Open Access Article
Open Access ArticleRecycling NR/SBR waste using probe sonication as a new devulcanizing method; study on influencing parameters
Marzieh Shabani and
Masoud Jamshidi *
*
Constructional Polymers and Composites Research Laboratory, School of Chemical, Petroleum and Gas Engineering, Iran University of Science and Technology (IUST), Tehran, Iran. E-mail: mjamshidi@iust.ac.ir; Fax: +98-21-77240495; Tel: +98-21-77240255
First published on 14th September 2022
Abstract
In this work, a vulcanized blend of natural rubber (NR) and styrene butadiene rubber (SBR) (i.e. at weight ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a model for tire rubber was devulcanized using probe sonication. The effect of processing parameters such as sonication media, power, temperature and time on sol/gel contents and devulcanization percent of rubbery samples was investigated. Moreover, the influence of pre-immersion of vulcanized NR/SBR samples in different liquids (i.e. water, oil and toluene) was assessed for different sonication times (i.e. 10 to 60 min) and powers (i.e. 30 to 60 W). It was found that pre-immersion of rubber particles in oil significantly increased the devulcanization percent. The optimum conditions for devulcanization of the NR/SBR blend via probe sonication were found to be 60 Watts, 20 min, oil and 24 h for sonication power, sonication time, pre-immersion/sonication media and pre-immersion time, respectively. The highest obtained devulcanization percent in this step was about 40%. The effects of two devulcanizing chemical agents (i.e. diphenyl disulfide and VitaX) on devulcanization performances of the samples were also studied. Results showed that higher devulcanization percent (i.e. about 52%) was obtained by using VitaX. It was also observed that VitaX significantly improved re-vulcanization speed (i.e. cure rate index) and decreased scorch time. It was also found that lower content of VitaX (i.e. 0.6 phr) caused better curing properties but lower mechanical properties compared to the higher content (i.e. 1.2 phr).
50) as a model for tire rubber was devulcanized using probe sonication. The effect of processing parameters such as sonication media, power, temperature and time on sol/gel contents and devulcanization percent of rubbery samples was investigated. Moreover, the influence of pre-immersion of vulcanized NR/SBR samples in different liquids (i.e. water, oil and toluene) was assessed for different sonication times (i.e. 10 to 60 min) and powers (i.e. 30 to 60 W). It was found that pre-immersion of rubber particles in oil significantly increased the devulcanization percent. The optimum conditions for devulcanization of the NR/SBR blend via probe sonication were found to be 60 Watts, 20 min, oil and 24 h for sonication power, sonication time, pre-immersion/sonication media and pre-immersion time, respectively. The highest obtained devulcanization percent in this step was about 40%. The effects of two devulcanizing chemical agents (i.e. diphenyl disulfide and VitaX) on devulcanization performances of the samples were also studied. Results showed that higher devulcanization percent (i.e. about 52%) was obtained by using VitaX. It was also observed that VitaX significantly improved re-vulcanization speed (i.e. cure rate index) and decreased scorch time. It was also found that lower content of VitaX (i.e. 0.6 phr) caused better curing properties but lower mechanical properties compared to the higher content (i.e. 1.2 phr).
1. Introduction
Elastomers are a specific category of polymeric materials whose applications have been progressive in the modern-day world.1–3 There are various applications for elastomers ranging from medical to the tire industry. Cross-linking through vulcanization induces suitable physical/mechanical properties in elastomers4–6Nowadays, automotives have become an undeniable human need. On this basis, the need for tire rubber has increased significantly in past decades. Millions of tires are produced annually in the world and they are turned into waste after usage. The tire waste, due to their huge content and negative effects on ecosystems, have become a major environmental problem. On this basis, recycling of waste tire rubber has been essential from environmental and economical points of view7,8
Some methods were used for elimination of waste tire rubbers that include burning as a heat source,9 landfilling,10,11 reclaiming12–14 etc. It has been known that burning and landfilling of tire rubbers impact environment by releasing greenhouse gases (i.e. SOx and COx) in the air and immigration of the toxic low molecular weight organic matters in to the soil, respectively.
Rubber reclaiming attracted attention of researchers in the beginning. However, reclaimed ground tire rubbers (GTRs), due to the very low mechanical properties, disappointed producers. In fact, during reclaiming a rubber, thermal/mechanical stresses are encountered to ground waste rubber particles that cause fracture in the polymer backbone and so significant decrement in the mechanical properties of the recycled product.15–17
On this basis, an effective method is needed for recycling waste rubbers. Literature review among published articles in this field shows that devulcanization (i.e. reverse process of vulcanization) is a promising method for this purpose.18–20 Different methods has been introduced for devulcanization of rubbers especially for ground tire rubbers (GTRs). For instance, mechanical,21 thermal,22 thermo-mechanical,23 chemo-thermomechanical,24 ultrasonic25 and microwave26,27 processes has been used for devulcanization of waste rubbers.
The aim of devulcanization is breaking in C–S and S–S bonds (i.e. cross-links) instead of C–C bonds (i.e. backbone) in the vulcanized rubber network. In theory, fracture in C–C bonds needs to higher energy than C–S and S–S bonds, but in real condition both chain scission and cross-link breakage occur during devulcanization.28 On this basis, the best devulcanization method is a process with higher cross-link breakage to chain scission ratio.
Using ultrasonic waves is one of the most considerate methods for waste rubber recycling. Two sonication based methods have been conventionally used for rubber recycling: extrusion-sonication of waste rubber and sonication of rubber particles in an ultrasonic bath. Liang and Isayev et al.29 used ultrasonic waves for devulcanization of rubbers by combination of sonication and extrusion methods. They assessed the effect of rubber compound and ultrasonic amplitude on devulcanization of unfilled and silica-filled natural rubber. Based on the results, ultrasonic amplitude had a direct relationship with power. However, the die pressure illustrated an inverse trend which is a consequence of rubber thixotropic properties and declining in the chain breakage. Also, sonication caused breaking in the NR chains that caused declining in the rubber–silica connections. The devulcanazation of SBR contained carbon black (CB) was also investigated.30–32 It was performed by an extruder equipped to an ultrasonic die. It was claimed that the revulcanized samples prepared from devulcanized SBRs had close properties to the virgin rubber. The devulcanization of BR and SBR/BR blend via sonication-extrusion process was also investigated by Liang et al.33,34 They evaluated effects of different fillers (i.e. carbon black, silica and silanized silica) by a single screw extruder and various ultrasonic amplitudes. It was found that carbon black promoted the mechanical properties of SBR/BR blend compared to silica filler. It was proposed that CB made some bridges in rubber structure which caused improved properties. Based on the experiments, silica containing BR that contained long side branches, showed strong rubber–filler interactions that caused better distribution of silica in rubber matrix but declined rolling resistance of the rubber.
Saputra et al.35 investigated effect of deep eutectic solvents and their content at different heating times on devulcanization of GTR in an ultrasonic bath. They assessed the relationship between devulcanization performances and solvents viscosity. It was found that low viscosity solvent led to success in de-cross-linking process. They realized that increase in the heating time caused decline in the cross-link density and enhancement of devulcanization percentage. The Horikx theory was also used to determine chain scission to cross-links breakage ratio. It was found that the most broken bonds belonged to cross-links which exposed to a selective devulcanization.
Kim et al.36 investigated devulcanization of waste tire rubber through probe sonication. They studied devulcanization of rubber through determination of prepared free sulfur content that formed by cross-links breakage. The rubber fragments were prepared by cryogenic processing of tire scrap (i.e. both the tread wear and sidewall). The fragmented samples were immersed in acetone for 24 h to extract plasticizers, paraffin's and antioxidants. The samples showed increased volume that confirmed suitable swelling. Thereafter, the samples were sonicated by a probe ultrasonic device and then refluxed and filtered to remove carbon black. The samples then dried and analyzed for sulfur content. It was found that sonication in presence of H2O2 effectively increased fracture in the sulfur bonding and cross-links.
Based on the literature review, it was found that sonication-based devulcanization of waste rubbers have been studied in few researches. Besides, in most cases ultrasonic devulcanization performed by altering mechanical stresses (i.e. in an extruder) along with sonication by a horn or ultrasonic bath.37 In one investigation, probe sonication was used for devulcanization of waste tire rubber but the devulcanization properties (gel/sol fractions and devulcanization percentage) and process parameters (sonication power, time and etc.) were not assessed. Due to the fact that probe sonication is a simple method and involve higher energies to the rubber particles (e.g. GTRs) compared to sonication bath, investigating the method and the influencing parameters would be a challenging subject.
In this study, probe sonication was used for devulcanization of a cured NR/SBR blend as a model tire rubber. For this purpose, NR/SBR blend (i.e. at NR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SBR ratio of 50
SBR ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) was prepared and vulcanized. The sample were ground to particles with mesh size of 16. The crumbed particles were devulcanized by probe sonication and also sonication in presence of chemical agent (DPDS and VitaX). Effect of sonication media, swelling media, sonication power-time and chemical agent content on devulcanization percent, cross-link density, sol/gel fractions, curing and mechanical properties of the devulcanized samples was evaluated. The samples characterized with oscillating disc rheometer (ODR), Fourier-transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC) and thermogravimetric (TGA) analysis. Finally the mechanical properties of the recycled rubbers in blend with pristine rubber was evaluated.
50) was prepared and vulcanized. The sample were ground to particles with mesh size of 16. The crumbed particles were devulcanized by probe sonication and also sonication in presence of chemical agent (DPDS and VitaX). Effect of sonication media, swelling media, sonication power-time and chemical agent content on devulcanization percent, cross-link density, sol/gel fractions, curing and mechanical properties of the devulcanized samples was evaluated. The samples characterized with oscillating disc rheometer (ODR), Fourier-transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC) and thermogravimetric (TGA) analysis. Finally the mechanical properties of the recycled rubbers in blend with pristine rubber was evaluated.
2. Experimental
2.1. Material
Masterbatches of styrene−butadiene rubber (SBR) and natural rubber (NR) were supplied from Mouj Andishe Novin Baspar Company (Tehran, Iran) that their components are listed in Table 1.| Sample | NR Masterbatch (phr) | SBR Masterbatch (phr) |
|---|---|---|
| Raw elastomeric gum | 100 | 100 |
| Carbon black (N550) | 60 | 60 |
| Process oil | 20 | 20 |
| ZnO | 5 | 5 |
| Stearic acid | 1 | 1 |
2.2. Preparation of vulcanized NR/SBR
SBR and NR compounds were blended at NR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SBR ratio of 50
SBR ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. The master batches were mixed by a two-roll mill at room temperature for 15 min. At this stage, 1.5 phr of sulfur and 1.5 phr of N-cyclohexyl-2-benzothiazole sulfonamide (CBS) were added to the rubber as vulcanizing agent and curing accelerator, respectively. After homogenization and 24 hours conditioning, the samples were molded in sheet shape and vulcanized at 160 °C for 15 min in a hydraulic molding hot-press.
50. The master batches were mixed by a two-roll mill at room temperature for 15 min. At this stage, 1.5 phr of sulfur and 1.5 phr of N-cyclohexyl-2-benzothiazole sulfonamide (CBS) were added to the rubber as vulcanizing agent and curing accelerator, respectively. After homogenization and 24 hours conditioning, the samples were molded in sheet shape and vulcanized at 160 °C for 15 min in a hydraulic molding hot-press.
2.3. Preparation of ground NR/SBR powder (GR)
After vulcanization of the NR/SBR sheets, they were ground in a two roll mill and then multi-function disintegrator (BEST 100 Industrial Grinder). The ground particles were sieved to obtain rubbery particles with mesh size of 16. The prepared samples and the codes are listed in Table 2.| Sample code | Condition | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-immersion media | Sonication media | Sonication power (W) | Sonication time (min) | Pre-immersion time (hr) | |||||
| Water | Oil | Toluene | Water | Oil | Toluene | ||||
| DGR-Ctrl | — | — | — | — | — | — | 0 | 0 | 0 |
| DGR-W(45-20) | — | — | — | √ | — | — | 45 | 20 | 0 |
| DGR-O(45-20) | — | — | — | — | √ | — | 45 | 20 | 0 |
| DGR-T(45-20) | — | — | — | — | — | √ | 45 | 20 | 0 |
| DGR-W24(45-20) | √ | — | — | √ | — | — | 45 | 20 | 24 |
| DGR-O24(45-20) | — | √ | — | — | √ | — | 45 | 20 | 24 |
| DGR-T24(45-20) | — | — | √ | — | — | √ | 45 | 20 | 24 |
| DGR-T24W(45-20) | — | — | √ | √ | — | — | 45 | 20 | 24 |
| DGR-O24W(45-20) | — | √ | — | √ | — | — | 45 | 20 | 24 |
| DGR-O24T(45-20) | — | √ | — | — | — | √ | 45 | 20 | 24 |
| DGR-O24(30-10) | — | — | — | — | √ | — | 30 | 10 | 24 |
| DGR-O24(30-20) | — | √ | — | — | √ | — | 30 | 20 | 24 |
| DGR-O24(30-30) | — | √ | — | — | √ | — | 30 | 30 | 24 |
| DGR-O24(60-20) | — | √ | — | — | √ | — | 60 | 20 | 24 |
| DGR-O24(60-25) | — | √ | — | — | √ | — | 60 | 25 | 24 |
| DGR-O24(60-30) | — | √ | — | — | √ | — | 60 | 30 | 24 |
2.4. Devulcanization process
The process oil, toluene and water were selected as sonication media in this investigation. The prepared GRs were suspended in media and then exposed to sonication using a probe ultrasonic device (BANDELIN SONOPULS HD3100, HF power of maximum 100 W, microtip MS 73, Italy). The sonication power changed ranging from 30 W to 60 W and sonication time altered between 10 and 30 min. A control sample was prepared by mechanical devulcanization via two roll milling of the GR sample for 20 min (i.e. DGR-Ctrl). To understand the swelling effect on devulcanization yield, GRs were also pre-immersed in oil, toluene and water for 0, 24 and 48 h.2.5. Chemical devulcanizing agents
Diphenyl disulfide (DPDS) were used as a conventional and effective chemical devulcanizing agent at 1.2 phr38–40 in this study. It was added directly to the NR/SBR powder on a two roll mill. VitaX was also used as a new complex devulcanizing agent at 0.6 and 1.2 phr.27 It was processed DPDS in presence of CaCO3 and ZnO. To devulcanize rubber samples in presence of VitaX, 80 g of ground rubbers (GRs) were soaked in 20 g of process oil contained VitaX (0.6 or 1.2 phr) in an oven at 70 °C for 12 h. This helped intrusion of Vitax into the rubber bulk by swelling process. After devulcanization, all the revulcanizable rubber compounds were rolled by a laboratory two roll mill for 5 min to form sheets with 2 mm thickness.2.6. Revulcanization process
Based on the devulcanization performances, some of the devulcanized GRs (i.e. DGR samples) were revulcanized to prepare rubber sheets for evaluating the tensile properties. To re-vulcanize the DGRs, they were mixed to virgin NR/SBR at ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 and then 1.5 phr of CBS and 1.5 phr of sulfur were added to compounds on a two roll mill and mixing continued for 10 min. Then the samples were molded and vulcanized with hot-press under 50 ton pressure at 160 °C for 15 min.27 After curing, dumbbell shape test specimens were prepared using a cutter and manual press from revulcanized samples.
50 and then 1.5 phr of CBS and 1.5 phr of sulfur were added to compounds on a two roll mill and mixing continued for 10 min. Then the samples were molded and vulcanized with hot-press under 50 ton pressure at 160 °C for 15 min.27 After curing, dumbbell shape test specimens were prepared using a cutter and manual press from revulcanized samples.
2.7. Estimating devulcanization percent (DP)
To evaluate sol and gel fractions of all devulcanized samples, they were extracted by acetone and then by toluene in a Soxhlet. The processes removed low molecular weight chains and oils from de-vulcanized samples.4,25 For this purpose, about 2 g of each sample was extracted in boiling acetone for 16 h and followed by drying in an oven at 70 °C for 12 h. The next extraction was performed for 24 h in boiling toluene and the sample was dried at 70 °C for 12 h in an oven. The sol and gel fractions of the DGRs was estimated by evaluating the soluble and insoluble contents in toluene, respectively.41To evaluate cross-link density (CLD) of the samples, they were immersed in toluene at room temperature for 72 h. The solvent was refreshed three times with pure solvent over the swelling time (each 24 h). Thereafter, the samples were removed from solvent and quickly dried by filter paper and weighted. Finally, the samples were dried in an oven at 70 °C for 12 h to evaporate the rest of solvent and then weighted again. The cross-link density (eqn (1)) was calculated using Flory–Rehner equation41 as following:
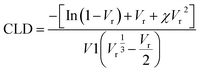 | (1) |
The volume fraction of polymer in swollen specimen (Vr) was measured as following:
 | (2) |
The density of dry samples was measured according to ASTM D6814 (ref. 42) using the eqn (3) as following:
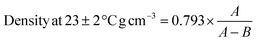 | (3) |
In which, A and B are weight of the specimen in air (g) and methanol (g), respectively. On this basis, the density of pristine NR/SBR sample was obtained 1.129 g cm−3.
The devulcanization percentages of the samples was also calculated using eqn (4):41
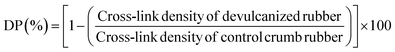 | (4) |
2.8. Characterization methods
Pyrolysis Fourier transform infrared spectroscopy (Pyrolysis-FTIR) analysis was performed by a PerkinElmer TGA 8000 instrument. Thermogravimetric analysis (TGA) was performed on the samples by NETZSCH STA 449 F3 Jupiter device (Germany) at heating rate of 10 °C min−1 to provide information about thermo-oxidative degradation of the samples.41 Differential scanning calorimetry was carried out in N2 environment at heating rate of 10 °C min−1 in the range of −100 to 300 °C by NETZSCH DSC 200F3 instrument to depict curing behavior and glass transition temperature of the samples before and after devulcanization. The curing behavior of samples were evaluated at 160 °C in an oscillating disk rheometer (ODR) (Monsanto, R-100 Oscillatory Disk rheometer) according to ASTM D 2084.43The tensile tests were carried out on dumbbell-shaped specimens (type C) using a SANTAM STM150 Universal Machine at room temperature (25 ± 2 °C) to evaluate tensile strength and elongation at break of the pristine and re-vulcanized samples based on the ASTM D 412.44 The uniform crosshead speed of 500 mm min−1 was applied in tension and the test was repeated 10 times for each sample.
3. Results and discussions
3.1. Effects of pre-immersion and sonication media
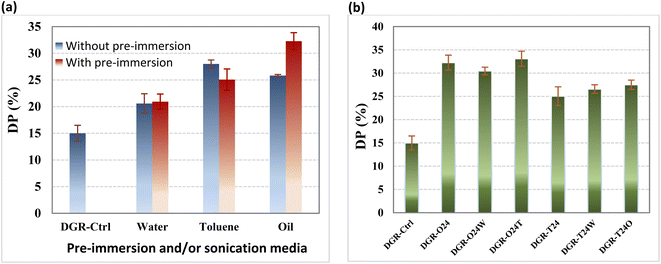
Three kinds of liquids (i.e. oil, water and toluene) and their mixture were used as sonication media in this study. To compare effect of different liquids, the ultrasonic power and time were kept constant (i.e. 45 Watt and 20 min), at the first step. Results are shown in Fig. 1.Fig (1a) shows the effect of sonication media on devulcanization percentage. It is clearly seen that type of sonication media affects the devulcanization percent (i.e. in the samples processes without pre-immersion). It is well known that breakage of these bubbles induce sonication energy to the ground rubber particles. However, due to the hydrophobic nature of rubber particles, they had not intimate contact to water (in contrary to toluene) that this decreased induced sonication energy to the particles. The low DP of the sample in oil media was attributed to its higher viscosity that dissipated induced sonication energy to the GTRs. The highest devulcanization percentage was obtained (i.e. about 28%) in the case of using toluene as sonication media. The devulcanization performances of the samples are illustrated in Table 3.
| Samples | CLD (×104) (±st. dev.) (%) | Sol fraction (±st. dev.) (%) | Gel fraction(±st. dev.) (%) |
|---|---|---|---|
a UB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ultrasonic bath. Ultrasonic bath. |
|||
| DGR-Ctrl | 1.95 (±0.08) | 10.5((±0.03) | 89.5 (±0.03) |
| DGR-T(45-20) | 1.4 (±0.095) | 18.95 (±0.019) | 81.05 (±0.019) |
| DGR-O(45-20) | 1.44 (±0.034) | 15 (±0.02) | 85 (±0.02) |
| DGR-W(45-20) | 1.54 (±0.035) | 13.5 (±0.015) | 86.5 (±0.015) |
| DGR-T24(45-20) | 1.46 (±0.039) | 13 (±0.01) | 87 (±0.01) |
| DGR-O24(45-20) | 1.32 (±0.031) | 20 (±0.02) | 80 (±0.02) |
| DGR-W24(45-20) | 1.54 (±0.028) | 15.4 (±0.025) | 84.6 (±0.025) |
| DGR-T24W(45-20) | 1.43 (±0.018) | 16 (±0.02) | 84 (±0.02) |
| DGR-O24W(45-20) | 1.35 (±0.015) | 18 (±0.03) | 82 (±0.03) |
| DGR-O24T(45-20) | 1.30 (±0.031) | 20 (±0.01) | 80 (±0.01) |
| DGR-O24(30-10) | 1.71 (±0.024) | 17.5 (±0.02) | 82.5 (±0.02) |
| DGR-O24(30-20) | 1.64 (±0.035) | 17 (±0.033) | 83 (±0.033) |
| DGR-O24(30-30) | 1.62 (±0.027) | 16.65 (±0.01) | 83.35 (±0.01) |
| DGR-O24(60-20) | 1.19 (±0.046) | 22 (±0.05) | 78 (±0.05) |
| DGR-O24(60-25) | 1.35 (±0.015) | 18.5 (±0.01) | 81.5 (±0.01) |
| DGR-O24(60-30) | 1.37 (±0.066) | 16.25 (±0.022) | 83.75 (±0.022) |
| DGR-O24 (60-20)-VitaX0.6 | 1.13 (±0.04) | 19.15 (±0.03) | 80.85 (±0.03) |
| DGR-O24 (60-20)-VitaX1.2 | 0.93 (±0.068) | 20 (±0.03) | 80 (±0.03) |
| DGR-O24 (60-20)-DPDS1.2 | 1.45 (±0.096) | 15.4 (±0.025) | 84.6 (±0.025) |
| DGR-O24 (30-10)-VitaX0.6 | 1.22 (±0.067) | 13.37 (±0.035) | 86.62 (±0.035) |
| DGR-O24 (30-10)-VitaX1.2 | 1.24 (±0.083) | 16.1 (±0.01) | 83.9 (±0.01) |
| DGR-O24 (30-10)-DPDS1.2 | 1.45 (±0.097) | 15 (±0.05) | 87 (±0.05) |
| DGR-O24 (60-20)-UBa | 1.35 (±0.099) | 15.83 (±0.084) | 84.17 (±0.084) |
| DGR-O24 (60-20)-VitaX1.2-UB | 1.32 (±0.019) | 17 (±0.02) | 83 (±0.02) |
It is clearly seen that cross-link density (CLD) decreased significantly in the devulcanized samples compared to the control sample (i.e. the mechanically devulcanized sample). It proves that probe sonication caused fracture in the cross-links properly as it was desired. The sol content shows that low molecular weight and soluble chains increased considerably in the sonicated samples. This was attributed to the chain scissions that occurred during devulcanization in the rubber matrix.
The figure also shows the effect of pre-immersion of the samples in the individual liquids for 24 h. Results showed that DP just increased in the case of using oil as pre-immersion media. It is also obviously seen that just using oil as pre-immersion media could influence CLD and sol/gel fractions of the sonicated samples (see Table 3). This was attributed to diffusion of the oil into the rubber bulk during pre-immersion step that swelled and expanded its network and helped effective devulcanization during sonication. In contrary, the CLD of samples was not affected after 24 h of immersing in water and toluene, however, their sol content changed significantly.
The pre-immersion of the samples in water caused increment in the sol content that means it increased the chain scission. In contrary, pre-immersion in toluene caused decrement in the chain scission. In fact, the chain scission represents unsuitable devulcanization of GTRs that decreases the mechanical properties.33,34
Based on the results, toluene and oil were selected as effective liquids for pre-immersion step. The effect of water, toluene and oil as sonication media was also evaluated. Results are shown in Fig. (1b) and Table 3. It is obviously seen that using different liquids for pre-immersion and sonication has negligible effect on devulcanization percent. On this basis, the DGR-O24 sample and oil were selected as the best devulcanized sample (32%) and the best media for pre-immersion/sonication, respectively.
3.2. Effect of sonication power and time
The effect of sonication energy (i.e. multiply of power and time) on DP of the samples was evaluated. Results are shown in Fig. 2 and Table 3. It was found that by increment in the sonication power from 30 to 60 W, the sol fraction and CLD enhanced at constant sonication time (i.e. 20 min). It was attributed to this fact that at higher sonication energies, bigger and more air bubbles are formed in the sonication media and higher energies induced to the rubber particles. Due to the particulate shape of the samples, it is normal to consider partial devulcanization as dominant mechanism for the recycling process.27 Based on the results, it was concluded that higher sonication energies caused deeper diffusion of the waves in to the particles core. Therefore, more breakage has been occurred in their rubber network.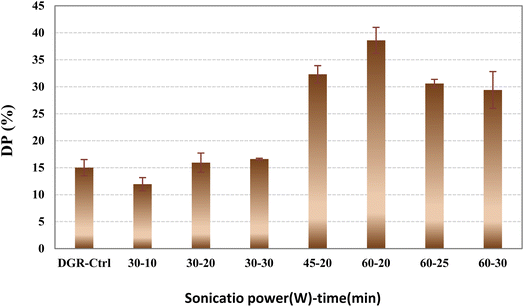 | ||
| Fig. 2 Devulcanization percent of the DGR-O24 samples sonicated at different powers and times in oil (after 24 h pre-immersion in oil). | ||
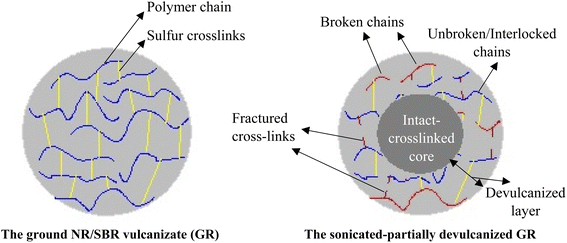
Negligible changes was observed in devulcanization percent of the samples at sonication power of 30 W (at all times). The highest DP was obtained at sonication power and time of 60 W and 20 min (38%), respectively. Similar to the CLD and sol fraction results, herein the DP increased with increment in the induced energy. The reverse results was obtained at sonication times of 25 and 30 min at amplitude of 60 W. It was postulated that due to the very high energies encountered to the particles, their surface scorched and formed an insulated layer which decreased wave intrusion toward the particles core. Fig. 3 shows a schematic for partial devulcanization of the rubber particles that happened under probe sonication.
In these schematic, the mechanism of partial devulcanization of ground rubber particle due to sonication was illustrated. The degree of cross-links breakage incredibly depends on ultrasonic amplitude, time and kind of liquid. The more sonication amplitude causes decrement in the area of intact cross-linked core and improvement in the DP and crosslinks breakage. In contrast, at low sonication powers, devulcanization just occurs at a thin surface layer of the particles.
3.3. Effect of chemicals agents
On the basis of the obtained results, DGR-O24(60-20) and DGR-O24(30-10) samples were selected as the best and worst devulcanized samples by probe sonication. The effect of chemical agents (i.e. DPDS and VitaX) on devulcanization performances was evaluated in these samples. Table 3 shows the CLD and sol/gel fraction results. It was found that using VitaX caused better devulcanization of the samples compared to DPDS at both low and high sonication energies. The CLD and sol fraction of the samples decreased using VitaX. In fact, it decreased chain scission and increased cross-links breakage. In contrary, DPDS caused significant decrease in the sol fraction while considerable increase in the CLD. It means that DPDS caused some new cross-linking in the rubber structure. It was occurred due to accelerating effect of DPDS during revulcanization process. It also enhanced the chain scission at the same time. The negative effect of DPDS was not observed at lower sonication energy and time (i.e. 30 W for 10 min).Fig. 4 shows that both chemical agents significantly increased devulcanization percent of the sonicated samples (DGR-O24), however, higher DPs were obtained using VitaX. Devulcanization percent was improved considerably at higher VitaX loading content (i.e. 1.2 phr). The VitaX content had negligible effect on devulcanization percent at lower sonication energies. Furthermore, DPDS had lower impact on devulcanization percent compared to VitaX at low and high sonication energies. The better results obtained by VitaX were related to its complex chemistry and its different application process.27 In fact, using processing oil along with VitaX caused more intrusion of the VitaX into the particles core. On the other hand, using VitaX increased the thickness of the devulcanized layer in the partially devulcanized particles. In the case of using DPDS, it just acted on the surface of the rubber particles this limited its devulcanizing performance. The results were in good correlation with CLD and sol fraction results.
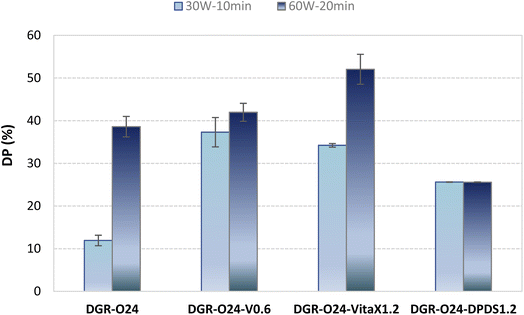 | ||
| Fig. 4 Devulcanization percent of the samples devulcanized in presence of different chemical agents. | ||
3.4. Effect of sonication method
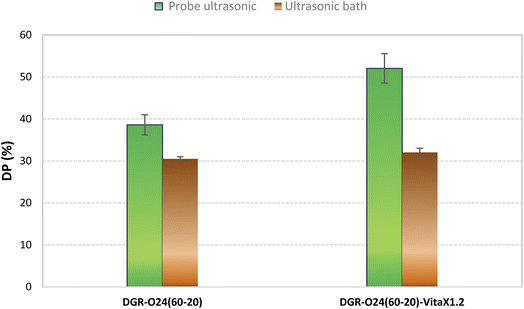
In previous researches, ultrasonic bath mostly used to devulcanize the crumbed rubber particles. In this study, the performance of probe sonication was compared to sonication bath. Results are given in Fig. 5. It is obvious that probe sonication illustrated higher devulcanization performances than ultrasonic bath. It was attributed to the higher energies induced to the particles in probe sonication and also direct contact between rubber particles and the sonication source (i.e. probe).3.5. Characterization
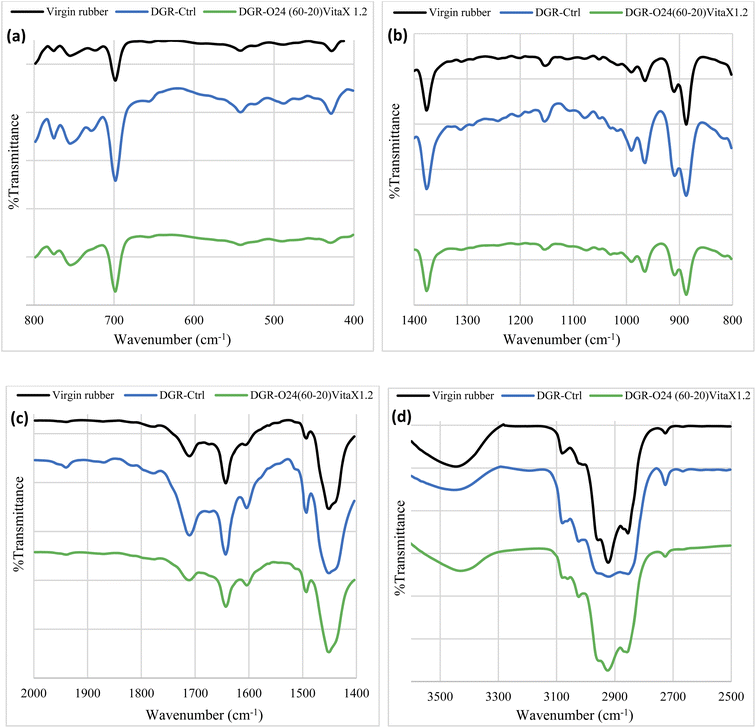 | ||
| Fig. 6 Pyrolysis-FTIR spectra of different samples; (a) from 400 to 600 cm−1, (b) from 600 to 1200 cm−1, (c) from 1200 to 1550 cm−1 and (d) from 1550 to 1800 cm−1. | ||
| Characterized peak | Wavenumber (cm−1) | Components | Ref. |
|---|---|---|---|
| S–S stretching | 540 | Crosslinks | 7,12,15 |
| C–S stretching | 603 | Crosslinks | 7,26 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending C bending |
870 | NR | 15 |
| C–C bending | 900–1000 | NR &SBR | 15 |
| C–C stretching | 1018 | Carbon black | 5,7,15 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching C stretching |
1645 | NR/SBR | 12,15,26 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
1720 | CBS-stearic acid | 5,9,12 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H C–H |
3070 | — | 15,21,26 |
Comparing the spectrums of the virgin NR/SBR and DGR-Ctrl samples showed that rubber grinding and then mechanical devulcanization affected the rubber network structure according to the peaks appeared at wavelength of 2800 to 3200 cm−1. It is apparent that the intensity of the peaks related to CH and CH2 groups are reduced and CH3 has been added after grinding. This change simply confirmed chain scission and creation of new CH3 groups at the end of the broken chains. The peak at 3500 cm−1 is related to tension vibration of hydroxyl groups of carbon black. Moreover, the presence of a sharp peak at 1650 cm−1 (bending vibration of OH groups) could also confirm the presence of moisture in the sample. The observed peak at 1690 cm−1 was related to –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– groups in the virgin and control rubbers. The twin peaks at 1450 and 1550 cm−1 should be attributed to the benzene rings in the SBR. Peaks in the range of 750 to 1200 cm−1 was corresponded to the flexural vibration of alkane compounds (–CH2–CH2–) which increased by grinding of vulcanized rubber. This confirmed occurrence of chain scission and formation of smaller chains in the mechanically devulcanized sample (DGR-Ctrl). The increase in the intensity of the peaks in DGR-O24(60-20)-VitaX1.2 sample confirmed more failure of the rubber network after sono-chemical devulcanization.
CH– groups in the virgin and control rubbers. The twin peaks at 1450 and 1550 cm−1 should be attributed to the benzene rings in the SBR. Peaks in the range of 750 to 1200 cm−1 was corresponded to the flexural vibration of alkane compounds (–CH2–CH2–) which increased by grinding of vulcanized rubber. This confirmed occurrence of chain scission and formation of smaller chains in the mechanically devulcanized sample (DGR-Ctrl). The increase in the intensity of the peaks in DGR-O24(60-20)-VitaX1.2 sample confirmed more failure of the rubber network after sono-chemical devulcanization.
The peak appeared at 720 cm−1 was related to the –C–SH bonds which increased in devulcanized samples. This confirmed that more breakage happened in the cross-links (i.e. –S–S– bonds) in presence of sonication.
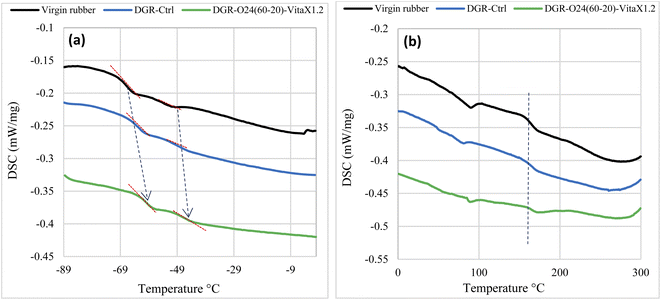
As it is seen, the Tg values for both NR (i.e. Tg1) and SBR (i.e. Tg2) parts were increased in the mechanically and sono-chemically devulcanized samples compared to the uncured virgin rubber. This was attributed to the networked structure that created due to the primary and secondary vulcanization steps that was performed on the devulcanized samples. The exothermic peak observed at about 160 °C in all samples was corresponded to the curing temperature of the rubbers. It is obviously seen that this peak weakened in the mechanically and sono-mechanically samples due to lower cross-links occurred in these samples compared to the virgin rubber.
According to the results, the glass transition temperature increased in the DGR-O24(60-20)-Vitax1.2 compared to the DGR-Ctrl sample. This was attributed to presence of higher amounts of low molecular weight chains (i.e. broken chains) in the mechanically devulcanized sample. This confirms more crosslinks breakage instead of chain scission in the sono-chemically devulcanized sample.
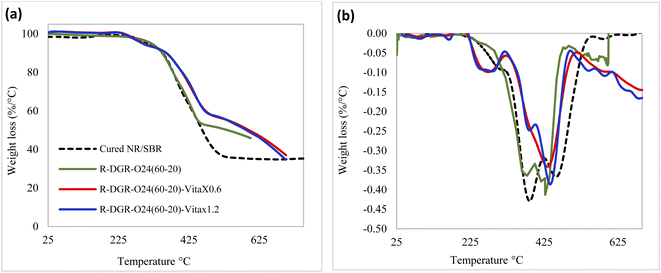 | ||
| Fig. 8 The effect of VitaX on thermogravimetric properties of the samples; (a) TGA curve and (b) DTG curve. | ||
| Compounds | Data | Low molecular weight organic mater | NR part | SBR part | Inorganic residues |
|---|---|---|---|---|---|
| ΔT [°C] | 35 to 320 | 320 to 425 | 425 to 540 | 540 to 750 | |
| Cured NR/SBR | Δm [%] | 5.90 | 34.21 | 27.24 | 35.8 |
| Tmax [°C] | — | 386 | 457 | — | |
| R-DGR-O24(60-20) | Δm [%] | 5.75 | 29.64 | 14.85 | 49.69 |
| Tmax [°C] | — | 383.03 | 437.27 | — | |
| R-DGR-O24(60-20)-Vitax0.6 | Δm [%] | 7.56 | 19.64 | 18.95 | 54.7 |
| Tmax [°C] | — | 389.96 | 433.92 | — | |
| R-DGR-O24(60-20)-Vitax 1.2 | Δm [%] | 7.9 | 18.62 | 20.26 | 54.53 |
| Tmax [°C] | — | 384.46 | 439.98 | — |
The temperature regions of 320–425 °C and 425–540 °C was attributed to degradation of NR and SBR parts of samples, respectively. It is obvious that both NR and SBR parts of sonicated samples showed lower weight losses compared to the cured NR/SBR sample due to the fractures occurred in polymers chains. It is also seen that the weight loss related to NR part was lower in the devulcanized samples in presence of VitaX compared to DGR-O24(60-20) sample. This shows that in presence of VitaX, fractures occurred mostly in the SBR network instead of NR structure.
Higher thermal stabilities were observed in revulcanized samples due to the presence of intact-crosslinked core of particles that acted as reinforcing filler in devulcanized matrices.
Fig. (9b) shows DTG curves for the samples. The results confirmed the higher thermal stabilities of the devulcanized samples compared to the cured NR/SBR sample due to the increment in the degradation temperatures of the NR and SBR parts.
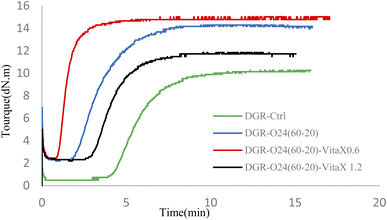
3.6. Curing properties
The curing properties of the samples was evaluated by ODR. Results are shown in Fig. 9 and Table 7. The results showed that MH − ML content which is a representative of cross-link density (CLD) increased for sonication assisted devulcanized samples compared to the mechanically devulcanized sample. It was attributed to the more breakages that happened in the cross-links in this sample that made more chances for revulcanization of the compound during ODR test. However, the curing rate index (CRI) was higher in the sonicated samples in presence of VitaX. It was also found that by addition of 0.6 phr of VitaX to the sonicated sample, the CRI increased considerably and the scorch time deceased very much. This finding proved the positive effect of lower contents of VitaX on desired devulcanization of NR/SBR compounds.| Compounds | ML [dN m] | MH [dN m] | MH − ML [dN m] | t90 [Minute] | CRI (%) |
|---|---|---|---|---|---|
| DGR-Ctrl | 0.49 | 10.26 | 9.8 | 8.09 | 29.54 |
| DGR-O24(60-20) | 2.2 | 14.28 | 12.1 | 5.5 | 27.72 |
| DGR-O24(60-20)-Vitax0.6 | 2.32 | 15.02 | 12.7 | 2.53 | 62.13 |
| DGR-O24(60-20)-Vitax1.2 | 2.2 | 11.84 | 9.7 | 5.3 | 36.03 |
3.7. Mechanical properties
Fig. 10 shows the comparative tensile properties of the rubber samples. It is clearly seen that the tensile strength of DGR-O24(60-20)-VitaX1.2 is so close to the virgin sample that illustrates effect of VitaX on suitable devulcanization of sonicated rubbers (i.e. it caused the least rubber chain scission). Moreover, this chemical agent increased cross-linking chance in the revulcanization process which caused increment in the tensile strength. On the other hand, in the samples which contained lower concentration of VitaX, the mechanical properties declined because of the high CLD and toughness and low flexibility.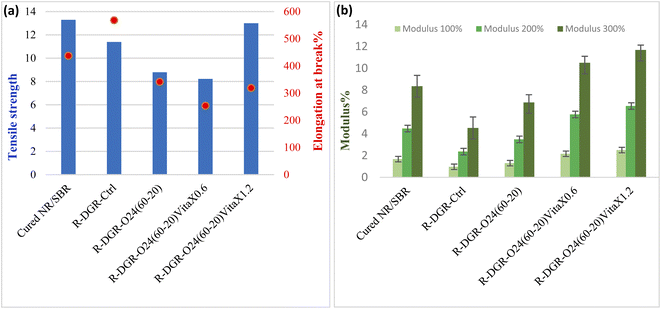 | ||
| Fig. 10 The tensile properties of the re-vulcanized samples; (a) tensile strength/elongation at break and (b) modulus. | ||
Based on the results, modulus of the samples were obtained. For the effectively devulcanized sample, the modulus content confirmed the TS results. In comparison to the virgin sample, the modulus of VitaX containing samples was considerably increased because of their higher CLD and toughness. Therefore the cross-links breakage were less selective in DGR-O24 (60-20) sample compared to the VitaX containing samples. With regards to elongation at breaks (EBs), it was vivid that DGR-O24 (60-20)-VitaX0.6 with non-selective devulcanization and high toughness resulted in lower EB (63%) than DGR-O24(60-20)-VitaX1.2. All in all, devulcanaized samples show acceptable mechanical properties and confirmed other tests results.
4. Conclusions
A NR/SBR compound was cured and devulcanized through probe sonication as model tire rubber. Chemical agents were also used along with sonication. Based on the results, the following conclusions were made:• It was claimed that probe sonication is a promising method for recycling of tire rubber due to its facility and effectiveness compared to other methods (e.g. microwave assisted devulcanization).
• It was found that devulcanization percent (DP) enhanced from 15 to 32% by pre-immersion of ground rubber particles in oil. However, sonication in all the liquids improved DP.
• There is a direct relationship between DP and sonication power, while no specific relation was found between DP and sonication time. Sonication at 60 W/20 min caused 6.34% increment in the DP compared to the sample sonicated for 45 W/20 min.
• Devulcanization percent was improved 100% by using 1.2 phr of VitaX instead of DPDS. It was also about 20% more than DP of the sample contained 0.6 phr of VitaX.
• It was found that probe sonication caused about 27% increment in the DP of the samples in absence of VitaX while it increased up to 65% in presence of VitaX.
• The highest cure rate index (more than 60%) and the lowest scorch time (about 1 min) was obtained in the case of using 0.6 phr of VitaX.
• It was found that devulcanization by sonication caused decrement in the tensile strength (more than 40%) of virgin rubber/devulcanized rubber blend (at 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 weight ratio), except for the sample contained 1.2 phr of VitaX that showed the same tensile strength as cured NR/SBR sample.
50 weight ratio), except for the sample contained 1.2 phr of VitaX that showed the same tensile strength as cured NR/SBR sample.
Conflicts of interest
It hereby declares that there is no conflict of interests with any person, research centers or companies regarding this article.Acknowledgements
The authors would like to thank Engineers Mohammad Amin Ghowsi and Mostafa Vahdatbin for their helps in this research.References
- A. Saikia, Modelling the Vulcanization Reaction of Devulcanized Rubber, Master's thesis, University of Waterloo, 2014.
- F. Valentini, A. Dorigato and A. Pegoretti, Evaluation of the Role of Devulcanized Rubber on the Thermo-mechanical Properties of Polystyrene, J. Polym. Environ., 2020, 28, 1737–1748 CrossRef CAS.
- A. J. Bowles, G. D. Fowler, C. O'Sullivan and K. Parker, Sustainable rubber recycling from waste tyres by waterjet: A novel mechanistic and practical analysis, Sustainable Mater. Technol., 2020, 25, e00173 CrossRef CAS.
- A. Fazli and D. Rodrigue, Waste rubber recycling: A review on the evolution and properties of thermoplastic elastomers, Materials, 2020, 13, 782 CrossRef CAS PubMed.
- O. Buitrago-Suescún and R. Britto, Devulcanization of ground tire rubber: Thermo-oxidation followed by microwave exposure in the presence of devulcanizing agent, Iran. Polym. J., 2020, 29, 553–567 CrossRef.
- T. Lin, J. Ke, J. Wang, C. Lin and X. Wu, Decrosslinking of zinc diacrylate cured epoxidized natural rubber via selective cleavage of carbon–oxygen bond, J. Appl. Polym. Sci., 2020, 137, 49175 CrossRef CAS.
- E. Akca, A. Gursel and N. Sen, A review on devulcanization of waste tire rubber, Period. Eng. Nat. Sci., 2018, 6, 154–160 Search PubMed.
- A. Fazli and D. Rodrigue, Recycling waste tires into ground tire rubber (GTR)/rubber compounds: a review, J. Compos. Sci., 2020, 4, 103 CrossRef CAS.
- M. R. Islam, M. N. Islam, N. N. Mustafi, M. A. Rahim and H. Haniu, Thermal recycling of solid tire wastes for alternative liquid fuel: the first commercial step in Bangladesh, Procedia Eng., 2013, 56, 573–582 CrossRef CAS.
- X. Xu, Z. Leng, J. Lan, W. Wang, J. Yu, Y. Bai and J. Hu, Sustainable practice in pavement engineering through value-added collective recycling of waste plastic and waste tyre rubber, Engineering, 2021, 7, 857–867 CrossRef CAS.
- K. Korniejenko, B. Kozub, A. Bąk, P. Balamurugan, M. Uthayakumar and G. Furtos, Tackling the Circular Economy Challenges—Composites Recycling: Used Tyres, Wind Turbine Blades, and Solar Panels, J. Compos. Sci., 2021, 5, 243 CrossRef CAS.
- S. Ramarad, C. T. Ratnam, Y. Munusamy, N. A. A. Rahim and M. Muniyadi, Thermochemical compatibilization of reclaimed tire rubber/poly (ethylene-co-vinyl acetate) blend using electron beam irradiation and amine-based chemical, J. Polym. Res., 2021, 28, 1–19 CrossRef.
- G. Xu, P. Kong, Y. Yu, J. Yang, M. Zhu and X. Chen, Rheological properties of rubber modified asphalt as function of waste tire rubber reclaiming degree, J. Cleaner Prod., 2022, 332, 130113 CrossRef CAS.
- M. Belhaj, P. Vacková and J. Valentin, Evaluation of an Asphalt Mixture Containing a High Content of Reclaimed Asphalt and Different Crumb Rubber Modified Binders, Slovak J. Civ. Eng., 2021, 29, 22–30 CrossRef.
- H. Wen, M. Wang, S. Luo, Y. Zhou and T. Liu, Mechanical Properties of Silicone Rubber Enhanced Microcellular EPDM Foams Based on Supercritical CO2 Foaming Technology, Macromol. Mater. Eng., 2021, 306, 2100310 CrossRef CAS.
- B. Algaily, W. Kaewsakul, S. S. Sarkawi and E. Kalkornsurapranee, Enabling reprocessability of ENR-based vulcanisates by thermochemically exchangeable ester crosslinks, Plast., Rubber Compos., 2021, 50, 315–328 CrossRef CAS.
- L. Wang, J. Zhang, Y. Sun, T. Zhang, L. Wang, J. Wang and Q. Fu, Green preparation and enhanced gas barrier property of rubber nanocomposite film based on graphene oxide-induced chemical crosslinking, Polymer, 2021, 225, 123756 CrossRef CAS.
- L. E. Alonso Pastor, K. C. Núñez Carrero, J. Araujo-Morera, M. Hernández Santana and J. M. Pastor, Setting Relationships between Structure and Devulcanization of Ground Tire Rubber and Their Effect on Self-Healing Elastomers, Polymers, 2022, 14, 11 CrossRef CAS PubMed.
- J. Huang, Engineering Dynamic Bonds into Elastomeric Materials-Reinforcement and Reprocessability, Doctoral dissertation, Ghent University, 2021.
- M. Zadshir, Modeling and Experimental Study of Thermal Management for Infrastructure Surface Materials, Columbia University, 2021 Search PubMed.
- E. Akca, A. Gursel and N. Sen, A review on devulcanization of waste tire rubber, Period. Eng. Nat. Sci., 2018, 6, 154–160 Search PubMed.
- L. Asaro, M. Gratton, S. Seghar and N. Hocine, Recycling of rubber wastes by devulcanization, Resour., Conserv. Recycl., 2018, 133, 250–262 CrossRef.
- S. Seghar, L. Asaro, M. Rolland-Monnet and N. Hocine, Thermo-mechanical devulcanization and recycling of rubber industry waste, Resour., Conserv. Recycl., 2019, 144, 180–186 CrossRef , ISSN 0921-3449.
- X. Zhang, P. Saha, L. Cao, H. Li and J. Kim, Devulcanization of waste rubber powder using thiobisphenols as novel reclaiming agent, Waste Manage., 2018, 78, 980–991 CrossRef CAS.
- S. O. Movahed, A. Ansarifar and S. Estagy, Review of the reclaiming of rubber waste and recent work on the recycling of ethylene–propylene–diene rubber waste, Rubber Chem. Technol., 2016 Mar, 89, 54–78 Search PubMed.
- K. Formela, A. Hejna, L. Zedler, X. Colom Fajula and F. J. Cañavate Ávila, Microwave treatment in waste rubber recycling–recent advances and limitations, eXPRESS Polym. Lett., 2019, 13, 565–588 CrossRef CAS.
- M. Vahdatbin and M. Jamshidi, Using chemical agent in microwave assisted devulcanization of NR/SBR blends: An effective recycling method, Resour., Conserv. Recycl., 2022, 179, 106045 CrossRef.
- Z. Tang, Y. Liu, Q. Huang, J. Zhao, B. Guo and L. Zhang, A real recycling loop of sulfur-cured rubber through transalkylation exchange of C–S bonds, Green Chem., 2018, 20, 5454–5458 RSC.
- T. Liang and A. I. Isayev, Ultrasonic Extrusion of NR Gum and Its Effect on the Structure and Properties of Unfilled and Silica Filled NR, Polym. Eng. Sci., 2020, 60, 925–934 CrossRef CAS.
- A. Isayev, S. P. Yushanov, S. H. Kim and V. Y. Levin, Ultrasonic devulcanization of waste rubbers: Experimentation and modeling, Rheol. Acta, 1996, 35, 616–630 CrossRef CAS.
- A. Isayev, Inventor; University of Akron, assignee. Single and twin screw extruders with ultrasound horns for decrosslinking and devulcanization, US pat., 10,465,059, 2019 Search PubMed.
- T. Liang and A. I. Isayev, Effect of ultrasonic extrusion of star styrene butadiene rubber on properties of carbon black and silica filled compounds and vulcanizates, J. Appl. Polym. Sci., 2019, 136, 47451 CrossRef.
- T. Liang and A. I. Isayev, Structure and properties of ultrasonically extruded SBR/BR blends and prepared compounds and vulcanizates with various fillers, J. Elastomers Plast., 2019, 51, 603–625 CrossRef CAS.
- T. Liang and A. I. Isayev, The effect of ultrasonic extrusion on the structure and properties of BR gum and prepared compounds and vulcanizates, Adv. Ind. Eng. Polym. Res., 2019, 2, 13–24 Search PubMed.
- R. Saputra, R. Walvekar, M. Khalid, K. Shahbaz and S. Ramarad, Effective devulcanization of ground tire rubber using choline chloride-based deep eutectic solvents, J. Environ. Chem. Eng., 2019, 7, 103151 CrossRef.
- D. Kim, F. J. Y. Shiu and T. F. Yen, Devulcanization of scrap tire through matrix modification and ultrasonication, Energy Sources, 2003, 25, 1099–1112 CrossRef CAS.
- I. Janajreh, M. Hussain, S. Elagroudy and K. Moustakas, Recycled tire granular for playground in hot regions: technical assessment, J. Mater. Cycles Waste Manage., 2021 Jan, 23, 107–120 Search PubMed.
- L. Gschwind, C. S. Jordan and N. Vennemann, Devulcanization of ethylene propylene diene monomer rubber waste. Effect of diphenyl disulfide derivate as devulcanizing agent on vulcanization, and devulcanization process, J. Appl. Polym. Sci., 2022, 10, 52141 CrossRef.
- S. B. B. Vega, L. Montero, S. Lincoln and N. Agullo, Control of vulcanizing/devulcanizing behavior of diphenyl disulfide with microwaves as the heating source, J. Appl. Polym. Sci., 2008, 108, 1969–1975 CrossRef.
- L. Asaro, M. Gratton, S. r. Segha and N. A. Hocine, Devulcanization of natural rubber industry waste in supercritical carbon dioxide combined with diphenyl disulfide, Waste Manage., 2020, 118, 647–654 CrossRef CAS PubMed.
- American Society for Testing and Materials, ASTM D6814, Standard Test Method for Determination of Percent Devulcanization of Crumb Rubber Based on Crosslink Density, ASTM International, 2018 Search PubMed.
- American Society for Testing and Materials, ASTM D297, Standard Test Methods for Rubber Products—Chemical Analysis, ASTM International, 2019 Search PubMed.
- American Society for Testing and Materials, ASTM D2084, Standard Test Method for Rubber Property—Vulcanization Using Oscillating Disk Cure Meter, ASTM International, 2016 Search PubMed.
- American Society for Testing and Materials, ASTM D412, Standard Test Methods for Vulcanized Rubber and Thermoplastic Elastomers—Tension, ASTM International, 2016 Search PubMed.
| This journal is © The Royal Society of Chemistry 2022 |