Y. Li, W. Fu, Z. Cui, J. Shen and M. Ye, J. Colloid Interface Sci., 2017, 505, 983–994 CrossRef CAS PubMed.
- Y. Feng, H. Zhang, B. Xin and J. Wu, J. Colloid Interface Sci., 2017, 506, 154–161 CrossRef CAS PubMed.
- T. Yao, W. Jia, X. Tong, Y. Feng, Y. Qi, X. Zhang and J. Wu, J. Colloid Interface Sci., 2018, 527, 214–221 CrossRef CAS PubMed.
- D. Varade and K. Haraguchi, eXPRESS Polym. Lett., 2018, 12, 996–1004 CrossRef CAS.
- Y. Feng, J. Yin, S. Liu, Y. Wang, B. Li and T. Jiao, ACS Omega, 2020, 5, 3725–3733 CrossRef CAS PubMed.
- K.-K. Kang, K. Shim and C.-S. Lee, Colloids Surf., A, 2020, 593, 124607 CrossRef CAS.
- R. Wang, M. Zhang, B. Ge, L. Zhang, J. Zhou, S. Liu and T. Jiao, J. Mol. Liq., 2020, 310, 113083 CrossRef CAS.
- M. Xia, S.-M. Kang, G.-W. Lee, Y. S. Huh and B. J. Park, J. Ind. Eng. Chem., 2019, 73, 306–315 CrossRef CAS.
- J. Zhu, X. Zhang, Z. Qin, L. Zhang, Y. Ye, M. Cao, L. Gao and T. Jiao, Colloids Surf., A, 2021, 611, 125889 CrossRef CAS.
- H. Liu, Y.-n. Qin, H.-y. Li, L.-x. Gai, Q.-d. An, S.-r. Zhai, Z.-y. Xiao and L. Cui, Int. J. Biol. Macromol., 2021, 173, 160–167 CrossRef CAS PubMed.
- C. Yang, C.-H. Choi, C.-S. Lee and H. Yi, ACS Nano, 2013, 7, 5032–5044 CrossRef CAS PubMed.
- P. Eghbali, B. Nişancı and Ö. Metin, Pure Appl. Chem., 2018, 90, 327–335 CrossRef CAS.
- I. Sadeghi, E. Y. Liu, H. Yi and A. Asatekin, ACS Appl. Nano Mater., 2019, 2, 5233–5244 CrossRef CAS.
- L. Minati, G. Speranza, V. Micheli, M. Dalla Serra and M. Clamer, Dalton Trans., 2020, 49, 3333–3340 RSC.
- B. Wang, M. Ran, G. Fang, T. Wu, Q. Tian, L. Zheng, L. Romero-Zerón and Y. Ni, Cellulose, 2020, 27, 6995–7008 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |