Preparation of an aminographene–aliphatic hydroxyl-terminated polysiloxane hybrid for synergistic enhancement of the mechanical and tribological performance of monomer casting nylon 6
Chengjie
Li
 *a,
Minghui
Guo
a,
Ying
Dai
a,
Peikuan
Xu
a,
Bin
Shi
a,
Dewang
Hou
a and
Ruiguang
Li
b
*a,
Minghui
Guo
a,
Ying
Dai
a,
Peikuan
Xu
a,
Bin
Shi
a,
Dewang
Hou
a and
Ruiguang
Li
b
aJiangsu Key Laboratory of Marine Bioresources and Environment, School of Environmental and Chemical Engineering, Jiangsu Ocean University, Lianyungang 222005, China. E-mail: licj_poly@jou.edu.cn
bState Key Laboratory of Polymer Materials Engineering, Polymer Research Institute of Sichuan University, Chengdu 610065, China
First published on 6th October 2021
Abstract
A UFG–AHPDMS hybrid with covalent linkages was prepared by chemical grafting and reduction reaction of GO and urea, and then through reaction with AHPDMS using TDI as a bridge in CL melt. MC PA6/UFG–AHPDMS nanocomposites were synthesized via in situ polymerization from the stable colloidal suspension of UFG–AHPDMS/CL melt. AHPDMS molecules were confirmed to be grafted onto UFG layers through the aliphatic hydroxyl groups of AHPDMS and amino groups of UFG, and the UFG–AHPDMS hybrid exhibited better distribution in matrix with strong interfacial bonding and no phase separation occurring. In comparison with neat MC PA6, the introduction of UFG–AHPDMS resulted in a 20% and 24% increase in tensile strength and impact strength for the MC PA6/UFG–AHPDMS nanocomposite, higher than those of the MC PA6/UFG and MC PA6/AHPDMS composites, indicating the reinforcing and toughening effect of UFG–AHPDMS. Meanwhile, the friction coefficient and specific wear rate respectively decreased by more than 58% and 49% with a relatively smooth worn surface and narrower worn depth distribution, confirming the excellent synergistic friction reduction and anti-wear effect of UFG–AHPDMS on MC PA6. Moreover, a uniform and continuous UFG–AHPDMS hybrid tribofilm was formed by tribochemical reaction during the friction process, and was responsible for the reduction of the friction coefficient and wear rate.
1. Introduction
Monomer casting nylon 6 (MC PA6) is an important type of engineering plastic, and was synthesized by anionic polymerization of the ε-caprolactam monomer. It possesses outstanding properties such as splendid mechanical performance and superior self-lubricating performance owing to its high molecular weight, which enables it to be widely used as gears, bearings and shaft sleeves in the mining, automotive and aerospace fields,1–3 gradually replacing metallic materials. However, the application of pure MC PA6 is limited by its low wear resistance and high friction coefficient under harsh working conditions at high load and friction velocity, especially in high-end fields.4 Therefore, efforts must be made to improve the tribological behavior to advance the application adaptability.Wear resistance and lubrication are two aspects of tribology. Recently, various inorganic fillers such as graphene, fibers, boron nitride, molybdenum disulfide and so forth have been added to improve the wear resistance of MC PA6,5–8 and liquid lubricants such as ionic liquids, paraffin wax and polysiloxane have been proved to reduce friction for enhancing the self-lubricity of MC PA6.9–11 However, it was difficult to balance both anti-wear and self-lubrication properties by direct addition of these solid or liquid components. In addition, the mechanical properties of the composites usually deteriorated due to the poor interfacial interactions caused by high fillings.
As a typical 2D material with a one-atom lamellar thickness of sp2 carbon atoms arranged in a hexagonal lattice, graphene currently attracts great attention because of its unique mechanical strength, excellent electrical conductivity and remarkable self-lubricating properties due to its atomically smooth surface.12,13 Graphene oxide (GO) is a derivative of graphene with many oxygen-containing groups such as hydroxyl, epoxy and carboxyl on the surface, which can be easily functionalized. Incorporation of graphene into polymer matrices has been highly pursued to improve tribological behavior. Nevertheless, several studies showed that the anti-wear properties of polymer/graphene composites were much lower than expected, and in some cases even decreased.14,15 Based on this, combining graphene and other liquid lubricants appears attractive to endow polymer composites with excellent tribological performance that is intrinsic to both components. Huang et al. prepared MC PA6 composites using melamine foam as a 3D template to adsorb GO and paraffin wax (PW), which showed an 82% and 84% decrease in friction coefficient and wear rate due to the synergistic lubrication effect of PW/GO.16 Ruan et al. prepared ionic liquid modified graphene (ILFG) by a π–π stacking strategy, which was added into a polyimide (PI) matrix to obtain ILFG/PI composites, and the friction coefficient and specific wear rate of the composite decreased by 38.2% and 25% at 0.4 wt% ILFG content compared with those of neat PI under dry sliding.17 Zhao et al. synthesized oil-impregnated MC PA6 reinforced with GO and lanthanum(III) chloride (LaCl3). GO was crosslinked through coordination and electrostatic interactions between La3+ and carboxyl on GO in the composites, which exhibited excellent mechanical and tribological properties, much better than those of composites from only the addition of oil or GO.18 Chen et al. prepared polyoxymethylene (POM)/graphene–perfluoropolyether (PFPE) composites via conventional extrusion processing, and POM/PFPE chains were intercalated into graphene layers by formation of hydrogen bonding interactions, contributing to improvement of lubricity and anti-wear performance.19 These examples clearly demonstrated the synergistic effect of both components in enhancing the tribological performance of composites. However, graphene suffers from aggregation due to strong van der Waals forces and phase separation occurs due to the immiscibility of liquid lubricants such as inert oil, resulting in poor dispersion in the polymer matrix.20
Aliphatic hydroxyl-terminated poly(dimethylsiloxane) (AHPDMS) is an environment-friendly material, exhibiting many intriguing properties including molecular flexibility, good hydrophobicity and heat resistance, and self-lubricating properties, which mainly arise from its Si–O–Si skeleton and inorganic Si–O bonds.21,22 Meanwhile, AHPDMS with aliphatic hydroxyl groups as end groups has high reactivity, which can be functionalized by reacting with amino, carboxyl and isocyanate groups.
In our work, firstly, amino groups were grafted onto the GO surface by a one-step chemical and reduction reaction with urea to obtain urea-functionalized GO (UFG), and then through reaction with AHPDMS by virtue of toluene-2,4-diisocyanate (TDI) as a covalent bridge in caprolactam (CL) melt, and a UFG–AHPDMS hybrid with covalent linkages was prepared. This feature simultaneously enables good dispersion of UFG–AHPDMS in MC PA6 matrix. Secondly, a stable colloidal suspension of UFG–AHPDMS in melted CL at 70 °C was obtained by ultrasonic dispersion, and MC PA6/UFG–AHPDMS nanocomposites were in situ synthesized by anionic ring-opening polymerization in the presence of TDI as a cocatalyst. The interfacial structure in the nanocomposite system and the synergistic reinforcing and friction-reducing effect of UFG–AHPDMS were explored. This work provided an effective method to achieve complementary performance with mechanical and tribological properties for polymer nanocomposites.
2. Experimental section
2.1 Materials
The caprolactam (CL) monomer was purchased from Sinopec Shijiazhuang Refining & Chemical Company. Graphene oxide (GO) was supplied by Angxing New Carbon Materials Co., Ltd Co. Ltd. (Changzhou, China). Aliphatic hydroxyl-terminated poly(dimethylsiloxane) (AHPDMS) (MW: 1750) was obtained from Mingyi Silicone Co. Ltd. (Anhui, China). Sodium hydroxide (NaOH) of analytical grade was purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). Toluene-2,4-diisocyanate (TDI) was obtained from Huaxia Chemical Reagent Co. Ltd. (Chengdu, China).2.2 Preparation of the UFG–AHPDMS hybrid
200 mg GO powder was dispersed in 100 ml deionized water under ultrasonication for 30 min to form a stable GO/H2O suspension. A certain amount of urea (200 wt% GO) was dissolved in deionized water to form a 5 mg ml−1 solution. Then, the above solution was mixed at 95 °C under stirring and refluxing for 3 h and the mixed solution turned black.23,24 Afterwards, the dispersion was centrifuged and washed to neutral to remove the residual urea. The resulting solids were dried at 50 °C, and finally urea-functionalized GO was obtained, which was designated as UFG.The preparation of UFG–AHPDMS was performed as follows: 4 mol caprolactam (CL) monomer was melted at 70 °C as a reaction solvent, and a certain amount of toluene-2,4-diisocyanate (TDI) (200 wt% UFG) was added and stirred. Then, UFG was added and further sonicated for 1 h to promote UFG dispersion and the reaction of TDI and UFG, and aliphatic hydroxyl-terminated poly(dimethylsiloxane) (AHPDMS) (the mass ratio of UFG and AHPDMS was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was introduced into the mixed system sonicated for 1 h to covalently react with UFG to form a UFG–AHPDMS hybrid using TDI as a bridge.
1) was introduced into the mixed system sonicated for 1 h to covalently react with UFG to form a UFG–AHPDMS hybrid using TDI as a bridge.
2.3 In situ synthesis of MC PA6/UFG-AHPDMS nanocomposites
The above CL melt/UFG–AHPDMS mixed dispersion was put into a flask and heated to 140 °C, and variable amounts of the UFG–AHPDMS hybrid (0.2 wt%, 0.4 wt%, 0.6 wt%, 0.8 wt% and 1.0 wt% based on CL) were added. The melt was refluxed under vacuum for 30 min to remove traces of water, and 0.03 mol NaOH as a catalyst was added. Then, the melt was vacuumed again for 20 min and 0.03 mol TDI as an initiator was added. Finally, the mixture was cast into a preheated mould at 170 °C, the reaction was maintained for 1 h, and thus MC PA6/UFG–AHPDMS nanocomposites were obtained.4,25,26 For comparison, neat MC PA6, MC PA6/UFG and MC PA6/AHPDMS composites were prepared as control experiments according to the above procedure.2.4 Characterization
Fourier-transform infrared (FT-IR) spectra of GO, UFG and UFG-AHPDMS were collected with a Nicolet iS10 spectrometer (U.S.A.). XPS analysis was conducted with a PE2400 Series II spectrometer (U.S.A.). Raman spectroscopy was performed using a Renishaw Invia Raman microprobe (Britain). TGA was performed on a STA 449 F3 Jupiter® analyzer (Germany). XRD analysis was conducted with a Rigaku D/max IIIB X-ray diffractometer (Japan) using Cu Kα radiation (λ = 0.154 nm). Atomic force microscopy (AFM) of GO, UFG and UFG–AHPDMS and the worn surfaces was performed using a Shimadzu SPM-9700 scanning probe microscope (Japan) in tapping mode. SEM and TEM were conducted using a JEOL JSM-5900LV SEM and a JEM 100CX II TEM (Japan), and energy-dispersive X-ray spectroscopy (EDS) was carried out at the same time.The mechanical properties were tested with a 5567 universal material testing machine from Instron Co. (U.S.A.) with an extension rate of 10 mm min−1. The notched Charpy impact strength was tested with a ZBC-4B impact testing machine from Xinsansi Co. (Shenzhen, China). The tribological performance was measured with a block sample size of 30 mm × 7 mm × 6 mm under dry sliding by using an M-200A machine from Guance Instrument Co. (China) according to the standard GB/T 3960-2017. A velocity of 200 r min−1 and load of 196 N were applied. The specific wear rate (W) was calculated according to the equations:27
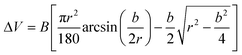 | (1) |
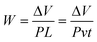 | (2) |
3. Results and discussion
3.1 Structure construction of the UFG–AHPDMS hybrid in MC PA6 matrix
UFG was firstly prepared by a one-step reaction via chemical grafting and reduction of GO with urea, with amino groups being introduced onto the UFG surface. The UFG–AHPDMS hybrid was ultrasonically prepared using TDI as a covalent bridge in CL melt. Afterwards, MC PA6/UFG–AHPDMS nanocomposites were fabricated through an in situ anionic ring-opening polymerization mechanism using NaOH as a catalyst and TDI as an initiator. The preparation procedure of the UFG–AHPDMS hybrid and MC PA6/UFG–AHPDMS nanocomposites is shown in Fig. 1.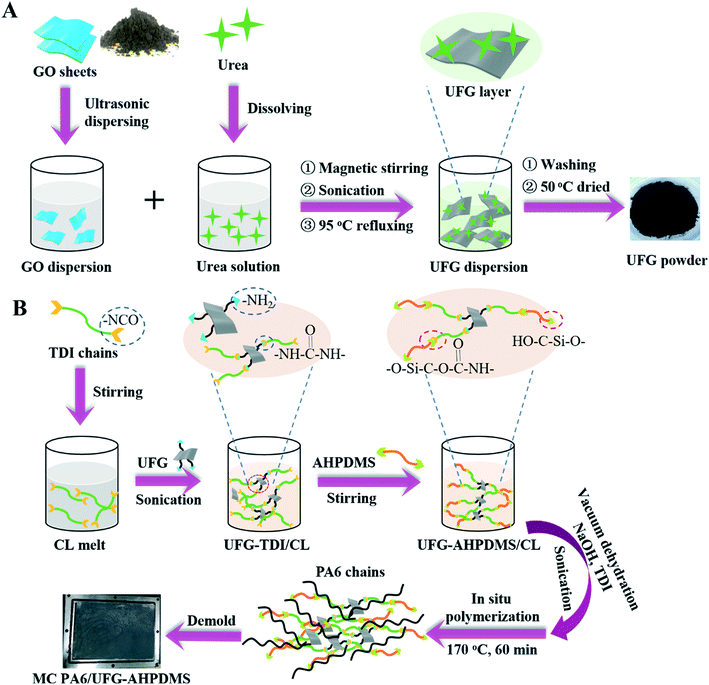 | ||
| Fig. 1 (A) Preparation process of the UFG–AHPDMS hybrid and (B) MC PA6/UFG–AHPDMS nanocomposites via in situ polymerization. | ||
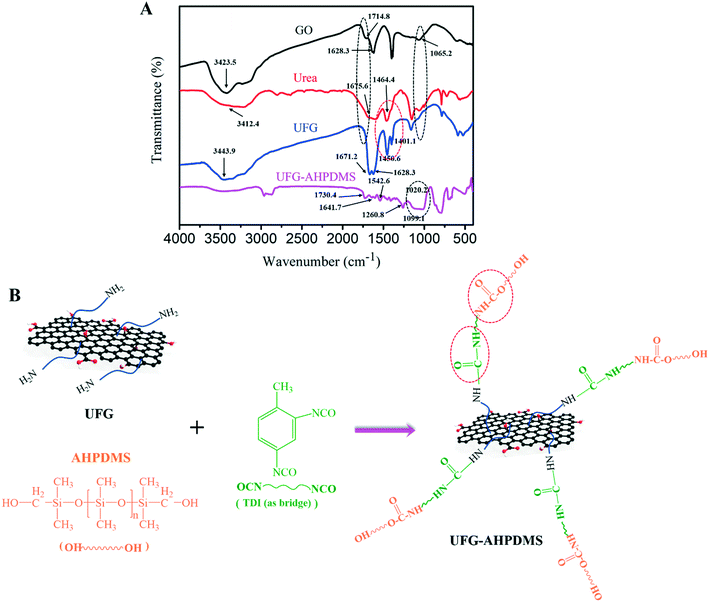
The structural features of GO, urea, UFG and UFG–AHPDMS were analyzed by FT-IR, as shown in Fig. 2A. GO exhibited strong peaks at 3423.5 cm−1, 1628.3 cm−1 and 1065.2 cm−1, which were attributed to O–H stretching vibrations, O–H bending vibrations/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C skeletal ring vibrations and C–O–C stretching vibrations,28 respectively, and the characteristic peak at 1727 cm−1 corresponded to C
C skeletal ring vibrations and C–O–C stretching vibrations,28 respectively, and the characteristic peak at 1727 cm−1 corresponded to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations at the edges of the GO layers, revealing the presence of oxygen-containing groups such as –OH, C
O stretching vibrations at the edges of the GO layers, revealing the presence of oxygen-containing groups such as –OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O–C on the GO surface. For urea, there were obvious absorption peaks at 3412.4 cm−1 and 1675.6 cm−1, which corresponded to N–H stretching vibrations of the primary amide group and C
O and C–O–C on the GO surface. For urea, there were obvious absorption peaks at 3412.4 cm−1 and 1675.6 cm−1, which corresponded to N–H stretching vibrations of the primary amide group and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations/N–H bending vibrations of the primary amine group, respectively.29 The characteristic peak at 1464.4 cm−1 was assigned to the stretching vibrations of C–N in urea chains. For UFG, the absorption peak at 3443.9 cm−1 attributed to –OH groups was remarkably weakened, and the peaks of C
O stretching vibrations/N–H bending vibrations of the primary amine group, respectively.29 The characteristic peak at 1464.4 cm−1 was assigned to the stretching vibrations of C–N in urea chains. For UFG, the absorption peak at 3443.9 cm−1 attributed to –OH groups was remarkably weakened, and the peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O–C stretching vibrations at 1714.8 cm−1 and 1065.2 cm−1 disappeared, indicating the decrease of oxygen groups due to the reduction effect of urea on GO. Meanwhile, new peaks at 1450.6 cm−1 and 1401.1 cm−1 were observed in comparison with GO, and were attributed to the symmetric and asymmetric stretching vibrations of C–N bonds, and an absorption peak at 1671.2 cm−1 ascribed to N–H bending vibrations of –NH2 was observed, suggesting the occurrence of reaction between GO and urea. The results undoubtedly indicated the chemical grafting and reduction effect of urea on GO. For UFG–AHPDMS, the characteristic peaks at 1730.4 cm−1, 1641.7 cm−1 and 1542.6 cm−1 were observed, which corresponded to the C
O and C–O–C stretching vibrations at 1714.8 cm−1 and 1065.2 cm−1 disappeared, indicating the decrease of oxygen groups due to the reduction effect of urea on GO. Meanwhile, new peaks at 1450.6 cm−1 and 1401.1 cm−1 were observed in comparison with GO, and were attributed to the symmetric and asymmetric stretching vibrations of C–N bonds, and an absorption peak at 1671.2 cm−1 ascribed to N–H bending vibrations of –NH2 was observed, suggesting the occurrence of reaction between GO and urea. The results undoubtedly indicated the chemical grafting and reduction effect of urea on GO. For UFG–AHPDMS, the characteristic peaks at 1730.4 cm−1, 1641.7 cm−1 and 1542.6 cm−1 were observed, which corresponded to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of carbamate, and the –NH–CO–NH– stretching vibrations and N–H bending vibrations of the secondary amide group, respectively. Meanwhile, the new peaks at 1260.8 cm−1, 1099.1 cm−1 and 1020.2 cm−1 were attributed to the structural vibrations of Si–CH3 groups and the stretching vibrations of Si–O–Si groups.30,31 The results demonstrated that the anchoring effect of AHPDMS molecules onto UFG layers was achieved through the reaction between the aliphatic hydroxyl groups of AHPDMS and the amino groups of UFG using TDI as a covalent bridge, as shown in Fig. 2B.
O stretching vibrations of carbamate, and the –NH–CO–NH– stretching vibrations and N–H bending vibrations of the secondary amide group, respectively. Meanwhile, the new peaks at 1260.8 cm−1, 1099.1 cm−1 and 1020.2 cm−1 were attributed to the structural vibrations of Si–CH3 groups and the stretching vibrations of Si–O–Si groups.30,31 The results demonstrated that the anchoring effect of AHPDMS molecules onto UFG layers was achieved through the reaction between the aliphatic hydroxyl groups of AHPDMS and the amino groups of UFG using TDI as a covalent bridge, as shown in Fig. 2B.
 | ||
| Fig. 2 (A) FTIR spectra of GO, urea, UFG and UFG–AHPDMS and (B) anchoring reactions between AHPDMS chains and UFG layers using TDI as a bridge. | ||
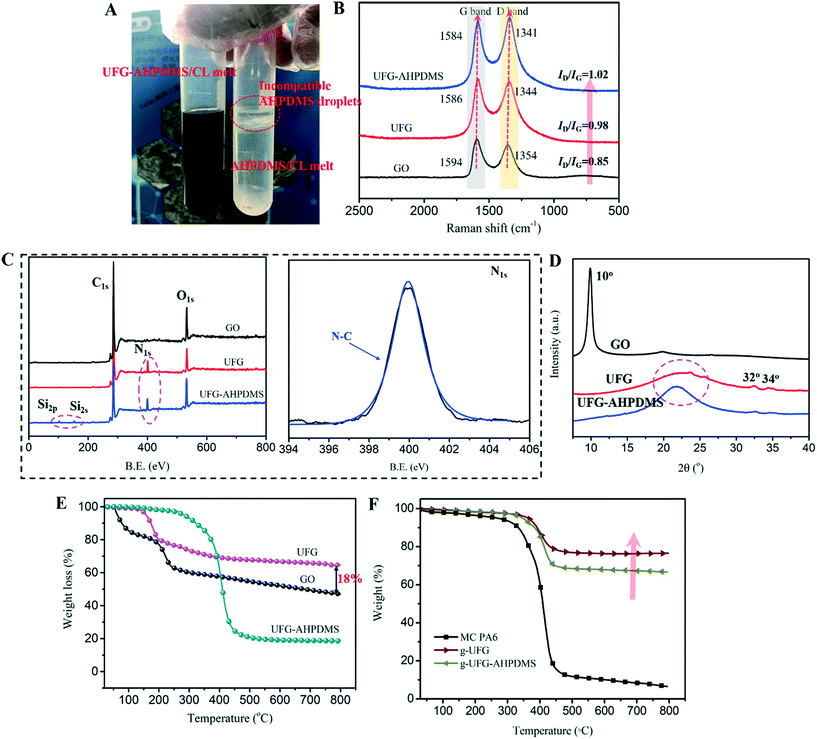
The dispersion of AHPDMS and UFG–AHPDMS in CL solvent is shown in Fig. 3A. It can be seen that AHPDMS was incompatible with CL and floated on the surface in the form of oil droplets, while UFG–AHPDMS was dispersed homogeneously in CL and no obvious oil droplets appeared, indicating that AHPDMS molecules were covalently linked with UFG layers, which promoted the dispersion of the hybrids in CL melt by ultrasonication. Fig. 3B shows the Raman spectra of the GO, UFG and UFG–AHPDMS samples. For GO, two characteristic peaks appeared at around 1354 cm−1 and 1594 cm−1, ascribed to the D band and G band due to lattice distortions/structural defects and the first-order scattering of the E2g vibration mode, respectively.32,33 For UFG and UFG–AHPDMS, the above D/G bands were still observed and the skeletal structure of GO was maintained. Meanwhile, the D/G bands shifted to 1341 and 1584 cm−1 from 1354 and 1594 cm−1 for GO, indicating that urea and AHPDMS chains exerted an influence on the lattice structure of GO with interfacial interactions after functionalization. The ID/IG values of UFG and UFG–AHPDMS were 0.98 and 1.02, slightly higher than the value of GO (0.85), indicating a slight increase of the disorder degree in the sp2 domain of lamellar GO by grafting of urea and AHPDMS.34–36
The elemental composition of the GO, UFG and UFG–AHPDMS samples was investigated by XPS, as shown in Fig. 3C. For GO, two typical peaks at 284.9 eV (C1s) and 533.4 eV (O1s) were detected. For UFG, besides the C and O elements, a new signal of N1s was also found at 401.2 eV, and the peak intensity of O1s significantly decreased, indicating the chemical grafting and reduction effect of urea on GO, resulting in the increase of the ratio of C/O elemental content (6.75), much higher than that of GO (5.62). For UFG–AHPDMS, besides the signals of C1s, O1s and N1s, two new peaks at 102.7 eV (Si2p) and 153.8 eV (Si2s) were observed, and the O1s signal peak intensity further increased, revealing the successful reaction of AHPDMS with UFG due to the covalent bridging effect of TDI, which agreed well with FT-IR analysis. The N1s spectra of UFG and UFG–AHPDMS presented an obvious N–C peak at 399.3 eV. The result confirmed that urea and AHPDMS molecular chains were grafted onto the GO surface. The XRD patterns of the GO, UFG and UFG–AHPDMS samples are illustrated in Fig. 3D. For GO, the peak at 2θ = 10° corresponded to the (001) plane. For UFG and UFG–AHPDMS, the (001) plane peak disappeared and a new characteristic diffraction peak at 2θ = 22–26° for the (002) plane of graphene was observed, which corresponded to the disordered peak of UFG,37 indicating the reduction effect of urea on GO. Meanwhile, the diffraction peaks of urea at 2θ = 32° and 34° were observed, indicating the chemical grafting of urea on GO layers.
The thermal properties of the GO, UFG and UFG–AHPDMS samples were analyzed, as shown in Fig. 3E. It was found that GO showed a weight loss of 20% in the range of 30–150 °C, which was attributed to the removal of water held between layers, and the second large weight loss of 30% in the range of 150–800 °C resulted from the degradation of oxygen groups such as –OH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –C–O–C groups on the GO surface. However, the weight loss of UFG remarkably decreased due to the reduction effect of urea, and the amount of oxygen groups obviously decreased. About a 20% weight loss was observed in the range of 150–200 °C, mainly ascribed to the degradation of urea on UFG layers, and finally the residual ratio increased by 18% at 800 °C in comparison with that of GO. For UFG–AHPDMS, an obvious weight loss in the range of 350–450 °C was observed due to the degradation of AHPDMS with high-energy Si–O–Si bonds, indicating the improvement of thermal stability of the UFG–AHPDMS hybrid. For analysis of the structure of MC PA6 nanocomposites, the MC PA6/UFG–AHPDMS sample was dissolved in formic acid, centrifuged and washed repeatedly with formic acid and toluene to remove free PA6 and AHPDMS molecules, and the resultant grafted sample was denoted as g-UFG–AHPDMS. For comparison, the grafted sample prepared from MC PA6/UFG was denoted as g-UFG. Fig. 3(F) shows the TGA curves of the MC PA6, g-UFG, and g-UFG–AHPDMS samples. It was found that the weight loss of MC PA6 occurred in the range of 350–450 °C, which was attributed to the degradation of the PA6 chain skeleton. In contrast, for g-UFG and g-UFG–AHPDMS, an obvious weight loss in the range of 350–450 °C was observed and the weight loss of g-UFG–AHPDMS was higher than that of g-UFG, which was attributed to the decomposition of the PA6 and AHPDMS components.
O and –C–O–C groups on the GO surface. However, the weight loss of UFG remarkably decreased due to the reduction effect of urea, and the amount of oxygen groups obviously decreased. About a 20% weight loss was observed in the range of 150–200 °C, mainly ascribed to the degradation of urea on UFG layers, and finally the residual ratio increased by 18% at 800 °C in comparison with that of GO. For UFG–AHPDMS, an obvious weight loss in the range of 350–450 °C was observed due to the degradation of AHPDMS with high-energy Si–O–Si bonds, indicating the improvement of thermal stability of the UFG–AHPDMS hybrid. For analysis of the structure of MC PA6 nanocomposites, the MC PA6/UFG–AHPDMS sample was dissolved in formic acid, centrifuged and washed repeatedly with formic acid and toluene to remove free PA6 and AHPDMS molecules, and the resultant grafted sample was denoted as g-UFG–AHPDMS. For comparison, the grafted sample prepared from MC PA6/UFG was denoted as g-UFG. Fig. 3(F) shows the TGA curves of the MC PA6, g-UFG, and g-UFG–AHPDMS samples. It was found that the weight loss of MC PA6 occurred in the range of 350–450 °C, which was attributed to the degradation of the PA6 chain skeleton. In contrast, for g-UFG and g-UFG–AHPDMS, an obvious weight loss in the range of 350–450 °C was observed and the weight loss of g-UFG–AHPDMS was higher than that of g-UFG, which was attributed to the decomposition of the PA6 and AHPDMS components.
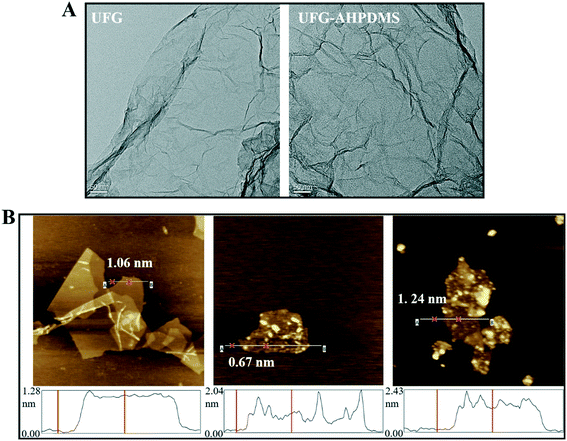
Fig. 4A illustrates the TEM images of the UFG and UFG–AHPDMS samples. It was evident that UFG exhibited a typical exfoliated layer structure, and the sheet edges scrolled and folded up slightly as part of the intrinsic nature of graphene due to the strong van der Waals forces between the layers through the reduction reaction. After grafting with AHPDMS, the layers became rough and the layered structure of the hybrid wrinkled more seriously than that of UFG, which was attributed to the covalent interactions between UFG and AHPDMS. The AFM images and 3D view images of the GO, UFG and UFG–AHPDMS samples are shown in Fig. 4B. An individual GO layer has an average thickness of 1.06 nm due to the epoxy, carboxyl and hydroxyl groups on both sides. For UFG, the thickness decreased to 0.67 nm, much larger than that of an individual graphene nanosheet with a monoatomic layer (∼0.34 nm),38 which was attributed to the grafting of urea on the GO layer accompanied by a reduction effect. However, for UFG–AHPDMS, the average thickness further increased to 1.24 nm, further confirming the successful grafting reaction of AHPDMS chains on the UFG surface.
3.2 Synergistic enhancement of the mechanical and tribological properties of MC PA6/UFG–AHPDMS nanocomposites
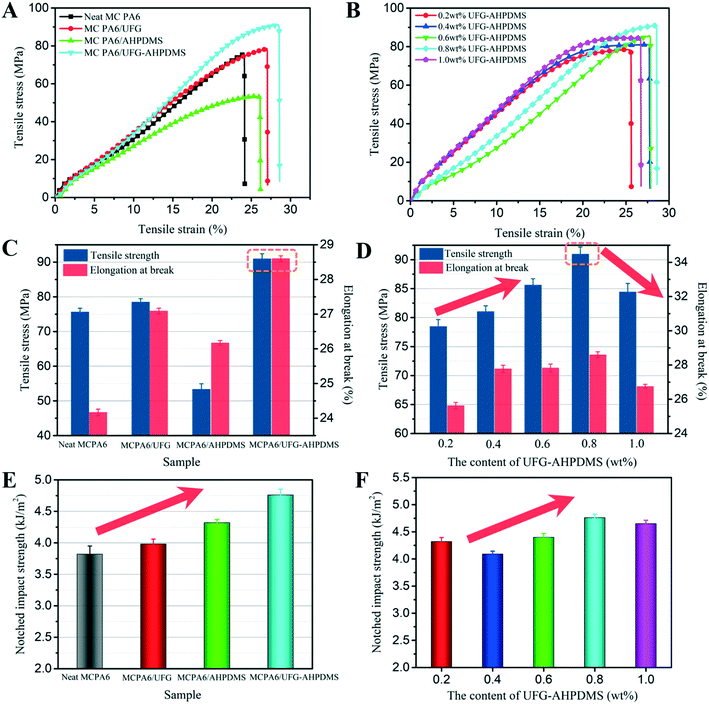
The effect of UFG–AHPDMS on the mechanical and tribological properties of MC PA6 was studied. The stress–strain curves of neat MC PA6 and the MC PA6/UFG, MC PA6/AHPDMS and MC PA6/UFG–AHPDMS nanocomposites are shown in Fig. 5A and B, and all samples exhibited an untypical stress yield behavior. Fig. 5C–F show the tensile strength, elongation at break and notched impact strength of neat MC PA6 and the composites. Neat MC PA6 showed a typical value of tensile strength of about 75.61 MPa. In comparison, for the MC PA6/UFG nanocomposite, the tensile strength and elongation at break exhibited a slight increase due to the reinforcing effect of UFG, but the impact strength had no remarkable improvement. For MC PA6/AHPDMS, the tensile strength obviously decreased while the impact strength increased, which was caused by phase separation due to the incompatibility of AHPDMS with the PA6 matrix. However, for MC PA6/UFG–AHPDMS, the tensile strength, elongation at break and impact strength all increased in comparison with those of neat MC PA6. With the increase of UFG–AHPDMS content, the tensile strength, elongation at break and impact strength of the nanocomposites first increased, reaching the maximum at 0.8 wt% UFG–AHPDMS content, and then decreased. A rough 20% increase in tensile strength from 75.61 MPa to 90.96 MPa was observed, while the impact strength increased by 24% relative to that of neat MC PA6, indicating the synergistic reinforcing and toughening effect of UFG–AHPDMS on MC PA6. Moreover, the UFG–AHPDMS hybrid with TDI molecules as a macro-initiator may participate in the polymerization reaction of MC PA6, which can promote the distribution of UFG–AHPDMS hybrids and increase the entanglement of PA6 molecular chains, leading to the formation of a UFG–AHPDMS–PA6 interlocking structure.39 However, the tensile strength, elongation at break and impact strength began to decline due to the inevitable stress concentration caused by excess UFG–AHPDMS nano-hybrids in the matrix.The tribological performance of neat MC PA6 and the MC PA6/UFG, MC PA6/AHPDMS and MC PA6/UFG–AHPDMS nanocomposites with different contents of UFG–AHPDMS was investigated, as shown in Fig. 6A and B. The friction coefficient of all the samples increased rapidly at the beginning, then gradually declined and became stable during the process of wearing. Relative to that of neat MC PA6, by incorporation of UFG, no apparent decrease of the friction coefficient was observed, which instead even slightly increased. However, the friction coefficient decreased drastically by addition of AHPDMS with a lubrication effect. For MC PA6/UFG–AHPDMS, the friction coefficient–sliding time curve was below that of the MC PA6/AHPDMS sample, and the running-in period was obviously shorter than that of neat MC PA6. With increasing UFG–AHPDMS content, the friction coefficient of the nanocomposites showed an obviously downward trend.
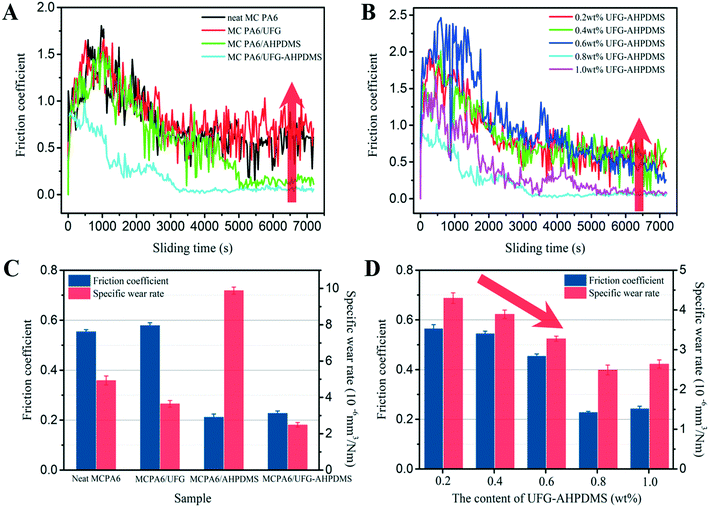 | ||
| Fig. 6 (A and B) Curves of friction coefficient-sliding time and (C and D) tribological properties of neat MC PA6, MC PA6/UFG, MC PA6/AHPDMS and MC PA6/UFG-AHPDMS with different UFG-AHPDMS contents. | ||
Fig. 6C shows the average friction coefficient and specific wear rate of the composites at a velocity of 200 r min−1 under 196 N for 2 h. Relative to neat MC PA6, addition of liquid AHPDMS led to a significant reduction of the friction coefficient from 0.55 to 0.21. Nevertheless, the specific wear rate increased significantly from 4.93 × 10−6 mm3 N−1 m−1 to 9.88 × 10−6 mm3 N−1 m−1, mainly resulting from the deterioration of mechanical properties because of the poor compatibility between AHPDMS and MC PA6.40 However, addition of UFG had a negligible effect on the friction coefficient of MC PA6/UFG, with a slight increase to 0.58 instead, while the specific wear rate obviously decreased to 3.65 × 10−6 mm3 N−1 m−1, which was attributed to the outstanding wear resistance of UFG layers. It is interesting that, for the MC PA6/UFG–AHPDMS nanocomposite, a significant improvement in the tribological properties was achieved, and the friction coefficient (0.23) and specific wear rate (2.49 × 10−6 mm3 N−1 m−1) decreased by more than 58% and 49%, respectively, in comparison with those of neat MC PA6, which indicated the excellent synergistic friction reduction and lubrication effect of UFG–AHPDMS on MC PA6. Fig. 6D shows the effect of the UFG–AHPDMS content on the tribological behavior of the MC PA6/UFG–AHPDMS nanocomposites. It is demonstrated that the friction coefficient and specific wear rate first decreased, reaching the minimum at 0.8 wt% UFG–AHPDMS content, and then further increased with increasing UFG–AHPDMS content, which was well consistent with the variation of the mechanical properties. The UFG–AHPDMS hybrid with both wear resistance and lubrication effects can enhance the load bearing capacity, thus protecting the matrix, while the AHPDMS molecules on the UFG surface can easily form a lubricating film and thus contribute to the reduction of the friction coefficient of the composites.
3.3 Mechanism of synergistic enhancement of the mechanical/tribological properties of the MC PA6/UFG–AHPDMS nanocomposites
To better explain the mechanical and tribological properties of the MC PA6/UFG–AHPDMS nanocomposites, the dispersion of the UFG–AHPDMS hybrid in MC PA6 matrix was observed by SEM and TEM, as shown in Fig. 7(A–C). For MC PA6/UFG, UFG layers were embedded in the matrix with certain agglomerates due to the strong van der Waals force between graphene nanosheets (Fig. 7A). For MC PA6/AHPDMS, it can be clearly seen that many segregated spherical AHPDMS particles were non-uniformly distributed in the matrix caused by the phase separation and weak interfacial adhesion during the polymerization process (Fig. 7B), which led to the defects in the composite and decline of the comprehensive performance. In comparison, for the MC PA6/UFG–AHPDMS nanocomposite, UFG covalently bonded with AHPDMS was observed, which was almost mono-dispersed in the PA6 matrix. Meanwhile, no phase separation occurred and the size of spherical AHPDMS particles was reduced (Fig. 7C), revealing the strong interfacial bonding and fine compatibility between the UFG–AHPDMS hybrid and PA6 matrix.41–43 The tensile fractured surface morphologies of the MC PA6/UFG, MC PA6/AHPDMS and MC PA6/UFG–AHPDMS nanocomposites are shown in Fig. 7(D–F). The tensile fractured surface of MC PA6/UFG was relatively flat, showing the typical characteristics of brittle fracture. For MC PA6/APDMS, the tensile fractured surface showed aggregated spherical AHPDMS particles with a phase separation structure. However, for the MC PA6/UFG–AHPDMS nanocomposite, the tensile fractured surfaces were relatively rough with large deformations, presenting obvious ductile fracture characteristics, indicating strong interfacial interaction in the nanocomposite system.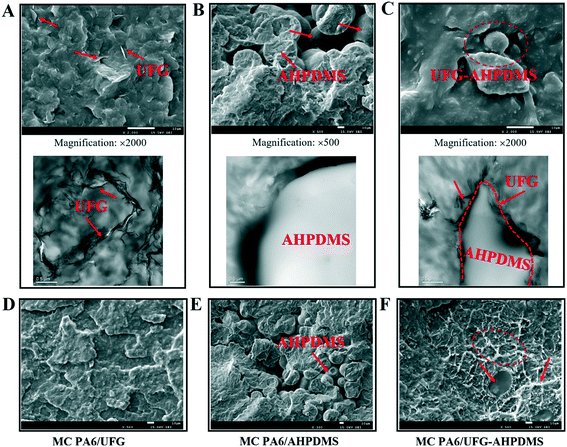 | ||
| Fig. 7 SEM and TEM images of the fractured surfaces of MC PA6/UFG (A and D), MC PA6/AHPDMS (B and E) and MC PA6/UFG–AHPDMS (C and F). | ||
It is essential to investigate the worn surface morphologies of the composites to explore the wear mechanism, as shown in Fig. 8. Neat MC PA6 showed rough wear cracks and obvious furrows and swarf, and a large amount wear debris was observed along the sliding direction (Fig. 8A). For the MC PA6/UFG sample, the worn surface displayed relatively smooth and flat features, but some scratches appeared on the surfaces, which were caused by the protection effect of UFG layers on the PA6 matrix (Fig. 8B), meaning an improvement of the anti-wear properties. For the MC PA6/AHPDMS sample, severer damage with many furrows and coarse wrinkles was observed on the worn surface, and the matrix was peeled off on the worn surface (Fig. 8C), indicating the sharp decline in abrasion resistance by incorporation of AHPDMS, which correlated well with the decrease of mechanical strength. In comparison, for MC PA6/UFG–AHPDMS, there were slight wear scratches and wear grooves on the worn surface without obvious rugged defects (Fig. 8D), indicating that the anti-friction properties were significantly improved due to the synergistic friction reduction and wear resistance effect of UFG–AHPDMS on the MC PA6 matrix.
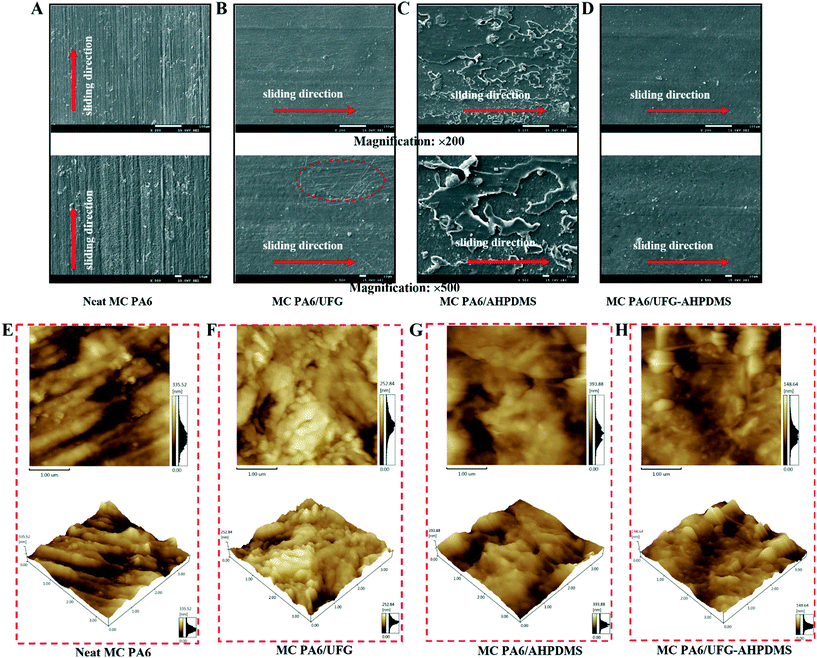 | ||
| Fig. 8 SEM and AFM images with depth distribution of the worn surfaces of neat MC PA6 (A and E), MC PA6/UFG (B and F), MC PA6/AHPDMS (C and G) and MC PA6/UFG–AHPDMS (D and H). | ||
In order to detect the roughness of the worn surfaces of the composites, the AFM images were further studied. For neat MC PA6, the worn surface was relatively rough and a depth distribution ranging from 64 nm to 232 nm was observed (Fig. 8E). In contrast, the worn surface of MC PA6/UFG exhibited relatively smooth features with a depth distribution ranging from 87 nm to 198 nm (Fig. 8F). For MC PA6/AHPDMS, the worn surface was very coarse and a serious matrix spalling phenomenon was observed, and the depth distribution ranged from 89 nm to 266 nm, as shown in Fig. 8G. However, for MC PA6/UFG–AHPDMS, the worn surface became smooth and flat, and a more uniform and narrower depth distribution ranging from 34 nm to 101 nm was obtained, as presented in Fig. 8H. The changes of AFM morphologies of the composites were in accordance with the SEM morphologies. Anti-wear UFG layers with low shear force and liquid AHPDMS with excellent self-lubrication contributed to the relatively flat worn surface of the MC PA6/UFG–AHPDMS nanocomposite. The above results demonstrated that the UFG–AHPDMS hybrid could resist the deformation of the PA6 matrix with friction through the synergistic tribological effect.
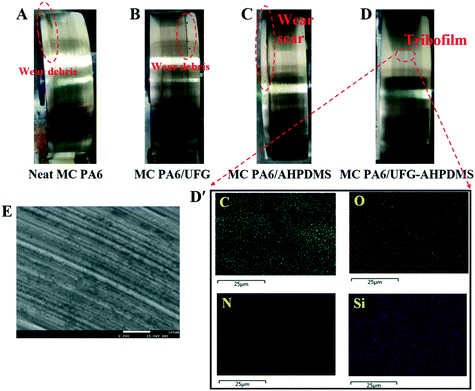
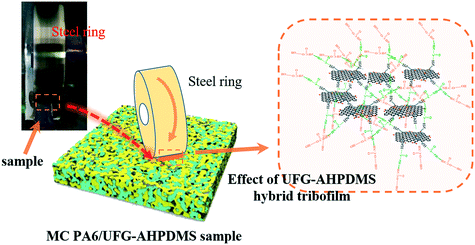
With friction, the polymer matrix was torn down and the deformed wear debris stuck together due to friction heat, and a tribochemical reaction occurred. The tribofilm (transfer film) was formed on the counterpart ring during the rubbing process, which played an important role in tribological performance under dry conditions.44Fig. 9 shows the photographs of the friction counterpart rings for neat MC PA6, MC PA6/UFG, MC PA6/AHPDMS and MC PA6/UFG–AHPDMS, respectively. It was visible that no obvious protective tribofilm was formed and only some rough wear debris scattered on the friction pair after wearing of neat MC PA6 due to the friction heat (Fig. 9A). For MC PA6/UFG, the tribofilm was thicker and more compact than that of pure MC PA6 with some wear debris, which contributed to the improvement of anti-wear properties due to the load-bearing capacity of UFG layers and suppression effect on generation of large wear debris (Fig. 9B). For MC PA6/AHPDMS, a deep and broad wear scar was observed on the steel ring, while the immiscible AHPDMS easily migrates on the friction surface to exhibit a lubricating function, which was beneficial for reduction of the friction coefficient (Fig. 9C). A much thicker, uniform and continuous transfer film formed on the friction pair was observed for the MC PA6/UFG–AHPDMS nanocomposite (Fig. 9D), serving as a protection barrier between the matrix and steel ring during the sliding process, which was believed to be responsible for the improved tribological properties. EDS elemental mapping as convincing evidence was further used to confirm the existence of elements in the tribofilm from regions of the counterpart ring surface. After the friction test of MC PA6/UFG–AHPDMS, the elements C, O, N and Si were found in the tribofilm on the steel ring, which was derived from the formation of a UFG–AHPDMS hybrid lubrication film between the matrix and counterpart ring during the friction process (Fig. 9D′). The hybrid tribofilm was beneficial for the remarkable reduction of the friction coefficient and wear rate.45Fig. 9(E) shows a microscopy image of the worn area of the counterpart metallic ring. It was found that a symmetrical transfer film was formed on the counterpart ring under dry sliding conditions, indicating that there was a mixture of MC PA6 and UFG–AHPDMS transferred on the surface of the ring with light color, which was beneficial for reduction of the friction coefficient.
The MC PA6/UFG–AHPDMS nanocomposites exhibited an attractive enhancement of mechanical/tribological properties in comparison with neat MC PA6. Based on the above analysis, the mechanism of mechanical/tribological performance of the MC PA6/UFG-AHPDMS nanocomposites can be explained as follows: the UFG–AHPDMS hybrid with TDI molecules as a macro-initiator may participate in the polymerization reaction of MC PA6, which can promote the uniform distribution of the UFG–AHPDMS hybrid in the matrix and the formation of a UFG–AHPDMS–PA6 interlocking structure with strong interfacial bonding. UFG covalently bonded with AHPDMS can utilize the synergistic tribological properties with excellent wear resistance of UFG layers and friction reduction effect of AHPDMS. A tribofilm was formed with high load-bearing/anti-wear capacity and self-lubrication by tribochemical reaction during the friction process, inhibiting the direct contact between the matrix and counterpart ring, and was responsible for the reduction of the friction coefficient and improvement of the wear resistance. A schematic model depicted the mechanism of synergistic enhancement of the tribological performance of the MC PA6/UFG–AHPDMS nanocomposites, as shown in Fig. 10.
4. Conclusions
In this work, a UFG–AHPDMS hybrid was prepared by chemical grafting and reduction reaction of GO and urea and then through reaction with AHPDMS using TDI as a covalent bridge in CL melt, and the anchoring effect of AHPDMS molecules onto UFG layers was achieved. MC PA6/UFG–AHPDMS nanocomposites were further in situ synthesized by anionic ring-opening polymerization. The UFG–AHPDMS hybrid with TDI molecules exhibited much better dispersibility than AHPDMS in CL melt, which participated in the polymerization of MC PA6 and promoted uniform distribution in matrix, revealing the formation of strong interfacial bonding interactions. In comparison with neat MC PA6, incorporation of UFG–AHPDMS resulted in a 20% increase in tensile strength from 75.61 MPa to 90.96 MPa and a 24% increase in impact strength, higher than those of MC PA6/UFG and MC PA6/AHPDMS. Moreover, the running-in period of the friction coefficient–sliding time curve was obviously shorter, and the friction coefficient and specific wear rate decreased by more than 58% and 49% in comparison with those of neat MC PA6, revealing the excellent synergistic reinforcing and friction reduction effect of UFG–AHPDMS. With increasing UFG–AHPDMS content, the friction coefficient and specific wear rate first decreased, reaching the minimum at 0.8 wt% UFG–AHPDMS content, and then further increased, which was well consistent with the variation of the mechanical properties. The worn surface of MC PA6/UFG–AHPDMS was relatively smooth without obvious rugged defects and a uniform depth distribution ranging from 34 nm to 101 nm was achieved. Meanwhile, a uniform and continuous UFG–AHPDMS hybrid tribofilm formed as a protection barrier between the matrix and steel ring, which was beneficial for the remarkable reduction of the friction coefficient and wear rate due to the synergistic tribological effect of anti-wear UFG layers and self-lubricating AHPDMS during the friction process. This work may open up new thinking for the development of high-performance polymer nanocomposites for a wide range of applications in the tribology field.Author contributions
Chengjie Li: conceptualization, methodology, data curation, writing – original draft preparation, writing – review and editing, and supervision. Minghui Guo: data curation, validation, and writing – review and editing. Ying Dai: data curation, formal analysis, and resources. Peikuan Xu: data curation and formal analysis. Bin Shi: project administration and investigation. Dewang Hou: project administration and investigation. Ruiguang Li: data curation, investigation, and supervision.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The authors gratefully acknowledge the Open-end Funds of Jiangsu Key Laboratory of Marine Bioresources and Environment (SH20201203) for financial support.References
- W. Wang, L. Meng and Y. Huang, Hydrolytic degradation of monomer casting nylon in subcritical water, Polym. Degrad. Stab., 2014, 110, 312–317 CrossRef CAS.
- B. Pan, S. Zhang, W. Li, J. Zhao, J. Liu, Y. Zhang and Y. Zhang, Tribological and mechanical investigation of MC nylon reinforced by modified graphene oxide, Wear, 2012, 294, 395–401 CrossRef.
- Y. Zhuang, X. Cao, J. Zhang, Y. Ma, X. Shang, J. Lu, S. Yang, K. Zheng and Y. Ma, Monomer casting nylon/graphene nanocomposite with both improved thermal conductivity and mechanical performance, Composites, Part A, 2019, 120, 49–55 CrossRef CAS.
- C. Li, M. Xiang and L. Ye, Intercalation structure and highly enhancing tribological performance of monomer casting nylon-6/graphene nano-composites, Composites, Part A, 2017, 95, 274–285 CrossRef CAS.
- C. Li, M. Xiang, X. Zhao and L. Ye, In situ synthesis of monomer casting nylon-6/graphene-polysiloxane nanocomposites: intercalation structure, synergistic reinforcing, and friction-reducing effect, ACS Appl. Mater. Interfaces, 2017, 9, 33176–33190 CrossRef CAS PubMed.
- L. Zheng, L. Zhao and J. Zhang, The effect of fiber oxidation on the tribological behavior of 3D-braided carbon fiber/nylon composites, Wear, 2007, 262, 1026–1030 CrossRef CAS.
- B. Pan, N. Li, G. Chu, F. Wei, J. Liu, J. Zhang and Y. Zhang, Tribological investigation of MC PA6 reinforced by boron nitride of single layer, Tribol. Lett., 2014, 54, 161–170 CrossRef CAS.
- J. Wang, M. Gu, B. Songhao and S. Ge, Investigation of the influence of MoS2 filler on the tribological properties of carbon fiber reinforced nylon 1010 composites, Wear, 2003, 255, 774–779 CrossRef CAS.
- H. Ruan, Q. Zhang, W. Liao, Y. Li, X. Huang, X. Xu and S. Lu, Enhancing tribological, mechanical, and thermal properties of polyimide composites by the synergistic effect between graphene and ionic liquid, Mater. Des., 2020, 189, 108527–108536 CrossRef CAS.
- B. Pan, S. Peng, S. Song, J. Chen, J. Liu, H. Liu, Y. Zhang and Q. Niu, The adaptive tribological investigation of polycaprolactam/graphene nanocomposites, Tribol. Lett., 2017, 65, 1–14 CrossRef CAS.
- L. Song, H. Yang, D. Li, Q. Jiang and D. Zeng, Polydimethylsiloxane/monomer casting nylon copolymers: Preparation, flame-retardant properties, and wear-resistant properties, J. Appl. Polym. Sci., 2020, 137, 48753–48760 CrossRef CAS.
- X. Huang, X. Qi, F. Boey and H. Zhang, Graphene-based composites, Chem. Soc. Rev., 2012, 41, 666–686 RSC.
- M. Arab and S. P. H. Marashi, Graphene nanoplatelet (GNP)-incorporated AZ31 magnesium nanocomposite: microstructural, mechanical and tribological properties, Tribol. Lett., 2018, 66, 1–11 CrossRef CAS.
- Q. Feng, F. Wang and Y. Wang, Effect of modified Mg2B2O5w on mechanical and friction properties of oil-containing monomer casting nylon, J. Appl. Polym. Sci., 2020, 137, 48856–48866 CrossRef CAS.
- Z. Tai, Y. Chen, Y. An, X. Yan and Q. Xue, Tribological behavior of UHMWPE reinforced with graphene oxide nanosheets, Tribol. Lett., 2012, 46, 55–63 CrossRef CAS.
- S. Huang, B. Pan, M. Xie, J. Cao, G. Zhao, Y. Niu and H. Wang, Synergistic effects of graphene oxide and paraffin wax on the tribological properties of monomer casting nylon-6 composites, Tribol. Int., 2021, 154, 106726–106734 CrossRef CAS.
- H. Ruan, Q. Zhang, W. Liao, Y. Li, X. Huang, X. Xu and S. Lu, Enhancing tribological, mechanical, and thermal properties of polyimide composites by the synergistic effect between graphene and ionic liquid, Mater. Des., 2020, 189, 108527–108536 CrossRef CAS.
- Y. Zhao, Q. Meng, Q. Zhang, F. Wang, Q. Wang and Y. Wang, Oil-impregnated monomer casting nylon composites reinforced by graphene oxide and Lanthanum (III) chloride, Polym. Eng. Sci., 2019, 59, 982–988 CrossRef CAS.
- C. Chen, Z. Zhang, X. Zhao and L. Ye, Polyoxymethylene/graphene oxide-perfluoropolyether nano-composite with ultra-low friction coefficient fabricated by formation of superior interfacial tribofilm, Composites, Part A, 2020, 132, 105856–105868 CrossRef CAS.
- C. Xue, Z. Zhang, J. Zhang and S. Jia, Lasting and self-healing superhydrophobic surfaces by coating of polystyrene/SiO2 nanoparticles and polydimethylsiloxane, J. Mater. Chem. A, 2014, 2, 15001–15007 RSC.
- Y. Zheng, J. Cheng, C. Zhou, H. Xing, X. Wen, P. Pi and S. Xu, Droplet motion on a shape gradient surface, Langmuir, 2017, 33, 4172–4177 CrossRef CAS PubMed.
- A. Dundua, S. Franzka and M. Ulbricht, Improved antifouling properties of polydimethylsiloxane films via formation of polysiloxane/polyzwitterion interpenetrating networks, Macromol. Rapid Commun., 2016, 37, 2030–2036 CrossRef CAS PubMed.
- H. Khojasteh, M. Salavati-Niasari, H. Safajou and H. Safardoust-Hojaghan, Facile reduction of graphene using urea in solid phase and surface modification by N-doped graphene quantum dots for adsorption of organic dyes, Diamond Relat. Mater., 2017, 79, 133–144 CrossRef CAS.
- X. Xu, Y. Zhou, T. Yuan and Y. Li, Methanol electrocatalytic oxidation on Pt nanoparticles on nitrogen doped graphene prepared by the hydrothermal reaction of graphene oxide with urea, Electrochim. Acta, 2013, 112, 587–595 CrossRef CAS.
- M. Xiang, C. Li and L. Ye, In situ synthesis of monomer casting nylon-6/reduced graphene oxide nanocomposites: Intercalation structure and electrically conductive properties, J. Ind. Eng. Chem., 2017, 50, 123–132 CrossRef CAS.
- C. Li, M. Xiang and L. Ye, Structure and tribological performance of monomer casting nylon-6/colloidal graphite composites synthesized through in situ polymerization, Polym.-Plast. Technol. Eng., 2017, 56, 1345–1357 CrossRef CAS.
- J. Xu, X. Kong, M. Chen, Q. Wang and F. Wang, High-entropy FeNiCoCr alloys with improved mechanical and tribological properties by tailoring composition and controlling oxidation, J. Mater. Sci. Technol., 2021, 82, 207–213 CrossRef.
- J. L. Suter, R. C. Sinclair and P. V. Coveney, Principles governing control of aggregation and dispersion of graphene and graphene oxide in polymer melts, Adv. Mater., 2020, 32, 2003213–2003219 CrossRef CAS PubMed.
- L. Sun, L. Wang, C. Tian, T. Tan, Y. Xie, K. Shi, M. Li and H. Fu, Nitrogen-doped graphene with high nitrogen level via a one-step hydrothermal reaction of graphene oxide with urea for superior capacitive energy storage, RSC Adv., 2012, 2, 4498–4506 RSC.
- A. S. Palsule and Y. Poojari, Enzymatic synthesis of silicone fluorinated aliphatic polyesteramides, Polymer, 2010, 51, 6161–6167 CrossRef CAS.
- F. Sun, S. Yang and Q. Wang, Selective decomposition process and mechanism of Si-O-Si cross-linking bonds in silane cross-linked polyethylene by solid-state shear milling, Ind. Eng. Chem. Res., 2020, 59, 12896–12905 CrossRef CAS.
- Y. Liu, D. He, O. Dubrunfaut, A. Zhang, H. Zhang, L. Pichon and J. Bai, GO-CNTs hybrids reinforced epoxy composites with porous structure as microwave absorbers, Compos. Sci. Technol., 2020, 200, 108450–108457 CrossRef CAS.
- Y. Yao, J. Li, H. Zhang, H. Tang, L. Fang, G. Niu, X. Sun and F. Zhang, Facile synthesis of a covalently connected RGO-COF hybrid material by in situ reaction for enhanced visible-light induced photocatalytic H2 evolution, J. Mater. Chem. A, 2020, 8, 8949–8956 RSC.
- J. Ji, G. Zhang, H. Chen, S. Wang, G. Zhang, F. Zhang and X. Fan, Sulfonated graphene as water-tolerant solid acid catalyst, Chem. Sci., 2011, 2, 484–487 RSC.
- J. M. Smolsky and A. V. Krasnoslobodtsev, Nanoscopic imaging of oxidized graphene monolayer using tip-enhanced Raman scattering, Nano Res., 2018, 11, 6346–6359 CrossRef CAS.
- G. Sun, H. Wu, Q. Liao and Y. Zhang, Enhanced microwave absorption performance of highly dispersed CoNi nanostructures arrayed on graphene, Nano Res., 2018, 11, 2689–2704 CrossRef CAS.
- A. Osman, A. Elhakeem, S. Kaytbay and A. Ahmed, Thermal, electrical and mechanical properties of graphene/nano-alumina/epoxy composites, Mater. Chem. Phys., 2021, 257, 123809–123818 CrossRef CAS.
- L. Chen, Z. Chen, X. Tang, W. Yan, Z. Zhou, L. Qian and S. H. Kim, Friction at single-layer graphene step edges due to chemical and topographic interactions, Carbon, 2019, 154, 67–73 CrossRef CAS.
- C. H. Cho, I. K. Shin, K. Y. Kim and Y. J. Choi, Enhancing adhesion properties between binder-free copper nanoink and flexible substrate using chemically generated interlocking structure, Appl. Surf. Sci., 2019, 485, 484–489 CrossRef CAS.
- P. Samyn, P. De Baets, G. Schoukens and I. Van Driessche, Friction, wear and transfer of pure and internally lubricated cast polyamides at various testing scales, Wear, 2007, 262, 1433–1449 CrossRef CAS.
- Z. Ma, S. Kang, J. Ma, L. Shao, Y. Zhang, C. Liu, A. Wei, X. Xiang, L. Wei and J. Gu, Ultraflexible and mechanically strong double-layered aramid nanofiber-Ti3C2Tx MXene/silver nanowire nanocomposite papers for high-performance electromagnetic interference shielding, ACS Nano, 2020, 14, 8368–8382 CrossRef CAS PubMed.
- Y. Zhang, K. Ruan, X. Shi, H. Qiu, Y. Yan and J. Gu, Ti3C2Tx/rGO porous composite films with superior electromagnetic interference shielding performances, Carbon, 2021, 175, 271–280 CrossRef CAS.
- C. Li, Z. Yuan and L. Ye, Facile construction of Zn2+-carboxyl salt-bonding as sacrificial unit in EPDM rubber toward mechanical and sealing resilience performance enhancement, Macromol. Mater. Eng., 2021, 2100184–2100196 CrossRef CAS.
- P. Zhang, L. Zhang, D. Wei, P. Wu, J. Cao, C. Shijia and X. Qu, Adjusting function of MoS2 on the high-speed emergency braking properties of copper-based brake pad and the analysis of relevant tribo-film of eddy structure, Composites, Part B, 2020, 185, 107779–107791 CrossRef CAS.
- Y. Guo, G. Liu, G. Li, F. Zhao, L. Zhang, F. Guo and G. Zhang, Solvent-free ionic silica nanofluids: smart lubrication materials exhibiting remarkable responsiveness to weak electrical stimuli, Chem. Eng. J., 2020, 383, 123202–123213 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |