Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effects of the reaction temperature and Ba/Ti precursor ratio on the crystallite size of BaTiO3 in hydrothermal synthesis†
Min Zhang a,
Joseph Falvey
a,
Joseph Falvey a,
Andrew L. Hector
a,
Andrew L. Hector *a and
Nuria Garcia-Araez
*a and
Nuria Garcia-Araez ab
ab
aSchool of Chemistry, University of Southampton, Highfield, Southampton SO17 1BJ, UK. E-mail: A.L.Hector@soton.ac.uk
bThe Faraday Institution, Quad One, Harwell Campus, Didcot OX11 0RA, UK
First published on 29th September 2022
Abstract
Nanocrystalline BaTiO3 has been prepared by hydrothermal synthesis from titanium isopropoxide and barium hydroxide octahydrate. Reaction conditions including synthesis temperature and Ba/Ti precursor ratio have been explored with the aim of producing BaTiO3 with small crystallites and a low concentration of defects. It has been found that the crystallite/particle size and tetragonality of the BaTiO3 samples increase as the synthesis temperature increases; and the crystallite/particle size of BaTiO3 is also affected by the Ba/Ti precursor ratio. The BaTiO3 sample synthesised using a Ba/Ti precursor ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at a reaction temperature of 120 °C exhibited homogeneous crystallites of the smallest size of 107 nm. Additionally, the Ba/Ti precursor ratio of 2
1 at a reaction temperature of 120 °C exhibited homogeneous crystallites of the smallest size of 107 nm. Additionally, the Ba/Ti precursor ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with synthesis temperature of 220 °C was found to produce a smaller concentration of defects in BaTiO3.
1 with synthesis temperature of 220 °C was found to produce a smaller concentration of defects in BaTiO3.
1. Introduction
Barium titanate (BaTiO3) is commonly used in multilayer capacitors, thermistors, transducers, electromechanical devices, electro-optical devices, dynamic random-access memory, field-effect transistors and actuators, etc., due to its excellent dielectric and ferroelectric properties.1 BaTiO3 is both mechanically and chemically stable, and exhibits different crystalline structures depending on the temperature.2,3 Above 120 °C, BaTiO3 exhibits the classical perovskite structure with Ba2+ at the corners of the cubic unit cell, O2− at the face centres and Ti4+ at the body centre; this structure is cubic and centrosymmetric and, therefore, the material does not exhibit a net dipole moment and hence behaves as a dielectric. Below 120 °C, BaTiO3 experiences a structural distortion: Ti4+ is displaced off its central position in the octahedron, in the direction of one of the O2−; this tetragonal distortion in the structure generates a net dipole moment, and hence the material behaves as a ferroelectric. BaTiO3 exhibits a very high dielectric constant, particularly at temperatures close to the ferroelectric–dielectric transition.The overriding goal in BaTiO3 synthesis in recent years is to create smaller and more uniform particles to allow for thinner layers of the ceramic material to be used in electronic device components such as capacitors.4 Therefore it is important to synthesise BaTiO3 crystallites of the smallest size possible. Manufacturers of multilayer capacitors use BaTiO3 because of its excellent dielectric properties, reliability, high breakdown voltage and low cost of production.5 Other uses for BaTiO3 in the electronics industry include temperature sensing (temperature trips), overcurrent protection (self-resetting fuses) and electronic timing circuitry. These devices use the positive temperature coefficient of resistivity (PTCR) property in BaTiO3 materials to control the resistance in a circuit as a function of temperature in the form of thermistors.6,7 This PTCR effect has also recently been shown to be able to prevent thermal runaway in batteries.8
There are multiple synthesis methods used in industry to produce BaTiO3, however manufacturers of multilayer capacitors and PTCR devices have specific requirements for the ceramic material if it is to be used in the preparation of dielectric layers. The method must produce a high purity product with homogeneous composition to ensure stable and consistent dielectric properties. A route that causes strong agglomeration will result in a non-uniform particle size, which is problematic. For the production of multilayer capacitors, in general, solution-based techniques such as hydrothermal synthesis are used to prepare very fine particles, as these techniques achieve a homogeneous, phase-pure stoichiometric BaTiO3 with synthesis temperatures far lower than solid state methods (>600 °C).5,9 Nanosized particles of BaTiO3 can be achieved by controlling reaction conditions such as synthesis temperature and time, Ba and Ti precursor categories and Ba/Ti precursor ratio.4 Zeng et al. prepared nanocrystalline BaTiO3 from barium hydroxide octahydrate and titanium isopropoxide at low temperature (<100 °C) and ambient pressure, and reported the impact of the reaction temperature and Ba/Ti precursor ratio on the morphology and size of BaTiO3.10,11 Then, Nahm et al. used the same precursors and hydrothermal conditions for the synthesis of nanocrystalline BaTiO3, thus producing a one-step reaction process with faster reaction times, but they only employed one temperature (220 °C) and Ba/Ti precursor ratio (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).12 In this work, we employ the same precursors and hydrothermal conditions for the synthesis of nanocrystalline BaTiO3, but we systematically explore the effect of the reaction temperature (from 80 to 220 °C) and Ba/Ti precursor ratio (from 4
1).12 In this work, we employ the same precursors and hydrothermal conditions for the synthesis of nanocrystalline BaTiO3, but we systematically explore the effect of the reaction temperature (from 80 to 220 °C) and Ba/Ti precursor ratio (from 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and we characterise the reaction product to identify the optimal reaction conditions in terms of small particle size and low concentration of defects.
1), and we characterise the reaction product to identify the optimal reaction conditions in terms of small particle size and low concentration of defects.
2. Experimental
2.1. Materials synthesis
The BaTiO3 samples were synthesized via a hydrothermal method,12 as illustrated in Scheme 1. As a Ti precursor, the Ti(OCH(CH3)2)4 (≥97% purity, Sigma Aldrich) was added dropwise to 15 mL of ice cold distilled water while being constantly stirred. Upon addition of the Ti(OCH(CH3)2)4, the distilled water became turbid then a white precipitate formed. The reaction mixture was stirred in ice bath for 1 h then at room temperature for a further 2 h to allow the reaction to reach completion. To make up the Ba precursor, an appropriate amount of Ba(OH)2·8H2O (≥98% purity, Emsure) was dissolved in 55 mL of distilled water. The exact concentration of each precursor used is specific to the reaction parameter being investigated and is specified in the results and discussion section. The Ba precursor solution was added to the Ti precursor suspension to make up the reaction mixture. The reaction mixture was then transferred to an autoclave where it was sealed and heated. Each reaction mixture was heated at a specific temperature in an oven for 16 h. The white solid that formed in the reaction was collected and washed in a centrifuge at 4500 rpm for 8 min. The solid was washed with diluted HCl once, then with distilled water 3 times. After that, the solid was dried at 80 °C for 12 h in a vacuum oven to obtain BaTiO3 samples.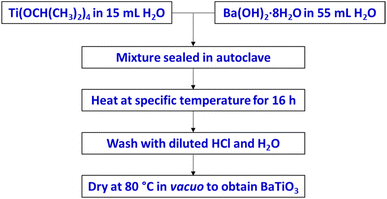 | ||
| Scheme 1 Hydrothermal synthesis to prepare BaTiO3, using various Ba/Ti precursor ratios/concentrations and reaction temperatures. | ||
2.2. Materials characterisation
X-ray diffraction (XRD) spectra were collected using a Bruker D2 Phaser with Cu-Kα radiation. Rietveld analysis was performed using the GSAS package.13 The crystallite sizes were calculated from the Lorentzian broadening components collected from the Rietveld fits to the XRD patterns, according to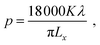 where p is the crystallite size (unit: nm), K is the Scherrer constant, λ is the wavelength of the incident X-rays (unit: nm), and Lx is the refined Lorentzian value.13 Scanning electron microscopy (SEM) micrographs were collected on a JEOL JSM-6500F operated at 10 kV, and images were analysed using the ImageJ software and Gaussian function. Transmission electron microscopy (TEM) used a Hitachi HT7700 (120 kV). Raman spectra were recorded using a Renishaw inVia confocal microscope, and the spectra were analysed using the WiRE 4.1 software. Dynamic light scattering (DLS) measurements were carried out with a Malvern Zetasizer Nano ZS, and three sequential measurements were performed for each sample. Wavelength dispersive X-ray analysis (WDS) used a Thermofisher Magnaray detector with Noran System 7 processing. Thermogravimetric analysis (TGA) was carried out using a NETZSCH TG 209 F1 Libra instrument, with a heating process of at 1 °C min−1 from 25 to 200 °C, then at 10 °C min−1 from 200 to 1000 °C under Ar atmosphere.
where p is the crystallite size (unit: nm), K is the Scherrer constant, λ is the wavelength of the incident X-rays (unit: nm), and Lx is the refined Lorentzian value.13 Scanning electron microscopy (SEM) micrographs were collected on a JEOL JSM-6500F operated at 10 kV, and images were analysed using the ImageJ software and Gaussian function. Transmission electron microscopy (TEM) used a Hitachi HT7700 (120 kV). Raman spectra were recorded using a Renishaw inVia confocal microscope, and the spectra were analysed using the WiRE 4.1 software. Dynamic light scattering (DLS) measurements were carried out with a Malvern Zetasizer Nano ZS, and three sequential measurements were performed for each sample. Wavelength dispersive X-ray analysis (WDS) used a Thermofisher Magnaray detector with Noran System 7 processing. Thermogravimetric analysis (TGA) was carried out using a NETZSCH TG 209 F1 Libra instrument, with a heating process of at 1 °C min−1 from 25 to 200 °C, then at 10 °C min−1 from 200 to 1000 °C under Ar atmosphere.
3. Results and discussion
3.1. Effects of the reaction temperature on the crystallite size, morphology and composition of BaTiO3 in hydrothermal synthesis
Table 1 summarises the experimental conditions that successfully led to the production of BaTiO3. Using a constant Ti precursor concentration of 0.045 mol L−1 and the Ba precursor concentration was varied from 0.045 to 0.18 mol L−1, thus Ba/Ti precursor ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 have been achieved. These three reaction mixtures were heated at various temperatures of 80, 120, 150, 180 and 220 °C to obtain different BaTiO3 samples.
1 have been achieved. These three reaction mixtures were heated at various temperatures of 80, 120, 150, 180 and 220 °C to obtain different BaTiO3 samples.
| Sample | Ba precursor concentration (mol L−1) | Ba/Ti precursor ratio | Synthesis temperature (°C) | Crystallite size (nm) |
|---|---|---|---|---|
a Note that samples synthesized using Ba/Ti precursor ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 80, 120 and 150 °C (as well as using 2 1 at 80, 120 and 150 °C (as well as using 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio at 80 °C) had very low yields, thus no further investigation proceeded. 1 ratio at 80 °C) had very low yields, thus no further investigation proceeded. |
||||
BTO 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 80 °C 1 – 80 °C |
0.18 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 | 128 (5) |
BTO 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 120 °C 1 – 120 °C |
120 | 202 (8) | ||
BTO 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 150 °C 1 – 150 °C |
150 | 218 (7) | ||
BTO 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 180 °C 1 – 180 °C |
180 | 230 (12) | ||
BTO 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 220 °C 1 – 220 °C |
220 | 355 (15) | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
BTO 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 120 °C 1 – 120 °C |
0.09 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
120 | 107 (4) |
BTO 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 150 °C 1 – 150 °C |
150 | 246 (9) | ||
BTO 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 180 °C 1 – 180 °C |
180 | 251 (10) | ||
BTO 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 220 °C 1 – 220 °C |
220 | 348 (15) | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
BTO 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 180 °C 1 – 180 °C |
0.045 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
180 | 334 (15) |
BTO 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 220 °C 1 – 220 °C |
220 | 371 (19) | ||
The XRD patterns of the obtained BaTiO3 samples (Fig. 1a) indicate that phase-pure BaTiO3 was obtained in all cases, since no impurity phases were detected. Habib et al. detected the formation of a BaCO3 impurity for BaTiO3 synthesised hydrothermally, since a BaCO3 peak was observed at a 2θ value of approximately 24°.14 BaCO3 forms in the heating step in a reaction between Ba2+ ions in the solution and any CO2 that may be in the air space above the reaction mixture. In our work, the BaTiO3 samples were washed with diluted HCl, which would remove BaCO3 impurities, and indeed, the XRD data do not show the presence of any BaCO3. The XRD results also do not show the presence of any unreacted rutile or anatase TiO2. While some previous work employed TiO2 as precursor for the BaTiO3 hydrothermal synthesis,14,15 in this work, a metal–organic [Ti(OCH(CH3)2)4] with higher reactivity is used as Ti precursor. This precipitates as amorphous TiO2 (Fig. S1†) in water improving the likelihood that it will fully react with the Ba precursor in hydrothermal synthesis, such that any unreacted TiO2 may not be observed.
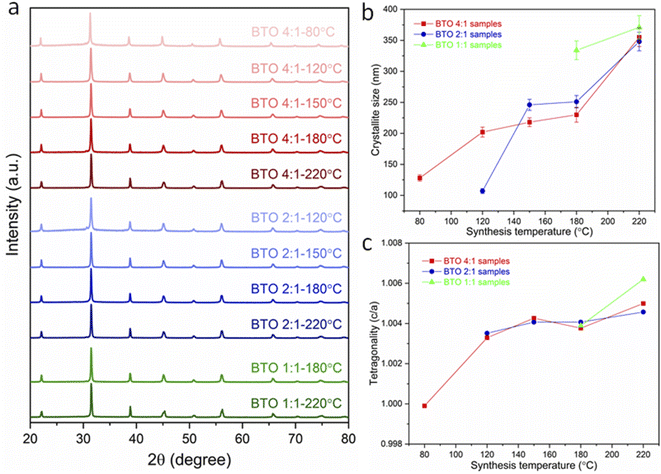 | ||
Fig. 1 (a) XRD patterns of BaTiO3 samples hydrothermally synthesised with Ba/Ti precursor ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1 1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, at various reaction temperatures of 80, 120, 150, 180 and 220 °C; (b) crystallite size and (c) tetragonality (c/a) of BaTiO3 samples plotted against reaction temperature in hydrothermal synthesis using Ba/Ti precursor ratios of 4 1, at various reaction temperatures of 80, 120, 150, 180 and 220 °C; (b) crystallite size and (c) tetragonality (c/a) of BaTiO3 samples plotted against reaction temperature in hydrothermal synthesis using Ba/Ti precursor ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1 1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, obtained from the Rietveld fits to the XRD patterns (sample labels explained in Table 1). 1, obtained from the Rietveld fits to the XRD patterns (sample labels explained in Table 1). | ||
From the Lorentzian values collected from the Rietveld fits (Fig. S2†) to the XRD patterns, the crystallite sizes of all samples have been calculated and presented in Table 1. The obtained lattice parameters (a and c), tetragonality (c/a), Lorentzian values (Lx and Ly) and reliability factors (Rwp and Rp) are listed in Table S1.† Fig. 1b shows the variation of the crystallite size of BaTiO3 versus the reaction temperature in hydrothermal synthesis with Ba/Ti precursor ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In all three Ba/Ti precursor ratios, there is a general decrease in crystallite size with decreasing synthesis temperature. Using a 4
1. In all three Ba/Ti precursor ratios, there is a general decrease in crystallite size with decreasing synthesis temperature. Using a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ba/Ti precursor ratio, the crystallite size of the obtained BaTiO3 reduces from 355 nm at 220 °C to 128 nm at 80 °C. At a 2
1 Ba/Ti precursor ratio, the crystallite size of the obtained BaTiO3 reduces from 355 nm at 220 °C to 128 nm at 80 °C. At a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio the crystallite size falls from 348 nm at 220 °C to 107 nm at 120 °C. The 1
1 ratio the crystallite size falls from 348 nm at 220 °C to 107 nm at 120 °C. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio shows a crystallite size that drops from 371 nm at 220 °C to 334 nm at 180 °C. The synthesis temperature has been found to have a profound effect on the crystallite size of the BaTiO3 samples with a lower synthesis temperature producing crystallites of a smaller size. The strong growth of BaTiO3 crystallites to their final size is typical of an Ostwald ripening process which involves the growth of large particles at the expense of smaller particles. An accelerated Ostwald ripening process with increasing temperature is likely to be the cause of the increase in crystallite size.15–17 BaTiO3 with crystallites as small as 107 nm have been produced using a 2
1 ratio shows a crystallite size that drops from 371 nm at 220 °C to 334 nm at 180 °C. The synthesis temperature has been found to have a profound effect on the crystallite size of the BaTiO3 samples with a lower synthesis temperature producing crystallites of a smaller size. The strong growth of BaTiO3 crystallites to their final size is typical of an Ostwald ripening process which involves the growth of large particles at the expense of smaller particles. An accelerated Ostwald ripening process with increasing temperature is likely to be the cause of the increase in crystallite size.15–17 BaTiO3 with crystallites as small as 107 nm have been produced using a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ba/Ti precursor ratio and reacted at 120 °C.
1 Ba/Ti precursor ratio and reacted at 120 °C.
The tetragonality (c/a) has been used to denote the degree of tetragonal–cubic distortion,18 and BaTiO3 samples with large tetragonality are essential to obtain multilayer capacitors with large capacitances.12 Fig. 1c presents the tetragonality variation of BaTiO3 against the reaction temperature in hydrothermal synthesis with Ba/Ti precursor ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The increasing c/a value suggests that the tetragonality of the crystal structure increases with the increasing synthesis temperature in all three Ba/Ti precursor ratios. In general, the tetragonality of the BaTiO3 is reduced by the presence of hydroxyl defects in the product.19 Therefore, the BaTiO3 samples synthesised at higher temperatures are considered to contain less hydroxyl defects. This can be confirmed by the TGA characterisation presented below.
1. The increasing c/a value suggests that the tetragonality of the crystal structure increases with the increasing synthesis temperature in all three Ba/Ti precursor ratios. In general, the tetragonality of the BaTiO3 is reduced by the presence of hydroxyl defects in the product.19 Therefore, the BaTiO3 samples synthesised at higher temperatures are considered to contain less hydroxyl defects. This can be confirmed by the TGA characterisation presented below.
The variations in tetragonality of hydrothermally synthesized BaTiO3 samples can also be confirmed by Raman characterisation. As shown in Fig. 2a, the Raman spectrum of tetragonal BaTiO3 presents the Raman active modes A1 (TO), E (TO) at ∼180 cm−1, A1 (TO) at ∼270 cm−1, B1, E (LO + TO) at ∼307 cm−1, A1 (TO), E (TO) at ∼515 cm−1 and A1 (LO), E (LO) at ∼720 cm−1.18 It has been reported that the sharp peak at ∼307 cm−1 (shaded in Fig. 2a) is particularly sensitive to the tetragonality of the crystal structure, with its intensity increasing when BaTiO3 becomes more tetragonal.20–22 Fig. 2b shows the variation of the relative integrated intensity of the peak at 307 cm−1 for BaTiO3 versus the reaction temperature in hydrothermal synthesis with Ba/Ti precursor ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The increasing integrated intensity of the peak at 307 cm−1 suggests that the tetragonality of the crystal structure increases with the increasing synthesis temperature in all three Ba/Ti precursor ratios. This trend is consistent with the results obtained from the XRD Rietveld analysis.
1. The increasing integrated intensity of the peak at 307 cm−1 suggests that the tetragonality of the crystal structure increases with the increasing synthesis temperature in all three Ba/Ti precursor ratios. This trend is consistent with the results obtained from the XRD Rietveld analysis.
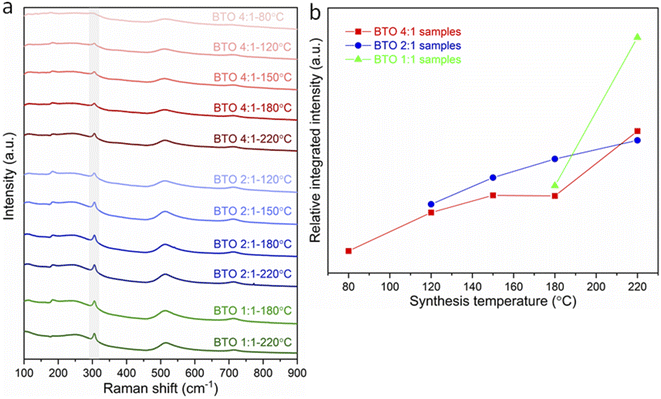 | ||
Fig. 2 (a) Raman spectra of BaTiO3 samples hydrothermally synthesised with Ba/Ti precursor ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1 1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, at various reaction temperatures of 80, 120, 150, 180 and 220 °C (peaks at ∼307 cm−1 being marked as shaded); (b) relative integrated intensities of the Raman peak at 307 cm−1 for BaTiO3 samples plotted against reaction temperature in hydrothermal synthesis using Ba/Ti precursor ratios of 4 1, at various reaction temperatures of 80, 120, 150, 180 and 220 °C (peaks at ∼307 cm−1 being marked as shaded); (b) relative integrated intensities of the Raman peak at 307 cm−1 for BaTiO3 samples plotted against reaction temperature in hydrothermal synthesis using Ba/Ti precursor ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1 1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (sample labels explained in Table 1). 1 (sample labels explained in Table 1). | ||
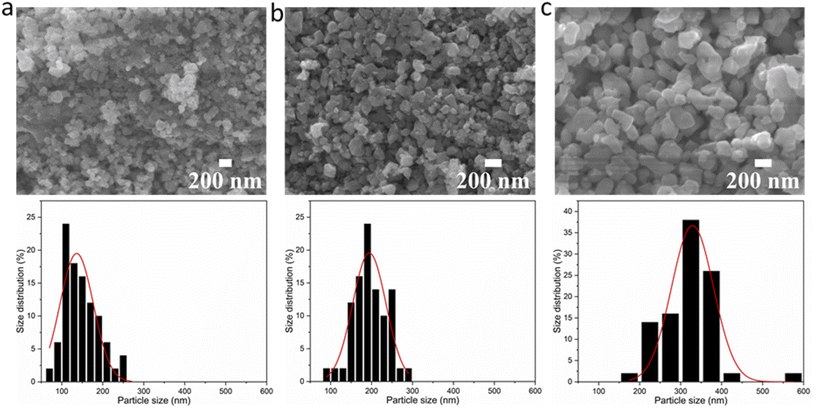
To investigate any trends between the size and morphology of individual particles and the reaction temperature, Fig. 3 shows the SEM images and particle size distributions of BaTiO3 samples synthesised using a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ba/Ti precursor ratio with reaction temperatures of 80, 150 and 220 °C in hydrothermal synthesis. The particle sizes of BaTiO3 samples were determined from analysing SEM images using the ImageJ software, and the size distributions were obtained by fitting with a Gaussian function. The BaTiO3 sample synthesised at 80 °C shows the smallest average particle size of 136 nm with ill-defined morphology, and the small particles agglomerated into clusters. As the synthesis temperature increases to 150 and 220 °C, the average particle size increases to 194 and 329 nm, respectively, and the edges of particles become well-defined. This can be confirmed by the DLS and TEM characterisations shown in Fig. 4. The average particle sizes obtained from the DLS measurements are 176, 203 and 335 nm for BaTiO3 samples synthesised at 80, 150 and 220 °C, respectively. Note that the smaller particles are more likely to agglomerate into clusters, thus the samples with larger particle sizes gave better accuracy during DLS measurements. According to the SEM, TEM and DLS characterisations, it can be concluded that the BaTiO3 particle size increases as the synthesis temperature is increased. This trend agrees with the result obtained from the Rietveld fits to the XRD data.
1 Ba/Ti precursor ratio with reaction temperatures of 80, 150 and 220 °C in hydrothermal synthesis. The particle sizes of BaTiO3 samples were determined from analysing SEM images using the ImageJ software, and the size distributions were obtained by fitting with a Gaussian function. The BaTiO3 sample synthesised at 80 °C shows the smallest average particle size of 136 nm with ill-defined morphology, and the small particles agglomerated into clusters. As the synthesis temperature increases to 150 and 220 °C, the average particle size increases to 194 and 329 nm, respectively, and the edges of particles become well-defined. This can be confirmed by the DLS and TEM characterisations shown in Fig. 4. The average particle sizes obtained from the DLS measurements are 176, 203 and 335 nm for BaTiO3 samples synthesised at 80, 150 and 220 °C, respectively. Note that the smaller particles are more likely to agglomerate into clusters, thus the samples with larger particle sizes gave better accuracy during DLS measurements. According to the SEM, TEM and DLS characterisations, it can be concluded that the BaTiO3 particle size increases as the synthesis temperature is increased. This trend agrees with the result obtained from the Rietveld fits to the XRD data.
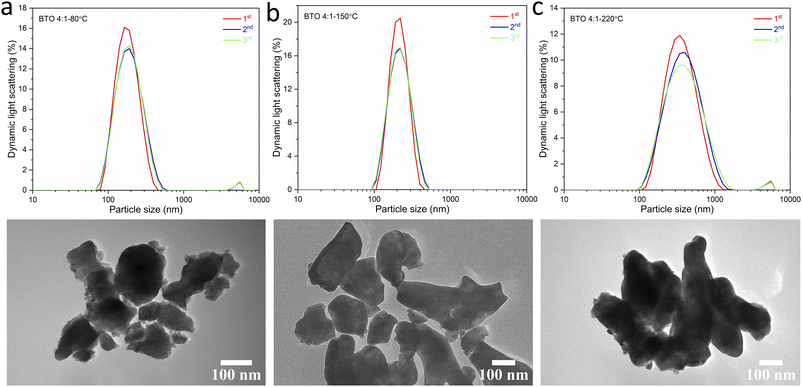 | ||
Fig. 4 DLS patterns (above) and TEM images (below) of BaTiO3 samples hydrothermally synthesised using a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ba/Ti precursor ratio with reaction temperatures of (a) 80 °C, (b) 150 °C and (c) 220 °C. 1 Ba/Ti precursor ratio with reaction temperatures of (a) 80 °C, (b) 150 °C and (c) 220 °C. | ||
TGA was carried out for BaTiO3 samples hydrothermally synthesised using a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ba/Ti precursor ratio with reaction temperatures of 80, 120, 150, 180 and 220 °C. Fig. 5a and Table 2 show the mass losses of the BaTiO3 samples in the TGA characterisations as the temperature is raised from 25 to 1000 °C in an inert Ar atmosphere. For the BaTiO3 samples that have been synthesised at 220, 180, 150, 120 and 80 °C, there are increasing mass losses of 0.3%, 0.6%, 1.6%, 1.8% and 3.4%, respectively, with the mass change attributed to loss of surface hydroxyl groups in the temperature range of 100–800 °C.23 The TGA analysis indicates that as the synthesis temperature of BaTiO3 decreases, there is a greater amount of mass lost when the BaTiO3 product is heated.
1 Ba/Ti precursor ratio with reaction temperatures of 80, 120, 150, 180 and 220 °C. Fig. 5a and Table 2 show the mass losses of the BaTiO3 samples in the TGA characterisations as the temperature is raised from 25 to 1000 °C in an inert Ar atmosphere. For the BaTiO3 samples that have been synthesised at 220, 180, 150, 120 and 80 °C, there are increasing mass losses of 0.3%, 0.6%, 1.6%, 1.8% and 3.4%, respectively, with the mass change attributed to loss of surface hydroxyl groups in the temperature range of 100–800 °C.23 The TGA analysis indicates that as the synthesis temperature of BaTiO3 decreases, there is a greater amount of mass lost when the BaTiO3 product is heated.
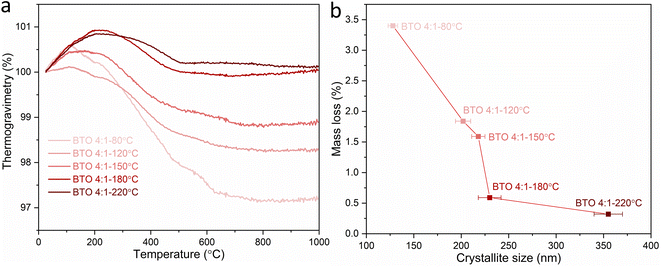 | ||
Fig. 5 (a) TGA curves and (b) mass losses against crystallite sizes of BaTiO3 samples hydrothermally synthesised using a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ba/Ti precursor ratio with reaction temperatures of 80, 120, 150, 180 and 220 °C, examined by heating in Ar with a heating process of at 1 °C min−1 from 25 to 200 °C, then at 10 °C min−1 from 200 to 1000 °C (sample labels explained in Table 1). 1 Ba/Ti precursor ratio with reaction temperatures of 80, 120, 150, 180 and 220 °C, examined by heating in Ar with a heating process of at 1 °C min−1 from 25 to 200 °C, then at 10 °C min−1 from 200 to 1000 °C (sample labels explained in Table 1). | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ba/Ti precursor ratio with reaction temperatures of 80, 120, 150, 180 and 220 °C, for the calculation of x in the form of
1 Ba/Ti precursor ratio with reaction temperatures of 80, 120, 150, 180 and 220 °C, for the calculation of x in the form of 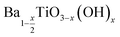
| Synthesis temperature (°C) | Mass loss (%) | Value of x |
|---|---|---|
| 220 | 0.3 | 0.08 |
| 180 | 0.6 | 0.15 |
| 150 | 1.6 | 0.37 |
| 120 | 1.8 | 0.41 |
| 80 | 3.4 | 0.70 |
The increase in surface hydroxyl defects can be linked to the increased surface area of crystallites when formed at lower temperatures.24 Table 2 shows the corrected stoichiometric formulas of the samples with the removal of hydroxyl defects in the form of 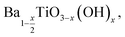 according to
according to 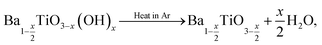 which exhibits increasing x values as the synthesis temperature decreases. The BaTiO3 samples synthesised using a 4
which exhibits increasing x values as the synthesis temperature decreases. The BaTiO3 samples synthesised using a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ba/Ti precursor ratio with decreasing reaction temperatures of 220, 180, 150, 120 and 80 °C exhibit decreasing crystallite sizes of 355, 230, 218, 202 and 128 nm, respectively. Decreasing the synthesis temperature results in a smaller crystallite size of BaTiO3 and a larger surface area resulting in a greater number of hydroxyl defects in the product, and therefore a greater mass loss when heated in the TGA characterisation, as shown in Fig. 5b.
1 Ba/Ti precursor ratio with decreasing reaction temperatures of 220, 180, 150, 120 and 80 °C exhibit decreasing crystallite sizes of 355, 230, 218, 202 and 128 nm, respectively. Decreasing the synthesis temperature results in a smaller crystallite size of BaTiO3 and a larger surface area resulting in a greater number of hydroxyl defects in the product, and therefore a greater mass loss when heated in the TGA characterisation, as shown in Fig. 5b.
3.2. Effects of the Ba/Ti precursor ratio/concentration on the crystallite size, morphology and composition of BaTiO3 in hydrothermal synthesis
As shown in Table 1, three different Ba/Ti precursor ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were employed in this work, and Fig. 1b shows that samples synthesised using Ba/Ti precursor ratios of 4
1) were employed in this work, and Fig. 1b shows that samples synthesised using Ba/Ti precursor ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 exhibit crystallites of smaller sizes, as obtained from the Rietveld fits to the XRD data, than those using a Ba/Ti precursor ratio of 1
1 exhibit crystallites of smaller sizes, as obtained from the Rietveld fits to the XRD data, than those using a Ba/Ti precursor ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
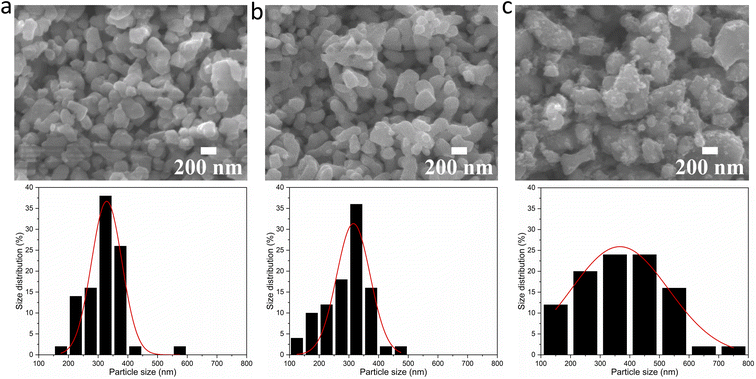
This result is supported by the SEM images of the BaTiO3 samples, and the particle size distributions obtained from the SEM images, as shown in Fig. 6. The BaTiO3 sample synthesised using Ba/Ti precursor ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, at 220 °C, exhibit average particle sizes of 329 and 315 nm, respectively. With the Ba/Ti precursor ratio of 1
1, at 220 °C, exhibit average particle sizes of 329 and 315 nm, respectively. With the Ba/Ti precursor ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the obtained BaTiO3 sample shows a larger average particle size of 367 nm and a wider size distribution. The particles are also less elliptical and much more irregular in shape with a more agglomerated structure. These results are corresponding with the results obtained from the DLS and TEM characterisations, as shown in Fig. 7. The average particle sizes obtained from the DLS measurements are 335, 324 and 379 nm for BaTiO3 samples synthesised using Ba/Ti precursor ratios of 4
1, the obtained BaTiO3 sample shows a larger average particle size of 367 nm and a wider size distribution. The particles are also less elliptical and much more irregular in shape with a more agglomerated structure. These results are corresponding with the results obtained from the DLS and TEM characterisations, as shown in Fig. 7. The average particle sizes obtained from the DLS measurements are 335, 324 and 379 nm for BaTiO3 samples synthesised using Ba/Ti precursor ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively.
1, respectively.
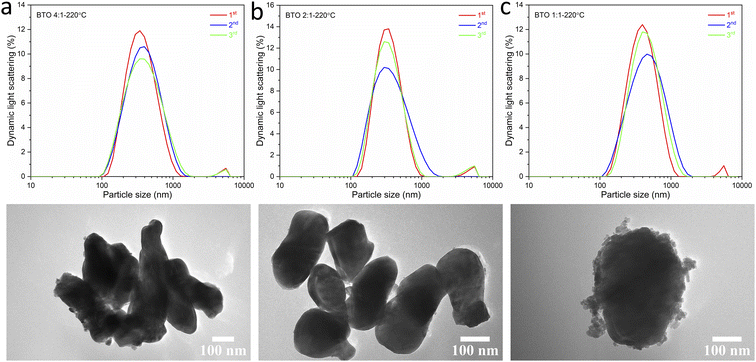 | ||
Fig. 7 DLS patterns (above) and TEM images (below) of BaTiO3 samples hydrothermally synthesised at 220 °C using Ba/Ti precursor ratios of (a) 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (b) 2 1, (b) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and (c) 1 1 and (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
The larger and more irregular particle size of BaTiO3 for the lower Ba/Ti precursor ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 can be understood by realising that the lower the concentration of Ba precursor in the initial reaction mixture will produce a lower number of nucleation points where the growth of BaTiO3 is initiated. Eckert et al. studied the mechanism for the hydrothermal formation of BaTiO3 from barium hydroxide octahydrate and anatase titania,25 and concluded that the growth of BaTiO3 particles is much slower than the formation of nuclei, however by reducing the concentration of Ba2+ in the reaction mixture, the rate of the particle growth could begin to compete with the rate of initial nuclei formation, leading to an uneven size distribution in the final BaTiO3 product, such as that seen in Fig. 6c.
1 can be understood by realising that the lower the concentration of Ba precursor in the initial reaction mixture will produce a lower number of nucleation points where the growth of BaTiO3 is initiated. Eckert et al. studied the mechanism for the hydrothermal formation of BaTiO3 from barium hydroxide octahydrate and anatase titania,25 and concluded that the growth of BaTiO3 particles is much slower than the formation of nuclei, however by reducing the concentration of Ba2+ in the reaction mixture, the rate of the particle growth could begin to compete with the rate of initial nuclei formation, leading to an uneven size distribution in the final BaTiO3 product, such as that seen in Fig. 6c.
In summary, the XRD, SEM, TEM and DLS results consistently show that the crystallite size and morphology of BaTiO3 are affected by the Ba precursor concentration and Ba/Ti precursor ratio in hydrothermal synthesis, and the BaTiO3 samples synthesised using Ba/Ti precursor ratios of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 exhibit homogeneous crystallites with smaller sizes.
1 exhibit homogeneous crystallites with smaller sizes.
WDS analysis was carried out on the standard BaTiO3 and BaTiO3 samples hydrothermally synthesised at 220 °C using Ba/Ti precursor ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S3†). The Ba–Lα and Ti–Kα peaks overlapped strongly in a standard energy dispersive X-ray spectroscopy (EDS). With WDS they were successfully resolved, however some degree of overlap remained. To determine the areas under the two separate peaks, they were fitted using Origin software (Fig. 8). The peak areas of the synthesised BaTiO3 samples were compared to a standard BaTiO3 sample made by solid-state synthesis which is known to have a Ba/Ti ratio of 1
1 (Fig. S3†). The Ba–Lα and Ti–Kα peaks overlapped strongly in a standard energy dispersive X-ray spectroscopy (EDS). With WDS they were successfully resolved, however some degree of overlap remained. To determine the areas under the two separate peaks, they were fitted using Origin software (Fig. 8). The peak areas of the synthesised BaTiO3 samples were compared to a standard BaTiO3 sample made by solid-state synthesis which is known to have a Ba/Ti ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The peak areas for the Ba-Lα and Ti-Kα peaks for the WDS spectra and the calculated stoichiometric Ba/Ti ratios of the BaTiO3 samples are presented in Table 3.
1. The peak areas for the Ba-Lα and Ti-Kα peaks for the WDS spectra and the calculated stoichiometric Ba/Ti ratios of the BaTiO3 samples are presented in Table 3.
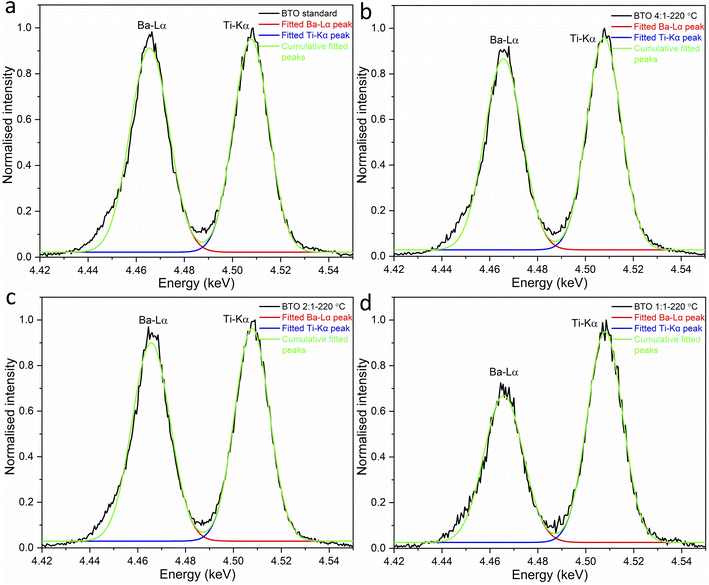 | ||
Fig. 8 Fit of the WDS spectra peaks for the Ba–Lα and Ti–Kα peaks of (a) the standard BaTiO3 and BaTiO3 samples hydrothermally synthesised at 220 °C using Ba/Ti precursor ratios of (b) 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (c) 2 1, (c) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and (d) 1 1 and (d) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (sample labels explained in Table 1). 1 (sample labels explained in Table 1). | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1
| Ba/Ti precursor ratio | Area of Ba-Lα peak | Area of Ti–Kα peak | Stoichiometric Ba/Ti ratio in BaTiO3 |
|---|---|---|---|
| Standard | 0.01806 | 0.01709 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.01658 | 0.01684 | 0.93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.01735 | 0.01705 | 0.96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.01324 | 0.01703 | 0.74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
As shown in Table 3, all hydrothermally synthesised samples are Ba deficient. This is to be expected as the acid washing step in the synthesis has the ability to leach Ba2+ from the surface of the particles.7 In addition, Ba2+ vacancies in the sample also occur to maintain electroneutrality when hydroxyl defects are present as described above.19 The sample synthesised using a Ba/Ti precursor ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 exhibits a far lower Ba content in the product with a stoichiometric Ba/Ti ratio of 0.74
1 exhibits a far lower Ba content in the product with a stoichiometric Ba/Ti ratio of 0.74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Raising the Ba/Ti precursor ratios to 2
1. Raising the Ba/Ti precursor ratios to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 increases the stoichiometric Ba/Ti ratios in the products to 0.96
1 increases the stoichiometric Ba/Ti ratios in the products to 0.96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 0.93
1 and 0.93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively.
1, respectively.
3.3. Effects of the Ti precursor concentration on the crystallite size of BaTiO3 in hydrothermal synthesis
Table 4 shows a second set of experimental conditions that successfully led to the production of BaTiO3. In this case, the Ba precursor concentration was kept constant at 0.09 mol L−1, while the Ti precursor concentration was varied from 0.0225 to 0.09 mol L−1, thus giving Ba/Ti precursor ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Note that the Ba/Ti precursor ratios are the same as for the first set of experiments, described in Table 1, and the reaction temperatures were also the same.
1. Note that the Ba/Ti precursor ratios are the same as for the first set of experiments, described in Table 1, and the reaction temperatures were also the same.
| Sample | Ti precursor concentration (mol L−1) | Ba/Ti precursor ratio | Synthesis temperature (°C) | Crystallite size (nm) |
|---|---|---|---|---|
a Note that samples synthesized using Ba/Ti precursor ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 80 and 120 °C (as well as using 2 1 at 80 and 120 °C (as well as using 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4 1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio at 80 °C) had very low yields, thus no further investigation proceeded. 1 ratio at 80 °C) had very low yields, thus no further investigation proceeded. |
||||
BTO* 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–120 °C 1–120 °C |
0.0225 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
120 | 156 (6) |
BTO* 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 150 °C 1 – 150 °C |
150 | 321 (15) | ||
BTO* 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 180 °C 1 – 180 °C |
180 | 337 (13) | ||
BTO* 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 220 °C 1 – 220 °C |
220 | 394 (26) | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
BTO* 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 120 °C 1 – 120 °C |
0.045 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
120 | 112 (3) |
BTO* 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 150 °C 1 – 150 °C |
150 | 303 (12) | ||
BTO* 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 180 °C 1 – 180 °C |
180 | 310 (11) | ||
BTO* 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 220 °C 1 – 220 °C |
220 | 386 (24) | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
BTO* 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 150 °C 1 – 150 °C |
0.09 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 380 (31) |
BTO* 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 180 °C 1 – 180 °C |
180 | 445 (37) | ||
BTO* 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 – 220 °C 1 – 220 °C |
220 | 483 (39) | ||
As before for the previous set of conditions, the XRD patterns of the obtained BaTiO3 samples (Fig. 9a) indicate that phase-pure BaTiO3 was prepared in all cases, with no impurity phases detected. This can be attributed to the diluted acid wash step in the hydrothermal synthesis to remove any BaCO3 impurities that may have formed in the reaction, as well as to the use of a metal–organic [Ti(OCH(CH3)2)4] as the Ti precursor to precipitate amorphous TiO2 with higher reactivity in hydrothermal synthesis to avoid any unreacted TiO2 impurities.14,15
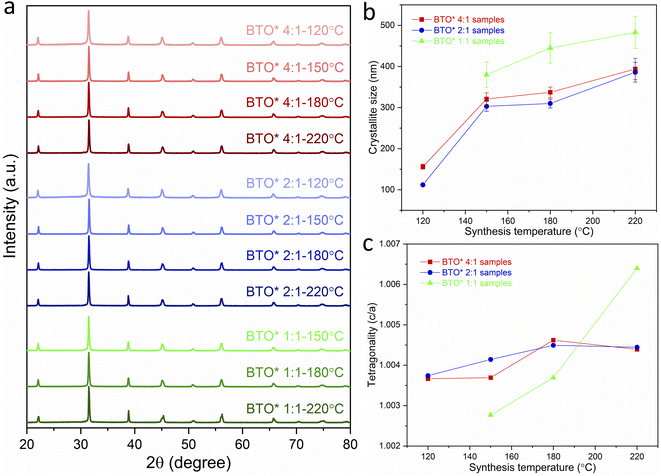 | ||
Fig. 9 (a) XRD patterns of BaTiO3 samples hydrothermally synthesised using Ba/Ti precursor ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1 1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with Ba precursor concentration kept constant at 0.09 mol L−1, and reacted at various synthesis temperatures of 120, 150, 180 and 220 °C; (b) crystallite size and (c) tetragonality (c/a) of BaTiO3 samples plotted against reaction temperature in hydrothermal synthesis using Ba/Ti precursor ratios of 4 1 with Ba precursor concentration kept constant at 0.09 mol L−1, and reacted at various synthesis temperatures of 120, 150, 180 and 220 °C; (b) crystallite size and (c) tetragonality (c/a) of BaTiO3 samples plotted against reaction temperature in hydrothermal synthesis using Ba/Ti precursor ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1 1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, obtained from the Rietveld fits to the XRD patterns (sample labels explained in Table 4). 1, obtained from the Rietveld fits to the XRD patterns (sample labels explained in Table 4). | ||
From the Lorentzian values collected from the Rietveld fits (Fig. S4†) to the XRD patterns, the crystallite sizes of all samples have been calculated and presented in Table 4. The obtained lattice parameters (a and c), tetragonality (c/a), Lorentzian values (Lx and Ly) and reliability factors (Rwp and Rp) are listed in Table S2.† Fig. 9b shows the variation of the crystallite size of BaTiO3 versus the reaction temperature in hydrothermal synthesis with Ba/Ti precursor ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Similar to the trend observed in Fig. 1b, in all three Ba/Ti precursor ratios, there is a general decrease in crystallite size with decreasing synthesis temperature. Using a 4
1. Similar to the trend observed in Fig. 1b, in all three Ba/Ti precursor ratios, there is a general decrease in crystallite size with decreasing synthesis temperature. Using a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ba/Ti precursor ratio, the crystallite size of the obtained BaTiO3 reduces from 394 nm at 220 °C to 156 nm at 120 °C. At a 2
1 Ba/Ti precursor ratio, the crystallite size of the obtained BaTiO3 reduces from 394 nm at 220 °C to 156 nm at 120 °C. At a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio the crystallite size falls from 386 nm at 220 °C to 112 nm at 120 °C. The 1
1 ratio the crystallite size falls from 386 nm at 220 °C to 112 nm at 120 °C. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio shows a crystallite size that drops from 483 nm at 220 °C to 380 nm at 150 °C. The synthesis temperature has been found to have a profound effect on the crystallite size of the BaTiO3 samples with a lower synthesis temperature producing crystallites of a smaller size, in agreement with previous work.15–17 The BaTiO3 samples synthesised using Ba/Ti precursor ratios of 4
1 ratio shows a crystallite size that drops from 483 nm at 220 °C to 380 nm at 150 °C. The synthesis temperature has been found to have a profound effect on the crystallite size of the BaTiO3 samples with a lower synthesis temperature producing crystallites of a smaller size, in agreement with previous work.15–17 The BaTiO3 samples synthesised using Ba/Ti precursor ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 exhibit crystallites of smaller sizes, comparing to that using a Ba/Ti precursor ratio of 1
1 exhibit crystallites of smaller sizes, comparing to that using a Ba/Ti precursor ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as a low Ba precursor concentration would result in a smaller amount of BaTiO3 nuclei generated in the initial stage of the reaction and a larger crystallite size, again in agreement with previous work.25
1, as a low Ba precursor concentration would result in a smaller amount of BaTiO3 nuclei generated in the initial stage of the reaction and a larger crystallite size, again in agreement with previous work.25
The tetragonality variation of BaTiO3 against the reaction temperature in hydrothermal synthesis with Ba/Ti precursor ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is presented in Fig. 9c. Similar to the trend observed in Fig. 1c, the increasing c/a value indicates that the tetragonality of the crystal structure increases with the increasing synthesis temperature in all three Ba/Ti precursor ratios.
1 is presented in Fig. 9c. Similar to the trend observed in Fig. 1c, the increasing c/a value indicates that the tetragonality of the crystal structure increases with the increasing synthesis temperature in all three Ba/Ti precursor ratios.
The changes in tetragonality of hydrothermally synthesized BaTiO3 samples can also be confirmed by Raman characterisation (Fig. 10a). The variation of the relative integrated intensity of the peak at 307 cm−1 for BaTiO3 versus the reaction temperature in hydrothermal synthesis with Ba/Ti precursor ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is presented in Fig. 10b. Similar to the trend observed in Fig. 2b, the increasing integrated intensity of the peak at 307 cm−1 indicates that the tetragonality of the crystal structure increases with the increasing synthesis temperature in all three Ba/Ti precursor ratios. This trend agrees with the result obtained from the Rietveld fits to the XRD patterns.
1 is presented in Fig. 10b. Similar to the trend observed in Fig. 2b, the increasing integrated intensity of the peak at 307 cm−1 indicates that the tetragonality of the crystal structure increases with the increasing synthesis temperature in all three Ba/Ti precursor ratios. This trend agrees with the result obtained from the Rietveld fits to the XRD patterns.
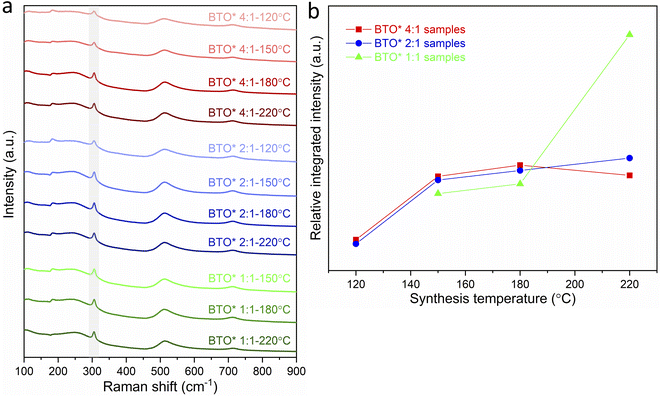 | ||
Fig. 10 (a) Raman spectra of BaTiO3 samples hydrothermally synthesised using Ba/Ti precursor ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1 1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with Ba precursor concentration kept constant at 0.09 mol L−1, and reacted at various synthesis temperatures of 120, 150, 180 and 220 °C (peaks at ∼307 cm−1 being marked as shaded); (b) relative integrated intensities of the Raman peak at 307 cm−1 for BaTiO3 samples plotted against reaction temperature in hydrothermal synthesis using Ba/Ti precursor ratios of 4 1 with Ba precursor concentration kept constant at 0.09 mol L−1, and reacted at various synthesis temperatures of 120, 150, 180 and 220 °C (peaks at ∼307 cm−1 being marked as shaded); (b) relative integrated intensities of the Raman peak at 307 cm−1 for BaTiO3 samples plotted against reaction temperature in hydrothermal synthesis using Ba/Ti precursor ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1 1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (sample labels explained in Table 4). 1 (sample labels explained in Table 4). | ||
Finally, in order to elucidate the effect of the reagent concentration, the results for the same Ba/Ti precursor ratio, but different reagent concentrations, are shown in Fig. S5.† This figure is obtained by combining the experimental results for the set of conditions in Tables 1 and 4, for the Ba/Ti precursor ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. When increasing the Ba and Ti precursor concentrations, the crystallite size of BaTiO3 shows an overall decrease for samples made by using the Ba/Ti precursor ratio of 4
1. When increasing the Ba and Ti precursor concentrations, the crystallite size of BaTiO3 shows an overall decrease for samples made by using the Ba/Ti precursor ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; however, the BaTiO3 crystallite size increases for samples using the Ba/Ti precursor ratio of 1
1; however, the BaTiO3 crystallite size increases for samples using the Ba/Ti precursor ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Comparing the effect of the precursor concentration (Fig. S5†) and the reaction temperature (Fig. 1b and 9b) on the crystallite size, it is seen that the effect of the reaction temperature is more pronounced.
1. Comparing the effect of the precursor concentration (Fig. S5†) and the reaction temperature (Fig. 1b and 9b) on the crystallite size, it is seen that the effect of the reaction temperature is more pronounced.
Hydrothermal synthesis to produce BaTiO3 nanoparticles has been investigated by many researchers, and it has been reported that nanosized particles of BaTiO3 can be achieved by controlling the reaction conditions.4 In our work, nanocrystalline BaTiO3 has been prepared by hydrothermal synthesis using barium hydroxide octahydrate and titanium isopropoxide as precursors. The same precursors have been used by Zeng et al. to prepared nanocrystalline BaTiO3 at low temperature (<100 °C), and the effects of the Ba/Ti precursor ratio and reaction temperature on the size and morphology of BaTiO3 were investigated.10,11 The same precursors have also been applied by Nahm et al. in hydrothermal synthesis to prepare nanocrystalline BaTiO3, but they only employed one reaction temperature (220 °C) and Ba/Ti precursor ratio (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).12 In this work, we systematically explored the effects of the reaction temperature (80, 120, 150, 180 and 220 °C) and Ba/Ti precursor ratio (4
1).12 In this work, we systematically explored the effects of the reaction temperature (80, 120, 150, 180 and 220 °C) and Ba/Ti precursor ratio (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in hydrothermal synthesis for preparing nanocrystalline BaTiO3, and we characterised the reaction product by XRD, Raman, SEM, TEM, DLS, TGA and WDS to identify the optimal hydrothermal synthesis conditions in terms of small particle size and low concentration of defects.
1) in hydrothermal synthesis for preparing nanocrystalline BaTiO3, and we characterised the reaction product by XRD, Raman, SEM, TEM, DLS, TGA and WDS to identify the optimal hydrothermal synthesis conditions in terms of small particle size and low concentration of defects.
4. Conclusion
Nanocrystalline BaTiO3 has been prepared via a hydrothermal synthesis. Reaction conditions including synthesis temperature and Ba/Ti precursor ratio have been controlled and adapted with the aim of producing small crystallites of BaTiO3, as well as to investigate the effects of hydrothermal synthesis conditions on the microstructure of BaTiO3. The XRD, SEM, TEM and DLS results show that the crystallite/particle size of the BaTiO3 samples increases with the increasing synthesis temperature; and the crystallite/particle size of BaTiO3 is also affected by the Ba/Ti precursor ratio. The BaTiO3 sample synthesised using a Ba/Ti precursor ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at a reaction temperature of 120 °C exhibited homogeneous crystallites of the smallest size of 107 nm. Moreover, the Raman and XRD results indicate that the tetragonality of the crystal structure increases with the increasing synthesis temperature. In addition, the TGA results suggest that the number of defects in BaTiO3 decreases as the synthesis temperature increases; and the WDS results indicate that the BaTiO3 with less defects was obtained when using a Ba/Ti precursor ratio of 2
1 at a reaction temperature of 120 °C exhibited homogeneous crystallites of the smallest size of 107 nm. Moreover, the Raman and XRD results indicate that the tetragonality of the crystal structure increases with the increasing synthesis temperature. In addition, the TGA results suggest that the number of defects in BaTiO3 decreases as the synthesis temperature increases; and the WDS results indicate that the BaTiO3 with less defects was obtained when using a Ba/Ti precursor ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank EPSRC for support under the Industrial Strategy Challenge Fund (EP/R021295/1) and for an early career fellowship to NGA (EP/N024303/1). MZ thanks Patricia Goggin for help in the TEM; thanks Priti Tiwana and Russel Torah for help in the DLS Training; and thanks Andrea Russell for access to the Raman. The raw data associated with figures in this article and in the ESI can be found at https://doi.org/10.5258/SOTON/D2382.References
- B. Jiang, J. Iocozzia, L. Zhao, H. Zhang, Y. W. Harn, Y. Chen and Z. Lin, Chem. Soc. Rev., 2019, 48, 1194–1228 RSC.
- G. H. Kwei, A. C. Lawson and S. J. L. Billinge, J. Phys. Chem., 1993, 97, 2368–2377 CrossRef CAS.
- A. R. West, Solid State Chemistry and its Applications, John Wiley & Sons Ltd, United Kingdom, 2014 Search PubMed.
- M. Z.-C. Hu, V. Kurian, E. A. Payzant, C. J. Rawn and R. D. Hunt, Powder Technol., 2000, 110, 2–14 CrossRef CAS.
- C. Pithan, D. Hennings and R. Waser, Int. J. Appl. Ceram. Technol., 2005, 2, 1–14 CrossRef CAS.
- M. Wegmann, R. Brönnimann, F. Clemens and T. Graule, Sens. Actuators, A, 2007, 135, 394–404 CrossRef CAS.
- P. Pinceloup, C. Courtois, A. Leriche and B. Thierry, J. Am. Ceram. Soc., 1999, 82, 3049–3056 CrossRef CAS.
- M. Zhang, S. Fop, D. Kramer, N. Garcia-Araez and A. L. Hector, J. Mater. Chem. A, 2022, 10, 11587–11599 RSC.
- H. S. Yun, B. G. Yun, S. Y. Shin, D. Y. Jeong and N. H. Cho, Nanomater, 2021, 11, 754 CrossRef CAS.
- M. Zeng, Appl. Surf. Sci., 2011, 257, 6636–6643 CrossRef CAS.
- M. Zeng, N. Uekawa, T. Kojima and K. Kakegawa, J. Mater. Res., 2011, 22, 2631–2638 CrossRef.
- J.-M. Han, M.-R. Joung, J.-S. Kim, Y.-S. Lee, S. Nahm, Y.-K. Choi, J.-H. Paik and E. Suvaci, J. Am. Ceram. Soc., 2014, 97, 346–349 CrossRef CAS.
- A. Larson, R. Von Dreele, L. Finger, M. Kroeker and B. Toby, J. Appl. Crystallogr., 2001, 34, 210–213 CrossRef.
- A. Habib, R. Haubner and N. Stelzer, Mater. Sci. Eng., B, 2008, 152, 60–65 CrossRef CAS.
- H.-J. Chen and Y.-W. Chen, Ind. Eng. Chem. Res., 2003, 42, 473–483 CrossRef CAS.
- D. Hennings, G. Rosenstein and H. Schreinemacher, J. Eur. Ceram. Soc., 1991, 8, 107–115 CrossRef CAS.
- H. Kumazawa, S. Annen and E. Sada, J. Mater. Sci., 1995, 30, 4740–4744 CrossRef CAS.
- M. B. Smith, K. Page, T. Siegrist, P. L. Redmond, E. C. Walter, R. Seshadri, L. E. Brus and M. L. Steigerwald, J. Am. Chem. Soc., 2008, 130, 6955–6963 CrossRef CAS PubMed.
- S. Wada, T. Suzuki and T. Noma, J. Ceram. Soc. Jpn., 1995, 103, 1220–1227 CrossRef CAS.
- M. El Marssi, F. Le Marrec, I. A. Lukyanchuk and M. G. Karkut, J. Appl. Phys., 2003, 94, 3307–3312 CrossRef CAS.
- Y. Shiratori, C. Pithan, J. Dornseiffer and R. Waser, J. Raman Spectrosc., 2007, 38, 1300–1306 CrossRef CAS.
- A. Mansuri, I. N. Bhatti, I. N. Bhatti and A. Mishra, J. Adv. Dielectr., 2018, 8, 1850024 CrossRef CAS.
- E.-W. Shi, C.-T. Xia, W.-Z. Zhong, B.-G. Wang and C.-D. Feng, J. Am. Ceram. Soc., 1997, 80, 1567–1572 CrossRef CAS.
- E. Ciftci, M. N. Rahaman and M. Shumsky, J. Mater. Sci., 2001, 36, 4875–4882 CrossRef CAS.
- J. O. Eckert, C. C. Hung-Houston, B. L. Gersten, M. M. Lencka and R. E. Riman, J. Am. Ceram. Soc., 1996, 79, 2929–2939 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra03707f |
| This journal is © The Royal Society of Chemistry 2022 |